Abstract
Background & Aims
Increasing evidence points towards a role of hepatitis C virus (HCV) infection in causing malignant lymphomas. We pooled case-control study data to provide robust estimates of the risk of non-Hodgkin’s lymphoma (NHL) subtypes after HCV infection.
Methods
The analysis included 7 member studies from the International Lymphoma Epidemiology Consortium (InterLymph) based in Europe, North America, and Australia. Adult cases of NHL (n = 4784) were diagnosed between 1988 and 2004 and controls (n = 6269) were matched by age, sex, and study center. All studies used third-generation enzyme-linked immunosorbent assays to test for antibodies against HCV in serum samples. Participants who were human immunodeficiency virus positive or were organ-transplant recipients were excluded.
Results
HCV infection was detected in 172 NHL cases (3.60%) and in 169 (2.70%) controls (odds ratio [OR], 1.78; 95% confidence interval [CI], 1.40–2.25). In subtype-specific analyses, HCV prevalence was associated with marginal zone lymphoma (OR, 2.47; 95% CI, 1.44–4.23), diffuse large B-cell lymphoma (OR, 2.24; 95% CI, 1.68–2.99), and lymphoplasmacytic lymphoma (OR, 2.57; 95% CI, 1.14–5.79). Notably, risk estimates were not increased for follicular lymphoma (OR, 1.02; 95% CI, 0.65–1.60).
Conclusions
These results confirm the association between HCV infection and NHL and specific B-NHL subtypes (diffuse large B-cell lymphoma, marginal zone lymphoma, and lymphoplasmacytic lymphoma).
Hepatitis C virus (HCV) infection has been reported to be a prevalent disease since the second half of the 20th century. The infection spread to the general population in some countries such as Japan, Italy, and Egypt, with prevalence estimates ranging from 5% to 10%. In other developed countries the infection largely has been limited to individuals who have received blood transfusions or are intravenous drug users with population prevalence estimates ranging from 1% to 2%.1, 2 and 3
A causal role of HCV infection in cirrhosis and hepatocellular carcinoma is well established. Also, HCV has been linked to lymphomagenesis in people with and without type II mixed cryoglobulinemia.4 However, in the majority of lymphoma studies, small sample sizes have prevented an analysis of the relationship between HCV and single lymphoma subtypes.
Increasing evidence indicates that the association between HCV infection and lymphoma may be owing to viral infection–related chronic antigenic stimulation similar to that reported for Helicobacter pylori and gastric mucosa-associated lymphoid tissue lymphoma.5 The chronic inflammation pathway would be consistent with the association between HCV and several types of lymphomas and with the regression of some lymphomas after eradicating the HCV infection.6 and 7
We present results from a large international pooled analysis of the association between non-Hodgkin lymphoma (NHL) and HCV in which HCV infection was determined using a third-generation enzyme-linked immunosorbent assay test to measure HCV antibodies. Our study includes data from 4784 NHL cases and 6269 controls from case-control studies participating in the International Lymphoma Epidemiology Consortium (InterLymph).
MATERIALS AND METHODS
Study Population
InterLymph was established in 2000 as a voluntary consortium to facilitate collaboration among epidemiologic studies of lymphoma (http://epi.grants.cancer.gov/InterLymph).8 and 9 Through the InterLymph Consortium, 7 case-control studies (3 were multicentric, for a total of 17 participating centers) conducted between 1988 and 2004 were identified as eligible for a pooled analysis. Studies were required to have used the third-generation enzyme-linked immunosorbent assay test for HCV. Detailed information on the association between HCV and NHL risk already has been published for 510, 11, 12, 13 and 14 of the 7 studies.
We hereafter refer to each contributing study as they have been published: Connecticut, North–South Italy, National Cancer Institute (NCI)-surveillance epidemiology end result (SEER), New South Wales (NSW), University of California San Francisco (UCSF), EpiLymph (includes 6 countries in Europe), and British Columbia (Table 1). Selected characteristics of each study, including acronym, study site, age range, selection criteria, and participation rates, are presented in Table 1. Of the 17 study centers, 11 used population-based controls and 6 used hospital-based controls. Cases and controls who were human immunodeficiency virus–positive or organ-transplant recipients were excluded from this analysis. With the exception of the North–South Italy study, all studies frequency-matched their cases and controls by age, sex, and study site. NCI-SEER also frequency-matched cases and controls by race. Local institutional review boards approved all studies and written informed consent was obtained from each participant.
Table 1.
Characteristics of Case-Control Studies Included in the Pooled Analysis
| Cases (n = 4784) | |||||
|---|---|---|---|---|---|
| Acronym | Country: Study center | Year | Age, y | n | Participation % |
| Connecticut | United States: Connecticut | 1995–2001 | 23–85 | 463 | 72 |
| North-South Italya | Italy: Aviano, Naples | 1999–2002 | 18–84 | 225 | >97 |
| NCI-SEER | United States: Detroit, MI; Iowa; Los Angeles, CA; Seattle, WA | 1998–2001 | 20–74 | 813 | 76 |
| NSW | Australia: New south Wales; Australian Capital Territory | 2000–2002 | 20–74 | 587 | 85 |
| UCSF | United States: San Francisco, CA | 1988–1995 | 21–74 | 554 | 72 |
| EpiLymph | Europe: Spain,a France,a Germany, Italy, Ireland,a and Czech Republica | 1998–2004 | 18–89 | 1346 | 82–93 |
| British Columbia | Canada: Greater Vancouver Regional District, Capital Regional District | 1996–2004 | 20–82 | 796 | 85 |
| Controls (n = 6269) | ||||
|---|---|---|---|---|
| Matching criteria | Source | n | Participation % | Study Reference |
| Age | RDD and CMMS | 534 | RDD, 69; CMMS, 47 | 11 |
| None | Hospital controls | 504 | >91 | 13 |
| Age, sex, and study site | RDD and CMMS | 684 | 52 | 10 |
| Age, sex, and area of residence | Random selection from electoral rolls | 518 | 61 | 14 |
| Age, sex, and country of residence | RDD | 1544 | 78 | |
| Age and sex and hospital study site when appropriate | Hospital-based (Spain, France, Ireland, and Czech Republic); population-based controls (Germany and Italy) | 1788 | 44–96 | 12 |
| Age, sex, and geographic region | Client Registry of the Ministry of Health | 697 | 50 | |
RDD, random digit dialing; CMMS, Centers for Medicare and Medicaid Services (official abbreviation is CMS).
Hospital-based case-control studies; all other studies were population-based (ie, cases were identified from hospitals and registries).
Classification of Non-Hodgkin Lymphoma Subtypes
Four studies, British Columbia, NCI-SEER, NSW, and EpiLymph, used the World Health Organization classification system to define lymphoid neoplasms.15 The studies conducted in North–South Italy and Connecticut used the Revised European American Lymphoma Classification (REAL) classification system to define NHL subtypes.16 The UCSF study used both the REAL and the Working Formulation, and cases were recategorized into the World Health Organization classification.
Classification systems from all studies were combined based on the International Classification of Diseases for Oncology17 and 18 and the World Health Organization classification-based categories developed within the InterLymph Pathology Working Group, with the participation of representative pathologists from each major study.19 Eleven subtypes were defined for subtypespecific analyses based on morphology and/or immunohistochemistry information: small lymphocytic lymphoma and chronic lymphocytic leukemia, mantle-cell lymphoma, diffuse large B-cell lymphoma (DLBCL), Burkitt lymphoma, marginal zone lymphoma (MZL), follicular lymphoma (FL), lymphoplasmacytic lymphoma (LPL), other B-cell lymphoma, Mycosis fungoides and Sézary syndrome, other T-cell lymphoma, as well as NHL not otherwise specified (NHL NOS).
Statistical Analysis
A preliminary evaluation of categoric exposure variables and the overall NHL risk was conducted using contingency tables analysis and the chi-square test of association.
Heterogeneity in risk estimates between study centers was assessed using the likelihood ratio test under a logistic regression model. The model of interaction between countries and exposure was compared with the model measuring the main effects only for outcomes categorized as dichotomous or polytomous.20 When the P value of the chi-squared statistic was less than .1021 the risk estimates were considered to be heterogeneous between study centers.
A 2-stage estimation method was followed for risk of overall NHL; such a model allows the control for confounding by individual studies and the consideration of random effects to measure the unexplained interstudy variability.22 Study-specific risk estimates were calculated using unconditional logistic regression adjusting for sex, age (<35, 35–44, 45–54, 55–64, and ≥65 y), and race (white, black, Asian, and other) because these variables were used for matching in most of the original studies. In addition, any other confounders identified in the preliminary analysis were included in the adjusted models. The study-specific risk estimates obtained after adjustment were weighted by the inverse of the sum of the variance of the individual study estimates to produce a second-stage pooled odds ratio (OR).
For the subtype-specific analysis a joint fixed-effects model was used to allow the inclusion of the studies that found zero HCV prevalence (ie, no cases of HCV infection) for a particular NHL subtype. The risk estimates for specific lymphoma histology subtypes were calculated using polytomous unconditional logistic regression models adjusted for the same variables used in the overall NHL models. The results of lymphoma subtypes that were found to be associated with HCV infection or those with 10 or more HCV-infected cases are presented graphically, plotting the study summary OR as a black square, whose size is inversely proportional to the variance of the estimate. A horizontal line represents the 95% confidence interval (CI). Diamonds are used to plot the summary OR (estimated using the models described previously) for subtypes. The center of the diamond represents the pooled OR and the extremes show the limits of the 95% CI. A log scale is used for the OR to preserve the symmetry of the CI.
Sensitivity analyses were performed to compare pooled risk estimates after systematically excluding each study to determine whether any single study unduly influenced the pooled estimates. Statistical analyses of the data were conducted using STATA version 9.2 (StataCorp, College Station, TX).
RESULTS
The pooled study included 11,053 participants, 4784 cases and 6269 controls from 7 case-control studies conducted in the United States, Europe, and Australia with information on HCV infection (Table 1). Table 2 describes the prevalence of HCV for cases and controls by sex, age, race, and history of blood transfusion. The pooled study population was predominantly Caucasian (91.79%) and there were differences in the distribution by sex, with a higher proportion of men compared with women (52.26% vs 47.74%). Among controls, HCV was more prevalent among men than among women (3.18% vs 2.15%; P = .012), and the prevalence was lower in Asians (0.0%), blacks (1.39%), and whites (2.61%) compared with other ethnic groups (5.01%). Among both cases and controls, HCV was related to transfusion history (ever transfusion vs never: P < .0001 and P = .003, respectively). The overall prevalence of HCV was stable across age groups in all the studies with the exception of the North–South Italy study that showed an increasing HCV prevalence with increasing age (data not shown).
Table 2.
HCV Prevalence by Status and Study Population Characteristics
| HCV prevalence | ||||
|---|---|---|---|---|
| Overall n (%) |
Controls/cases | Controls n (%) |
Cases n (%) |
|
| Included subjects | 11,053 | 6269/4784 | 169(2.70) | 172(3.60) |
| Sex | ||||
| Male | 5776 (52.26) | 3333/2443 | 106 (3.18) | 97 (3.97) |
| Female | 5277 (47.74) | 2936/2341 | 63 (2.15) | 75 (3.2) |
| P valuea | .01 | .15 | ||
| Age, y | ||||
| <35 | 860 (7.78) | 594/266 | 11 (1.85) | 7 (2.63) |
| 35–44 | 1280 (11.58) | 792/488 | 23 (2.90) | 23 (4.71) |
| 45–54 | 1959 (17.72) | 1073/886 | 22 (2.05) | 39 (4.40) |
| 55–64 | 2677 (24.22) | 1415/1262 | 43 (3.04) | 47 (3.72) |
| ≥65 | 4277 (38.70) | 2395/1882 | 70 (2.92) | 56 (2.98) |
| P valuea | .34 | .18 | ||
| Race | ||||
| White | 10,146 (91.79) | 5795/4351 | 151 (2.61) | 157 (3.61) |
| Asian | 126 (1.14) | 63/90 | 0 (0.0) | 0 (0.0) |
| Black | 153 (1.38) | 72/54 | 1 (1.39) | 3 (5.56) |
| Others | 628 (5.68) | 339/289 | 17 (5.01) | 12 (4.15) |
| P valuea | .03 | .24 | ||
| History of blood transfusion | ||||
| No | 9001 (82.10) | 5125/3876 | 123 (2.40) | 117 (3.02) |
| Yes | 1899 (17.32) | 1067/832 | 43 (4.03) | 51 (6.13) |
| P valuea | .003 | <0.0001 | ||
Chi-squared association P value.
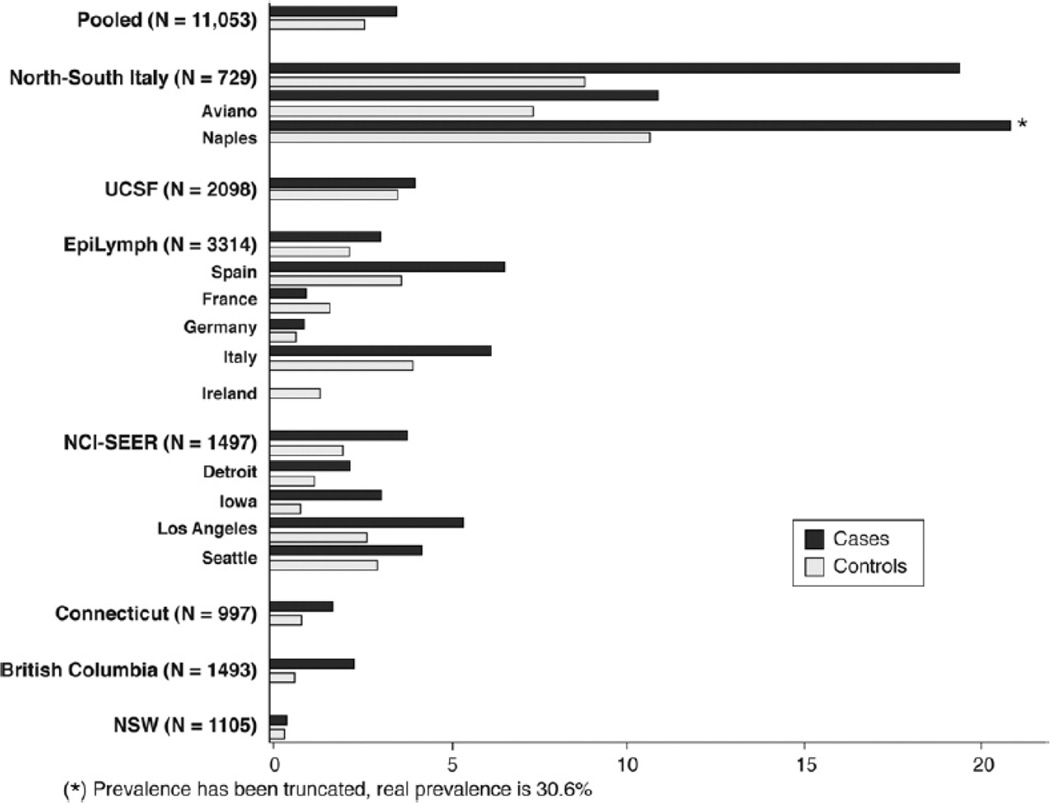
The pooled HCV prevalence was estimated to be 2.70% (range, 0.39%–10.0%) among controls and 3.59% (range, 0%–30.6%) among cases (Figure 1). The study-specific association between HCV and risk of NHL is presented in Table 3. In the pooled dataset, NHL risk was increased in association with HCV positivity (OR, 1.78; 95% CI, 1.40–2.25). The study-specific associations between HCV and risk of NHL are presented in Table 3; no heterogeneity between the studies was identified (χ2 = 7.44; df = 6; P = .28). History of transfusion was not related independently to lymphoma risk.
Figure 1.
Prevalence of antibodies against HCV among NHL cases and their respective controls by study.
Table 3.
Risk Estimates (OR, 95% CI) for NHL Associated With HCV by Study
| Prevalence | |||||||
|---|---|---|---|---|---|---|---|
| Control | Case | Control | Case | ||||
| n | n | n | % | n | % | OR (95% CI)a | |
| Connecticut | 534 | 463 | 5 | 0.94 | 8 | 1.73 | 2.80 (0.65–6.64) |
| Italy | 504 | 225 | 45 | 8.93 | 44 | 19.56 | 2.67 (1.66–4.29) |
| NCI | 684 | 813 | 14 | 2.05 | 32 | 3.94 | 1.89 (0.99–3.61) |
| NSW | 518 | 587 | 2 | 0.39 | 3 | 0.51 | 1.15 (0.19–7.00) |
| UCSF | 1544 | 554 | 57 | 3.69 | 23 | 4.15 | 1.24 (0.75–2.05) |
| EpiLymph | 1788 | 1346 | 41 | 2.29 | 43 | 3.19 | 1.44 (0.93–2.24) |
| British Columbia | 697 | 796 | 5 | 0.72 | 19 | 2.39 | 3.28 (1.20–8.98) |
| Pooled data | 6269 | 4784 | 169 | 2.7 | 172 | 3.59 | 1.78 (1.40–2.25)b |
NOTE. Test of heterogeneity between studies; P = .28, χ2 = 7.44; df = 6.
OR and 95% CI were estimated using unconditional logistic regression models adjusted for age, sex, race, and study center.
Two-stage logistic regression model.
There was a wide range of HCV prevalence between the NHL subtypes, with the highest value for Burkitt lymphoma (7.50%) and the lowest value for mantle-cell lymphoma (0.84%) (Table 4). The effect of HCV positivity on risk of NHL varied within the B-cell NHL subtype with the lowest risk noted for mantle-cell lymphoma and the highest for MZL. In particular the risk estimate for DLBCL (OR, 2.24; 95% CI, 1.68–2.99) was statistically different from the one for follicular lymphoma (OR, 1.02; 95% CI, 0.65–1.60). HCV positivity was associated significantly with a statistically significant increase in risk of MZL (OR, 2.47; 95% CI, 1.44–4.23), LPL (OR, 2.57; 95% CI, 1.14–5.79), DLBCL (OR, 2.24; 95% CI, 1.68–2.99), and “other B-cell lymphoma” (OR, 2.36; 95% CI, 1.11–5.01). The “other B-cell lymphoma” category is a mixture of different subentities; the increased risk was restricted to the B NHL NOS group. The risk of Burkitt lymphoma was increased nonsignificantly 2-fold. HCV positivity did not appear to increase the risk of T-cell lymphomas.
Table 4.
Risk (OR and 95% Cls) of NHL Subtypes by HCV Infection
| Total | Prevalence | |||||
|---|---|---|---|---|---|---|
| n | % | n | % | OR (95% CI)a | P valueb | |
| Lymphoma subtype | ||||||
| Burkitt | 40 | 0.84 | 3 | 7.50 | 2.42 (0.71–8.27) | .75 |
| Diffuse large B-cell lymphoma | 1494 | 31.24 | 76 | 5.09 | 2.24 (1.68–2.99) | .07 |
| LPL | 144 | 3.01 | 7 | 4.86 | 2.57 (1.14–5.79) | .83 |
| MZL | 383 | 8.00 | 17 | 4.44 | 2.47 (1.44–4.23) | .03 |
| Chronic lymphocytic leukemia/small lymphocytic lymphoma | 608 | 12.71 | 22 | 3.62 | 1.48 (0.92–2.38) | .31 |
| Other B-cell lymphomac | 244 | 5.10 | 8 | 3.28 | 2.36 (1.11–5.01) | .14 |
| Other T-cell lymphoma | 206 | 4.31 | 6 | 2.91 | 1.41 (0.60–3.29) | .71 |
| NHL NOS | 248 | 5.18 | 7 | 2.82 | 1.50 (0.67–3.33) | .90 |
| FL | 1181 | 24.68 | 23 | 1.95 | 1.02 (0.65–1.60) | .10 |
| M fungoides/Sézary syndrome | 117 | 2.45 | 2 | 1.71 | 0.74 (0.18–3.08) | .41 |
| Mantle cell lymphoma | 119 | 2.49 | 1 | 0.84 | 0.60 (0.08–4.41) | NA |
NOTE. Subtypes are ordered by HCV prevalence.
OR and 95% CI were estimated using unconditional logistic regression and a joint fixed-effects model, adjusted for age, sex, race, and study center.
Test of heterogeneity between study.
Other B-cell lymphoma includes small B-lymphocytic NOS (n = 30), mediastinal large B-cell lymphoma (n = 28), large B-cell immunoblastic (n = 1), B-cell NOS (n = 87), Precursor B NHL (n = 27), other B NHL (n = 60), precursor B-lymphoblastic leukemia or lymphoma (n = 2), hairy cell leukemia (n = 8), and lymphoblastic lymphoma (n = 1).
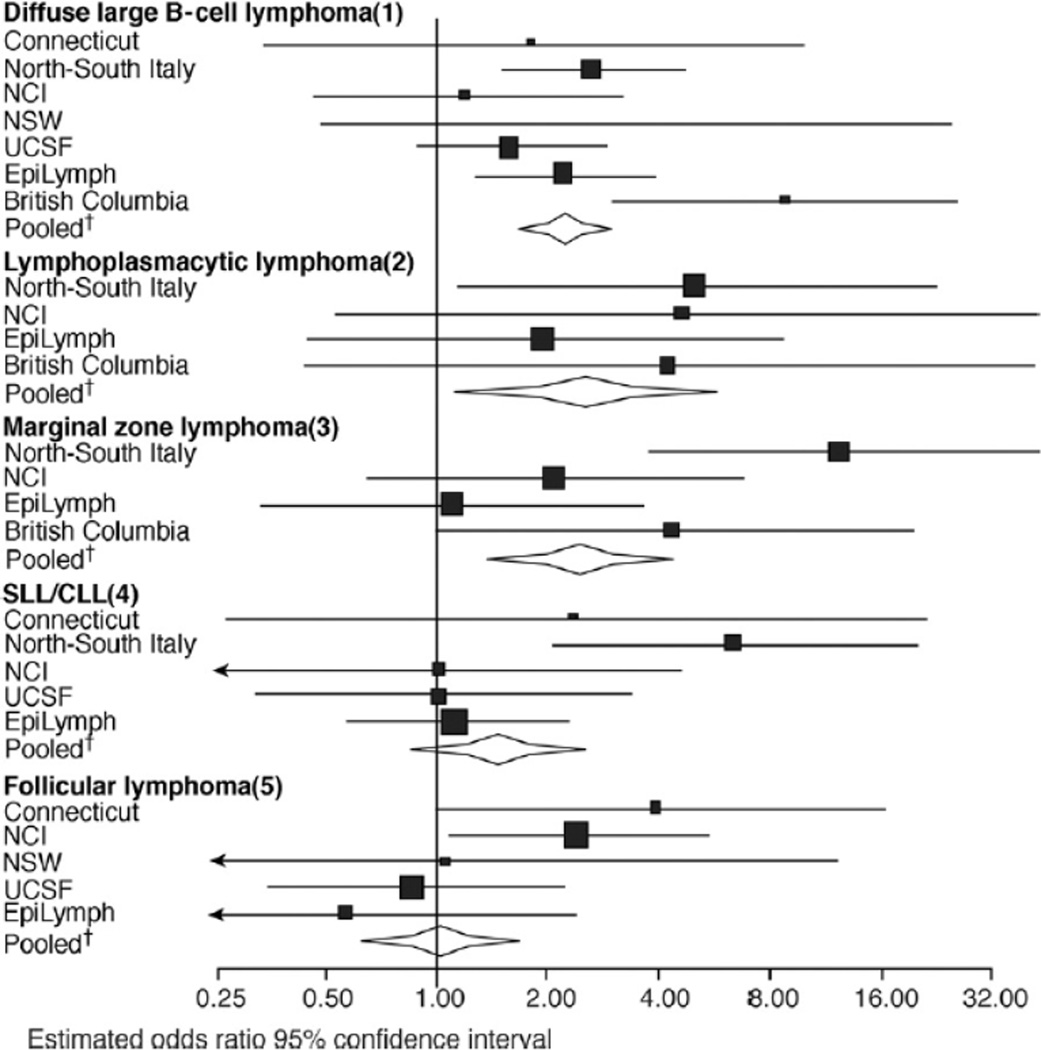
Study-specific analyses were performed for those lymphoma subtypes with a statistically significant association with HCV (except the heterogeneous group of “other B-cell lymphomas”) or those with 10 or more cases exposed to HCV (Figure 2). Risk of LPL, chronic lymphocytic leukemia, and FL associated with HCV infection did not differ among studies whereas study differences were observed for DLBCL and MZL. The strongest association between HCV and DLBCL was observed in British Columbia; however, the sensitivity analysis showed no influence of any single study in the overall estimation of risk, although the overall estimate was decreased by the absence of HCV infection among MZL patients in the Connecticut and the NSW study populations. For MZL, a slightly increased risk was observed when the EpiLymph study was excluded (OR, 4.84; 95% CI, 2.30–10.19) and the opposite was observed when the North–South Italy study was excluded (OR, 1.96; 95% CI, 0.94–4.12). In Figure 2, no estimates are available for those lymphoma subtypes with no HCV-positive cases.
Figure 2.
Risk estimates for lymphoma sub-types associated with HCV by study. SLL/CLL, small-cell lymphocytic lymphoma/chronic lymphocytic leukemia. (1) Heterogeneity test: χ2 = 11.69; df = 6; P = .07. (2) Heterogeneity test: χ2 = 0.90; df = 3; P = .83. (3) Heterogeneity test: χ2 = 8.96; df = 6; P = .03. (4) Heterogeneity test: χ2 = 4.80; df = 6; P = .31. (5) Heterogeneity test: χ2 = 7.79; df = 4; P = .10. †OR and 95% CI obtained from the polytomous unconditional logistic regression using a joint fixed-effects model adjusted for age, sex, race, and study center.
DISCUSSION
This pooled analysis to explore the association between HCV infection and risk of NHL subtypes included mostly countries with low background HCV prevalence with the exception of Italy. Our results show increased risks of DLBCL, MZL, and LPL associated with HCV infection. These risk estimates were particularly robust for DLBCL with a 2-fold increased risk overall and a statistically significant increased risk observed in 3 of the 7 studies. Our results are in agreement with several studies mainly from Italy, a high-prevalence country, which also found an association between HCV and DLBCL,10, 11, 12, 13 and 23 MZL,24, 25 and 26 and LPL.27, 28 and 29
The association observed between HCV and MZL was strongest in the study conducted in North–South Italy, with a 12-fold increased risk. However, sensitivity analyses showed that the overall estimate was decreased by the absence of HCV infection among MZL patients in the Connecticut and the NSW study populations. MZL is a subtype that was not defined formally before the REAL classification30 and therefore the global incidence of MZL may be underestimated when pooling studies using different classifications. Although the pooled analysis included 385 MZL, with an overall 3-fold increase in risk, a more precise evaluation of this association may require pooling of studies that each used the World Health Organization classification to characterize MZL.
HCV infection was associated with a 3-fold increased risk of LPL. This association was consistent in 4 of 6 studies with LPL cases. LPL includes immunocytoma and Waldenström’s disease, both repeatedly identified as related to HCV infection in case-control studies27, 28 and 29 and in a large cohort of US veterans.31 LPLs produce an immunoglobulin M paraprotein with autoantibody or cryoglobulin activity. Interestingly, these immunoglobulin Ms also may be present in MZL.15
Unlike 3 previous studies10, 11 and 25 and a recent meta-analysis,2 our pooled data did not show an increased risk of FL associated with HCV infection, although risk of FL was significantly lower than that of DLBCL. Differences may be owing to the different study inclusion criteria. In the study presented here, we included only case-control studies that used third-generation enzyme-linked immunosorbent assay testing and that provided the individual study records. Other lymphoma subtypes that do not originate from germinal center or postgerminal center B cells, such as mantle-cell lymphoma, Burkitt lymphoma, and T-cell lymphoma, were not related consistently to HCV infection. Our results support the hypothesis that proliferation of specific B-cell clones caused by chronic antigenic stimulation sustained by HCV is a likely mechanism that drives the HCV-mediated pathogenesis of B-cell lymphoma, in particular MZL and DLBCL.
No clear mechanism of direct induction of malignancy by HCV in the lymphocyte population has been shown consistently in vivo. Although earlier work6 showed lymphoma remission after successful treatment of HCV in patients with splenic lymphoma with villous lymphocytes, there are not yet any follow-up data from well-planned randomized trials to clarify whether antiviral treatment is effective for the remission of lymphoproliferative malignancy in hematologic HCV-positive patients.32 Such confirmation would imply a benefit from a proven therapeutic tool, and confirm a strong association between HCV and NHL.
Another line of evidence supporting the association between HCV and lymphoma is the consistent association between HCV and mixed cryoglobulinemia. Chronic infection with HCV is strongly associated with mixed cryoglobulinemia type II, which can evolve into overt lymphoma in some patients.29, 33 and 34 Increasing evidence indicates that MZL of mucosa-associated lymphoid tissue, both nodal and splenic types, are associated with chronic antigenic stimulation by autoantigens and/or bacterial or viral pathogens. These associations could not be tested in this study because no information was collected about history of cryoglobulinemia. Our data warrant further investigation of this issue.
HCV does not integrate into the host genome and it does not contain an obvious oncogene. Little is known about the mechanism of HCV in the activation and alterations of B-cell functions leading to lymphomas. HCV envelope protein E2 interacts with CD81 on the surface of B lymphocytes35 and with the B-cell receptor of HCV-associated lymphomas.36 This may jeopardize B-cell function and promote lymphomagenesis. New evidence suggests an important role of HCV infection in the up-regulation of B-lymphocyte stimulator (BLyS).37 BLyS is expressed by a variety of immune cells and acts on mature B lymphocytes promoting differentiation, proliferation, and survival.38 and 39 BLyS transgenic mice develop a lupus-like syndrome and finally B-cell lymphoma.40 and 41 Furthermore, BLyS deregulation has been observed in patients with NHL42 as well as in several autoimmune diseases that predispose to lymphomas including systemic lupus erythromatosus,43 Sjögren’s syndrome,44 and rheumatoid arthritis,45 and in mixed cryoglobulinemia syndrome.37 Interestingly, BLyS serum levels during chronic HCV infection are correlated significantly with B-cell proliferation.46 Other contributing factors for which some evidence exists include the following: (1) HCV-mediated induction of a mutator phenotype with a substantial increase in mutation frequency in immunoglobulins and proto-oncogenes,47 and 48 (2) down-regulation of major histocompatibility complex class II molecules and apoptotic factors,49 and (3) activation of proinflammatory cytokine production.50 and 51
As previously reported,52 the InterLymph consortium has allowed investigators to pool data from contributing studies, providing adequate statistical power to test hypotheses using fully adjusted models among participants without known immune suppression. These types of initiatives are an excellent alternative when individual studies have low power to test hypotheses related to rare exposures and within small subgroups.
Our results confirm the association between HCV infection and specific B-NHL subtypes (DLBCL, MZL, and LPL). This study had sufficient statistical power to confirm these associations in populations with low HCV prevalence. Our study further emphasizes the need to implement strategic measures to prevent HCV infection and to treat HCV infection adequately.
Abbreviations used in this paper
- BLyS
B-lymphocyte stimulator
- CI
confidence interval
- DLBCL
diffuse large B-cell lymphoma
- FL
follicular lymphoma
- HCV
hepatitis C virus
- InterLymph
International Lymphoma Epidemiology Consortium
- LPL
lymphoplasmacytic lymphoma
- MZL
marginal zone lymphoma
- NCI
National Cancer Institute
- NHL
non-Hodgkin lymphoma
- NOS
not otherwise specified
- NSW
New South Wales
- OR
odds ratio
- SEER
Surveillance epidemiology end result
- UCSF
University of California San Francisco
REFERENCES
- 1.Strickland GT. Liver disease in Egypt: hepatitis C superseded schistosomiasis as a result of iatrogenic and biological factors. Hepatology. 2006;43:915–922. doi: 10.1002/hep.21173. [DOI] [PubMed] [Google Scholar]
- 2.Dal Maso L, Franceschi S. Hepatitis C virus and risk of lymphoma and other lymphoid neoplasms: a meta-analysis of epidemiologic studies. Cancer Epidemiol Biomarkers Prev. 2006;15:2078–2085. doi: 10.1158/1055-9965.EPI-06-0308. [DOI] [PubMed] [Google Scholar]
- 3.Cowgill KD, Loffredo CA, Eissa SA, et al. Case-control study of non-Hodgkin’s lymphoma and hepatitis C virus infection in Egypt. Int J Epidemiol. 2004;33:1034–1039. doi: 10.1093/ije/dyh183. [DOI] [PubMed] [Google Scholar]
- 4.Saadoun D, Landau DA, Calabrese LH, et al. Hepatitis C-associated mixed cryoglobulinaemia: a crossroad between autoimmunity and lymphoproliferation. Rheumatology (Oxford) 2007;46:1234–1242. doi: 10.1093/rheumatology/kem132. [DOI] [PubMed] [Google Scholar]
- 5.Isaacson PG. Update on MALT lymphomas. Best Pract Res Clin Haematol. 2005;18:57–68. doi: 10.1016/j.beha.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Hermine O, Lefrere F, Bronowicki JP, et al. Regression of splenic lymphoma with villous lymphocytes after treatment of hepatitis C virus infection. N Engl J Med. 2002;347:89–94. doi: 10.1056/NEJMoa013376. [DOI] [PubMed] [Google Scholar]
- 7.Vallisa D, Bernuzzi P, Arcaini L, et al. Role of anti-hepatitis C virus (HCV) treatment in HCV-related, low-grade, B-cell, non-Hodgkin’s lymphoma: a multicenter Italian experience. J Clin Oncol. 2005;23:468–473. doi: 10.1200/JCO.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 8.Boffetta P, Linet MS, Armstrong BK. The InterLymph collaboration: a consortium of molecular epidemiological studies of non- Hodgkin’s lymphoma. Proc Am Assoc Cancer Res. 2003;44:308. (Abstract 1579). [Google Scholar]
- 9.Boffetta P, Armstrong B, Linet M, et al. Consortia in cancer epidemiology: lessons from InterLymph. Cancer Epidemiol Biomarkers Prev. 2007;16:197–199. doi: 10.1158/1055-9965.EPI-06-0786. [DOI] [PubMed] [Google Scholar]
- 10.Engels EA, Chatterjee N, Cerhan JR, et al. Hepatitis C virus infection and non-Hodgkin lymphoma: results of the NCI-SEER multi-center case-control study. Int J Cancer. 2004;111:76–80. doi: 10.1002/ijc.20021. [DOI] [PubMed] [Google Scholar]
- 11.Morton LM, Engels EA, Holford TR, et al. Hepatitis C virus and risk of non-Hodgkin lymphoma: a population-based case-control study among Connecticut women. Cancer Epidemiol Biomarkers Prev. 2004;13:425–430. [PubMed] [Google Scholar]
- 12.Nieters A, Kallinowski B, Brennan P, et al. Hepatitis C and risk of lymphoma: results of the European multicenter case-control study EPILYMPH. Gastroenterology. 2006;131:1879–1886. doi: 10.1053/j.gastro.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 13.Talamini R, Montella M, Crovatto M, et al. Non-Hodgkin’s lymphoma and hepatitis C virus: a case-control study from northern and southern Italy. Int J Cancer. 2004;110:380–385. doi: 10.1002/ijc.20137. [DOI] [PubMed] [Google Scholar]
- 14.Vajdic CM, Grulich AE, Kaldor JM, et al. Specific infections, infection-related behavior, and risk of non-Hodgkin lymphoma in adults. Cancer Epidemiol Biomarkers Prev. 2006;15:1102–1108. doi: 10.1158/1055-9965.EPI-06-0078. [DOI] [PubMed] [Google Scholar]
- 15.Jaffe ES, Harris NL, Stein H, et al. World Health Organization Classification of Tumours. Lyon: IARC Press; 2001. [Google Scholar]
- 16.Herrinton LJ. Epidemiology of the revised European-American lymphoma classification subtypes. Epidemiol Rev. 1998;20:187–203. doi: 10.1093/oxfordjournals.epirev.a017980. [DOI] [PubMed] [Google Scholar]
- 17.Fritz A, Percy C, Jack A, et al. International classification of diseases for oncology. Geneva: World Health Organization; 2000. [Google Scholar]
- 18.Percy C, Van Holten V, Muir C. International Classification of diseases for oncology. Geneva: World Heath Organization; 1990. [Google Scholar]
- 19.Morton LM, Turner JJ, Cerhan JR, et al. Proposed classification of lymphoid neoplasms for epidemiologic research from the Pathology Working Group of the International Lymphoma Epidemiology Consortium (InterLymph) Blood. 2007;110:695–708. doi: 10.1182/blood-2006-11-051672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clayton D, Hills M. Statistical models in epidemiology. Oxford: Oxford University Press; 1993. [Google Scholar]
- 21.Engels EA, Schmid CH, Terrin N, et al. Heterogeneity and statistical significance in meta-analysis: an empirical study of 125 meta-analyses. Stat Med. 2000;19:1707–1728. doi: 10.1002/1097-0258(20000715)19:13<1707::aid-sim491>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 22.Stukel TA, Demidenko E, Dykes J, et al. Two-stage methods for the analysis of pooled data. Stat Med. 2001;20:2115–2130. doi: 10.1002/sim.852. [DOI] [PubMed] [Google Scholar]
- 23.Colin C, Lanoir D, Touzet S, et al. Sensitivity and specificity of third-generation hepatitis C virus antibody detection assays: an analysis of the literature. J Viral Hepat. 2001;8:87–95. doi: 10.1046/j.1365-2893.2001.00280.x. [DOI] [PubMed] [Google Scholar]
- 24.Germanidis G, Haioun C, Pourquier J, et al. Hepatitis C virus infection in patients with overt B-cell non-Hodgkin’s lymphoma in a French center. Blood. 1999;93:1778–1779. [PubMed] [Google Scholar]
- 25.Luppi M, Longo G, Ferrari MG, et al. Clinico-pathological characterization of hepatitis C virus-related B-cell non-Hodgkin’s lymphomas without symptomatic cryoglobulinemia. Ann Oncol. 1998;9:495–498. doi: 10.1023/a:1008255830453. [DOI] [PubMed] [Google Scholar]
- 26.Silvestri F, Pipan C, Barillari G, et al. Prevalence of hepatitis C virus infection in patients with lymphoproliferative disorders. Blood. 1996;87:4296–4301. [PubMed] [Google Scholar]
- 27.Mele A, Pulsoni A, Bianco E, et al. Hepatitis C virus and B-cell non-Hodgkin lymphomas: an Italian multicenter case-control study. Blood. 2003;102:996–999. doi: 10.1182/blood-2002-10-3230. [DOI] [PubMed] [Google Scholar]
- 28.Silvestri F, Barillari G, Fanin R, et al. Hepatitis C virus infection among cryoglobulinemic and non-cryoglobulinemic B-cell non-Hodgkin’s lymphomas. Haematologica. 1997;82:314–317. [PubMed] [Google Scholar]
- 29.Zuckerman E, Zuckerman T. Hepatitis C and B-cell lymphoma: the hemato-hepatologist linkage. Blood Rev. 2002;16:119–125. doi: 10.1054/blre.2002.0194. [DOI] [PubMed] [Google Scholar]
- 30.Harris NL, Jaffe ES, Stein H, et al. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood. 1994;84:1361–1392. [PubMed] [Google Scholar]
- 31.Giordano TP, Henderson L, Landgren O, et al. Risk of non- Hodgkin lymphoma and lymphoproliferative precursor diseases in US veterans with hepatitis C virus. JAMA. 2007;297:2010–2017. doi: 10.1001/jama.297.18.2010. [DOI] [PubMed] [Google Scholar]
- 32.Bruni L, de Sanjose S. Hepatitis C infection and lymphomas: is there any benefit in viral treatment? Gastroenterology. 2006;131:685–686. doi: 10.1053/j.gastro.2006.06.032. [DOI] [PubMed] [Google Scholar]
- 33.Misiani R, Bellavita P, Fenili D, et al. Hepatitis C virus infection in patients with essential mixed cryoglobulinemia. Ann Intern Med. 1992;117:573–577. doi: 10.7326/0003-4819-117-7-573. [DOI] [PubMed] [Google Scholar]
- 34.Pozzato G, Mazzaro C, Crovatto M, et al. Low-grade malignant lymphoma, hepatitis C virus infection, and mixed cryoglobulinemia. Blood. 1994;84:3047–3053. [PubMed] [Google Scholar]
- 35.Pileri P, Uematsu Y, Campagnoli S, et al. Binding of hepatitis C virus to CD81. Science. 1998;282:938–941. doi: 10.1126/science.282.5390.938. [DOI] [PubMed] [Google Scholar]
- 36.Quinn ER, Chan CH, Hadlock KG, et al. The B-cell receptor of a hepatitis C virus (HCV) associated non-Hodgkin lymphoma binds the viral E2 envelope protein, implicating HCV in lymphomagenesis. Blood. 2001;98:3745–3749. doi: 10.1182/blood.v98.13.3745. [DOI] [PubMed] [Google Scholar]
- 37.Fabris M, Quartuccio L, Sacco S, et al. B-lymphocyte stimulator (BLyS) up-regulation in mixed cryoglobulinaemia syndrome and hepatitis C virus infection. Rheumatology (Oxford) 2007;46:37–43. doi: 10.1093/rheumatology/kel174. [DOI] [PubMed] [Google Scholar]
- 38.Batten M, Groom J, Cachero TG, et al. BAFF mediates survival of peripheral immature B lymphocytes. J Exp Med. 2000;192:1453–1466. doi: 10.1084/jem.192.10.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Do RK, Hatada E, Lee H, et al. Attenuation of apoptosis underlies B lymphocyte stimulator enhancement of humoral immune response. J Exp Med. 2000;192:953–964. doi: 10.1084/jem.192.7.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Batten M, Fletcher C, Ng LG, et al. TNF deficiency fails to protect BAFF transgenic mice against autoimmunity and reveals a predisposition to B cell lymphoma. J Immunol. 2004;172:812–822. doi: 10.4049/jimmunol.172.2.812. [DOI] [PubMed] [Google Scholar]
- 41.Mackay F, Woodcock SA, Lawton P, et al. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med. 1999;190:1697–1710. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Novak AJ, Grote DM, Stenson M, et al. Expression of BLyS and its receptors in B-cell non-Hodgkin lymphoma: correlation with disease activity and patient outcome. Blood. 2004;104:2247–2253. doi: 10.1182/blood-2004-02-0762. [DOI] [PubMed] [Google Scholar]
- 43.Dorner T, Putterman C. B cells, BAFF/zTNF4, TACI, and systemic lupus erythematosus. Arthritis Res. 2001;3:197–199. doi: 10.1186/ar299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Groom J, Kalled SL, Cutler AH, et al. Association of BAFF/BLyS overexpression and altered B cell differentiation with Sjogren’s syndrome. J Clin Invest. 2002;109:59–68. doi: 10.1172/JCI14121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheema GS, Roschke V, Hilbert DM, et al. Elevated serum B lymphocyte stimulator levels in patients with systemic immunebased rheumatic diseases. Arthritis Rheum. 2001;44:1313–1319. doi: 10.1002/1529-0131(200106)44:6<1313::AID-ART223>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 46.Sene D, Limal N, Ghillani-Dalbin P, et al. Hepatitis C virus-associated B-cell proliferation—the role of serum B lymphocyte stimulator (BLyS/BAFF) Rheumatology (Oxford) 2007;46:65–69. doi: 10.1093/rheumatology/kel177. [DOI] [PubMed] [Google Scholar]
- 47.Machida K, Cheng KT, Sung VM, et al. Hepatitis C virus infection activates the immunologic (type II) isoform of nitric oxide synthase and thereby enhances DNA damage and mutations of cellular genes. J Virol. 2004;78:8835–8843. doi: 10.1128/JVI.78.16.8835-8843.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Machida K, Cheng KT, Pavio N, et al. Hepatitis C virus E2-CD81 interaction induces hypermutation of the immunoglobulin gene in B cells. J Virol. 2005;79:8079–8089. doi: 10.1128/JVI.79.13.8079-8089.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu CG, Budhu A, Chen S, et al. Effect of hepatitis C virus core protein on the molecular profiling of human B lymphocytes. Mol Med. 2006;12:47–53. doi: 10.2119/2006-00020.Wu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Machida K, Cheng KT, Sung VM, et al. Hepatitis C virus induces toll-like receptor 4 expression, leading to enhanced production of beta interferon and interleukin-6. J Virol. 2006;80:866–874. doi: 10.1128/JVI.80.2.866-874.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yoshida H, Kato N, Shiratori Y, et al. Hepatitis C virus core protein activates nuclear factor kappa B-dependent signaling through tumor necrosis factor receptor-associated factor. J Biol Chem. 2001;276:16399–16405. doi: 10.1074/jbc.M006671200. [DOI] [PubMed] [Google Scholar]
- 52.Wang SS, Slager SL, Brennan P, et al. Family history of hematopoietic malignancies and risk of non-Hodgkin lymphoma (NHL): a pooled analysis of 10 211 cases and 11 905 controls from the International Lymphoma Epidemiology Consortium (InterLymph) Blood. 2007;109:3479–3488. doi: 10.1182/blood-2006-06-031948. [DOI] [PMC free article] [PubMed] [Google Scholar]