Abstract
An α-helical model peptide (Ac-EAEKAAKE-X-EKAAKEAEK-amide) was used as a template to examine the efficacy of conventional reversed-phase high-performance liquid chromatography (RP-HPLC) in separating peptide analogs with single substitutions (at position X) of diasteromeric amino acids Ile, allo-Ile, D-Ile and D-allo-Ile. We compared differences of peptide retention behavior on a C8 column and a C18 column at different temperatures. We demonstrated how subtle differences in peptide secondary structure affected by the different substitutions of amino acids with identical overall hydrophobicity enabled effective resolution of these peptide analogs. We also demonstrated the ability of RP-HPLC to separate Ile- and allo-Ile-substituted analogs of a 26-residue α-helical antimicrobial peptide (AMP), with the substitution site towards the C-terminus of the α-helix. These peptides show different values of antibacterial activity and hemolytic activity, and different selectivity against bacteria and human cells. Our results underline the ability of RP-HPLC to resolve even difficult diasteromeric peptide mixtures as well as its value in monitoring very subtle hydrophobicity changes in de novo-designed AMP.
Keywords: α-helical peptide, chiral stereoisomer, secondary structure, hydrophobicity
Introduction
Molecular chirality frequently plays an important role in many important areas, including the pharmaceutical, food and beverage and agrochemical industries, as well as the environment (Bai et al., 2010; Kasprzyk-Hordern, 2010). With the exception of glycine, the remaining 19 proteinogenic amino acids contain at least one chiral carbon atom, with isoleucine (Ile) and threonine also containing a second chiral carbon atom. Such chiral centers allow the existence of amino acid enantiomers(Andreetto et al., 2006; Ilisz et al., 2010). It is well known that L- and D-enantiomeric forms of amino acids substituted into peptides and proteins can have profoundly different effects on stability and biological activity (Chen et al., 2005; Kondejewski et al., 1999; Lee et al., 2004; Oren and Shai, 1997; Papo and Shai, 2005). In addition, racemization of amino acids, as well as the occurrence of small amounts of alternate enantiomers in commercial amino acids, may be found during peptide synthesis. Hence, a requirement for effective resolution of peptide diastereomers is clearly important and frequently challenging.
Approaches to separating chiral compounds include enzymatic degradation, crystallization, membrane-based spectroscopy, capillary electrophoresis and, most commonly, high-performance liquid chromatography (HPLC) methods (Hrobonova et al., 2002; Hutt et al., 1999; Scaloni et al., 1991).
Generally, two major strategies have been applied for chiral separations. The first is based on the formation of diastereomers in the reactions of a chiral derivatizing agent with chiral compounds and using an achiral stationary phase to separate the diastereomeric derivatives. This method is not suitable for the analysis of amino acid enantiomers in a standard sample or in pharmaceutical preparations, where a low amount of enantiomer (at a level of 0.1 or 0.05%) is to be determined. The second strategy is based on the formation of diastereomers on a chiral stationary phase or with a chiral selector in the mobile phase coupled with an achiral stationary phase. This latter method requires a suitable chiral stationary phase or chiral selector with high chiral purity to achieve better separation or higher detection sensitivity (Ilisz et al., 2008; Ilisz et al., 2010; Lammerhofer, 2010).
The most widely used HPLC method to separate peptides, including closely-related peptides, has long been reversed-phase HPLC (RP-HPLC) (Mant et al., 2007; Mant et al., 1997). Indeed, conventional RP-HPLC, i.e., with achiral stationary phases and in the absence of any chiral selector in the mobile phase, has been shown to be able to resolve peptide diastereomers, either due to the disruption of peptide secondary structure by the presence of D-amino acids in a peptide otherwise comprised of L-amino acids (Chen et al., 2002) or due to different nearest-neighbour interactions of L- or D- chains (Kovacs et al., 2006). The former may be viewed as a structure-based mechanism of separation and is particularly relevant when one considers the growing application of D-amino acids in the modulation of biological activity of such biologically important molecules as antimicrobial peptides (Chen et al., 2005; Kondejewski et al., 1999; Lee et al., 2004; Oren and Shai, 1997; Papo and Shai, 2005).
We believe that conventional RP-HPLC may prove to be an even more potent tool for resolution of difficult diastereomeric peptide mixtures than has hitherto been demonstrated, making it ideal for routine separations of such molecules. Thus, we set out to test the capability of RP-HPLC to separate mixtures of peptides with just single substitutions of one of the four stereoisomers of isoleucine. This paper describes our initial results in developing an RP-HPLC approach to the separation of the four isoleucine-substituted analogs of a model amphipathic α-helical peptide and its subsequent application to a biologically active synthetic peptide with antimicrobial activity.
Experimental
Reagents and materials
Rink amide 4-methylbenzhydrylamine resin (0.8 mmol/g), N-α-Fmoc protected amino acids, coupling reagents for peptide synthesis (O-Benzotriazole-1-yl-N,N,N′,N′-tetramethyl-uronium hexafluorophosphate (HBTU); l-hydroxybenzotriazole (HOBt); N,N′-diisopropylethylamine (DIEA) and trifluoroacetic acid (TFA) were purchased from GL Biochem (Shanghai, China). Dichloromethane (DCM), N,N-dimethylformamide (DMF), piperidine and 2,2,2-trifluoroethanol (TFE) were analytical grade and purchased from JinTai Chemicals (Changchun, China). Acetonitrile (HPLC grade) was obtained from Fisher (Beijing, China).
Instruments
Peptide samples were analyzed on a Shimadzu LC-20A liquid chromatographic system coupled with a UV detector and thermostated column compartment; a Shimadzu LC-6A preparative liquid chromatographic system was used to purify the crude peptides.
Molecular mass of peptides was confirmed by VG Quattro electrospray mass spectrometry (Fisons, Pointe-Claire, Quebec, Canada).
Amino acid analyses of the purified peptides were carried out on an Agilent 1200 Liquid Chromatography using a Zorbax Eclipse-AAA HPLC column (150 × 4.6-mm I.D., 5-μm particle size). Online derivatization was applied using o-phthalaldehyde (OPA) for primary amino acids and 9-fluorenylmethyl chloroformate (Fmoc) for secondary amino acids.
Peptide synthesis and purification
The peptides were synthesized by solid-phase peptide synthesis (Chen et al., 2006). The crude peptides were purified by preparative RP-HPLC using a semi-preparative Zorbax 300 SB-C8 column (250 × 9.4-mm I.D., 5-μm particle size, 300 Å pore size) from Agilent Technologies with a linear AB gradient (0.1% acetonitrile/min) at a flow rate of 2 ml/min, where eluent A was 0.1% aqueous TFA and eluent B was 0.1% TFA in acetonitrile. The purity of peptides was verified by analytical RP-HPLC as described below. The peptides were further characterized by electrospray mass spectrometry and amino acid analysis.
Characterization of peptide secondary structure
Circular dichroism spectra were acquired with a 0.02-cm path length quartz cuvette on a Jasco J-810 spectropolarimeter (Jasco, Easton, MD) at 30°C and 65°C as described previously (Chen et al., 2005; Chen et al., 2006) using a concentration of 75 μM peptides in benign buffer (50 mM KH2PO4/K2HPO4, 100 mM KCl, pH 7) or benign buffer with 50% 2,2,2-trifluoroethanol (TFE) at 30°C and 65°C. The mean residue molar ellipticities were calculated by the equation [θ] = θ/101cMn. The values of mean residue molar ellipticities of the peptide analogs at 222 nm were used to represent the relative helicity of the peptides.
Analytical RP-HPLC conditions
Peptide and amino acid samples were analyzed on a Shimadzu LC-20A HPLC with linear AB gradients, where eluent A was 0.1% aqueous TFA and eluent B was 0.1% TFA in acetonitrile on a Zorbax 300 SB-C8 column (150 × 4.6-mm I.D., 5-μm particle size, 300-Å pore size) and a Zorbax 300 SB-C18 column (250 × 4.6-mm I.D., 5-μm particle size, 300-Å pore size) from Agilent Technologies. C8 and C18 columns (with SB denoting StableBond) were chosen in this study due to the excellent stability to high temperatures at low pH (Chen et al., 2004, 2007b). Signals were detected at 210nm. For runs of Fmoc-amino acids, the linear AB gradient started at 30% acetonitrile with a subsequent gradient of 1% acetonitrile/min at a flow-rate of 1 ml/min; for runs of model peptides, the linear AB gradient started at 5% acetonitrile with a subsequent gradient of 1% acetonitrile/min at a flow-rate of 1 ml/min; for runs of antimicrobial peptides, the linear AB gradient started at 5% acetonitrile with a subsequent gradient of 1% acetonitrile/min or at 30% acetonitrile with a subsequent gradient of 0.1% acetonitrile/min at a flow-rate of 0.5 ml/min.
Measurement of antibacterial activity
Minimal inhibitory concentrations (MIC) were determined using a broth dilution method (Stark et al., 2002). Briefly, bacteria were grown overnight at 37 °C in Mueller-Hinton (MH) broth, diluted in the same medium and transferred into 96-well microtiter plates (90 μl/well). Peptides were serially diluted by 0.2% bovine serum albumin containing 0.01% acetic acid and added to the microtiter plates in a volume of 10 μl of each well to give a final concentration of 5×105 colony-forming units/ml. MICs were determined as the lowest peptide concentration that inhibited bacterial growth after incubation for 24 h at 37 °C.
Measurement of hemolytic activity
Peptide samples were serially diluted by phosphate-buffered saline (PBS) in 96-well plates (round-bottom) to give a volume of 70 μl sample solution in each well. Human erythrocytes anticoagulated by EDTAK2 were collected by centrifugation (1000 rpm) for 5 min, and washed twice by PBS, then diluted to a concentration of 2% in PBS. 70 μl of 2% erythrocytes were added to each well to give final 1% human erythrocytes in each well and reactions were incubated at 37 °C for 1h. The plates were then centrifuged for 10 min at 2000 rpm and supernatant (90 μl) was transferred to a 96-well plate (flat-bottom). The release of hemoglobin was determined by measuring the absorbance of the supernatant at 578 nm. Peptide samples were diluted in a 2-fold series in order to determine the minimum concentration with no hemolysis. Erythrocytes in PBS and distilled water were used as controls of 0 and 100% hemolysis, respectively.
Results and discussion
Isoleucine stereoisomers
There are two chiral centers in isoleucine, one at the α-carbon and one at the β-carbon on the side-chain. Therefore, four stereoisomers are possible. These isomers are: L-Ile ((2S,3S)-2-amino-3-methyl-valeric acid), L-allo-Ile ((2S,3R)-2-amino-3-methyl-valeric acid), D-Ile ((2R,3R)-2-amino-3-methyl-valeric acid) and D-allo-Ile ((2R,3S)-2-amino-3-methyl-valeric acid) (Andreetto et al., 2006). The structures of the four Fmoc-protected Ile configurational isomers used in this study are shown in Fig. 1A. It should be noted that the four stereoisomers comprise two pairs of enantiomers (L-Ile/D-Ile and L-allo-Ile/D-allo-Ile) and two pairs of diastereomers (L-Ile/L-allo-Ile and D-Ile/D-allo-Ile), as discussed further. In addition, the overall hydrophobicities of all four isomers are identical.
Fig. 1.
A: Structure of Fmoc-protected isoleucine stereoisomers. B: Helical net representations of model synthetic α-helical peptide (left) and synthetic α-helical antimicrobial peptide (right); the amino acid residues on the non-polar faces of these amphipathic peptides are circled, while the substitution sites for the four isoleucine stereoisomers are in squares (X9, left, and X24, right). C: Sequences of the four synthetic model α-helical peptide analogues (I9, a-I9, I9d and a-I9d) and two of the antimicrobial peptide analogues (V13K and a-V13K). Ac- denotes Nα-acetyl and amide denotes Cα-amide. One-letter codes are used for the amino acid residues.
Synthetic model amphipathic α-helical peptide
An 18-residue amphipathic α-helical model peptide with the sequence Ac-EAEKAAKEXEKAAKEAEK-amide (substitution position X) (Chen et al., 2002; Zhou et al., 1994a) was chosen for an initial assessment of the ability of RP-HPLC to resolve multiple stereoisomer peptide mixtures. Substitution position X ensures the greatest influence of amino acid substitutions on peptide retention behavior due to this being at the center of the preferred binding domain of the non-polar face of the amphipathic α-helix (see helical net in Fig. 1B, left) (Chen et al., 2005; Chen et al., 2002). As noted previously, this amphipathic α-helical model peptide exhibits the following important features: (1) the helix is single-stranded and non-interacting, which highlights the effect of amino acid substitutions; (2) the substitution site in the center of the non-polar face (position 9) is surrounded by alanine residues, thus creating a uniform interaction environment; (3) the surrounding alanine residues ensure minimal interaction with the “guest” amino acid residues due to the small size of the alanine side-chain methyl group; (4) the small size of the peptide maximizes the effects of single amino acid substitutions (Chen et al., 2002, 2007b; Zhou et al., 1994b). Four peptide analogs denoted I9, a-I9, I9d, and a-I9d were synthesized with amino acid substitutions of L-Ile, L-allo-Ile, D-Ile and D-allo-Ile, respectively. Sequences of the synthetic peptide analogs are shown in Fig. 1C. Note that the L-Ile/L-allo-Ile and D-Ile/D-allo-Ile substituted diastereomeric analogs were designed to pose a particularly challenging separation problem, differing as they do solely in the configuration of a single methyl group substitution at the side-chain β-carbon, whilst maintaining identical overall side-chain hydrophobicity based on structure.
Synthetic amphipathic α-helical antimicrobial peptide (AMP)
An α-helical AMP, denoted V13K (Ac-KWKSFLKTFKSAKKTVLHTALKAISS-amide) (Chen et al., 2007a; Chen et al., 2005) was also used as a template for substitutions of L-Ile and L-allo-Ile. These substitutions were made at position 24 of the sequence since the original amino acid at this position was L-Ile. Sequences of the two analogs are shown in Fig. 1C with a helical net representation of the peptide sequence shown in Fig. 1B, right.
In contrast to the model peptides described in the section of synthetic model amphipathic α-helical peptide, the substitution position in V13K is close to the C-terminal of the original peptide sequence at position 24 of the 26-residue peptide (Fig. 1B, right, and 1C), i.e., not in the center of the preferred binding domain of this amphipathic α-helical anti-microbial peptide. This sequence position, coupled with the very subtle difference between the L-Ile and L-allo-Ile side-chains, was a challenge to the resolving ability of RP-HPLC.
Peptide secondary structure
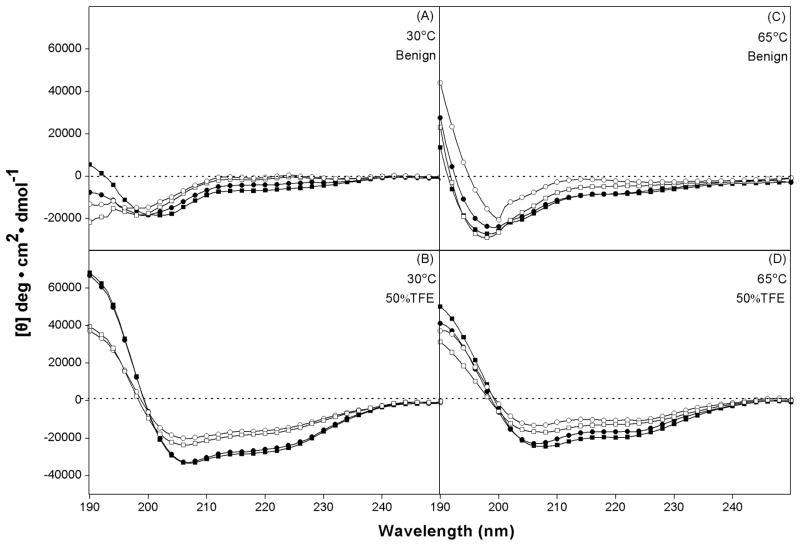
In order to show the effect of amino acid substitutions on peptide secondary structure, circular dichroism (CD) spectra of the peptide analogs were measured under benign conditions (50 mM KH2PO4/K2HPO4, 100 mM KCl, pH 7) and also in the presence of 50% TFE, an α-helix-inducing solvent and a mimic of the hydrophobic environment characteristic of membranes and hydrophobicity of the reversed-phase columns at 30 °C and 65 °C, respectively. From Fig. 2 and Table 1, it is clear that, in benign conditions the peptide analogs showed negligible helical structures. In contrast, in the presence of 50% TFE, the L-substituted analogs exhibit full helical structure at different temperatures. The D-substituted analogs, while considerably more helical than in benign conditions, did not exhibit full helical structure in the presence of 50% TFE (Fig. 2B; Table 1) due to the presence of a D-amino acid substituted into an otherwise all-L peptide. Temperature showed strong denaturing effect on helical structure of peptides since peptides inevitably exhibited lower helicity at 65 °C than at 30 °C (Fig. 2B, 2D and Table 1). From Table 1, the relative helicity of the peptides is in the order a-I9d<I9d<a-I9<I9 at different temperatures.
Fig. 2.
CD spectra of model peptides in benign medium (50mM PO4/100 mM KCl, pH 7.4) at 30 °C (A) and at 65 °C (C), and in the presence of 50% TFE at 30 °C (B) and at 65 °C (D). Symbols used are ■ for I9, ● for a-I9, □ for I9d, ○ for a-I9d.
Table 1.
Biophysical data of peptides and protected amino acids
| Peptides or protected amino acids | tR (min)a | [θ]222 (deg.cm2.dmol−1)b | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| 30 °C | 65°C | 30°C | 65°C | |||||
|
| ||||||||
| C8 | C18 | C8 | C18 | Benign | 50%TFE | Benign | 50%TFE | |
| Amino acidsc | 17.6 | — | 15.2 | — | — | — | — | — |
| I9 | 25.0 | 27.5 | 22.9 | 25.8 | −6300 | −26650 | −8250 | −19450 |
| a-I9 | 24.3 | 26.9 | 22.1 | 25.1 | −3950 | −25200 | −7850 | −16550 |
| I9d | 19.8 | 22.7 | 17.8 | 21.0 | −900 | −17000 | −4550 | −12600 |
| a-I9d | 19.3 | 22.3 | 17.3 | 20.6 | −100 | −15450 | −2600 | −10350 |
| V13K | 30.3 | 33.3 | 17.4 | 26.3 | −3300 | −26300 | −4050 | −22750 |
| a-V13K | 28.0 | 33.3 | 16.3 | 26.3 | −2950 | −24600 | −3000 | −20350 |
The retention time of peptides or protected amino acids during RP-HPLC on a C8 or C18 column.
The mean residue molar ellipticities, [θ]222(degree.cm2.dmol−1) at wavelength 222 nm were measured at 30°C and 65°C in benign conditions (100 mM KCl, 50 mM PO4, pH 7.4) and in the presence of 50% TFE, respectively.
Denotes Fmoc-protected isoleucine amino acid stereoisomers of Fmoc-Ile, Fmoc-allo-Ile, Fmoc-D-Ile and Fmoc-D-allo-Ile.
CD spectra of antimicrobial peptides V13K and a-V13K were also measured under benign conditions and in the presence of 50% TFE at 30 °C and 65 °C, respectively. Allo-Ile-substituted V13K showed lower helicity than V13K in the presence of 50% TFE, indicating that the stereoisomer amino acids contributed differently to the peptide helical structure. Temperature showed similar denaturing effect on the structure of antimicrobial peptides compared to that of model peptides (Table 1).
RP-HPLC of Fmoc-protected amino acid stereoisomers
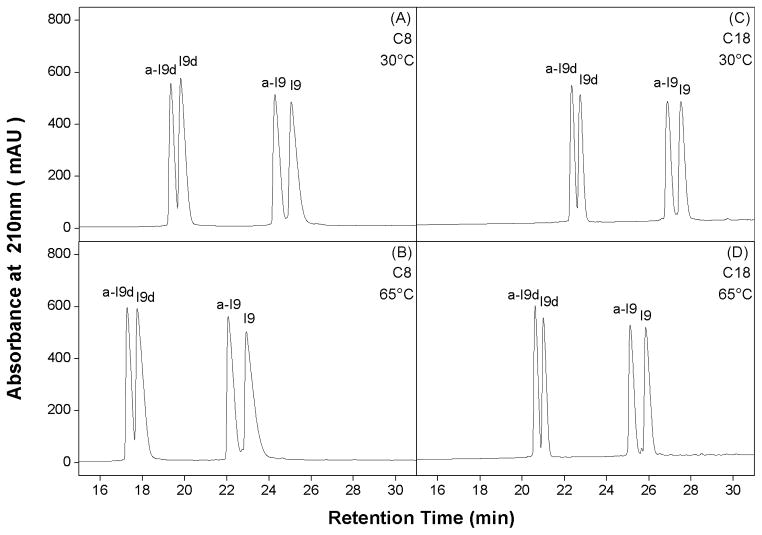
The four Fmoc-protected Ile amino acid stereoisomers (Fig. 1A) were subjected to RP-HPLC on a C8 column and a C18 column at 30 °C and 65 °C. All four protected amino acids were coeluted at 17.6 min (Fig. 3A) and 15.2 min (Fig. 3B) at 30 °C and 65 °C on a C8 column, and 19.8 min (Fig. 3C) and 18.1 min (Fig. 3D) at 30 °C and 65 °C on a C18 column, respectively, with the lower retention time at the higher temperature likely being due to enhanced mass transfer between the stationary and mobile phases with a temperature increase(Dolan, 2002), as well as increased solubility of the solutes at the higher temperature(Cohen et al., 1984; Hancock et al., 1986; Parker et al., 1986). These results indicate that all four Fmoc-protected stereomeric amino acids have the same intrinsic side-chain hydrophobicity and, thus, are clearly difficult to separate by RP-HPLC due to full exposure of these side-chains to the stationary phase no matter their configuration.
Fig. 3.
RP-HPLC profiles of Fmoc-protected Ile amino acid stereoisomers on a C8 column at 30°C (A) and 65°C (B), and on a C18 column at 30°C (C) and 65°C (D). Columns and conditions are described in the section of analytical RP-HPLC conditions.
RP-HPLC of model amphipathic α-helical peptide substituted with amino acid stereoisomers
In contrast to the results of the Fmoc-protected amino acids, when the four amino acid stereoisomers were substituted into the center of the non-polar face (position X9) of the model amphipathic α-helical peptide (Fig. 1B, left), the four resulting analogs were readily separated on a C8 or a C18 column at both 30°C (Fig. 4A, 4C) and 65°C (Fig. 4B, 4D). Except the greater retention time values of peptides, runs on a more frequently used C18 column did not show better resolution than those runs on a C8 column. Unsurprisingly, peptides were more retentive on a C18 column than on a C8 column due to the stronger hydrophobic interaction between the stationary phase of column and peptides (Fig. 4 and Table 1).
Fig. 4.
RP-HPLC profiles of model peptides on a C8 column at 30°C (A) and 65°C (B), and on a C18 column at 30°C (C) and 65°C (D). Column and conditions are described in section of analytical RP-HPLC conditions. The sequences of the peptides are shown in Fig. 1.
It is well known that the chromatography conditions characteristic of RP-HPLC (hydrophobic stationary phase, non-polar eluting solvent) induce helical structure in potentially helical polypeptides (Blondelle et al., 1995; Zhou et al., 1990). Polypeptides which are thus induced into an amphipathic α-helix on interaction with a hydrophobic RP-HPLC stationary phase will exhibit preferred binding of their non-polar face with the stationary phase. From Fig. 1B, left, it can be seen that the substitution site at position 9, in the center of the non-polar face of the helix, ensures intimate interaction of the substituting side-chain with the reversed-phase stationary phase; concomitantly, this is designed to maximize any observed effects on RP-HPLC retention behavior when substituting different residues at this site. Significantly, the order of elution of the four peptide analogs was a-I9d<I9d<a-I9<I9 on either a C8 column or a C18 column at different temperatures, i.e., the same as was observed for increasing α-helicity of these peptides (Table 1), i.e., the four peptides were separated due to effects of different amino acid substitutions on peptide secondary structure. The most subtle effects can be seen for the D- and L- amino acid-substituted peptide pairs of a-I9d/I9d and a-I9/I9, with the allo-analogs being eluted just prior to the respective non-allo-analogs, reflecting the lower helicities of the former compared to the latter (Table 1). This result is consistent with helicity data measured by CD. From Fig. 4 and Table 1, it can also be seen that the D-amino acid-substituted peptide pairs are eluted significantly earlier than their L-substituted counterparts due to the aforementioned helix-disrupting properties of D-amino acids in an otherwise all-L α-helix (Aguilar et al., 1993), which is consistent with CD spectra of the peptides in Fig. 2. Also from Fig. 4, the only effect of an increase in temperature from 30 °C to 65 °C on both C8 and C18 columns was a decrease in peptide retention time with negligible differences in resolution of peptide pairs between the two temperatures.
RP-HPLC of α-helical antimicrobial peptides substituted with amino acid diastereomers
The results shown in Fig. 4 for the model α-helical peptides now prompted the separation of antimicrobial peptide (AMP) analogs with an Ile to allo-Ile substitution near the C-terminal of the 26-residue peptide sequence (Fig. 1B, right and Fig. 1C). The greatest effect of an amino acid substitution in amphipathic α-helical peptides on their RP-HPLC retention times occurs with substitution in the center of the non-polar face (Hartmann et al., 2003; Hodges et al., 2004), allowing the resolution of the a-I9d/I9 and a-I9/I9 pairs seen in Fig. 4. However, substitution of amino acids with identical overall hydrophobicity towards the termini of such helices, where their effect on the interaction of the preferred binding domain represented by the non-polar face with the hydrophobic stationary phase would be much less pronounced, would pose an even more potent test for RP-HPLC. Further, the native AMP under consideration denoted V13K (Fig. 1C), is a well-studied α-helical cationic AMP with broad spectrum antimicrobial activity against Gram-negative and Gram-positive bacteria with negligible cytotoxicity against eukaryotic cells (Chen et al., 2005; Chen et al., 2006; Jiang et al., 2008a; Jiang et al., 2008b). Refinement of the activity of such AMPs often involves just subtle variations in peptide structure through amino acid substitutions. Thus, evidence that RP-HPLC is able to monitor the effects of such minimal variations as afforded by an Ile to allo-Ile substitution at the terminus of V13K also has wider implications for AMP development generally.
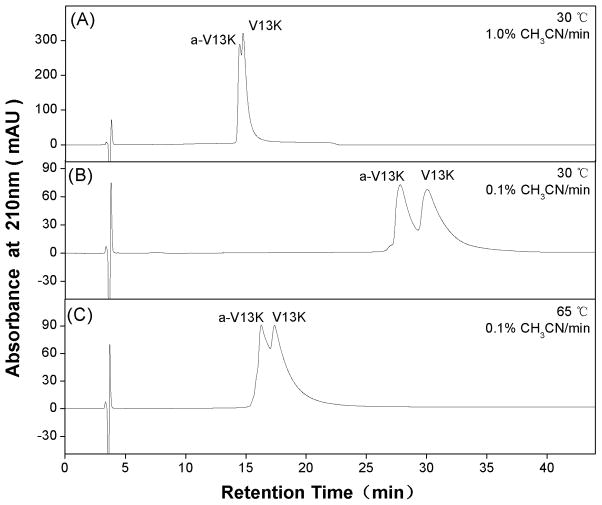
From Fig. 5A, a 1% acetonitrile/min gradient at 30°C on a C8 column provided only a limited separation of V13K and a-V13K. However, lowering the gradient rate to 0.1% acetonitrile/min on the same column (Fig. 5B) produced a much improved resolution of the two peptides. Interestingly, in a similar manner to the model amphipathic α-helical peptides in Fig. 4, the allo-Ile-substituted analog (a-V13K) was again eluted prior to the Ile-substituted peptide (V13K). From Fig. 5C, a rise in temperature to 65°C did not improve the resolution at the 0.1%/min gradient rate. Indeed, the separation was markedly/inferior to that achieved at 30°C (Fig. 5B). This was likely due to an unfolding of α-helical structure at the higher temperature, since temperature showed dramatic denaturing effect on the helicity of V13K serial peptides(Chen et al., 2005). Such an unfolding would also disrupt the non-polar preferred binding domain of the peptides which, coupled with the general effects of a temperature increase described above, would result in the observed decrease in retention time compared to 30°C (Fig. 5B). It was surprising that the antimicrobial peptides could not be separated on a C18 column at 30°C and 65°C at a 1% acetonitrile/min gradient and even at a very low gradient rate of 0.1% acetonitrile/min (Table 1). In general, peptides with amino acid substitutions towards the termini of helices showed less effect on peptide helicity, thus, had more difficulty on RP-HPLC separation.
Fig. 5.
RP-HPLC profiles of antimicrobial peptides on a C8 column at 1% acetonitrile/min at 30°C (A), or 0.1% acetonitrile/min at 30°C (A) and 65°C (B). Column and conditions are described in section of analytical RP-HPLC conditions. The sequences of the peptides are shown in Fig. 1.
Biology activities of α-helical antimicrobial peptides
Biology activities of α-helical antimicrobial peptides V13K and a-V13K were tested against various Gram-positive and Gram-negative bacterial strains (antibacterial activities) and human red blood cells (cytotoxicity). As shown in Table 2, minimal inhibitory concentration (MIC) was used to evaluate the antibacterial ability of peptides against bacteria and minimal hemolytic concentration (MHC) was used to evaluate the cytotoxicity of the peptides against human cells. It is interesting to see that antimicrobial peptides V13K and a-V13K exhibited subtle difference both on MIC and MHC values. Compared to V13K, peptide a-V13K displaysed stronger antimicrobial activity against all tested Gram-negative and Gram-positive bacteria. In contrast, a-V13K exhibited lower toxicity against human red blood cells than V13K, showing a stronger selectivity on bacteria. These results also prove that conventional RP-HPLC could be valuable in the preparation of peptide drugs with subtle differences on peptide secondary structure but dramatic differences on biological activities in pharmaceutical manufacture.
Table 2.
Biological activities of antimicrobial peptides
| Peptide | MHCa (μg/ml) | MICb (μg/ml) | |||
|---|---|---|---|---|---|
|
| |||||
|
E. coli ATCC25922 |
P. aeruginosa ATCC27853 |
S. aureus ATCC25923 |
B. subtilis ATCC49619 |
||
| V13K | 250 | 16 | 8 | 16 | 8 |
| a-V13K | 500 | 8 | 4 | 8 | 4 |
Hemolytic activity (minimal hemolytic concentration) was determined on human red blood cells after incubating with peptides for 1 hour (hRBC).
Antimicrobial activity (minimal inhibitory concentration) was determined as the minimal concentration of peptide to inhibit microbial growth.
Conclusions
In this study, we examined the capability of RP-HPLC to separate mixtures of peptides with just single substitutions of one of the four stereoisomers of isoleucine. RP-HPLC has proved to be an effective method to separate peptides with substitutions of single amino acid stereoisomers based on subtle difference on peptide secondary structure. Thus, conventional RP-HPLC has the capacity to resolve even difficult diastereomeric peptide mixtures. The helix-inducing characteristics of the hydrophobic matrix and eluting solvents during RP-HPLC play an important role in separating diastereomeric peptides. In addition, conventional RP-HPLC has shown its value as a monitor of the effects of subtle amino acid substitutions in α-helical antimicrobial peptides resulting in different biological activities, which could be valuable in the preparation of peptide drugs in pharmaceutical manufacture.
Acknowledgments
This work was supported by Grants for Natural Science Foundation of Jilin Province 201015103 (Y.C.), Innovative team of Peptide Drugs of Jilin Province 20121807 (Y.C.), Basic Scientific Research Grant of Jilin University (Y.C.), The Youth Foundation of Jilin Province 20100126 (Y.B.H.), and an NIH grant to RSH (R01GM061855).
Abbreviations
- RP-HPLC
Reversed-phase high-performance liquid chromatography
- AMP
Antimicrobial peptide
- L-Ile
(2S,3S)-2-amino-3-methyl-valeric acid
- L-allo-Ile
(2S,3R)-2-amino-3-methyl-valeric acid
- D-Ile
(2R,3R)-2-amino-3-methyl-valeric acid
- D-allo-Ile
(2R,3S)-2-amino-3-methyl-valeric acid
- HBTU
O-Benzotriazole-1-yl-N,N,N′,N′-tetramethyl-uronium hexafluorophosphate
- HOBt
l-hydroxybenzotriazole
- DIEA
N,N′-diisopropylethylamine
- TFA
trifluoroacetic acid
- DCM
Dichloromethane
- DMF
N,N-dimethylformamide
- TFE
2,2,2-trifluoroethanol
References
- Aguilar MI, Mougos S, Boublik J, Rivier J, Hearn MT. High-performance liquid chromatography of amino acids, peptides and proteins. CXXVIII. Effect of D-amino acid substitutions on the reversed-phase high-performance liquid chromatography retention behaviour of neuropeptide Y[18–36] analogues. J Chromatogr. 1993;646:53–65. doi: 10.1016/s0021-9673(99)87007-1. [DOI] [PubMed] [Google Scholar]
- Andreetto E, Peggion C, Crisma M, Toniolo C. Handedness control of peptide helices by amino acid side-chain chirality: Ile/aIle peptides. Biopolymers. 2006;84:490–501. doi: 10.1002/bip.20534. [DOI] [PubMed] [Google Scholar]
- Bai J, Ching CB, Chowbay B, Chen WN. Secreted protein profile from HepG2 cells incubated by S(−) and R(+) enantiomers of chiral drug warfarin - An analysis in cell-based system and clinical samples. Proteomics Clin Appl. 2010;4:808–815. doi: 10.1002/prca.201000027. [DOI] [PubMed] [Google Scholar]
- Blondelle SE, Ostresh JM, Houghten RA, Perez-Paya E. Induced conformational states of amphipathic peptides in aqueous/lipid environments. Biophys J. 1995;68:351–359. doi: 10.1016/S0006-3495(95)80194-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Guarnieri MT, Vasil AI, Vasil ML, Mant CT, Hodges RS. Role of peptide hydrophobicity in the mechanism of action of alpha-helical antimicrobial peptides. Antimicrob Agents Chemother. 2007a;51:1398–1406. doi: 10.1128/AAC.00925-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Mant CT, Farmer SW, Hancock RE, Vasil ML, Hodges RS. Rational design of alpha-helical antimicrobial peptides with enhanced activities and specificity/therapeutic index. J Biol Chem. 2005;280:12316–12329. doi: 10.1074/jbc.M413406200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Mant CT, Hodges RS. Determination of stereochemistry stability coefficients of amino acid side-chains in an amphipathic alpha-helix. J Pept Res. 2002;59:18–33. doi: 10.1046/j.1397-002x.2001.10994.x. [DOI] [PubMed] [Google Scholar]
- Chen Y, Mant CT, Hodges RS. Selectivity differences in the separation of amphipathic alpha-helical peptides during reversed-phase liquid chromatography at pHs 2.0 and 7.0: effects of different packings, mobile phase conditions and temperature. J Chromatogr A. 2004;1043:99–111. doi: 10.1016/j.chroma.2004.04.028. [DOI] [PubMed] [Google Scholar]
- Chen Y, Mant CT, Hodges RS. Preparative reversed-phase high-performance liquid chromatography collection efficiency for an antimicrobial peptide on columns of varying diameters (1mm to 9.4mm I.D.) J Chromatogr A. 2007b;1140:112–120. doi: 10.1016/j.chroma.2006.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Vasil AI, Rehaume L, Mant CT, Burns JL, Vasil ML, Hancock RE, Hodges RS. Comparison of biophysical and biologic properties of alpha-helical enantiomeric antimicrobial peptides. Chem Biol Drug Des. 2006;67:162–173. doi: 10.1111/j.1747-0285.2006.00349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen KA, Schellenberg K, Benedek K, Karger BL, Grego B, Hearn MT. Mobile-phase and temperature effects in the reversed phase chromatographic separation of proteins. Anal Biochem. 1984;140:223–235. doi: 10.1016/0003-2697(84)90158-1. [DOI] [PubMed] [Google Scholar]
- Dolan JW. Temperature selectivity in reversed-phase high performance liquid chromatography. J Chromatogr A. 2002;965:195–205. doi: 10.1016/s0021-9673(01)01321-8. [DOI] [PubMed] [Google Scholar]
- Hancock WS, Knighton DR, Napier JR, Harding DR, Venable R. Determination of thermodynamic parameters for the interaction of a lipid-binding peptide and insulin with a reversed-phase column. J Chromatogr. 1986;367:1–8. doi: 10.1016/s0021-9673(00)94811-8. [DOI] [PubMed] [Google Scholar]
- Hartmann E, Chen Y, Mant CT, Jungbauer A, Hodges RS. Comparison of reversed-phase liquid chromatography and hydrophilic interaction/cation-exchange chromatography for the separation of amphipathic alpha-helical peptides with L- and D-amino acid substitutions in the hydrophilic face. J Chromatogr A. 2003;1009:61–71. doi: 10.1016/s0021-9673(03)00620-4. [DOI] [PubMed] [Google Scholar]
- Hodges RS, Chen Y, Kopecky E, Mant CT. Monitoring the hydrophilicity/hydrophobicity of amino acid side-chains in the non-polar and polar faces of amphipathic alpha-helices by reversed-phase and hydrophilic interaction/cation-exchange chromatography. J Chromatogr A. 2004;1053:161–172. [PubMed] [Google Scholar]
- Hrobonova K, Lehotay J, Cizmarik J, Armstrong DW. In vitro study of enzymatic hydrolysis of diperodon enantiomers in blood serum by two-dimensional LC. J Pharm Biomed Anal. 2002;30:875–880. doi: 10.1016/s0731-7085(02)00347-3. [DOI] [PubMed] [Google Scholar]
- Hutt LD, Glavin DP, Bada JL, Mathies RA. Microfabricated capillary electrophoresis amino acid chirality analyzer for extraterrestrial exploration. Anal Chem. 1999;71:4000–4006. doi: 10.1021/ac9903959. [DOI] [PubMed] [Google Scholar]
- Ilisz I, Berkecz R, Peter A. Application of chiral derivatizing agents in the high-performance liquid chromatographic separation of amino acid enantiomers: a review. J Pharm Biomed Anal. 2008;47:1–15. doi: 10.1016/j.jpba.2007.12.013. [DOI] [PubMed] [Google Scholar]
- Ilisz I, Pataj Z, Aranyi A, Peter A. High-performance liquid chromatography of biologically important, small epimeric peptides and their L, D-amino acid content. Mini Rev Med Chem. 2010;10:287–298. doi: 10.2174/138955710791330981. [DOI] [PubMed] [Google Scholar]
- Jiang Z, Kullberg BJ, van der Lee H, Vasil AI, Hale JD, Mant CT, Hancock RE, Vasil ML, Netea MG, Hodges RS. Effects of hydrophobicity on the antifungal activity of alpha-helical antimicrobial peptides. Chem Biol Drug Des. 2008a;72:483–495. doi: 10.1111/j.1747-0285.2008.00728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z, Vasil AI, Hale JD, Hancock RE, Vasil ML, Hodges RS. Effects of net charge and the number of positively charged residues on the biological activity of amphipathic alpha-helical cationic antimicrobial peptides. Biopolymers. 2008b;90:369–383. doi: 10.1002/bip.20911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasprzyk-Hordern B. Pharmacologically active compounds in the environment and their chirality. Chem Soc Rev. 2010;39:4466–4503. doi: 10.1039/c000408c. [DOI] [PubMed] [Google Scholar]
- Kondejewski LH, Jelokhani-Niaraki M, Farmer SW, Lix B, Kay CM, Sykes BD, Hancock RE, Hodges RS. Dissociation of antimicrobial and hemolytic activities in cyclic peptide diastereomers by systematic alterations in amphipathicity. J Biol Chem. 1999;274:13181–13192. doi: 10.1074/jbc.274.19.13181. [DOI] [PubMed] [Google Scholar]
- Kovacs JM, Mant CT, Kwok SC, Osguthorpe DJ, Hodges RS. Quantitation of the nearest-neighbour effects of amino acid side-chains that restrict conformational freedom of the polypeptide chain using reversed-phase liquid chromatography of synthetic model peptides with L- and D-amino acid substitutions. J Chromatogr A. 2006;1123:212–224. doi: 10.1016/j.chroma.2006.04.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammerhofer M. Chiral recognition by enantioselective liquid chromatography: mechanisms and modern chiral stationary phases. J Chromatogr A. 2010;1217:814–856. doi: 10.1016/j.chroma.2009.10.022. [DOI] [PubMed] [Google Scholar]
- Lee DL, Powers JP, Pflegerl K, Vasil ML, Hancock RE, Hodges RS. Effects of single D-amino acid substitutions on disruption of beta-sheet structure and hydrophobicity in cyclic 14-residue antimicrobial peptide analogs related to gramicidin S. J Pept Res. 2004;63:69–84. doi: 10.1046/j.1399-3011.2003.00106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mant CT, Chen Y, Yan Z, Popa TV, Kovacs JM, Mills JB, Tripet BP, Hodges RS. HPLC analysis and purification of peptides. Methods Mol Biol. 2007;386:3–55. doi: 10.1007/978-1-59745-430-8_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mant CT, Kondejewski LH, Cachia PJ, Monera OD, Hodges RS. Analysis of synthetic peptides by high-performance liquid chromatography. Methods Enzymol. 1997;289:426–469. doi: 10.1016/s0076-6879(97)89058-1. [DOI] [PubMed] [Google Scholar]
- Oren Z, Shai Y. Selective lysis of bacteria but not mammalian cells by diastereomers of melittin: structure-function study. Biochemistry. 1997;36:1826–1835. doi: 10.1021/bi962507l. [DOI] [PubMed] [Google Scholar]
- Papo N, Shai Y. A molecular mechanism for lipopolysaccharide protection of Gram-negative bacteria from antimicrobial peptides. J Biol Chem. 2005;280:10378–10387. doi: 10.1074/jbc.M412865200. [DOI] [PubMed] [Google Scholar]
- Parker JM, Guo D, Hodges RS. New hydrophilicity scale derived from high-performance liquid chromatography peptide retention data: correlation of predicted surface residues with antigenicity and X-ray-derived accessible sites. Biochemistry. 1986;25:5425–5432. doi: 10.1021/bi00367a013. [DOI] [PubMed] [Google Scholar]
- Scaloni A, Simmaco M, Bossa F. Determination of the chirality of amino acid residues in the course of subtractive Edman degradation of peptides. Anal Biochem. 1991;197:305–310. doi: 10.1016/0003-2697(91)90396-b. [DOI] [PubMed] [Google Scholar]
- Stark M, Liu LP, Deber CM. Cationic hydrophobic peptides with antimicrobial activity. Antimicrob Agents Chemother. 2002;46:3585–3590. doi: 10.1128/AAC.46.11.3585-3590.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou NE, Kay CM, Hodges RS. The net energetic contribution of interhelical electrostatic attractions to coiled-coil stability. Protein Eng. 1994a;7:1365–1372. doi: 10.1093/protein/7.11.1365. [DOI] [PubMed] [Google Scholar]
- Zhou NE, Kay CM, Hodges RS. The role of interhelical ionic interactions in controlling protein folding and stability. De novo designed synthetic two-stranded alpha-helical coiled-coils. J Mol Biol. 1994b;237:500–512. doi: 10.1006/jmbi.1994.1250. [DOI] [PubMed] [Google Scholar]
- Zhou NE, Mant CT, Hodges RS. Effect of preferred binding domains on peptide retention behavior in reversed-phase chromatography: amphipathic alpha-helices. Pept Res. 1990;3:8–20. [PubMed] [Google Scholar]