Abstract
Mouse model is ideal for investigating the genetic and developmental etiology of congenital heart disease. However, cardiovascular phenotyping for the precise diagnosis of structural heart defects in mice remain challenging. With rapid advances in imaging techniques, there are now high throughput phenotyping tools available for the diagnosis of structural heart defects. In this review, we discuss the efficacy of four different imaging modalities for congenital heart disease diagnosis in fetal/neonatal mice, including noninvasive fetal echocardiography, micro-computed tomography (micro-CT), micro-magnetic resonance imaging (micro-MRI), and episcopic fluorescence image capture (EFIC) histopathology. The experience we have gained in the use of these imaging modalities in a large-scale mouse mutagenesis screen have validated their efficacy for congenital heart defect diagnosis in the tiny hearts of fetal and newborn mice. These cutting edge phenotyping tools will be invaluable for furthering our understanding of the developmental etiology of congenital heart disease.
Keywords: imaging, congenital heart defect
INTRODUCTION
Congenital heart disease (CHD) is one of the most common birth defects, affecting up to 1% of live births, making it imperative to gain insight into the developmental etiology of CHD. This can be carried out with the use of animal models to experimentally investigate the cardiovascular developmental processes disrupted in CHD. Mice have proven to be especially effective for such studies, as mice, like humans, have four chamber hearts with separate systemic and pulmonary circulation essential for oxygenation of blood. These are the anatomical structures disrupted in CHD. Furthermore, production of mouse models with a wide spectrum of CHD has been made possible with a combination of forward genetic approaches with chemical mutagenesis and gene trap strategies, and reverse genetic approaches involving gene targeting to generate knockout/knockin mouse models. Together, these different experimental approaches have provided a plethora of CHD mouse models that will undoubtedly advance our understanding of the developmental mechanisms and molecular etiology of CHD.
One of the current challenges in experimental modeling of CHD is the development of high resolution and high throughput cardiovascular phenotyping tools for the precise diagnosis of structural heart defects in these mutant mouse models. This requires imaging modalities that can interrogate the tiny hearts of fetal mice that are only 1–2 mm at midgestation, growing to just 3.5 mm at birth. The detailed characterization of the structural heart malformations is therefore technically challenging from an imaging perspective. Typically, such studies have classically relied on the use of paraffin histology analysis. While this can be highly informative, it is time consuming, and often the sectioning plane limits the anatomical assessments that can be made. This problem is particularly acute with analysis of structural heart malformations, as the assessment for atrial or ventricular septal defects, outflow tract malalignment, and atrioventricular valve abnormalities each require histological sections generated in different orientation. As a result, it is usually not possible to obtain a complete characterization of the totality of structural heart defects in any one specimen. This limitation has often resulted in poor or less than complete characterization of the structural heart defects in many mutant mouse models.
In this review, we briefly summarize the application of four different imaging modalities, which either individually or in combination can provide accurate diagnosis of structural heart defects in fetal/newborn mice: fetal echocardiography, micro computed tomography (micro-CT), micro magnetic resonance imaging (microMRI), and episcopic fluorescence image capture (EFIC) histopathology. The development of these four different imaging modalities for analysis of structural heart defects in fetal/neonatal mice emerged from our pursuit of a large scale mouse ethylnitroso urea (ENU) mutagenesis screen to recover mutant mouse models with CHD. Pregnant female mice were ultrasound scanned by noninvasive fetal echocardiography to identify fetuses with CHD. This was followed by further cardiovascular phenotyping using microMRI/microCT, and EFIC histopathology examinations for definitive CHD diagnosis. Below we briefly discuss each of the four imaging modalities in turn, and summarize the capabilities of each imaging modality. These are presented through the perspective we gained from our large scale mouse mutagenesis screen, and we expect that this cumulative experience will aid others interested in interrogating for congenital heart defects in mice.
ULTRASOUND PHENOTYPING
Ultrasound imaging, given its high temporal and spatial resolution, is a powerful tool for the analysis of cardiovascular development and disease in mice. This can be used for analysis of cardiovascular development and function from embryonic to fetal stages of development. Similar to the clinical pre-term assessment of human pregnancies, it is possible to conduct noninvasive real time interrogation of both cardiovascular structure and function using fetal echocardiography. As with clinical echocardiography, echocardiography in fetal mice is very high throughput, and allows longitudinal studies that can yield invaluable information on developmental changes in cardiovascular structure and functions (Phoon, 2006). At present, there are two major ultrasound platforms used for fetal mouse ultrasound imaging: clinical ultrasound systems and a research system referred to as the ultrasound biomicroscopy. The latter system has higher imaging resolution, particularly advantageous for imaging small fetal mouse hearts.
CARDIOVASCULAR PHENOTYPING FETAL MICE WITH CLINICAL ULTRASOUND
The clinical ultrasound system extensively used for imaging fetal mice is the Acuson Sequoia (Spurney et al., 2006; Tobita et al., 2010). This clinical ultrasound machine when used with a 15 MHz linear array vascular transducer can provide two dimensional (2D) imaging with axial resolution of 440 μm and lateral resolution of 630 μm. Together with the use of color flow and spectral Doppler imaging modalities, fetal mice can be readily detected in the uterine horns as early as embryonic day 8.5 (E8.5), when the linear heart tube begins to beat. Using imaging depths of 20–30 mm and image frame size of 25 mm, the location of multiple fetuses in the uterine horn can be visualized and their relative orientation determined. Measurements can be obtained to assess growth with measurement of the fetus crown to rump length and fetus area. The 2D imaging can readily detect hydrops or edema, also referred to clinically as nuchal translucency, which is highly associated with pending death and possible cardiac defects. Similarly, pericardial effusion can be easily detected, which often is observed in fetuses with failing hearts associated with cardiac anomalies and is indicative of pending death (Yu et al., 2004; Shen et al., 2005).
While the clinical ultrasound’s spatial resolution is limited, the use of color flow combined with spectral Doppler imaging provides assessment of hemodynamic function that can greatly aid in identifying structural cardiovascular anomalies, including assessment of the relative position of inflow and outflow tracts for malalignment defects and identification of abnormal blood flow indicative for assessment of possible valvular abnormalities, such as with increased flow velocity, valvular regurgitation, and abnormal shunt (Yu et al., 2004; Shen et al., 2005). Spectral Doppler is a useful tool for obtaining quantitative measurement of heart function by measuring blood flow profiles which include the velocity time integral (VTI), the ratio of early (E) to late (A) filling velocities across mitral valve, the deceleration time of the E wave, the time for the pressure/velocity to decrease by 50% (pressure half-time) across valve, myocardial performance index by calculating ejection time (ET), isovolumic relaxation time (IRT), isovolumic contraction time (ICT) (Moran et al., 2013; Phoon and Turnbull, 2003; Scherrer-Crosbie and Kurtz, 2010). There was good reproducibility in these spectral Doppler measurements after E14.5 (Yu et al., 2008). While M-mode measurements also can be obtained, our previous study showed (Yu et al., 2008) that due to the difficulty in achieving a true short axis view in ultrasound imaging of fetal mice, M-mode data for measurement of ventricular wall thickness and chamber dimensions were obtained for only 25% of the fetuses after embryonic day 14.5. Ventricular contractility indices, including fractional shortening (FS), and ejection fraction (EF), were derived from these measurements. An alternative means for measuring contractility entailed tracing the left ventricular area in diastole and systole using 2D images, which can be obtained in 50% of fetuses, but given the limited 2D resolution, the endocardial borders are often not clearly delineated within the ventricles, making the measurements problematic.
Overall, clinical ultrasound imaging performed with a 15-MHz transducer is well suited for high throughput phenotypic screening of fetal mice to monitor fetal growth, measure heart rates and flow velocities, assess cardiac contractility, and detect valvular regurgitation, pericardial effusion, and fetal hydrops. However, given the limited 2D imaging resolution of clinical ultrasound systems, the assessment of cardiovascular anatomy is largely inferred from the Doppler color flow imaging, spectral Doppler assessments, and M-mode measurements. The definitive diagnosis of specific cardiac defects still requires follow up analysis of the fetuses using other imaging modalities. Nevertheless, our experience has shown the Acuson clinical ultrasound system is an outstanding platform for high throughput cardiovascular phenotyping of fetal mice, although definitive diagnosis of the structural heart defects will require further analysis (see below) (Yu et al., 2004; Shen et al., 2005).
CARDIOVASCULAR PHENOTYPING FETAL MICE WITH ULTRASOUND BIOMICROSCOPY
Ultrasound biomicroscopy (UBM) has emerged as one of the most exciting imaging modalities for noninvasive interrogation of living mouse embryos. With the much higher 2D imaging resolution of the Visualsonics Vevo2100 UBM, mouse fetuses can be interrogated from much earlier developmental stages, allowing detailed 2D imaging of the cardiovascular system prior to cardiac and outflow tract septation at E12.5. UBM can provide 30 μm axial × 68 μm lateral resolution and ultrasound transducers are available with different ultrasound frequencies (30–50 MHz), suitable for in utero imaging of embryos requiring different penetration depths. Together with the availability of color flow spectral Doppler imaging for hemodynamic assessments, it is now possible to directly visualize and assess for structural cardiac anomalies, even in mouse embryos at E11.5 or younger (Moran et al., 2013; Tobita et al., 2010).
Using the UBM, a wide spectrum of CHD can be identified, which included outflow tract malalignment defects such as double outlet right ventricle (DORV) (Fig. 1), transposition of great arteries and persistent truncus arteriosus (PTA)/pulmonary atresia (PA) (Fig. 2), a variety of cardiac septation defects, including ventricular septum defect, atrioventricular septa defects (Fig. 1), left or right heart obstructive lesions comprising pulmonary stenosis, tricuspid stenosis or atresia, aortic stenosis or atresia, mitral stenosis or atresia, coarctation, hypoplastic right heart syndrome and hypoplastic left heart syndrome. Also detected by UBM are coronary artery fistula and cardiac situs anomalies (dextrocardia, heterotaxy). However, UBM imaging cannot distinguish between PTA versus PA, DORV versus overriding aorta, secundum atrial septal defect versus foramen ovale. Also difficult to assess by UBM are aortic arch anomalies.
Figure 1.
DORV with AVSD diagnosed by ultrasound imaging. Ultrasound phenotyping using the clinical ultrasound (Acuson) system identified a mouse fetus with hydrops (A, arrowhead). More detailed analysis using the higher resolution UBM revealed aorta arising from the right ventricle (RV), pulmonary artery (PA) overriding on the ventricular septum, and a subpulmonary artery ventricular setpal defect (VSD) (B, arrowhead). Together this suggested double outlet right ventricle (DORV) of the Taussig–Bing subtype. In addition, UBM scanning in the transverse view showed the presence of an atrioventricular septal defect (AVSD) (C, arrowhead) accompanied by common valve regurgitation (D, arrowhead). The finding of DORV (E) and AVSD (F) were confirmed by EFIC histopathology. The position of the fetus is as denoted by the bidirectional arrows: anterior (A), posterior (P), left (L), right (R), cranial (Cr), Caudal (Cd). Scale bar = 1 mm.
Figure 2.

Ultrasound diagnosis of persistent truncus arteriosus with mesocardia, biventricular hypertrophy. Outflow tract regurgitation was detected by color flow imaging using the Acuson (A), which was confirmed by spectral Doppler analysis (B). Higher resolution imaging with the UBM revealed mesocardia (C), biventricular hypertrophy (C, D), VSD and persistent truncus arteriosus (PTA) overriding on the ventricular septum (D). UBM findings were confirmed by subsequent EFIC histopathology which showed a muscular VSD (E), PTA and (F) and biventricular hypertrophy (E, F). RV, right ventricle; LV, left ventricle; VSD, ventricular septal defect; OFT, outflow tract; PTA, persistant truncus arteriosus; mVSD, muscular VSD; LPA, left pulmonary artery. AVSD, atrioventricular septal defect; Ao, aorta; PA, pulmonary artery. Scale bar = 1 mm.
Using the higher spatial resolution of the UBM, it is possible to obtain quantitative measurements for assessing fetal mouse cardiac function in utero (Moran et al., 2013; Phoon and Turnbull, 2003). Thus using the UBM acquired high-resolution 2D images, the internal cavity and endocardial border of the ventricular chamber can be visualized, allowing direct measurement of ejection fraction and fractional shortening to assess contractility. Moreover, the UBM comes equipped with much higher frame rate of up to 1,000 frame sec−1, as compared to the maximum 200 frame sec−1 in clinical ultrasound systems. With this higher frame rate, it is possible to quantitatively assess myocardial wall motion with speckle tracking and strain analysis (in both fetal and adult mice), which are sensitive indicators of early myocardial dysfunction (Peng et al., 2009; Frank et al., 2010; Tobita et al., 2010).
Overall, ultrasound scanning with the UBM, while much higher resolution, is also more time consuming compared to the clinical ultrasound systems, making it less suitable for high throughput screening applications. A further limitation is the fact that nucleated red blood cells in the embryo/fetus is echogenic at the higher Doppler frequencies of the UBM. This obscures the endocardial border and ventricular lumen until after E15.5, when hematopoiesis transitions to the mature enucleated erythrocytes. Nevertheless, our extensive experience using the UBM shows this ultrasound system is a highly sensitive phenotyping tool for comprehensive assessment of mouse fetal cardiovascular structure and function. Its noninvasive imaging capability makes it ideally suited for assessing and tracking cardiovascular development and function in the living mouse embryo, allowing analysis of the emergence of cardiovascular anomalies over the course of development.
PHENOTYPING BY MICRO-COMPUTED TOMOGRAPHY
Micro-computed tomography (micro-CT) is an imaging modality that uses ionizing radiation to achieve high imaging resolution. This imaging technique has been extensively used in rodent animal research (Schambach et al., 2010; Tobita et al., 2010; Ritman, 2011). Like ultrasound phenotyping, it is noninvasive and thus can be conducted without compromising the ability to further pursue downstream histological analysis (Degenhardt et al., 2010; Schambach et al., 2010; Tobita et al., 2010) Furthermore, the micro-CT data allows ready 3D visualization of the whole animal anatomy. Recently, high-resolution micro-CT systems have been developed with spatial resolution at near cellular levels (<1 μm) (Schambach et al., 2010; Cheng et al., 2011), but micro-CT imaging resolution of 15–45 μm is adequate for the visualization and analysis of fetal and newborn mouse hearts. Visualization of soft tissue using micro-CT does require staining with a contrast agent, such as iodine, osmium, uranium, zinc, or tungsten. Differences in the efficacy of sample staining can cause variability in the quality and imaging resolution achieved (Cheng et al., 2011; Hiraiwa et al., 2013).
Micro-CT systems currently available are used mostly to examine postmortem fixed tissue samples, as the imaging cannot be acquired in real-time (Tobita et al., 2010; Gregg and Butcher, 2012). We have found micro-CT scanning of developing mouse fetus and newborn animals fixed and stained with iodine provides high resolution images suitable for cardiovascular phenotyping. Micro-CT imaging, with its capability for rapid 3D image reconstruction, provides the means to perform virtual autopsies. Only fetuses/pups identified to have structural heart defects by micro-CT are then further examined with more time consuming phenotyping analysis involving necropsy and histopathology examination (Degenhardt et al., 2010; Tobita et al., 2010). We have used micro-CT imaging as a secondary imaging modality in our mouse ENU mutagenesis screen to recover mutants with CHD. Using contrast enhanced micro-CT imaging, a wide spectrum of CHD can be reliably detected in such post-mortem examinations of fetal/newborn mice (Kim et al., 2013). This can be high throughput, with up to 12 pups scanned simultaneously, with time required for image acquisition varying depending on the desired image resolution. Typically a single 1-h scan can be used to image four embryos (embryonic day 12 to 16) at 15 μm resolution or 12 newborn pups at 45 μm resolution. The acquired images can be viewed in arbitrary imaging planes and rapidly 3D reconstructed, thereby allowing a full assessment of structural heart anomalies without sample destruction (Fig. 3).
Figure 3.
Micro-CT scan of newborn pups reconstructed in different imaging planes. Micro-CT image was acquired at 45-μm resolution. Acquired CT image files were reconstructed using the OsirIX image software. Individual sample images were further processed for its own body axis and heart orientation for phenotype diagnosis. Left upper panel: sagittal view, left lower panel: transverse view, right panel: coronal (anterior–posterior) view.
Most of the major CHDs observed clinically can be visualized by micro-CT imaging in mouse embryos and newborn pups, with 15 μm required for examining the fetal samples and 45 μm for the newborn pup. Cardiac defects that can be detected by micro-CT imaging include all varieties of outflow tract anomalies, atrioventricular and ventricular septal defects, and ventricular hypoplasia or hypertrophy (Fig. 4). Even larger coronary artery fistulas, and aortic arch anomalies can be readily detected including interrupted aortic arch, right-sided aortic arch, and hypoplastic aortic arch. However, micro-CT imaging does not allow detailed visualization of the cardiac valves and papillary muscle chordae tendineae architecture, nor small coronary artery/vein, or hypoplastic or abnormal collateral vessels. While currently available higher resolution micro-CT imaging system can visualize these tiny cardiovascular structures, there is a significant increase in image acquisition/image processing time and also the acquired image file size is much larger as well. Overall, micro-CT is useful as a phenotyping tool to identify fetal/newborn mice with CHD, but histopathological assessment is still required to confirm the specific CHD diagnoses.
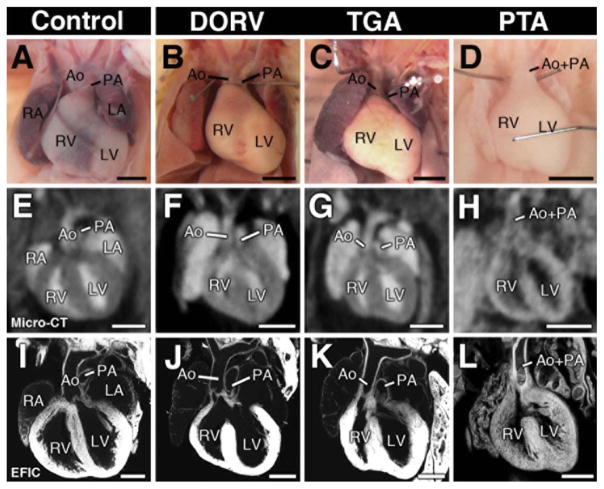
Figure 4.
Micro-CT detection of outflow tract defects in newborn mice. Micro-CT in the coronal imaging plane revealed OFT malalignment defects, including double outlet right ventricle (DORV; F), transposition of the great arteries (TGA;G) and persistent truncus arteriosus (PTA;H). Necropsy and EFIC image of each heart is shown in (A–D) and (E–L). Scale bars indicate 1 mm (A–H), 0.5 mm (I–L).
PHENOTYPING USING MICRO-MAGNETIC RESONANCE IMAGING
Micro-MRI is another very powerful nondestructive imaging technology suitable for phenotyping fetal/newborn mice (Berrios-Otero et al., 2009; Hogers et al., 2009; Nieman and Turnbull, 2010). MRI detects changes in the magnetic field of hydrogen atoms (and other) within the target tissue, converting this information into 2D or 3D images of the sample. MRI systems with high-magnetic field strengths (micro-MRI) with 7 to 14-Tesla magnets suitable for small animal imaging are commercially available (Yelbuz et al., 2004; Petiet et al., 2008; Berrios-Otero et al., 2009; Cleary et al., 2009; Hogers et al., 2009; Pallares et al., 2009; Parnell et al., 2009; French et al., 2010; Nieman and Turnbull, 2010; Tobita et al., 2010; Yamada et al., 2010; Zouagui et al., 2010). Some of these high-magnetic field systems can reach imaging resolution of ~20 μm, equivalent to the resolution of the UBM (Zouagui et al., 2010). In contrast to micro-CT, MRI can provide high contrast resolution for visualizing soft tissues without the need for staining with a contrast agent. MRI is also advantageous, as it can obviate the use of harmful ionizing radiation as in micro-CT.
Typically, we conduct MRI scan of 6 to 8 fetal/newborn mouse samples simultaneously with image acquisition set at 60–80 μm resolution; this can provide similar phenotype information as micro-CT scans conducted at 45-μm resolution (Fig. 5). A single MRI scan requires considerably longer scan time of 4–5 h, but the image file size is ~200 MB, which is less than 1/10 the file size of CT data acquired at 45-μm image resolution. The smaller image file size allows rapid image transfer and offline image processing. Therefore, total time required for CHD phenotyping by MRI is equivalent to that of micro-CT.
Figure 5.

Micro-MRI detection of ventricular septal defect and overriding aorta. Micro-MRI (A) accurately detects prominent ventricular septal defect (asterisk in A) and overriding aorta, but does not allow assessment of fine structures. In contrast, EFIC imaging provides detailed intracardiac anatomy including visualization of the aortic, mitral, and tricuspid valve leaflets and papillary muscle morphology, which could not be identified by micro-MRI. MRI: T1 weighted image sequence (TE: 24 msec, TR: 1300 msec). Scale bar indicates 200 μm.
While the currently available high-resolution MRI is limited to postmortem samples due to the high sensitivity to motion artifacts and the requisite long image acquisition time, live cardiac imaging of newborn mice can be conducted with MRI when advanced electrocardiogram and respiratory gating techniques are used together with a special animal cradle (Fig. 6) (Wiesmann et al., 2000). Although current micro-MRI has less overall image resolution compared to contrast enhanced micro-CT imaging, the image quality is more consistent across different samples, given the MRI signal is not dependent on contrast agent staining. Overall, the detection sensitivity for structural heart defects with micro-MRI is very similar to that of micro-CT, and as with micro-CT cardiac valve, papillary muscle chordae, and small vessel morphology, are not well visualized. While MRI has longer scan times when compared to micro-CT, image processing of MRI data is simpler and much faster.
Figure 6.

Micro-MRI cardiac cine mode acquisition (FLASH cine mode) of neonate day 0 normal newborn mouse. Cardiac cine mode image was acquired using FLASH cine (TE: 2.5 msec, TR: 8 msec, 256 × 256 matrix) image sequence with electrocardiogram and respiratory gating in a day 0 newborn mouse. Ao: aorta; RV: right ventricle; LV: left ventricle; mPA: main pulmonary artery; Diast: diastole; Syst: systole. Scale bar indicates 200 μm.
HISTOPATHOLOGY WITH EPISCOPIC FLUORESCENCE IMAGE CAPTURE
Histopathology examination remains the gold standard for the precise diagnosis of congenital heart defects. Thus even after ultrasound, micro-CT or micro-MRI phenotyping, histopathology examination is required to confirm any CHD diagnosis generated by these other imaging modalities. However, the complex anatomy of the cardiovascular system makes it difficult to arrive at a complete CHD diagnosis for each specimen, since the plane of sectioning of the paraffin embedded sample will ultimately determine what structures in the heart can be visualized. For example, sections in the transverse plane would not be suitable for assessment of atrioventricular septal or outflow tract malalignment defects. This problem is exacerbated by the fact that with complex congenital heart disease, structures in the heart are not predictably visualized in the expected imaging plane. Observation of more subtle defects (e.g., small septal defects) is entirely dependent on sample orientation during sectioning, and more complex defects, such as double outlet right ventricle, transposition of the great arteries, or major aortopulmonary collateral arteries can be difficult or impossible to diagnose depending on the sectioning plane. This contrasts with MRI or CT imaging, where the imaging data obtained can be readily reconstructed in 3D or resliced in any imaging plane.
To address this major shortcoming of histology, various algorithms have been developed with varying degrees of success to align and generate registered Z-stacks for 3D reconstructions (Feuerstein et al., 2011; Saalfeld et al., 2012). One of the major difficulties with reregistering paraffin sections is the mechanical deformation of the section as it is sliced off the paraffin block. To accommodate such deformations, registration algorithms often sacrifice resolution to allow alignment of serial sections into a single Z-stack. An ingenious solution to this problem was arrived at with the development of episcopic fluorescence image capture (EFIC) pioneered by Weninger et al. (1998). With EFIC imaging, the paraffin block face of the embedded sample is imaged as each section is cut, with the imaging carried out using tissue autofluorescence for visualizing the embedded tissue. Using this unconventional approach, the Z-stack collected of the specimen is perfectly registered so long as the paraffin block remained in a fixed position (Rosenthal et al., 2004; Weninger and Mohun, 2002). Moreover, with imaging of the block face, there is no distortion arising from deformation of the paraffin section and perfectly registered Z-stacks can be generated that allow rapid 3D reconstructions and also virtual resectioning of the Z-image stack in any orientation.
When EFIC imaging was first developed, the samples were embedded in paraffin infiltrated with Sudan IV, a dye that acts as a strong light-blocking compound. This allowed epifluorescent images to be captured from the block face with minimal bleed through of tissue autofluorescence from the underlying tissue in the block. While this worked effectively, the embedding process was difficult, requiring a very high temperature. The high temperature and messy red wax together made it necessary to process such paraffin embedding manually, making the process labor intensive and problematic, often with variable results. Despite this, excellent results can be achieved, with images of a quality better than those obtained with high-resolution micro-MRI or micro-CT. In contrast to MRI or CT, fine intracardiac anatomy can be visualized with EFIC imaging, such as valvular morphology, papillary muscles and ventricular trabeculation (Fig. 5). Since the early days of EFIC imaging, we have developed the use of confocal-enhanced EFIC imaging. With confocal EFIC, the sample can be processed using automatic paraffin processors without any light blocking compound and image at the block face is captured by confocal imaging. This new confocal enhanced EFIC imaging generates 2D image stacks of even higher resolution than those obtained with the red wax embedded samples (Figs. 7 and 8). Moreover, as the samples are not heated to high temperature, the paraffin sections can be collected for further analysis with immunohistochemistry or other types of analysis.
Figure 7.
Visualization of detailed cardiac anatomy by EFIC imaging. EFIC imaging allows the visualization of fine intracardiac anatomy important for the diagnosis of structural heart defects including semilunar and atrioventricuclar valvular morphology, outflow tract and atrioventricular valve alignment, venous drainage, as well as the atrial and ventricular septum, and the compact and trabeculated ventricular myocardium. (A) The 3D reconstruction of EFIC image stack showing heart and surrounding tissues in situ within the chest cavity. (B) RV-pulmonary valve connection with semilunar valve leaflets. (C) LV-aortic valve connection with the aortic valve leaflets. Prominent trabeculae can be observed in the LV. (D) Atrioventricular connection delineated by tricuspid and mitral valves in the right and left ventricle. Also visible is the foramen ovale (FO), and right and left superior vena cava (RSVC, LSVC), and right and left pulmonaroy artrery (RPA, LPA). (E) Superior and inferior vena cava drainage into the right atria. (F) Pulmonary venous drainage into the left atria. Scale bars = 0.5 mm. Ao: Aorta; DA: Ductus arteriosus; PT: Pulmonary trunk; LPA: Left pulmonary artery; RPA: Right pulmonary artery; FO: foramen ovale; RA: Right atrium; LA: Left atrium; RV: Right ventricle; LV: Left ventricle; T: Trachea; RSVC: Right superior vena cava; LSVC: Left superior vena cava; IVC: Inferior vena cava.
Figure 8.
Diagnosis of congenital heart defects by EFIC imaging. (A–B) Coronal 3D and 2D reconstructions show isolated double outlet right ventricle in a newborn mouse. (C–D) Coronal 3D and 2D reconstructions of a newborn mouse heart showing transposition of the great arteries. (E–F) Coronal 3D reconstructions of a newborn mouse heart exhibiting persistent truncus arteriosus (PTA). Scale bars = 0.5mm. Ao: Ascending Aorta; tAo: transverse Aorta; PTA: Persistent truncus arteriosus; IA: Innominate artery; CC: common carotid artery; DA: Ductus arteriosus; PT: Pulmonary trunk; PA: Pulmonary artery; T: Trachea; O: Oesophagus; RSVC: Right superior vena cava; LSVC: Left superior vena cava; RV: Right ventricle; LV: Left ventricle; RA: Right atrium; LA: Left atrium.
While no commercial instrumentation package exists for EFIC imaging, this can be easily put together using commercially available components. This includes a Leica SM2500 sledge microtome, customized with a MZFLIII stereomicroscope equipped for epifluorescence imaging or a Lecia LSI confocal microscope mounted over the sample block. An additional software interface is used to automatically trigger and integrate image acquisition with the automated sectioning. The resulting completely registered Z-image stacks can be analyzed in the same manner and using the same software routinely used to analyze micro-CT/micro-MRI datasets. Amongst many different software options, the open-source PACS workstation DICOM viewer Osirix has major advantages, being inexpensive and user friendly (Rosset et al., 2004). Using Osirix, it is possible to virtually resection the 2D image stacks in any orientation as well as generate high resolution 3D reconstructions with ease.
EFIC DIAGNOSIS OF CONGENITAL HEART DEFECTS
We routinely use EFIC imaging as our gold standard to confirm CHD diagnosis in fetal/newborn mice. The utility of EFIC for visualizing cardiac anatomy is highlighted in Figure 7. All the images presented in Figure 7 are generated from a single EFIC Z-stack from a wildtype newborn mouse heart. Examination of images from these different imaging orientations allow the assessment of all the major cardiac components, including the right ventricular-pulmonary valve connection (Fig. 7B), left ventricular-aortic valve connection (Fig. 7C), atrioventricular connections (Fig. 7D), and systemic/pulmonary venous drainage into the atria (Figs. 7E and 7F). The outflow tract connections can be particularly difficult to classify by necropsy examination or standard histology, which can only provide 2D slices from a single orientation dependent on sample placement within the paraffin block. However, the perfectly registered Z-stacks generated by EFIC allow for the easy reslicing of the sample in any orientation, making it possible to precisely diagnose any structural heart defects. Some examples of outflow tract defects visualized by EFIC imaging is shown Figure 8, including double outlet right ventricle (DORV; Figs. 8A and 8B), transposition of the great arteries (TGA; Figs. 8C and 8D), and persistent truncus arteriosus (PTA; Figs. EFIC 8E and 8F). Moreover the ability to generate 3D reconstructions from EFIC generated Z-image stacks not only allows detection of atrioventricular septal defects (AVSD) (Fig. 9), but it can readily differentiate between partial (Figs. 9C and 9D), and complete AVSD (Figs. 9E and 9F), which would be difficult to discern by standard paraffin histology.
Figure 9.
Ventricular and atrioventricular septal defects visualized by EFIC imaging in Dnai1 mutant mouse hearts. EFIC image stacks of three Dnaic1 mutant mouse hearts show normal cardiac anatomy in one animal (A, B), while the other two (C–F) exhibited complex congenital heart defects. Shown are coronal 2D images (A, C, E) and transverse 3D reconstructions (B, D, F) generated from the EFIC image stacks. The 3D reconstructions are viewed from the atrium into the AV junction. A, B) Dnai1 mutant shows normal heart with D-looped ventricles, normal positioning of the great arteries and atrioventricular septum. (C, D) L-looped heart with partial AVSD as demonstrated by the presence of a large inter-atrial communication (*) below an intact foramen ovale (FO) and above two discrete AV valves connected to an intact VS. (E, F) L-looped heart with perimembranous VSD forming part of a complete AVSD, muscular VSD within the ventricular septum, and noncompaction of the ventricular myocardium (Figure originally published in (Tan et al., 2007)). Scale bars = 0.2 mm. Ao: Ascending Aorta; PT: Pulmonary trunk; FO: foramen ovale; ASD: atrial septal defect; VSD: ventricular septal defect; RA: Right atrium; LA: Left atrium; RV: Right ventricle; LV: Left ventricle; CA: common atrium; mLA: morphological left atrium; mRA: morphological right atrium; mLV: morphological left ventricle; mRV: morphological right ventricle; RSVC: Right superior vena cava; LSVC: Left superior vena cava.
SUMMARY
We discussed the efficacy of four different imaging modalities, ultrasonography, micro-CT, micro-MRI and EFIC imaging for phenotyping congenital heart disease in fetal/newborn mice. Ultrasound imaging has the distinct advantage in allowing the interrogation of living embryos, while micro-MRI/micro-CT provide high throughput postmortem examination of fetal/newborn mice for structural heart defect assessments. Ultimately the finding of CHD obtained by ultrasound, or micro-CT/micro-MRI imaging requires confirmation by histopathology examination. We show that EFIC imaging provides a powerful new approach for histopathology analysis that allows the complete assessment of structural heart defect in every specimen regardless of embedding or sectioning orientation. With the combined use of these imaging modalities, it should be possible to identify CHD in every mutant. Further technical improvements of these imaging technologies will no doubt further transform and accelerate our understanding of the developmental etiology of congenital heart disease. We expect refinement of confocal EFIC imaging will allow detection and analysis of fluorescent protein expression for tracking cell lineage and defining gene expression domains in 3D. Moreover, further development of live MRI imaging of fetal and newborn mice may provide new avenues for interrogating the pathology and mechanism(s) of disease underlying structural heart anomalies.
Acknowledgments
Supported by a grant from the NIH (U01-HL098180)
References
- Berrios-Otero CA, Wadghiri YZ, Nieman BJ, Joyner AL, Turnbull DH. Three-dimensional micro-MRI analysis of cerebral artery development in mouse embryos. Magn Reson Med. 2009;62:1431–1439. doi: 10.1002/mrm.22113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng KC, Xin X, Clark DP, La Riviere P. Whole-animal imaging, gene function, and the Zebrafish Phenome Project. Curr Opin Genet Dev. 2011;21:620–629. doi: 10.1016/j.gde.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary JO, Price AN, Thomas DL, Scambler PJ, Kyriakopoulou V, McCue K, Schneider JE, Ordidge RJ, Lythgoe MF. Cardiac phenotyping in ex vivo murine embryos using microMRI. NMR Biomed. 2009;22:857–866. doi: 10.1002/nbm.1400. [DOI] [PubMed] [Google Scholar]
- Degenhardt K, Wright AC, Horng D, Padmanabhan A, Epstein JA. Rapid 3D phenotyping of cardiovascular development in mouse embryos by micro-CT with iodine staining. Circ Cardiovasc Imaging. 2010;3:314–322. doi: 10.1161/CIRCIMAGING.109.918482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuerstein M, Heibel H, Gardiazabal J, Navab N, Groher M. Reconstruction of 3-D histology images by simultaneous deformable registration. Med Image Comput Comput Assist Interv. 2011;14 (Part 2):582–589. doi: 10.1007/978-3-642-23629-7_71. [DOI] [PubMed] [Google Scholar]
- Frank LH, Yu Q, Francis R, Tian X, Samtani R, Sahn DJ, Leatherbury L, Lo CW. Ventricular rotation is independent of cardiac looping: a study in mice with situs inversus totalis using speckle-tracking echocardiography. J Am Soc Echocardiogr. 2010;23:315–323. doi: 10.1016/j.echo.2009.11.024. [DOI] [PubMed] [Google Scholar]
- French J, Gingles N, Stewart J, Woodhouse N. Use of magnetic resonance imaging (MRI) and micro-computed tomography (micro-CT) in the morphological examination of rat and rabbit fetuses from embryo-fetal development studies. Reprod Toxicol. 2010;30:292–300. doi: 10.1016/j.reprotox.2010.04.016. [DOI] [PubMed] [Google Scholar]
- Gregg CL, Butcher JT. Quantitative in vivo imaging of embryonic development: opportunities and challenges. Differ Res Biol Diversity. 2012;84:149–162. doi: 10.1016/j.diff.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraiwa N, Ishimoto M, Yasue H. Examination of the mouse embryo by micro-CT. Exp Anim Jpn Assoc Lab Anim Sci. 2013;62:57–61. doi: 10.1538/expanim.62.57. [DOI] [PubMed] [Google Scholar]
- Hogers B, van der Weerd L, Olofsen H, van der Graaf LM, DeRuiter MC, Gittenberger-de Groot AC, Poelmann RE. Non-invasive tracking of avian development in vivo by MRI. NMR Biomed. 2009;22:365–373. doi: 10.1002/nbm.1346. [DOI] [PubMed] [Google Scholar]
- Moran CM, Thomson AJ, Rog-Zielinska E, Gray GA. High-resolution echocardiography in the assessment of cardiac physiology and disease in preclinical models. Exp Physiol. 2013;98:629–644. doi: 10.1113/expphysiol.2012.068577. [DOI] [PubMed] [Google Scholar]
- Nieman BJ, Turnbull DH. Ultrasound and magnetic resonance microimaging of mouse development. Methods Enzymol. 2010;476:379–400. doi: 10.1016/S0076-6879(10)76021-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallares P, Fernandez-Valle ME, Gonzalez-Bulnes A. In vivo virtual histology of mouse embryogenesis by ultrasound biomicroscopy and magnetic resonance imaging. Reprod Fertil Dev. 2009;21:283–292. doi: 10.1071/rd08124. [DOI] [PubMed] [Google Scholar]
- Parnell SE, O’Leary-Moore SK, Godin EA, Dehart DB, Johnson BW, Allan Johnson G, Styner MA, Sulik KK. Magnetic resonance microscopy defines ethanol-induced brain abnormalities in prenatal mice: effects of acute insult on gestational day 8. Alcohol Clin Exp Res. 2009;33:1001–1011. doi: 10.1111/j.1530-0277.2009.00921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y, Popovic ZB, Sopko N, Drinko J, Zhang Z, Thomas JD, Penn MS. Speckle tracking echocardiography in the assessment of mouse models of cardiac dysfunction. Am J Physiol Heart Circ Physiol. 2009;297:H811–H820. doi: 10.1152/ajpheart.00385.2009. [DOI] [PubMed] [Google Scholar]
- Petiet AE, Kaufman MH, Goddeeris MM, Brandenburg J, Elmore SA, Johnson GA. High-resolution magnetic resonance histology of the embryonic and neonatal mouse: a 4D atlas and morphologic database. Proc Natl Acad Sci USA. 2008;105:12331–12336. doi: 10.1073/pnas.0805747105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phoon CK. Imaging tools for the developmental biologist: ultrasound biomicroscopy of mouse embryonic development. Pediatr Res. 2006;60:14–21. doi: 10.1203/01.pdr.0000219441.28206.79. [DOI] [PubMed] [Google Scholar]
- Phoon CK, Turnbull DH. Ultrasound biomicroscopy-Doppler in mouse cardiovascular development. Physiol Genom. 2003;14:3–15. doi: 10.1152/physiolgenomics.00008.2003. [DOI] [PubMed] [Google Scholar]
- Ritman EL. Current status of developments and applications of micro-CT. Annu Rev Biomed Eng. 2011;13:531–552. doi: 10.1146/annurev-bioeng-071910-124717. [DOI] [PubMed] [Google Scholar]
- Rosenthal J, Mangal V, Walker D, Bennett M, Mohun TJ, Lo CW. Rapid high resolution three dimensional reconstruction of embryos with episcopic fluorescence image capture. Birth Defects Res C Embryo Today. 2004;72:213–223. doi: 10.1002/bdrc.20023. [DOI] [PubMed] [Google Scholar]
- Rosset A, Spadola L, Ratib O. OsiriX: an open-source software for navigating in multidimensional DICOM images. J Digit Imaging. 2004;17:205–216. doi: 10.1007/s10278-004-1014-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saalfeld S, Fetter R, Cardona A, Tomancak P. Elastic volume reconstruction from series of ultrathin microscopy sections. Nat Methods. 2012;9:717–720. doi: 10.1038/nmeth.2072. [DOI] [PubMed] [Google Scholar]
- Schambach SJ, Bag S, Schilling L, Groden C, Brockmann MA. Application of micro-CT in small animal imaging. Methods. 2010;50:2–13. doi: 10.1016/j.ymeth.2009.08.007. [DOI] [PubMed] [Google Scholar]
- Scherrer-Crosbie M, Kurtz B. Ventricular remodeling and function: insights using murine echocardiography. J Mol Cell Cardiol. 2010;48:512–517. doi: 10.1016/j.yjmcc.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Leatherbury L, Rosenthal J, Yu Q, Pappas MA, Wessels A, Lucas J, Siegfried B, Chatterjee B, Svenson K, Lo CW. Cardiovascular phenotyping of fetal mice by noninvasive high-frequency ultrasound facilitates recovery of ENU-induced mutations causing congenital cardiac and extracardiac defects. Physiol Genom. 2005;24:23–36. doi: 10.1152/physiolgenomics.00129.2005. [DOI] [PubMed] [Google Scholar]
- Spurney CF, Lo CW, Leatherbury L. Fetal mouse imaging using echocardiography: a review of current technology. Echocardiography. 2006;23:891–899. doi: 10.1111/j.1540-8175.2006.00335.x. [DOI] [PubMed] [Google Scholar]
- Tan SY, Rosenthal J, Zhao XQ, Francis RJ, Chatterjee B, Sabol SL, Linask KL, Bracero L, Connelly PS, Daniels MP, Yu Q, Omran H, Leatherbury L, Lo CW. Heterotaxy and complex structural heart defects in a mutant mouse model of primary ciliary dyskinesia. J Clin Invest. 2007;117:3742–3752. doi: 10.1172/JCI33284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobita K, Liu X, Lo CW. Imaging modalities to assess structural birth defects in mutant mouse models. Birth Defects Res C Embryo Today Rev. 2010;90:176–184. doi: 10.1002/bdrc.20187. [DOI] [PubMed] [Google Scholar]
- Weninger WJ, Meng S, Streicher J, Muller GB. A new episcopic method for rapid 3-D reconstruction: applications in anatomy and embryology. Anat Embryol (Berl) 1998;197:341–348. doi: 10.1007/s004290050144. [DOI] [PubMed] [Google Scholar]
- Weninger WJ, Mohun T. Phenotyping transgenic embryos: a rapid 3-D screening method based on episcopic fluorescence image capturing. Nat Genet. 2002;30:59–65. doi: 10.1038/ng785. [DOI] [PubMed] [Google Scholar]
- Wiesmann F, Ruff J, Hiller KH, Rommel E, Haase A, Neubauer S. Developmental changes of cardiac function and mass assessed with MRI in neonatal, juvenile, and adult mice. Am J Physiol Heart Circ Physiol. 2000;278:H652–H657. doi: 10.1152/ajpheart.2000.278.2.H652. [DOI] [PubMed] [Google Scholar]
- Yamada S, Samtani RR, Lee ES, Lockett E, Uwabe C, Shiota K, Anderson SA, Lo CW. Developmental atlas of the early first trimester human embryo. Dev Dyn. 2010;239:1585–1595. doi: 10.1002/dvdy.22316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelbuz TM, Wessel A, Kirby ML. Studies on morphogenesis and visualization of the early embryonic heart with regard to the development of conotruncal heart defects. Z Kardiol. 2004;93:583–594. doi: 10.1007/s00392-004-0107-z. [DOI] [PubMed] [Google Scholar]
- Yu Q, Leatherbury L, Tian X, Lo CW. Cardiovascular assessment of fetal mice by in utero echocardiography. Ultrasound Med Biol. 2008;34:741–752. doi: 10.1016/j.ultrasmedbio.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Shen Y, Chatterjee B, Siegfried BH, Leatherbury L, Rosenthal J, Lucas JF, Wessels A, Spurney CF, Wu YJ, Kirby ML, Svenson K, Lo CW. ENU induced mutations causing congenital cardiovascular anomalies. Development. 2004;131:6211–6223. doi: 10.1242/dev.01543. [DOI] [PubMed] [Google Scholar]
- Zouagui T, Chereul E, Janier M, Odet C. 3D MRI heart segmentation of mouse embryos. Comput Biol Med. 2010;40:64–74. doi: 10.1016/j.compbiomed.2009.11.001. [DOI] [PubMed] [Google Scholar]