Abstract
Seasonal or chronic vitamin D deficiency and/or insufficiency is highly prevalent in human population. Receptors for 1,25-dihydroxyvitamin D3, the hormonal metabolite of vitamin D, are found throughout the brain. To provide further information on the role of this hormone on brain function, we analyzed the transcriptomic profiles of mixed neuron-glial cell cultures in response to 1,25-dihydroxyvitamin D3. 1,25-dihydroxyvitamin D3 treatment increases the mRNA levels of 27 genes by at least 1.9 fold. Among them, 17 genes were related to neurodegenerative and psychiatric diseases, or brain morphogenesis. Notably, 10 of these genes encode proteins potentially limiting the progression of Alzheimer’s disease. These data provide support for a role of 1,25-dihydroxyvitamin D3 in brain disease prevention. The possible consequences of circannual or chronic vitamin D insufficiencies on a tissue with a low regenerative potential such as the brain should be considered.
Introduction
Vitamin D is either obtained by dietary intake or produced in the skin following exposure to ultra violet B radiation (UVB) (290–315 nm). It is now clearly established that the human dietary intake of vitamin D is not sufficient because of the paucity of this compound in non fortified food [1]. Therefore, the major source of vitamin D is provided by the exposure of the skin to solar UVB which is influenced by season, latitude, urban air pollution, personal behaviour and skin colour [2]. Consequently, it has been repeatedly pointed out that a non negligible portion of the population suffers from a seasonal or even chronic vitamin D insufficiency that is defined by a circulating 25-hydroxyvitamin D (25D) level between 30 nmol/l and 50 nmol/l [1]. Importantly, vitamin D is a pro-hormone [1,3]. It is metabolized in the organism by two successive hydroxylation steps to generate a hormone named 1,25-dihydroxyvitamin D3 (1,25D3). In a way similar to steroid hormones, the primary mode of action of 1,25D3 is to regulate gene expression by interacting with a nuclear receptor named Vitamin D Receptor (VDR), which recognizes specific genomic DNA responsive elements named VDREs (Vitamin D responsive Element) [3,4]. VDR is found in almost all of the cells of the organism, including neurons and glial cells [1,4–6]. In view of the prevalence of vitamin D deficiency and/or insufficiency in human and of its pharmacological potential, determining the response of brain cells to 1,25D3 is a relevant field of investigation.
Recently, an emerging body of evidence has suggested that vitamin D may have previously-unrecognized effects on neurodegenerative or psychiatric diseases [7–9]. VDR is identified as a candidate gene for Parkinson’s disease (PD) [10] and at least five reports describe an association between vitamin D receptor gene polymorphism and Alzheimer’s disease (AD) [11–15]. Accordingly, and even if association does not necessarily mean causation, low serum 25(OH)-hydroxyvitamin D are associated with increased odds of cognitive impairment [16] and AD [17,18]. Conversely higher vitamin D dietary intake is associated with a lower risk of developing AD among older women [19].
Several experimental models have been developed to investigate how 1,25D3 affects brain function. In vivo, the effects of vitamin D on the nervous system are studied using rodent vitamin D deficiency models [20,21], or by investigating the neuroprotective or behavioural effects of vitamin D on brain lesion models or with VDR knock-out animals [22,23]. The results obtained in these different experimental models demonstrate that vitamin D deficiency during pregnancy affects brain morphology [21], alters dopamine turnover [24], and behaviour [25–29]. In addition the vitamin D prohormone or the 1,25D3 hormone have neuroprotective effects in several rodent experimental models of disease or injury including Alzheimer’s disease [30], Parkinson’s disease [31,32,33], traumatic brain injury [34–37], multiple sclerosis and demyelination [38–43]. A possible drawback of these in vivo experimental models is linked to the extreme complexity of the vitamin D endocrine system which encompasses many different physiological functions. Hence, some behavioural effects observed in vivo with vitamin D deficiency or knock out experimental models might be indirect and related for example to some effects of vitamin D depletion on bone and muscle [44]. In addition to these in vivo studies, the effects of vitamin D on nervous system have also been investigated in vitro on glial or neuronal cell cultures. For example 1,25D3 regulates the expression of VDR, and 1,25-dihydroxyvitamin D(3) 24-hydroxylase (Cyp24A) in astrocytes [45,46]. 1,25D3 also regulates the expression of VDR in oligodendrocytes, Schwann cell and cortical neuron cultures [47–49]. However, a consequence of using pure cell cultures, either glial or neuronal, is the disruption of the paracrine interactions existing between these different cell types in brain tissue. Such interactions can be highly relevant for understanding 1,25D3 function in the nervous system. Therefore, a complementary experimental approach is to study the effect of 1,25D3 treatment on mixed brain cell population. As vitamin D deficiency during pregnancy affects brain development [21], we used in the present study neuron-glial mixed cell cultures issued from neural stem cell cultures. In view of the prevalence of chronic or circannual vitamin D deficiency and/or insufficiency in human [1], the aim of this study was to characterize the transcriptomic response of mixed brain neuron-glial cell cultures issued from neural stem cells and chronically exposed to 1,25D3 to determine if this response includes genes related to neurodegenerative or psychiatric disease.
Material and Methods
Cell culture
Primary cultures of neural stem cells (NSCs) were established from the brain of mouse embryos at 17 days of gestation. The procedure was approved by Animal Welfare and Ethics Committee of Grenoble institute of neuroscience with the number MO36. Briefly, tissues freed of meninges were dissociated by mechanical trituration, aggregates were removed by filtration through a 40 μm grid and isolated cells were collected by centrifugation. Cells were plated onto uncoated dishes in basal culture medium composed of Dulbecco’s modified Eagle’s medium and Ham’s F-12 (DMEM)/F12 (1/1, v/v) (Life Technologies 10565-018, France) supplemented with N2 (Life Technologies 17502-048), B27 (Life Technologies 17504-044), 20 ng/ml basic fibroblast growth factor (FGF-2) (Peprotech AF-100-18B, France) and 20 ng/ml epidermal growth factor (EGF) (Peprotech AF-100-15) to allow the formation of floating neurospheres. For 1,25D3 treated cell cultures, 1,23D3 (Santa Cruz sc202877, USA) was present from the first day to the end of the experiment. Because 1,25D3 was dissolved in ethanol, control sister cultures were similarly treated with the same amount of ethanol (0.05μl/ml; 0.86 mM). At day 1, 2, 4, neurospheres were dissociated and 2/3 of the medium were replaced with fresh medium supplemented or not with 1,25D3. At day 6 neural stem cell differentiation was induced by culturing cells in DMEM medium supplemented with 10% fetal calf serum (Life Technologies 10270-106). Cells were then cultured for six additional days in DMEM supplemented with 10% fetal calf serum (Life Technologies 10270-106), with a medium change performed three days after the induction of differentiation. During the differentiation step, 1,25D3 at a final concentration of 10−8M was added to cells that have been previously treated with 1,25D3 and control cells were treated with vehicle alone. Thereafter, cells were cultured in serum-free medium (DMEM/F12 (1/1, v/v) supplemented with N2 and B27 without growth factors and with 1,25D3 (1,25D3 treated cultures) or ethanol (control cultures) for four additional days, with a medium change after three days, and then processed for RNA extraction. By doing this we ensured that 1,25-D3 treated cells were continuously exposed to 1,25D3 from the beginning to the end of the experiment. Cell cultures phenotyping was performed using the Neural 3-Color Immunocytochemistry kit from RD systems (R&D Europe, SC024).
Cytotoxicity assay
The absence of cytotoxicity of the different treatment was controlled by measuring the lactate dehydrogenase (LDH) released into the medium by cells cultured without ethanol or treated with ethanol or 1,25D3. The cytotoxicity levels of the groups were determined at day 1, 6, 12, 14 and 15 with the LDH-Cytotoxicity Assay Kit II (Abcam, UK, ab 65393) according to the manufacturer protocol. Each sample was tested in triplicate and assays were replicated twice.
Gene expression profiling
Total RNAs were extracted from cells with the MirVana isolation kit™ (Life Technologies AM1561) and further controlled for quality and concentration (Bio-Analyser, Agilent Technologies, Palo Alto, USA). For microarrays experiments, 200 ng of total RNA were amplified with the Ambion® WT Expression kit (Foster City, USA) and labeled with the GeneChip® WT Terminal Labeling kit (Affymetrix®, Santa Clara, USA). According to Affymetrix specifications, biotinylated targets were hybridized on Affymetrix® GeneChip® Whole Transcript (WT) expression array, Mouse Gene 1.0 ST. Then, the arrays were labelled with streptavidin-phycoerythrin and scanned. Fluorescence intensities for each probe were recorded and the expression values reported in arbitrary units. Data were processed and normalized using Robust Multi-array Average (RMA) algorithm. Three independent analyses using independent cell cultures issued from different pregnant mice were performed. Probe sets with a signal value of less than 60 in both conditions were excluded from analyses as this value corresponds to the median value of the internal negative control probe sets of the chip microarray. Only variation folds equal to or higher than 1.9 were considered for analysis and the expression changes between control and 1,25D3 treated cells were further validated with a statistical non parametric Mann-Whitney test with a p ≤ 0.05 considered as significant. The transcriptomic data have been deposited in NCBI’s Gene Expression Omnibus and are accessible through the Gene Expression Omnibus (GEO) accession number GSE41184.
RT-qPCR
2 μg of total RNA were transcribed into cDNA using Promega Reverse Transcription reagents (Promega M3683, France) with random dN6 primers. PCR primers (Eurogentec, France) for each gene were designed using the Universal ProbeLibrary Assay Design Center (https://www.roche-applied-science.com/sis/rtpcr/upl/ezhome.html) and sequences of the primers used are given as supplementary table 1. Then real-time PCR were performed according to the SYBR Green methodology on a CFX96 Touch™ Real-Time PCR Detection System (Bio-Rad Laboratories, Inc. France). The reference genes were βactin and succinate dehydrogenase complex subunit A (SDHA) whose expression were not affected by 1,25D3 according to our Affymetrix data (data not shown). The geometric mean Ct values of the reference genes were calculated for each individual sample and used to normalize expression levels using ΔΔCt method. Additional analyzes were performed with the CFX Manager ™ software (Biorad Laboratories, Inc. France) and compared by the ΔΔCt method [50]. Each qPCR was performed in triplicate for PCR yeld validation and all reactions were performed on three different biological samples. Finally, the statistical validation of gene changes was checked using the student t test with a p ≤ 0.05 considered as significant.
Results
To investigate whether 1,25D3 was able to modulate the mRNA levels of genes associated to neurodegenerative or psychiatric disorders, we investigated the transcriptomic response of mixed neuron-glial cell cultures to 1,25D3. Cell cultures were composed of glial fibrillary acidic protein positive astrocytes (GFAP+), neurons (βIII-tubulin+) and oligodendrocytes (O4+) (Supplementary Material Fig. 1 A,B). Since, 1,25D3 was resuspended in ethanol, the absence of cytotoxicity of 1,25D3 and ethanol was controlled. Addition of ethanol or 1,25D3 did not induce any statistically significant difference for LDH release in cell cultures compared to cultures without ethanol or hormone (Supp. Mat. Fig. 2). Transcriptomic analyses were performed on three independent mixed neuron-glial cell cultures and processed for transcriptomic analyses. Global transcriptomic analysis showed no gene down-regulated (cut-off value for significant change < 0.6), but identified 27 genes up-regulated in the presence of 1,25D3 (cut off value for significant fold change ≥1.9; Mann-Whitney test p ≤ 0.05) (Table 1). Regarding the 27 up-regulated genes, seven of them have been previously identified as 1,25D3 targets. Among these genes, the up-regulation by 1,25D3 of VDR in glia [45–48] and neurons [49], and of CYP24A in glial cells [46] was already documented. This validates the responsiveness of our cell culture system to 1,25D3. The five other genes already described as 1,25D3-regulated but in cell types others than neuron or glial cells were connector enhancer of kinase suppressor of Ras 2 (CNKSR2) [51], S100 calcium binding protein G (S100G) [52], lipoprotein lipase LPL [53,54], solute carrier family 1 (neuronal/epithelial high affinity glutamate transporter, system Xag), member 1 (Slc1a1), and the third component of complement C3 (C3) [55–57]. Five of these genes (VDR, CNKSR2, LPL, Slc1a1, C3) have been reported to be involved in brain functions related to neurodegenerative diseases (Fig. 1).
Table 1. List of differentially expressed genes in mixed neuron-glial cell cultures in the presence of 1,25D3 (induction fold ≥ 1.9).
Fold changes are the average of three independent experiments and indicate fold increases in gene expression for 1,25D3-treated cells compared to control cells. Published references for genes previously reported to be modulated by 1,25D3 are indicated.
| Probe set ID | Gene name | Gene symbol | Variation fold | Ref |
|---|---|---|---|---|
| 10607712 | gastrin releasing peptide receptor | Grpr | 8.7 | |
| 10490080 | cytochrome P450, family 24, subfamily a, polypeptide 1 | Cyp24a1 | 7.1 | 46 |
| 10605986 | solute carrier family 7 | Slc7a3 | 6.9 | |
| 10585438 | cellular retinoic acid binding protein I | Crabp1 | 5.9 | |
| 10495613 | RIKEN cDNA 4833424O15 gene | 4833424O15Rik | 5.1 | |
| 10432032 | vitamin D receptor | Vdr | 3.8 | 47–49 |
| 10480090 | integrin alpha 8 | Itga8 | 3.3 | |
| 10567995 | nuclear protein 1 | Nupr1 | 3.2 | |
| 10607562 | connector enhancer of kinase suppressor of Ras 2 | Cnksr2 | 3.0 | 51 |
| 10607705 | S100 calcium binding protein G | S100g | 3.0 | 52 |
| 10500283 | carbonic anhydrase 14 | Car14 | 2.7 | |
| 10473384 | solute carrier family 43, member 3 | Slc43a3 | 2.6 | |
| 10418434 | inter-alpha trypsin inhibitor, heavy chain 3 | Itih3 | 2.5 | |
| 10457183 | thioredoxin-related transmembrane protein 3 | Tmx3 | 2.5 | |
| 10572130 | lipoprotein lipase | Lpl | 2.5 | 53,54 |
| 10425945 | fibulin 1 | Fbln1 | 2.3 | |
| 10462313 | solute carrier family 1member 1 | Slc1a1 | 2.3 | 55 |
| 10419525 | RIKEN cDNA A930018M24 gene | A930018M24Rik | 2.2 | |
| 10449712 | cystathionine beta-synthase | Cbs | 2.2 | |
| 10399465 | family with sequence similarity 84, member A | Fam84a | 2.1 | |
| 10588592 | calcium channel, voltage-dependent, alpha 2 | Cacna2d2 | 2.1 | |
| 10452316 | complement component 3 | C3 | 2.0 | 56,57 |
| 10524955 | tescalcin | Tesc | 2.0 | |
| 10538852 | RIKEN cDNA A430010J10 gene | A430010J10Rik | 2.0 | |
| 10581388 | lecithin cholesterol acyltransferase | Lcat | 2.0 | |
| 10401841 | deiodinase, iodothyronine, type II | Dio2 | 1.9 | |
| 10454015 | tetratricopeptide repeat domain 39C | Ttc39c | 1.9 |
Fig. 1. Genes associated with CNS and up-regulated in the presence of 1,25D3 in mixed neuron-glial cell cultures.
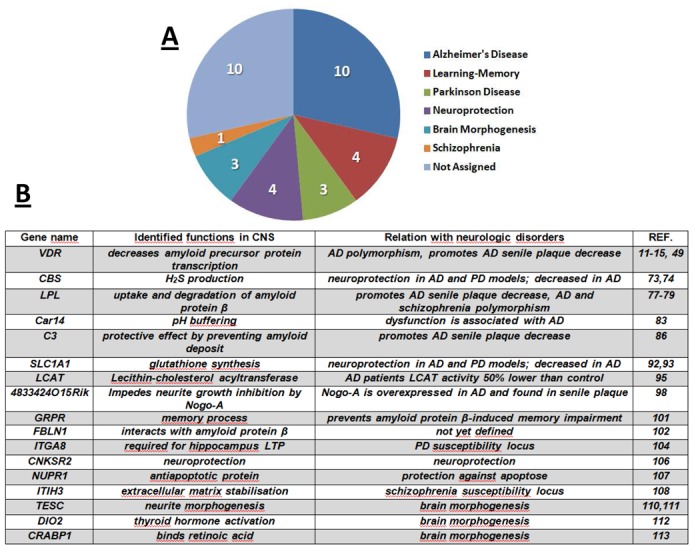
A. The 27 genes that are overexpressed in mixed neuron-glial cell cultures in the presence of 1,25D3 can be ascribed to the brain pathology or brain function-related classes indicated (several genes belong to more than one class).
B. Known roles of 17 of the 1,25D3-overexpressed genes in neurological disorders or brain morphogenesis (abbreviations used: AD, Alzheimer’s Disease; PD, Parkinson’s Disease).
Among the 22 remaining genes not previously associated with the vitamin D endocrine system, we identified 12 genes described as potentially involved in neurodegenerative, psychiatric diseases or brain morphogenesis (Fig. 1). These genes are gastrin-releasing peptide receptor (GRPR), cellular retinoic acid binding protein 1 (CRABP1), RIKEN cDNA 4833424O15, integrin, alpha 8 (ITGA8), nuclear protein, transcriptional regulator 1 (NUPR1), carbonic anhydrase XIV (CAR14), inhibitor heavy chain 3 (ITIH3), inter-alpha-trypsin fibulin 1 (FBLN1), cystathionine-beta-synthase(CBS), tescalcin (TESC), lecithin-cholesterol acyltransferase (LCAT), and deiodinase, iodothyronine, type II (DIO2) (Table 1). Confirmatory RT-qPCR analyses were performed for these 12 genes. They validated the transcriptomic data (Suppl. Mat. Fig. 3). According to these results, an analysis of the biological functions of the 27 genes up-regulated by 1,25D3 carried out with the Ingenuity Pathway Analysis software identified “Neurological disease” as the Top Bio Function with 6 genes associated with tauopathy (C3, CBS, CNKSR2, LPL, SLC1A1, VDR; p = 1.5 × 10−4), and 5 genes associated with AD (C3, CBS, CNKSR2, LPL, VDR; p = 1.0 × 10−3). Finally, 1,25D3 treatment did not affect the expression of genes coding for specific markers for neurons, astrocytes or oligodendrocytes (Supplementary Material Fig. 1C).
Discussion
The epidemiological evidence for a widespread vitamin D deficiency and/or insufficiency in the population is of major concern since it suggests that the health sequelae of the chronic or seasonal mild hypovitaminosis D could impact any tissue expressing VDR [3]. This point is particularly relevant for a tissue of low regenerative potential such as the brain. The repetition year after year and throughout the lifetime of the seasonal vitamin D deficiency and/or insufficiency cycle known to occur during winter and spring could be a cofactor in the occurrence of some neurodegenerative diseases during aging. Likewise, it has been proposed that a seasonal vitamin D deficiency occurring during pregnancy could be a cofactor associated with schizophrenia epidemiology [58]. On the other hand, a pharmacological potential for 1,25D3 is suggested by several experimental data demonstrating a neuroprotective effect for this hormone [30–43,59–65]. Hence, the characterization of the effects of 1,25D3 on gene expression is a relevant field of investigation. One experimental approach to grasp this problem is to characterize genes whose expression is regulated in the presence of 1,25D3 in mixed neurone-glial cell cultures and then to investigate if these genes are involved in neurodegenerative or psychiatric diseases. Since in vivo cells are chronically exposed to 1,25D3, we used an experimental model in which 1,25D3-treated cells were exposed to the hormone during all the course of the study. The relevance of our transcriptomic data is assessed by the finding that several genes characterized in this study (CYP24A, VDR, S100G, CNKSR2, LPL, Slc1a1, C3) have already been identified as up-regulated in the presence of 1,25D3 in other cell types. Regarding the other genes identified in this study, we restricted the analysis to those related to brain functions or neurodegenerative or psychiatric pathologies and validated their expression levels by RT-qPCR. Surprisingly, the NGF or GDNF genes which were previously reported as up-regulated by 1,25D3 in glial cells [45,66] were not detected as regulated by 1,25D3 in this study. This could reflect the differences in the cell culture protocols used in different studies. Here, we used mixed neuron-glial cell cultures issued from neural stem cell cultures and cells were chronically exposed to 1,25D3 for all the duration of the experiment (15 days). This contrasts with previous experiments performed either on glial or on neuron cultures, only transiently exposed to 1,25D3.
Among the 27 genes found up-regulated in the presence of 1,25D3 in this transcriptomic study, 17 genes code for proteins connected to neurodegenerative or psychiatric diseases, or brain morphogenesis and are listed in Fig. 1B.
Genes related to Alzheimer’s or Parkinson’s diseases in our study
The Vitamin D receptor (VDR)
VDR plays a pivotal role in the response to 1,25D3 as it mediates the genomic effects of 1,25D3. The finding that VDR gene expression is up-regulated in brain cells following 1,25D3 exposure has been reported [45,47,49,67]. In addition to the association between VDR gene polymorphism and PD or AD previously reported [10–15], overexpression of VDR or vitamin D treatment suppressed amyloid precursor protein transcription in neuroblastoma cells [11]. Accordingly, Vitamin D3-enriched diet correlates with a decrease of amyloid plaques in the brain of AβPP transgenic mice [30]. On the other hand, amyloid-β treatment decreases VDR gene expression in cultured cortical neurons [49]. In keeping with this, a reduction of vitamin D hormone receptor mRNA levels in Alzheimer as compared to Huntington hippocampus is also reported [68]. Hence, a relationship exists between VDR and amyloid-β levels. The finding that in cell culture each protein decreases the level of the other one [49] suggests the existence of a pathologic positive feed-back loop in which any down-regulation of the VDR pathway increases amyloid-β which in turn aggravates the VDR signaling deficiency and increases amyloid-β levels. If such a mechanism is operative in AD it could limit the therapeutic potential of vitamin D in late AD stages [49].
Cystathionine beta-synthase (CBS)
CBS is a hydrogen sulfide (H2S) producing enzyme in the brain. H2S is considered as a neuromodulator [69–71]. It enhances the activity of NMDA receptors and facilitates the induction of hippocampal long-term potentiation (LTP). Importantly H2S is an endogenous anti-inflammatory and neuroprotective agent [72]. It protects cells against oxidative stress caused by glutamate, beta-amyloid, or 1-methyl-4-phenylpyridinium ion (MPP+) a drug used to generate a Parkinson’s disease model in animals [73]. Moreover, brain H2S is severely decreased in AD patients [74]. The H2S therapeutic potential in neurodegenerative disorders of aging such as Alzheimer’s disease and Parkinson’s diseases is currently investigated [75].
Lipoprotein lipase (LPL)
LPL is the key enzyme of triglyceride hydrolysis and is expressed in the brain regions functionally relevant to learning and memory [76]. LPL was identified as a 1,25D3-regulated gene in adipocytes [53,54]. Association between single nucleotide polymorphisms in this gene and schizophrenia is observed in a Han Chinese population [77]. A polymorphism in LPL gene also affects the severity of Alzheimer’s disease pathophysiology [78]. Moreover LPL colocalizes with senile plaques and promotes the uptake and degradation of amyloid protein β by astrocytes [79].
Carbonic anhydrase 14 (Car14)
CAR14 is a membrane bound synaptic protein that catalyses the buffering of activity-dependent extracellular pH shift in the nervous system. By doing that it is indirectly implicated in the modulation of the NMDAR-mediated current and Ca2+ influx in hippocampal CA1 pyramidal neurons [80–82]. Carbonic anhydrase dysfunction impairs cognition and is associated with mental retardation, Alzheimer’s disease and aging [83].
Complement component 3 (C3) is found 1,25D3-responsive in osteoblastic cells [56,57]. It is detected in AD senile plaques and neurodegeneration is partially mediated by complement activation [84]. Accordingly C3 is identified as a plasma biomarker of brain atrophy in AD [85]. Complement activation products are generally considered as mediators between amyloid deposits found in senile plaques and the inflammatory response leading to neurotoxicity. Importantly, a notable exception is for complement C3 factor which has also a beneficial role in amyloid plaque clearance and protection against neuronal death [86]. Hence, although activation of the complement is usually considered as detrimental in AD pathology, C3 should paradoxically have a protective effect by preventing the accumulation of amyloid deposit, in the general context of the 1,25D3 anti-inflammatory action [87,88]
Solute carrier family 1 (neuronal/epithelial high affinity glutamate transporter, system Xag), member 1 (SLC1A1 also named EAAT3 or EAAC1)
SLC1A1 is a neuronal and epithelial glutamate transporter that is identified as 1,25D3-responsive in osteoblastic cells [55]. Several genetic studies have found an association between this gene and obsessive-compulsive disorder [89–91]. In addition, experiments performed on Slc1a1 deficient mouse have shown that this protein also transport cysteine an obligate precursor for the neuronal synthesis of the anti-oxidant glutathione [92]. Therefore, in Slc1a1 deficient mouse, impaired neuronal glutathione metabolism leads to oxidative stress and age-dependent neurodegeneration [92]. This point is of particular relevance for neurodegenerative disease in regard to the key role of glutathione as an antioxidant in AD and PD. Indeed, one of the earliest biochemical changes seen in PD is a reduction in the levels of total glutathione [93].
Lecithin-cholesterol acyltransferase (LCAT)
LCAT esterifies cholesterol on glial-derived apoE-lipoproteins, and influences cerebrospinal fluid (CSF) apolipoprotein E and apolipoprotein A-I levels. [94]. In AD patients LCAT activity was 50% lower than in CSF from normal controls [95].
4833424O15Rik also named Lppr5 or PRG-5
PRG-5 for Plasticity-related gene 5 protein belongs to the Plasticity-related genes (PRGs) also referred to as lipid phosphate phosphatase-related genes family (LPPRs). PRG-5 is specifically expressed in brain and spinal cord and regulated during brain development. It induces filipodia formation, neurite growth and drives axon elongation in neurons [96]. This protein impedes the RhoA-dependent neurite retractation mediated by the neurite growth inhibitors Nogo-A and lysophosphatidic acid (LPA) [96]. This point is important as Nogo-A contributes to the failure of CNS axons to regrow and reconnect after damage [97]. Importantly, Nogo-A is over-expressed by hippocampal neurons in AD and associated with beta-amyloid deposits in senile plaques [98]. The up-regulation of PRG-5 in presence of 1,25D3 provides additional evidence for a protective effect of 1,25D3 in neuro-regenerative processes or neurodegenerative diseases such as AD.
The Gastrin-releasing peptide receptor (GRPR)
GRPR is implicated in memory processes in amygdale and hippocampus [99,100]. Moreover, stimulating this receptor in rats prevents amyloid peptide-induced memory impairment [101]. In addition to memory, GRPR affects grooming and social behavior in rodents suggesting that it could be a molecular target for psychiatric and neurological disorders [99].
Fibulin 1 (FBLN1)
FBLN1 is an extracellular matrix interacting with the amyloid precursor protein [102]. However the consequence of this interaction in the pathogenesis of AD has not yet been elucidated.
Integrin α8 (ITGA8)
ITGA8 is required for hippocampal long-term potentiation [103]. This gene is identified as one novel PD susceptibility locus [104].
Connector enhancer of kinase suppressor of Ras 2 (CNKSR2)
CNKSR2 is induced by vitamin D and inhibits apoptosis in certain cancer cells by protecting against ROS-producing and DNA-damaging agents [105]. In neurons, CNKSR2 is a key participant in NGF signaling through its coupling to ERK activation [106].
Nuclear protein, transcriptional regulator, 1 (NUPR1 also named P8)
NUPR1 is an antiapoptotic protein when interacting with prothymosin α [107].
Gene related to psychiatric diseases
Inter-alpha-trypsin inhibitor heavy chain 3 (ITIH3)
ITIH3 is involved in extracellular matrix stabilization. A recent genome-wide study finds a significant association in schizophrenia to ITIH3/4 variants [108].
Genes related to brain morphogenesis
Tescalcin (TESC)
TESC is essential in megakaryocyte for the coupling of ERK cascade activation with the expression of E-twenty six (Ets) family genes [109]. However, it remains to be determined if a similar function is observed in nervous system. This point is critical in view of the role of Ets family genes in morphogenesis [110]. In addition Tescalcin promotes the maturation, transport and function of the Na+/H+ exchanger NHE1 isoform which plays a permissive role in neurite morphogenesis [111]. Notably functional NHE1 is required for the stimulation of neurite morphogenesis by neutrin-1 [111].
2 iodothyronine deiodinase (DIO2)
DIO2 catalyzes the conversion of the thyroid hormone T4 to T3 that leads to its activation. Dio2 is particularly important to provide intracellular T3 to brain cells [112]. The finding that 1,25D3 could be involved in the fine tuning of thyroid hormone signaling in brain cell is of great interest in view of the role of T3 in brain development and function.
Cellular retinoic acid binding protein 1 (CRABP1)
CRABP1 is assumed to serve as a regulator of retinoic acid function [113]. Hence our results establish a link between 1,25D3 and thyroid hormone and retinoic acid, two critical brain morphogens. This enlightens the complexity of the 1,25D3 effects and could explain some of the effects observed during pregnancy on brain morphology in the vitamin D deficiency models.
Taken together, these data add to the growing list of experimental and clinical findings identifying brain as a vitamin D target [5–9]. Our results show that more than half of the genes whose expression is increased in the presence of 1,25D3 are involved in brain function or connected to neurodegenerative or psychiatric diseases, notably AD. These results are in agreement with data showing that vitamin D-enriched diet correlates with a decrease of amyloid plaques in the brain of AβPP transgenic mice [30]. Recent meta-analyses also suggest that lower vitamin D concentrations are associated with a higher risk of AD [17,18]. In this regard it is noteworthy that 1,25D3 promotes the recovery of amyloid-β phagocytosis by AD macrophages [114]. Therefore, the question of the role of a vitamin D deficiency and/or insufficiency, or of a vitamin D system malfunction as a cofactor in the prevalence of neurological diseases such as AD is increasingly timely [115–117]. Because of the complex, multifactorial and progressive nature of such pathologies the answer might remain elusive for a long time. Expecting a univocal proof such as the one obtained with vitamin D deficiency and rickets is probably unrealistic as vitamin D probably acts as a protective cofactor. It is worth mentioning that the history of vitamin D in human health did not end with the discovery of its role in rickets prevention about one century ago. Accumulating data demonstrate a role for this hormone in the prevention of cancer, infectious, cardiovascular and autoimmune diseases [3]. These findings lead to suggest a therapeutic role for adjunctive vitamin D supplementation for these diseases. The potential interest of vitamin D in the treatment of neurodegenerative disease has been suggested at least 20 years ago [118]. Data presented in the present study raise the possibility that neurodegenerative diseases could soon join the cohort of diseases for which a vitamin D supplementation might be beneficial [119].
Supplementary Material
Acknowledgments
We thank Dr Boudewijn van der Sanden for critical reading of the manuscript.
References
- 1.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 2.Kimlin MG. Geographic location and vitamin D synthesis. Mol Aspects Med. 2008;29:453–61. doi: 10.1016/j.mam.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 3.Norman AW. From vitamin D to hormone D: fundamentals of the vitamin D endocrine system essential for good health. Am J Clin Nutr. 2008;88:491S–499S. doi: 10.1093/ajcn/88.2.491S. [DOI] [PubMed] [Google Scholar]
- 4.Bouillon R, Carmeliet G, Verlinden L, van Etten E, Verstuyf A, Luderer HF, Lieben L, Mathieu C, Demay M. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev. 2008;29:726–76. doi: 10.1210/er.2008-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcion E, Wion-Barbot N, Montero-Menei CN, Berger F, Wion D. New clues about vitamin D functions in the nervous system. Trends Endocrinol Metab. 2002;13:100–5. doi: 10.1016/s1043-2760(01)00547-1. [DOI] [PubMed] [Google Scholar]
- 6.Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat. 2005;29:21–30. doi: 10.1016/j.jchemneu.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Harms LR, Burne TH, Eyles DW, McGrath JJ. Vitamin D and the brain. Best Pract Res Clin Endocrinol Metab. 2011;25:657–69. doi: 10.1016/j.beem.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 8.Nimitphong H, Holick MF. Vitamin D, neurocognitive functioning and immunocompetence. Curr Opin Clin Nutr Metab Care. 2011;14:7–14. doi: 10.1097/MCO.0b013e3283414c38. [DOI] [PubMed] [Google Scholar]
- 9.Buell JS, Dawson-Hughes B. Vitamin D and neurocognitive dysfunction: preventing “D”ecline? Mol Aspects Med. 2008;29:415–22. doi: 10.1016/j.mam.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Butler MW, Burt A, Edwards TL, Zuchner S, Scott WK, Martin ER, Vance JM, Wang L. Vitamin D receptor gene as a candidate gene for Parkinson disease. Ann Hum Genet. 2011;75:201–210. doi: 10.1111/j.1469-1809.2010.00631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang L, Hara K, Van Baaren JM, Price JC, Beecham GW, Gallins PJ, Whitehead PL, Wang G, Lu C, Slifer MA, Züchner S, Martin ER, Mash D, Haines JL, Pericak-Vance MA, Gilbert JR. Vitamin D receptor and Alzheimer’s disease: a genetic and functional study. Neurobiol Aging. 2012;33:1844.e1–1844e.9. doi: 10.1016/j.neurobiolaging.2011.12.038. [DOI] [PubMed] [Google Scholar]
- 12.Lehmann DJ, Refsum H, Warden DR, Medway C, Wilcock GK, Smith AD. The vitamin D receptor gene is associated with Alzheimer’s disease. Neurosci Lett. 2011;504:79–82. doi: 10.1016/j.neulet.2011.08.057. [DOI] [PubMed] [Google Scholar]
- 13.Gezen-Ak D, Dursun E, Ertan T, Hanagasi H, Gurvit H, Emre M, Eker E, Ozturk M, Engin F, Yilmazer S. Association between vitamin D receptor gene polymorphism and Alzheimer’s disease. Tohoku J Exp Med. 2007;212:275–82. doi: 10.1620/tjem.212.275. [DOI] [PubMed] [Google Scholar]
- 14.Luedecking-Zimmer E, DeKosky ST, Nebes R, Kamboh MI. Association of the 3′ UTR transcription factor LBP-1c/CP2/LSF polymorphism with late-onset Alzheimer’s disease. Am J Med Genet B Neuropsychiatr Genet. 2003;117B:114–117. doi: 10.1002/ajmg.b.10026. [DOI] [PubMed] [Google Scholar]
- 15.Gezen-Ak D, Dursun E, Bilgic B, Hanagasi H, Ertan T, Gürvit H, Emre M, Eker E, Ulutin T, Uysal O, Yilmazer S. Vitamin d receptor gene haplotype is associated with late-onset Alzheimer’s disease. Tohoku J Exp Med. 2012;228:189–196. doi: 10.1620/tjem.228.189. [DOI] [PubMed] [Google Scholar]
- 16.Llewellyn DJ, Lang KM, Langa IA. Serum 25-hydroxyvitamin D concentration and cognitive impairment. J Geriatr Psychiatry Neurol. 2009;22 :188–195. doi: 10.1177/0891988708327888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Annweiler C, Llewellyn DJ, Beauchet O. Low Serum Vitamin D Concentrations in Alzheimer’s Disease: A Systematic Review and Meta-Analysis. J Alzheimers Dis. 2013 doi: 10.3233/JAD-2012-121432. in press. [DOI] [PubMed] [Google Scholar]
- 18.Balion C, Griffith LE, Strifler L, Henderson M, Patterson C, Heckman G, Llewellyn DJ, Raina P. Vitamin D, cognition, and dementia: A systematic review and meta-analysis. Neurology. 2012;79:1397–405. doi: 10.1212/WNL.0b013e31826c197f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Annweiler C, Rolland Y, Schott AM, Blain H, Vellas B, Hermann FR, Beauchet O. Higher Vitamin D Dietary Intake Is Associated With Lower Risk of Alzheimer’s Disease: A 7-Year Follow-up. J Gerontol A Biol Sci Med Sci. 2012;67:1205–1211. doi: 10.1093/gerona/gls107. [DOI] [PubMed] [Google Scholar]
- 20.Feron F, Burne TH, Brown J, Smith E, McGrath JJ, Mackay-Sim A, Eyles DW. Developmental Vitamin D3 deficiency alters the adult rat brain. Brain Res Bull. 2005;65:141–8. doi: 10.1016/j.brainresbull.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 21.Eyles DW, Feron F, Cui X, Kesby JP, Harms LH, Ko P, McGrath JJ, Burne TH. Developmental vitamin D deficiency causes abnormal brain development. Psychoneuroendocrinology. 2009;34:S247–257. doi: 10.1016/j.psyneuen.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 22.Kalueff AV, Keisala T, Minasyan A, Kuuslahti M, Miettinen S, Tuohimaa P. Behavioural anomalies in mice evoked by “Tokyo” disruption of the Vitamin D receptor gene. Neurosci Res. 2006;54:254–260. doi: 10.1016/j.neures.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 23.Zou J, Minasyan A, Keisala T, Zhang Y, Wang JH, Lou YR, Kalueff A, Pykkö I, Tuohimaa P. Progressive hearing loss in mice with a mutated vitamin D receptor gene. Audiol Neurootol. 2008;13:219–230. doi: 10.1159/000115431. [DOI] [PubMed] [Google Scholar]
- 24.Kesby JP, Cui X, O’Loan J, McGrath JJ, Burne TH, Eyles DW. Developmental vitamin D deficiency alters dopamine-mediated behaviors and dopamine transporter function in adult female rats. Psychopharmacology (Berl) 2009;208:159–68. doi: 10.1007/s00213-009-1717-y. [DOI] [PubMed] [Google Scholar]
- 25.Becker A, Eyles DW, McGrath JJ, Grecksch G. Transient prenatal vitamin D deficiency is associated with subtle alterations in learning and memory functions in adult rats. Behav Brain Res. 2005;161:306–12. doi: 10.1016/j.bbr.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 26.Eyles DW, Rogers F, Buller K, McGrath JJ, Ko P, French K, Burne TH. Developmental vitamin D (DVD) deficiency in the rat alters adult behaviour independently of HPA function. Psychoneuroendocrinology. 2006;31:958–64. doi: 10.1016/j.psyneuen.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 27.Harms LR, Eyles DW, McGrath JJ, Mackay-Sim A, Burne TH. Developmental vitamin D deficiency alters adult behaviour in 129/SvJ and C57BL/6J mice. Behav Brain Res. 2008;187:343–50. doi: 10.1016/j.bbr.2007.09.032. [DOI] [PubMed] [Google Scholar]
- 28.Burne TH, O’Loan J, Splatt K, Alexander S, McGrath JJ, Eyles DW. Developmental vitamin D (DVD) deficiency alters pup-retrieval but not isolation-induced pup ultrasonic vocalizations in the rat. Physiol Behav. 2011;102:201–4. doi: 10.1016/j.physbeh.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 29.Kesby JP, O’Loan JC, Alexander S, Deng C, Huang XF, McGrath JJ, Eyles DW, Burne TH. Developmental vitamin D deficiency alters MK-801-induced behaviours in adult offspring. Psychopharmacology (Berl) 2012;220:455–63. doi: 10.1007/s00213-011-2492-0. [DOI] [PubMed] [Google Scholar]
- 30.Yu J, Gattoni-Celli M, Zhu H, Bhat NR, Sambamurti K, Gattoni-Celli S, Kindy MS. Vitamin D3-enriched diet correlates with a decrease of amyloid plaques in the brain of AbetaPP transgenic mice. J Alzheimers Dis. 2011;25:295–307. doi: 10.3233/JAD-2011-101986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanchez B, Relova JL, Gallego R, Ben-Batalla I, Perez-Fernandez R. 1,25-Dihydroxyvitamin D3 administration to 6-hydroxydopamine-lesioned rats increases glial cell line-derived neurotrophic factor and partially restores tyrosine hydroxylase expression in substantia nigra and striatum. J Neurosci Res. 2009;87:723–32. doi: 10.1002/jnr.21878. [DOI] [PubMed] [Google Scholar]
- 32.Smith MP, Fletcher-Turner A, Yurek DM, Cass WA. Calcitriol protection against dopamine loss induced by intracerebroventricular administration of 6-hydroxydopamine. Neurochem Res. 2006;31:533–9. doi: 10.1007/s11064-006-9048-4. [DOI] [PubMed] [Google Scholar]
- 33.Wang JY, Wu JN, Cherng TL, Hoffer BJ, Chen HH, Borlongan CV, Wang Y. Vitamin D(3) attenuates 6-hydroxydopamine-induced neurotoxicity in rats. Brain Res. 2001;904:67–75. doi: 10.1016/s0006-8993(01)02450-7. [DOI] [PubMed] [Google Scholar]
- 34.Hua F, Reiss JI, Tang H, Wang J, Fowler X, Sayeed I, Stein DG. Progesterone and low-dose vitamin D hormone treatment enhances sparing of memory following traumatic brain injury. Horm Behav. 2012;61:642–51. doi: 10.1016/j.yhbeh.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malcok UA, Sengul G, Kadioglu HH, Aydin IH. Therapeutic effect of vitamin D3 in a rat diffuse axonal injury model. J Int Med Res. 2005;33:90–95. doi: 10.1177/147323000503300109. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Chiang YH, Su TP, Hayashi T, Morales M, Hoffer BJ, Lin SZ. Vitamin D(3) attenuates cortical infarction induced by middle cerebral arterial ligation in rats. Neuropharmacology. 2000;39:873–80. doi: 10.1016/s0028-3908(99)00255-5. [DOI] [PubMed] [Google Scholar]
- 37.Balden R, Selvamani A, Sohrabji F. Vitamin D deficiency exacerbates experimental stroke injury and dysregulates ischemia-induced inflammation in adult rats. Endocrinology. 2012;153:2420–35. doi: 10.1210/en.2011-1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wergeland S, Torkildsen O, Myhr KM, Aksnes L, Mork SJ, Bo L. Dietary vitamin D3 supplements reduce demyelination in the cuprizone model. PLoS One. 2011;6:e26262. doi: 10.1371/journal.pone.0026262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nataf S, Garcion E, Darcy F, Chabannes D, Muller JY, Brachet P. 1,25 Dihydroxyvitamin D3 exerts regional effects in the central nervous system during experimental allergic encephalomyelitis. J Neuropathol Exp Neurol. 1996;55:904–914. doi: 10.1097/00005072-199608000-00006. [DOI] [PubMed] [Google Scholar]
- 40.Garcion E, Sindji L, Nataf S, Brachet P, Darcy F, Montero-Menei CN. Treatment of experimental autoimmune encephalomyelitis in rat by 1,25-dihydroxyvitamin D3 leads to early effects within the central nervous system. Acta Neuropathol. 2003;105:438–448. doi: 10.1007/s00401-002-0663-0. [DOI] [PubMed] [Google Scholar]
- 41.Joshi S, Pantalena LC, Liu XK, Gaffen SL, Liu H, Rohowsky-Kochan C, Ichiyama K, Yoshimura A, Steinman L, Christakos S, Youssef S. 1,25-dihydroxyvitamin D(3) ameliorates Th17 autoimmunity via transcriptional modulation of interleukin-17A. Mol Cell Biol. 2011;31:3653–3669. doi: 10.1128/MCB.05020-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cantorna MT, Hayes CE, DeLuca HF. 1,25-Dihydroxyvitamin D3 reversibly blocks the progression of relapsing encephalomyelitis, a model of multiple sclerosis. Proc Natl Acad Sci U S A. 1996;93:7861–7864. doi: 10.1073/pnas.93.15.7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wergeland S, Torkildsen O, Myhr KM, Aksnes L, Mork SJ, Bo L. Dietary vitamin D3 supplements reduce demyelination in the cuprizone model. PLoS One. 2011;6:e26262. doi: 10.1371/journal.pone.0026262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McCann JC, Ames BN. Is there convincing biological or behavioral evidence linking vitamin D deficiency to brain dysfunction? FASEB J. 2008;22:982–1001. doi: 10.1096/fj.07-9326rev. [DOI] [PubMed] [Google Scholar]
- 45.Neveu I, Naveilhan P, Jehan F, Baudet C, Wion D, De Luca HF, Brachet P. 1,25-dihydroxyvitamin D3 regulates the synthesis of nerve growth factor in primary cultures of glial cells. Brain Res Mol Brain Res. 1994;24:70–6. doi: 10.1016/0169-328x(94)90119-8. [DOI] [PubMed] [Google Scholar]
- 46.Naveilhan P, Neveu I, Baudet C, Ohyama KY, Brachet P, Wion D. Expression of 25(OH) vitamin D3 24-hydroxylase gene in glial cells. Neuroreport. 1993;5:255–7. doi: 10.1097/00001756-199312000-00018. [DOI] [PubMed] [Google Scholar]
- 47.Baas D, Prufer K, Ittel ME, Kuchler-Bopp S, Labourdette G, Sarlieve LL, Brachet P. Rat oligodendrocytes express the vitamin D(3) receptor and respond to 1,25-dihydroxyvitamin D(3) Glia. 2000;31:59–68. doi: 10.1002/(sici)1098-1136(200007)31:1<59::aid-glia60>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 48.Cornet A, Baudet C, Neveu I, Baron-Van Evercooren A, Brachet P, Naveilhan P. 1,25-Dihydroxyvitamin D3 regulates the expression of VDR and NGF gene in Schwann cells in vitro. J Neurosci Res. 1998;53:742–746. doi: 10.1002/(SICI)1097-4547(19980915)53:6<742::AID-JNR11>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 49.Dursun E, Gezen-Ak D, Yilmazer S. A novel perspective for Alzheimer’s disease: vitamin D receptor suppression by amyloid-beta and preventing the amyloid-beta induced alterations by vitamin D in cortical neurons. J Alzheimers Dis. 2011;23:207–19. doi: 10.3233/JAD-2010-101377. [DOI] [PubMed] [Google Scholar]
- 50.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–8. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 51.Wang X, Patel R, Studzinski GP. hKSR-2, a vitamin D-regulated gene, inhibits apoptosis in arabinocytosine-treated HL60 leukemia cells. Mol Cancer Ther. 2008;7:2798–806. doi: 10.1158/1535-7163.MCT-08-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cao LP, Bolt MJ, Wei M, Sitrin MD, Chun Li Y. Regulation of calbindin-D9k expression by 1,25-dihydroxyvitamin D(3) and parathyroid hormone in mouse primary renal tubular cells. Arch Biochem Biophys. 2002;400:118–24. doi: 10.1006/abbi.2002.2775. [DOI] [PubMed] [Google Scholar]
- 53.Querfeld U, Hoffmann MM, Klaus G, Eifinger F, Ackerschott M, Michalk D, Kern PA. Antagonistic effects of vitamin D and parathyroid hormone on lipoprotein lipase in cultured adipocytes. J Am Soc Nephrol. 1999;10:2158–64. doi: 10.1681/ASN.V10102158. [DOI] [PubMed] [Google Scholar]
- 54.Vu D, Ong JM, Clemens TL, Kern PA. 1,25-Dihydroxyvitamin D induces lipoprotein lipase expression in 3T3-L1 cells in association with adipocyte differentiation. Endocrinology. 1996;137:1540–4. doi: 10.1210/endo.137.5.8612483. [DOI] [PubMed] [Google Scholar]
- 55.Tarroni P, Villa I, Mrak E, Zolezzi F, Mattioli M, Gattuso C, Rubinacci A Microarray analysis of 1,25(OH)(2)D(3) regulated gene expression in human primary osteoblasts. J Cell Biochem. 2012;113:640–9. doi: 10.1002/jcb.23392. [DOI] [PubMed] [Google Scholar]
- 56.Hong MH, Jin CH, Sato T, Ishimi Y, Abe E, Suda T. Transcriptional regulation of the production of the third component of complement (C3) by 1 alpha,25-dihydroxyvitamin D3 in mouse marrow-derived stromal cells (ST2) and primary osteoblastic cells. Endocrinology. 1991;129:2774–9. doi: 10.1210/endo-129-5-2774. [DOI] [PubMed] [Google Scholar]
- 57.Sato T, Hong MH, Jin CH, Ishimi Y, Udagawa N, Shinki T, Abe E, Suda T. The specific production of the third component of complement by osteoblastic cells treated with 1 alpha,25-dihydroxyvitamin D3. FEBS Lett. 1991;285:21–4. doi: 10.1016/0014-5793(91)80715-f. [DOI] [PubMed] [Google Scholar]
- 58.McGrath J. Hypothesis: is low prenatal vitamin D a risk-modifying factor for schizophrenia? Schizophr Res. 1999;40:173–7. doi: 10.1016/s0920-9964(99)00052-3. [DOI] [PubMed] [Google Scholar]
- 59.Taniura H, Ito M, Sanada N, Kuramoto N, Ohno Y, Nakamichi N, Yoneda Y. Chronic vitamin D3 treatment protects against neurotoxicity by glutamate in association with upregulation of vitamin D receptor mRNA expression in cultured rat cortical neurons. J Neurosci Res. 2006;83 :1179–1189. doi: 10.1002/jnr.20824. [DOI] [PubMed] [Google Scholar]
- 60.Brewer LD, Thibault V, Chen KC, Langub MC, Landfield PW, Porter NM. Vitamin D hormone confers neuroprotection in parallel with downregulation of L-type calcium channel expression in hippocampal neurons. J Neurosci. 2001;21:98–108. doi: 10.1523/JNEUROSCI.21-01-00098.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cass WA, Smith MP, Peters LE. Calcitriol protects against the dopamine- and serotonin-depleting effects of neurotoxic doses of methamphetamine. Ann N Y Acad Sci. 2006;1074 :261–271. doi: 10.1196/annals.1369.023. [DOI] [PubMed] [Google Scholar]
- 62.Moore ME, Piazza A, McCartney Y, Lynch MA. Evidence that vitamin D3 reverses age-related inflammatory changes in the rat hippocampus. Biochem Soc Trans. 2005;33:573–577. doi: 10.1042/BST0330573. [DOI] [PubMed] [Google Scholar]
- 63.Moore M, Piazza A, Nolan Y, Lynch MA. Treatment with dexamethasone and vitamin D3 attenuates neuroinflammatory age-related changes in rat hippocampus. Synapse. 2007;61:851–861. doi: 10.1002/syn.20433. [DOI] [PubMed] [Google Scholar]
- 64.Shinpo K, Kikuchi S, Sasaki H, Moriwaka F, Tashiro K. Effect of 1,25-dihydroxyvitamin D(3) on cultured mesencephalic dopaminergic neurons to the combined toxicity caused by L-buthionine sulfoximine and 1-methyl-4-phenylpyridine. J Neurosci Res. 2000;62:374–382. doi: 10.1002/1097-4547(20001101)62:3<374::AID-JNR7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 65.Masoumi A, Goldenson B, Ghirmai S, Avaqyan H, Zaghi J, Abel K, Zheng X, Espinosa-Jeffrey A, Mahanian M, Liu PT, Hewison M, Mizwickie M, Cashman J, Fiala M. 1alpha,25-dihydroxyvitamin D3 interacts with curcuminoids to stimulate amyloid-beta clearance by macrophages of Alzheimer’s disease patients. J Alzheimers Dis. 2009;17:703–717. doi: 10.3233/JAD-2009-1080. [DOI] [PubMed] [Google Scholar]
- 66.Naveilhan P, Neveu I, Wion D, Brachet P. 1,25-Dihydroxyvitamin D3, an inducer of glial cell line-derived neurotrophic factor. Neuroreport. 1996;7:2171–2175. doi: 10.1097/00001756-199609020-00023. [DOI] [PubMed] [Google Scholar]
- 67.Gezen-Ak D, Dursun E, Yilmazer S. The effects of vitamin D receptor silencing on the expression of LVSCC-A1C and LVSCC-A1D and the release of NGF in cortical neurons. PLoS One. 2011;6:e17553. doi: 10.1371/journal.pone.0017553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sutherland MK, Somerville MJ, Yoong LK, Bergeron C, Haussler MR, McLachlan DR. Reduction of vitamin D hormone receptor mRNA levels in Alzheimer as compared to Huntington hippocampus: correlation with calbindin-28k mRNA levels. Brain Res Mol Brain Res. 1992;13:239–50. doi: 10.1016/0169-328x(92)90032-7. [DOI] [PubMed] [Google Scholar]
- 69.Abe K, Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci. 1996;16:1066–1071. doi: 10.1523/JNEUROSCI.16-03-01066.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kimura H. Hydrogen sulfide: from brain to gut. Antioxid Redox Signal. 2010;12:1111–23. doi: 10.1089/ars.2009.2919. [DOI] [PubMed] [Google Scholar]
- 71.Qu K, Lee SW, Bian JS, Low CM, Wong PT. Hydrogen sulfide: neurochemistry and neurobiology. Neurochem Int. 2008;52:155–65. doi: 10.1016/j.neuint.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 72.Lee M, Schwab C, Yu S, McGeer E, McGeer PL. Astrocytes produce the antiinflammatory and neuroprotective agent hydrogen sulfide. Neurobiol Aging. 2009;30:1523–34. doi: 10.1016/j.neurobiolaging.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 73.Yin WL, He JQ, Hu B, Jiang ZS, Tang XQ. Hydrogen sulfide inhibits MPP(+)-induced apoptosis in PC12 cells. Life Sci. 2009;85:269–75. doi: 10.1016/j.lfs.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 74.Eto K, Asada T, Arima K, Makifuchi T, Kimura H. Brain hydrogen sulfide is severely decreased in Alzheimer’s disease. Biochem Biophys Res Commun. 2002;293:1485–8. doi: 10.1016/S0006-291X(02)00422-9. [DOI] [PubMed] [Google Scholar]
- 75.Gong QH, Shi XR, Hong ZY, Pan LL, Liu XH, Zhu YZ. A new hope for neurodegeneration: possible role of hydrogen sulfide. J Alzheimers Dis. 2011;24(Suppl 2):173–82. doi: 10.3233/JAD-2011-110128. [DOI] [PubMed] [Google Scholar]
- 76.Xian X, Liu T, Yu J, Wang Y, Miao Y, Zhang J, Yu Y, Ross C, Karasinska JM, Hayden MR, Liu G, Chui D. Presynaptic defects underlying impaired learning and memory function in lipoprotein lipase-deficient mice. J Neurosci. 2009;29:4681–5. doi: 10.1523/JNEUROSCI.0297-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xie C, Wang ZC, Liu XF, Wang L, Yang MS. Association between schizophrenia and single nucleotide polymorphisms in lipoprotein lipase gene in a Han Chinese population. Psychiatr Genet. 2011;21:307–14. doi: 10.1097/YPG.0b013e32834acc85. [DOI] [PubMed] [Google Scholar]
- 78.Blain JF, Aumont N, Theroux L, Dea D, Poirier J. A polymorphism in lipoprotein lipase affects the severity of Alzheimer’s disease pathophysiology. Eur J Neurosci. 2006;24:1245–51. doi: 10.1111/j.1460-9568.2006.05007.x. [DOI] [PubMed] [Google Scholar]
- 79.Nishitsuji K, Hosono T, Uchimura K, Michikawa M. Lipoprotein lipase is a novel amyloid beta (Abeta)-binding protein that promotes glycosaminoglycan-dependent cellular uptake of Abeta in astrocytes. J Biol Chem. 2011;286:6393–401. doi: 10.1074/jbc.M110.172106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Parkkila S, Parkkila AK, Rajaniemi H, Shah GN, Grubb JH, Waheed A, Sly WS. Expression of membrane-associated carbonic anhydrase XIV on neurons and axons in mouse and human brain. Proc Natl Acad Sci U S A. 2001;98:1918–23. doi: 10.1073/pnas.98.4.1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Svichar N, Waheed A, Sly WS, Hennings JC, Hubner CA, Chesler M. Carbonic anhydrases CA4 and CA14 both enhance AE3-mediated Cl--HCO3- exchange in hippocampal neurons. J Neurosci. 2009;29:3252–8. doi: 10.1523/JNEUROSCI.0036-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fedirko N, Avshalumov M, Rice ME, Chesler M. Regulation of postsynaptic Ca2+ influx in hippocampal CA1 pyramidal neurons via extracellular carbonic anhydrase. J Neurosci. 2007;27:1167–75. doi: 10.1523/JNEUROSCI.3535-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sun MK, Alkon DL. Carbonic anhydrase gating of attention: memory therapy and enhancement. Trends Pharmacol Sci. 2002;23:83–9. doi: 10.1016/s0165-6147(02)01899-0. [DOI] [PubMed] [Google Scholar]
- 84.Shen Y, Meri S. Yin and Yang: complement activation and regulation in Alzheimer’s disease. Prog Neurobiol. 2003;70:463–72. doi: 10.1016/j.pneurobio.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 85.Thambisetty M, Simmons A, Hye A, Campbell J, Westman E, Zhang Y, Wahlund LO, Kinsey A, Causevic M, Killick R, Kloszewska I, Mecocci P, Soininen H, Tsolaki M, Vellas B, Spenger C, Lovestone S. Plasma biomarkers of brain atrophy in Alzheimer’s disease. PLoS One. 2011;6:e28527. doi: 10.1371/journal.pone.0028527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Maier M, Peng Y, Jiang L, Seabrook TJ, Carroll MC, Lemere CA. Complement C3 deficiency leads to accelerated amyloid beta plaque deposition and neurodegeneration and modulation of the microglia/macrophage phenotype in amyloid precursor protein transgenic mice. J Neurosci. 2008;28:6333–41. doi: 10.1523/JNEUROSCI.0829-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Krishnan AV, Feldman D. Mechanisms of the anti-cancer and anti-inflammatory actions of vitamin D. Annu Rev Pharmacol Toxicol. 2011;51:311–336. doi: 10.1146/annurev-pharmtox-010510-100611. [DOI] [PubMed] [Google Scholar]
- 88.Smolders J, Moen SM, Damoiseaux J, Huitinga I, Holmoy T. Vitamin D in the healthy and inflamed central nervous system: access and function. J Neurol Sci. 2011;311:37–43. doi: 10.1016/j.jns.2011.07.033. [DOI] [PubMed] [Google Scholar]
- 89.Stewart SE, Fagerness JA, Platko J, Smoller JW, Scharf JM, Illmann C, Jenike E, Chabane N, Leboyer M, Delorme R, Jenike MA, Pauls DL. Association of the SLC1A1 glutamate transporter gene and obsessive-compulsive disorder. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:1027–33. doi: 10.1002/ajmg.b.30533. [DOI] [PubMed] [Google Scholar]
- 90.Arnold PD, Sicard T, Burroughs E, Richter MA, Kennedy JL. Glutamate transporter gene SLC1A1 associated with obsessive-compulsive disorder. Arch Gen Psychiatry. 2006;63:769–76. doi: 10.1001/archpsyc.63.7.769. [DOI] [PubMed] [Google Scholar]
- 91.Dickel DE, Veenstra-VanderWeele J, Cox NJ, Wu X, Fischer DJ, Van Etten-Lee M, Himle JA, Leventhal BL, Cook EH, Jr, Hanna GL. Association testing of the positional and functional candidate gene SLC1A1/EAAC1 in early-onset obsessive-compulsive disorder. Arch Gen Psychiatry. 2006;63:778–85. doi: 10.1001/archpsyc.63.7.778. [DOI] [PubMed] [Google Scholar]
- 92.Aoyama K, Suh SW, Hamby AM, Liu J, Chan WY, Chen Y, Swanson RA. Neuronal glutathione deficiency and age-dependent neurodegeneration in the EAAC1 deficient mouse. Nat Neurosci. 2006;9:119–26. doi: 10.1038/nn1609. [DOI] [PubMed] [Google Scholar]
- 93.Martin HL, Teismann P. Glutathione-a review on its role and significance in Parkinson’s disease. FASEB J. 2009;23:3263–72. doi: 10.1096/fj.08-125443. [DOI] [PubMed] [Google Scholar]
- 94.Hirsch-Reinshagen V, Donkin J, Stukas S, Chan J, Wilkinson A, Fan J, Parks JS, Kuivenhoven JA, Lutjohann D, Pritchard H, Wellington CL. LCAT synthesized by primary astrocytes esterifies cholesterol on glia-derived lipoproteins. J Lipid Res. 2009;50:885–93. doi: 10.1194/jlr.M800584-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Demeester N, Castro G, Desrumaux C, De Geitere C, Fruchart JC, Santens P, Mulleners E, Engelborghs S, De Deyn PP, Vandekerckhove J, Rosseneu M, Labeur C. Characterization and functional studies of lipoproteins, lipid transfer proteins, and lecithin:cholesterol acyltransferase in CSF of normal individuals and patients with Alzheimer’s disease. J Lipid Res. 2000;41:963–74. [PubMed] [Google Scholar]
- 96.Broggini T, Nitsch R, Savaskan NE. Plasticity-related gene 5 (PRG5) induces filopodia and neurite growth and impedes lysophosphatidic acid- and nogo-A-mediated axonal retraction. Mol Biol Cell. 2010;21:521–37. doi: 10.1091/mbc.E09-06-0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pernet V, Schwab ME. The role of Nogo-A in axonal plasticity, regrowth and repair. Cell Tissue Res. 2012;349:97–104. doi: 10.1007/s00441-012-1432-6. [DOI] [PubMed] [Google Scholar]
- 98.Gil V, Nicolas O, Mingorance A, Urena JM, Tang BL, Hirata T, Saez-Valero J, Ferrer I, Soriano E, del Rio JA. Nogo-A expression in the human hippocampus in normal aging and in Alzheimer disease. J Neuropathol Exp Neurol. 2006;65:433–44. doi: 10.1097/01.jnen.0000222894.59293.98. [DOI] [PubMed] [Google Scholar]
- 99.Roesler R, Henriques JA, Schwartsmann G. Gastrin-releasing peptide receptor as a molecular target for psychiatric and neurological disorders. CNS Neurol Disord Drug Targets. 2006;5:197–204. doi: 10.2174/187152706776359673. [DOI] [PubMed] [Google Scholar]
- 100.Roesler R, Lessa D, Venturella R, Vianna MR, Luft T, Henriques JA, Izquierdo I, Schwartsmann G. Bombesin/gastrin-releasing peptide receptors in the basolateral amygdala regulate memory consolidation. Eur J Neurosci. 2004;19:1041–5. doi: 10.1111/j.0953-816x.2004.03175.x. [DOI] [PubMed] [Google Scholar]
- 101.Roesler R, Luft T, Oliveira SH, Farias CB, Almeida VR, Quevedo J, Dal Pizzol F, Schroder N, Izquierdo I, Schwartsmann G. Molecular mechanisms mediating gastrin-releasing peptide receptor modulation of memory consolidation in the hippocampus. Neuropharmacology. 2006;51:350–7. doi: 10.1016/j.neuropharm.2006.03.033. [DOI] [PubMed] [Google Scholar]
- 102.Ohsawa I, Takamura C, Kohsaka S. Fibulin-1 binds the amino-terminal head of beta-amyloid precursor protein and modulates its physiological function. J Neurochem. 2001;76:1411–20. doi: 10.1046/j.1471-4159.2001.00144.x. [DOI] [PubMed] [Google Scholar]
- 103.Chan CS, Chen H, Bradley A, Dragatsis I, Rosenmund C, Davis RL. alpha8-integrins are required for hippocampal long-term potentiation but not for hippocampal-dependent learning. Genes Brain Behav. 2010;9:402–10. doi: 10.1111/j.1601-183X.2010.00569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lill CM, Roehr JT, McQueen MB, Kavvoura FK, Bagade S, Schjeide BM, Schjeide LM, Meissner E, Zauft U, Allen NC, Liu T, Schilling M, Anderson KJ, Beecham G, Berg D, Biernacka JM, Brice A, DeStefano AL, Do CB, Eriksson N, Factor SA, Farrer MJ, Foroud T, Gasser T, Hamza T, Hardy JA, Heutink P, Hill-Burns EM, Klein C, Latourelle JC, Maraganore DM, Martin ER, Martinez M, Myers RH, Nalls MA, Pankratz N, Payami H, Satake W, Scott WK, Sharma M, Singleton AB, Stefansson K, Toda T, Tung JY, Vance J, Wood NW, Zabetian CP, Young P, Tanzi RE, Khoury MJ, Zipp F, Lehrach H, Ioannidis JP, Bertram L. Comprehensive research synopsis and systematic meta-analyses in Parkinson’s disease genetics: The PDGene database. PLoS Genet. 2012;8:e1002548. doi: 10.1371/journal.pgen.1002548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang X, Patel R, Studzinski GP. hKSR-2, a vitamin D-regulated gene, inhibits apoptosis in arabinocytosine-treated HL60 leukemia cells. Mol Cancer Ther. 2008;7:2798–806. doi: 10.1158/1535-7163.MCT-08-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bumeister R, Rosse C, Anselmo A, Camonis J, White MA. CNK2 couples NGF signal propagation to multiple regulatory cascades driving cell differentiation. Curr Biol. 2004;14:439–45. doi: 10.1016/j.cub.2004.02.037. [DOI] [PubMed] [Google Scholar]
- 107.Malicet C, Giroux V, Vasseur S, Dagorn JC, Neira JL, Iovanna JL. Regulation of apoptosis by the p8/prothymosin alpha complex. Proc Natl Acad Sci U S A. 2006;103:2671–6. doi: 10.1073/pnas.0508955103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ripke S, Sanders AR, Kendler KS, Levinson DF, Sklar P, Holmans PA, Lin DY, Duan J, Ophoff RA, Andreassen OA, Scolnick E, et al. Genome-wide association study identifies five new schizophrenia loci. Nat Genet. 2011;43:969–76. doi: 10.1038/ng.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Levay K, Slepak VZ. Tescalcin is an essential factor in megakaryocytic differentiation associated with Ets family gene expression. J Clin Invest. 2007;117:2672–83. doi: 10.1172/JCI27465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Maroulakou IG, Bowe DB. Expression and function of Ets transcription factors in mammalian development: a regulatory network. Oncogene. 2000;19:6432–42. doi: 10.1038/sj.onc.1204039. [DOI] [PubMed] [Google Scholar]
- 111.Sin WC, Moniz DM, Ozog MA, Tyler JE, Numata M, Church J. Regulation of early neurite morphogenesis by the Na+/H+ exchanger NHE1. J Neurosci. 2009;29:8946–59. doi: 10.1523/JNEUROSCI.2030-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev. 2002;23:38–89. doi: 10.1210/edrv.23.1.0455. [DOI] [PubMed] [Google Scholar]
- 113.Chen AC, Yu K, Lane MA, Gudas LJ. Homozygous deletion of the CRABPI gene in AB1 embryonic stem cells results in increased CRABPII gene expression and decreased intracellular retinoic acid concentration. Arch Biochem Biophys. 2003;411:159–73. doi: 10.1016/s0003-9861(02)00732-4. [DOI] [PubMed] [Google Scholar]
- 114.Mizwicki MT, Menegaz D, Zhang J, Barrientos-Duran A, Tse S, Cashman JR, Griffin PR, Fiala M. Genomic and nongenomic signaling induced by 1alpha,25(OH)2-vitamin D3 promotes the recovery of amyloid-beta phagocytosis by Alzheimer’s disease macrophages. J Alzheimers Dis. 2012;29:51–62. doi: 10.3233/JAD-2012-110560. [DOI] [PubMed] [Google Scholar]
- 115.Soni M, Kos K, Lang IA, Jones K, Melzer D, Llewellyn DJ. Vitamin D and cognitive function. Scand J Clin Lab Invest Suppl. 2012;243:79–82. doi: 10.3109/00365513.2012.681969. [DOI] [PubMed] [Google Scholar]
- 116.Farid K, Volpe-Gillot L, Petras S, Plou C, Caillat-Vigneron N, Blacher J. Correlation between serum 25-hydroxyvitamin D concentrations and regional cerebral blood flow in degenerative dementia. Nucl Med Commun. 2012;33:1048–52. doi: 10.1097/MNM.0b013e32835674c4. [DOI] [PubMed] [Google Scholar]
- 117.Dickens AP, Lang IA, Langa KM, Kos K, Llewellyn DJ. Vitamin D, cognitive dysfunction and dementia in older adults. CNS Drugs. 2011;25:629–39. doi: 10.2165/11593080-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wion D, MacGrogan D, Neveu I, Jehan F, Houlgatte R, Brachet P. 1,25-Dihydroxyvitamin D3 is a potent inducer of nerve growth factor synthesis. J Neurosci Res. 1991;28:110–4. doi: 10.1002/jnr.490280111. [DOI] [PubMed] [Google Scholar]
- 119.Annweiler C, Herrmann FR, Fantino B, Brugg B, Beauchet O. Effectiveness of the combination of memantine plus vitamin d on cognition in patients with Alzheimer disease: a pre-post pilot study. Cogn Behav Neurol. 2012;25:121–7. doi: 10.1097/WNN.0b013e31826df647. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


