Abstract
Posttraumatic Stress Disorder (PTSD) and Major Depressive Disorder (MDD) are two highly comorbid and debilitating disorders experienced by more than half of intimate partner violence victims (IPV; Johnson, Delahanty, & Pinna, 2008). Hypothalamic Pituitary Adrenal (HPA) abnormalities are common in both disorders, though the direction of abnormalities often differs. The present study examined the relationship between comorbid PTSD and MDD, and the (salivary) cortisol waking response in 104 recently abused IPV victims. Waking cortisol levels, Area Under the Waking Curve with respect to ground (AUCg), and AUC with respect to increase (AUCi) were examined to determine the relation of HPA dynamics to comorbidity for basal versus more dynamic measures. Prior to accounting for comorbidity, women with PTSD or MDD showed significantly greater AUCi than women without the respective disorder. Accounting for comorbidity, PTSD only did not differ from other groups, while MDD only and PTSD+MDD showed greater AUCi than women with neither disorder. Results were nonsignificant for waking cortisol levels or AUCg. Results suggest that MDD drives elevated waking cortisol response, but not basal cortisol activity in recently abused IPV victims. Results demonstrate the importance of examining comorbid diagnoses and HPA activity from a dynamic perspective. Therapeutic implications are discussed.
Keywords: IPV, PTSD, depression, comorbidity, cortisol
Introduction
Intimate partner violence (IPV) is a traumatic stressor that often occurs over extended periods of time (DeBellis et al., 1999; Humphreys, Cooper, & Miaskowki, 2010; Johnson, Delahanty, & Pinna, 2008) and places victims at high risk for posttraumatic stress disorder (PTSD) and major depressive disorder (MDD; Humphreys et al., 2010; Sabri et al., 2013). Rates of PTSD in victims of IPV have been estimated to be as high as 75% (Griffin, Resick, & Yehuda, 2005; Johnson et al., 2008) and approximately half of IPV victims with PTSD suffer from comorbid MDD (Griffin et al., 2005; Nixon, Resick, & Nishith, 2004). Such comorbidity is associated with increased symptom severity and associated functional impairment (Ginzburg, 2006; Green et al., 2006). While biological studies have revealed hypothalamic-pituitary-adrenal (HPA) axis alterations associated with both PTSD (Lauc, Zvonar, Vusic-Mihaljevic, & Flogel, 2004; Neylan et al., 2005; Rohleder, Joksimovic, Wolf, & Kirschbaum, 2004; Wessa et al., 2006) and MDD (Bhagwagar, Hafizi, & Cowen, 2005; Johnson et al., 2008; Pruessner et al., 2003; Ulrike, Reinhold, & Dirk, 2013; see also Huber et al., 2006; Stetler & Miller, 2005), the direction of alterations often differs. Research examining comorbid PTSD and MDD in a range of populations has suggested that such comorbidity may alter the profile of HPA dynamics (de Kloet et al., 2008; Halbreich et al., 1988; Griffin et al., 2005; Oquendo et al., 2003; Yehuda et al., 2004; Young & Breslau, 2004). A clearer understanding of the biological basis of these two highly comorbid and highly debilitating disorders may enhance outcomes of intervention efforts targeting PTSD and depression in IPV victims. The present study examined the relation of HPA activity to PTSD, MDD, and their comorbidity in a sample of IPV victims.
HPA Dynamics
The HPA axis represents one of the primary biological stress systems, activation of which is believed to be necessary for successful adaptation in response to stress (e.g. McEwen, 2000; Rasmusson et al., 2004). HPA axis activity begins with secretion of corticotropin releasing hormone (CRH) from the hypothalamus. CRH stimulates the release of adrenocorticotropic hormone (ACTH) from the pituitary, which elicits cortisol excretion from the adrenal gland. HPA axis dynamics are characterized by both a circadian rhythm and responsivity to both real and perceived threat. In healthy adults, the circadian rhythm follows a predictable pattern of sharp increase in activity in the first 30 minutes after waking and a gradual decline over the course of the day. Activity enters nadir shortly after sleep onset and remains low until shortly before waking again the following morning. This pattern of activity is believed to activate physiological resources required to meet the challenges of the day, while allowing the body to recuperate from its expenditures while asleep.
Thus, the HPA axis is a dynamic system, adequate functioning of which is believed necessary to manage both the daily challenges of life and to overcome real and perceived threat. While rapid increases in cortisol may be necessary to overcome threat, chronic elevations in cortisol levels may be neurotoxic, particularly to structures involved in cognition and emotional/behavioral regulation (Burghy et al., 2012; Schuhmacher et al., 2012; Vachon-Presseau et al., 2013). Such impact may be mechanistic in the development of PTSD and/or MDD.
A Common Pathways Model
The importance of understanding mechanisms through which PTSD and MDD develop is particularly clear when considering the increased severity and functional impairment associated with their comorbidity. For example, Ginzburg (2006) reported that heart attack patients who had comorbid PTSD and MDD had the lowest levels of psychosocial functioning compared to patients with PTSD only, MDD only, and patients with neither disorder. Neria & Bromet (2000) have suggested a common underlying pathway to the development of both disorders following trauma. This would be consistent with noted similarities in risk factors for both disorders (e.g. female gender, low socioeconomic status, chronic stress/trauma; Breslau, Chilcoat, Kissler, & Davis, 1999; Buydens-Branchey, Noumair, & Branchey, 1990; Goto, Wilson, Kahana, & Slane, 2002; Patton, Coffey, Posterino, Carlin, & Bowes, 2003). Evidence that trauma exposure increases risk for the onset of depression in individuals who later develop PTSD, but not in trauma-exposed individuals who do not develop PTSD (Breslau, Davis, Peterson, & Schultz, 2000), also supports a model in which a common pathway leads to the development of both depression and PTSD following exposure to traumatic events such as IPV. The impact of trauma on HPA dynamics may reflect such a common pathway via associated cognitive and behavioral responses.
HPA Dynamics & Trauma
Understanding HPA dynamics and associated cognitive and behavioral sequelae may be critical to developing a better understanding of the potentially mechanistic relation of HPA activity to PTSD and MDD. Historically, basal cortisol levels were examined in MDD (Deuschle, Weber, Colla, Depner, & Heuser, 1998; Scott & Dinan, 1998), and PTSD (see Meewisse et al., 2007 for review). More recently, the limitations inherent in interpreting results focusing on basal measures have been noted, leading to a call to assess HPA axis functioning dynamically (de Kloet et al., 2006; Meewisse et al., 2007). Assessing HPA dynamically may enhance our ability to translate results into the cognitive and behavioral responses relevant to daily and threat-related adjustment. Further, such dynamic specificity may lead to differentiating paths common to both PTSD and MDD versus paths that may be specific to one disorder.
HPA Dynamics in PTSD
Past research has found elevated, decreased, or no difference in basal cortisol levels in adults with PTSD versus traumatized adults who did not develop PTSD (Klaasens etal., 2012; Meewisse et al., 2007). Results of meta-analyses have suggested that, while basal cortisol levels do not differ consistently across studies in general (Klaasens et al., 2012; Meewisse et al., 2007), lower cortisol is consistently observed when women are included in samples, abuse is the trauma of index, and when afternoon cortisol levels are assessed (Meewisse et al., 2007).
Several studies have examined naturally occurring HPA dynamics associated with the cortisol waking curve. Two studies – one with veterans and one with police officers – found lower waking cortisol responses in individuals with PTSD, compared to similarly traumatized groups without PTSD (Lauc et al., 2004; Neylan et al., 2005). Similarly, Rohleder et al. (2004) found lower cortisol response to waking in male and female war refugees with PTSD, compared to a mixed sample of refugees without PTSD and laboratory staff. Lower cortisol response to waking was also found in men and women with PTSD associated with mixed acute traumas (accidents, violent crimes, sexual assault), compared to both traumatized and non-traumatized controls (Wessa et al., 2006).
Only one study has examined the cortisol waking response associated with PTSD in recently abused IPV victims living in battered women’s shelters (Johnson et al., 2008; 2012). Results of this study found elevated, rather than blunted, cortisol response to waking associated with PTSD. The high levels of stress, recency of the trauma, and frequent trauma reminders in this population may explain why these findings differed from those of prior research. It is also possible that differential rates of comorbid depression are responsible for these discrepant findings. Specifically, prior research either excluded or had minimal representation of comorbid MDD (Neylan et al., 2005; Rohleder et al., 2004) or did not report the presence/absence of depression (Lauc et al., 2004). In the one study to conduct analyses with and without excluding individuals with comorbid MDD (Wessa et al., 2006), PTSD continued to be associated with lower waking cortisol after excluding participants with comorbid MDD. However, in this study, focal traumas were acute in nature, versus the highly chronic nature of IPV.
Thus, evidence largely suggests that PTSD is associated with a blunted cortisol response to waking. This may reflect decreased cognitive and behavioral activation which are generally believed to be required for optimal daily functioning. In recently abused IPV victims residing in battered women’s shelters, the opposite pattern of cortisol waking response has been noted. The frequent reminders of trauma (e.g. the sight of one’s own and other women’s injuries) may contribute to enhanced cognitive processing and associated readiness to react (i.e. hyperarousal), reflected in the enhanced waking cortisol response noted in this sample. Other factors such as comorbid MDD may also contribute to this noted difference in the cortisol waking response.
HPA Dynamics in MDD
MDD is consistently associated with elevated basal cortisol, though these elevations are largely limited to the latter half of the circadian rhythm (see Burke et al., 2005 for meta-analytic review). When considering the cortisol waking response, a blunted response is typically associated with PTSD, while significantly enhanced cortisol waking responses have been identified in several studies of depression (Bhagwagar et al., 2005; Johnson et al., 2008; Pruessner et al., 2003; Ulrike et al., 2013; see Huber et al., 2006 and Stetler & Miller, 2005 for exceptions). This would suggest an enhanced activation of cognitive and behavioral resources beyond that which is noted in well-adjusted individuals. Such enhanced cortisol response to waking, coupled with the general elevation reflected in basal cortisol levels, may become neurotoxic over time and lead to degradation of neurological structures associated with emotion regulation (McEwen, 2001; Schuhmacher et al., 2012; Vachon-Presseau et al., 2013). Such emotion regulation, of course, has cognitive and behavioral components at its core and is clearly noted in both MDD and PTSD.
However, this does not clearly explain the discrepant relation of PTSD versus MDD to HPA dynamics, despite the high rates of comorbidity between the two disorders. Consideration of HPA dynamics as they relate to cases of comorbid depression and PTSD may clarify this confusing state of the literature.
Comorbid PTSD/MDD & HPA Dynamics
Two studies have examined the role of comorbidity in circadian HPA dynamics. In the first, Young & Breslau (2004) examined the role of comorbid MDD in cortisol levels both upon waking and at 7 p.m. Adults with PTSD were compared to traumatized and non-traumatized controls. Only evening cortisol levels differed between groups. Elevated evening cortisol in the PTSD group, compared to both the traumatized and non-traumatized controls, was specific to PTSD comorbid with MDD. This is consistent with evidence that baseline cortisol elevations in MDD are particularly pronounced in the evening (see Burke et al., 2005 for meta-analytic review). The second study accounting for HPA circadian dynamics in comorbid PTSD and MDD examined baseline cortisol levels at multiple time points from 9 a.m. to 3 p.m. in a sample of MDD patients (Oquendo et al., 2003). Patients with MDD only were compared to those with comorbid MDD and PTSD and healthy controls. The MDD only group had higher cortisol levels across the day, compared to healthy controls. In contrast, the comorbid group had lower cortisol levels across the day, compared to controls. While these two studies may appear to be in conflict, similar waking values between comorbid and healthy controls (Young & Bresleau, 2004), followed by subsequently lower cortisol across the day (9 a.m. to 3 p.m.: Oquendo et al., 2003), and elevated evening values (Young & Bresleau, 2004) suggest an overall blunting of the circadian rhythm in individuals with comorbid PTSD and MDD.
Clearly, such an assertion requires further research examining the full circadian rhythm within a single study. However, such evidence, taken together, suggests the possibility that comorbidity may be associated with blunted cognitive and behavioral activation following waking and ongoing blunted activation throughout the course of the day.
In summary, comorbid PTSD and MDD appear to moderate HPA activity as measured by a range of methods. Lack of replication and specificity to time of waking limits conclusions that may be drawn from existing studies assessing basal measures across the circadian rhythm. However, the studies, taken together, suggest a blunted circadian rhythm associated with comorbidity. Research examining comorbidity as it relates to the cortisol waking response may help clarify results of prior circadian research (Oquendo et al., 2003; Young & Breslau, 2004), and set the stage for investigation of cognitive and behavioral translations.
The present study sought to extend prior literature examining the relation of comorbid PTSD/MDD to HPA circadian dynamics and specifically extend the literature examining HPA dynamics associated with IPV. We consider the natural dynamics of the cortisol response to waking in a sample of IPV victims currently residing in battered women’s shelters. Analyses expand upon our previous report in which elevated cortisol waking response was associated with PTSD and symptoms of depression in a sample of IPV victims (Johnson et al., 2008; 2012).
We expected that both PTSD and MDD would independently be associated with enhanced cortisol response to waking, replicating our previous report. Further, we expected that women with comorbid PTSD and MDD would show the greatest cortisol response. Based on results of prior research examining the relation of the cortisol waking response to PTSD (Lauc et al., 2004; Neylan et al., 2005; Rholeder et al., 2004; Wessa et al., 2006) or MDD (Bhagwager et al., 2005; Johnson et al., 2008; Pruessner et al., 2003), we expected that any enhanced cortisol waking response noted in PTSD would be driven by comorbid MDD. Specifically, we expected MDD, absent comorbid PTSD, to be associated with enhanced cortisol waking response, while we expected PTSD, absent comorbid MDD, to be associated with blunted cortisol response to waking, as has consistently been noted in previous research. Given evidence that comorbidity does not consistently impact basal cortisol activity in PTSD (Klaasens et al., 2012; Meewisse et al., 2007), we expected group differences in the cortisol response to waking, but not in basal measures of cortisol activity. Such evidence for the importance of considering the full range of HPA dynamics is expected to be critical to the ongoing clarification of apparent discrepancies in the relation of PTSD versus MDD to HPA activity and may contribute to emerging evidence for common pathways to both disorders. Perhaps most importantly, it is expected that results of the present study will contribute to enhancement of cognitive and behavioral interventions for comorbid PTSD and/or MDD in IPV victims.
Materials & Methods
The following procedures were approved by the Institutional Review Boards of Summa Health System and Kent State University. One hundred fifty-four women were recruited from battered women’s shelters between 2004 and 2007. Women who reported abuse by a romantic partner in the past month were eligible for the study. Eligible women provided informed consent, and collected saliva samples in shelter during the first hour after waking in order to determine their cortisol response to waking. Women were interviewed in-shelter with the Clinician Administered PTSD Scale (CAPS) and Structured Clinical Interview for DSM-IV (SCID). The Beck Depression Inventory (BDI) and Conflict Tactics Scale – 2 (CTS-2) were also completed to provide self-report of depression severity and characteristics of recent abuse.
Measures
CAPS
The Clinician Administered PTSD Scale (CAPS; Blake et al., 1990) is a semi-structured interview used to assess diagnostic criteria for PTSD, based on DSM-IV criteria. Criterion symptoms of PTSD specific to the index trauma are assessed for frequency and severity, and the extent to which they cause functional impairment and have been present for at least one month. The CAPS is one of the most-widely used measures for the assessment of PTSD and has well-established validity (Weathers, Keane, & Davidson, 2001). Current PTSD was assessed with respect to the abusive relationship that led to participants’ shelter admission. Inter-rater reliability was high when calculated for 21 randomly selected interviews (κ = .83). Internal reliability for symptom frequency and intensity was excellent α = .93.
SCID
Major Depressive Disorder was assessed with the Structured Clinical Interview for DSM-IV-TR Axis I Disorders (SCID; First, Gibbon, Spitzer, & Williams, 2001). The SCID is a standardized semi-structured clinical interview for the diagnosis of Axis I disorders, based on DSM-IV-TR criteria. The presence and duration of depressed mood and anhedonia are assessed first. Similar assessments of remaining criterion symptoms follow when one or both of these symptoms are positive, followed by assessment of functional impairment. Inter-rater reliability calculated for a subset of SCIDs for the current study was high (κ = .87, N = 16).
BDI
The Beck Depression Inventory (BDI; Beck, Ward, Mendelson, Mock, & Erbaugh, 1961) was used to assess severity of depression symptoms for descriptive purposes only. The BDI is a 21-item self-report measure of characteristic attitudes and symptoms of depression with established reliability and validity. Scores on the BDI are highly correlated with interview-based diagnosis of MDD (Ambrosini et al., 1991). Internal reliability in the present sample was good α = .88.
CTS-2
Injury inflicted on participants by recent (past month) IPV was assessed using the Conflict Tactics Scale – 2 (CTS-2), which is a 78-item self-report measure with established validity and reliability (Straus, Hamby, Boney-McCoy, & Sugarman, 1996). Items assess the extent to which acts of psychological aggression, physical assault, sexual coercion, and injury were perpetrated against participants, in addition to assessing acts of negotiation. Participant report of romantic partner abuse on the CTS-2 served as the basis for assessing inclusion criteria for the present study. Participants who endorsed at least one item on one of the following subscales were invited to participate in the present study: psychological aggression, physical assault, sexual coercion, or injury the month prior to shelter admission. Severity of total injuries in the past month was also examined as a possible control variable in present analyses. Chronicity of abuse was defined as duration of most recent abusive relationship in months, as measured by participant report. Internal reliability was excellent α = .95.
Salivary Cortisol
Waking cortisol samples were collected per recommendations by Pruessner et al. (2003) at four points over the first hour after waking – immediately upon waking, and at 30, 45, and 60 minutes after waking. Samples were collected in-shelter, and women were instructed to refrain from eating, drinking, smoking, or brushing their teeth during the sampling period. Women recorded their waking time, as well as the timing of each sample in an attempt to ensure proper sampling. Current medication use and whether an alarm was used to wake up were also recorded. Saliva samples were collected in Salivettes and stored at −80 degrees C until assayed. Saliva samples were processed at the Center for Psychobiology and Psychosomatic Research (Trier, Germany) using a time resolved immunoassay with fluorescence detection (DELPIA; Dressendorfer, Kirchbaum, Rohede, Stahl, & Strasburger, 1992). Samples were assayed in three batches, based on data analytic phase of the study (i.e. preliminary baseline, follow-up, and final analyses). All inter- and intra-assay coefficients of variations were less than 10%.
Statistical Analyses
Waking cortisol levels were examined (i.e. single sample at time of waking), and area under the curve with respect to ground (AUCg), and area under the curve with respect to increase (AUCi) were calculated according to recommendations by Pruessner et al. (2003). AUCg represents the total cortisol output in the first hour after waking, while AUCi represents HPA reactivity to waking (Vedhara et al., 2007). All measures of cortisol activity were normally distributed. Initial independent samples t-tests, one-way ANOVAs, and bivariate correlations were conducted to identify potential covariates. One-way ANCOVAs were conducted to determine the extent to which cortisol measures differed between the PTSD, MDD, and comorbid groups. Significant ANCOVAs for comorbidity groups were followed up with individual ANCOVAs to determine the extent to which group pairs differed significantly from one another.
Results
Preliminary Analyses
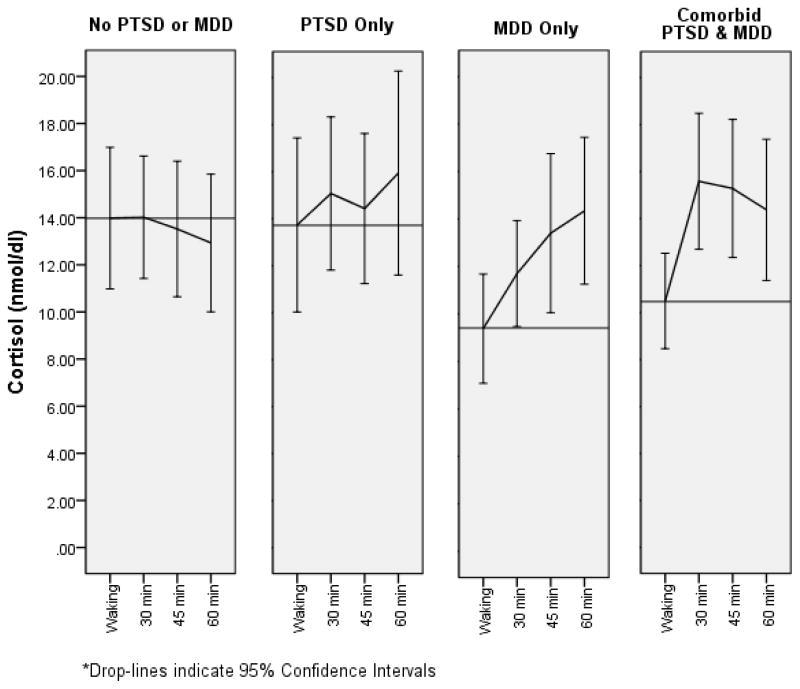
Of the original sample of 163 women, those who failed to provide sufficient samples for cortisol measurement and subsequent calculation of AUCg and AUCi (N = 28) were excluded from analyses, as were women reporting beginning their sampling 30 or more minutes following reported waking time (N = 14). Women reporting that they smoked during the sampling period (N = 15), a woman who reported corticosteroid use, and a woman who was identified as a multivariate outlier were also excluded. Excluded women did not differ significantly from women included in analyses in age t(161) = 1.42, p = .16, minority status χ2(1) = .22, p = .64, or diagnosis of PTSD χ2(1) = .97, p = .32 or MDD χ2(1) = .21, p = .65. The final sample included 104 women who provided complete and valid waking cortisol samples. Of these, the mean timing of the waking saliva sample was 3.58 minutes following waking SD = 5.86. Timing of waking saliva sample was not significantly correlated with waking cortisol levels r = .06, p = .56, AUCg r = −.02, p = .81, or AUCi r = −.11, p = .26. Waking cortisol curves are depicted by comorbidity group in Figure 1.
Figure 1.
Waking Cortisol Curve by Comorbidity Status
Demographics and rates of PTSD and MDD for the final sample are provided in Table 1. Rates of comorbidity between PTSD and MDD are noted in Table 2. Symptoms of PTSD averaged 71.19 (15.49) in the PTSD group, compared to 33.31 (15.49) in the non-PTSD group. Average duration of PTSD symptoms was 37.96 months (SD = 69.87; range = 1 month to 36 years) for those who met full criteria for the disorder.
Table 1.
Demographics
| Characteristic | Total Sample (N = 104) | PTSD +/− (N = 104) | MDD +/− (N = 104) | ||
|---|---|---|---|---|---|
|
| |||||
| PTSD+ (N = 68) | PTSD− (N = 36) | MDD+ (N = 55) | No MDD− (N = 49) | ||
|
| |||||
| Age | 18–52 33.84 (9.32) |
18–52 32.53 (8.92) |
19–52 36.31 (9.67) |
18–52 33.69 (8.92) |
19–52 34.00 (9.83) |
| Race | |||||
| African American | 46.2% | 48.5% | 41.7% | 40% | 53.1% |
| Caucasian | 41.3% | 39.7% | 44.4% | 49.1% | 32.7% |
| Biracial | 8.7% | 8.3% | 8.3% | 9.1% | 8.2% |
| Native American | 3.8% | 2.9% | 5.6% | 1.8% | 6.1% |
| Length of Index Abusive | 1 mo.–25 yrs. | 1 month–25 yrs. | 2 months–24 yrs. | 1 month–25 yrs. | 2 months–24 yrs. |
| Relationship (yrs.) | 5.22 (5.99) | 5.11 (5.59) | 4.9 (6.06) | 5.87 (6.31) | 4.11 (4.89) |
Table 2.
Mean (SD) HPA Activity by Group
| Variable | Total Sample (N = 104) | PTSD+/− | MDD+/− | Comorbidity Classification | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PTSD+ (N = 68) | PTSD− (N = 36) | F-test (df) p-value |
MDD+ (N = 55) | MDD− (N = 49) | F-test (df) p-value |
No PTSD or MDD (N = 24) | MDD Only (N = 12) | PTSD Only (N = 25) | PTSD & MDD (N = 43) | F-test (df) p-value |
||
| Waking | 11.95 (7.25) | 11.69 (7.66) | 12.45 (6.50) | .74 (8,95) .39 |
10.25 (6.06) | 13.86 (8.04) | 5.11 (8,95) .03 |
13.99 (7.12) | 9.38 (3.62) | 13.73 (8.98) | 10.50 (6.60) | 1.69 (10,93) .18 |
| AUCg | 833.88 (406.40) | 858.39 (449.30) | 787.59 (310.22) | 1.14 (8,95) .29 |
816.28 (413.50) | 853.63 (401.62) | .06 (8,95) .80 |
825.03 (352.23) | 712.72 (193.97) | 881.10 (449.56) | 845.19 (453.94) | .58 (10,93) .63 |
| AUCi | 116.81 (317.37) | 157.23 (339.24) | 40.46 (258.74) | 6.75 (8,95) .01 |
201.05 (319.35) | 22.24 (290.08) | 8.44 (8,95) .01 |
−14.17 (271.42) | 149.72 (198.60) | 57.20 (308.38) | 215.38 (346.16) | 3.62 (10,93) .02 |
Waking = waking cortisol (nmol/dl); AUCg = Area Under the Curve with Respect to Ground (nmol/dl); AUCi = Area Under the Curve with Respect to Increase (nmol/dl).
Note: All F-values control for age, chronicity, minority status, anti-anxiety medication, other Anxiety Disorders, Bipolar Spectrum Disorder and time between waking and first saliva sample.
Women with PTSD were significantly younger than women without PTSD t(102) = 2.00, p = .05, but age did not differ by MDD status, and was not related to measures of cortisol ps > .15. Due to the small number of Biracial and Native American women, race was dichotomized to examine minority status. Minority status did not differ significantly by PTSD diagnosis χ2(1) = .45, p = .50, MDD χ2(1) = 1.78, p = .18, or comorbidity group status χ2(3) = 4.25, p = .24. Waking cortisol also did not differ significantly by minority status t(102) = .77, p = .45, though minority status was associated with significantly lower AUCg t(102) = 3.15, p < .01, and higher AUCi t(102) = 2.92, p < .01. Use of an alarm clock to wake up was not correlated with cortisol activity [Waking: t(102) = 1.32, p = .19; AUCg: t(102) = .28, p = .78; AUCi: t(102) = 1.45, p = .15]. Total injuries in the past month were also not related to severity of PTSD r = .07, p = .49 or depression r = .10, p = .35, did not relate to any measure of cortisol activity (Waking: r = −.03, p = .73; AUCg: r = −.03, p = .80; AUCi: r = .02, p = .88), and did not differentiate between PTSD t(102) = .23, p = .82, MDD t(102) = 1.04, p = .30, or comorbidity groups F(3,100) = .38, p = .77. Chronicity of abuse did not differ between PTSD groups t(102) = .18, p = .86, MDD groups t(102) = 1.57, p = .12, or among comorbidity groups F(3,100) = 1.67, p = .18. Chronicity of abuse was not associated with waking cortisol r = −.17, p = .08 or AUCi r = .09, p = .36, but was negatively correlated with AUCg r = −.26, p = .01. Women with a Bipolar Spectrum Disorder N = 13 were significantly more likely to have PTSD than women without a Bipolar Spectrum Disorder χ2 = 4.76, p = .03. Diagnosis of at least one anxiety disorder other that PTSD N = 56 was associated with more severe symptoms of depression t = 2.01, p = .05. Use of psychiatric medications was similar by PTSD diagnosis χ2(1) = 1.13, p = .29, MDD diagnosis χ2(1) = 1.34, p = .25, comorbidity group χ2(3) = 4.39, p = .22. Symptoms of PTSD and depression were also similar between women who did N = 15 and did not use psychiatric medications t(102) = .45, p = .65 and t(98) = .13, p = .90, respectively. Furthermore, all measures of cortisol activity were similar between those using and not using psychiatric medications [waking cortisol: t(102) = .40, p = .69; AUCg: t(102) = .01, p > .99, AUCi: t(102) = .56, p = .58]. When examining specific psychiatric medications, similar results were found for antidepressants and mood stabilizers. Use of antidepressants N = 11 were similar by PTSD diagnosis χ2(1) = 29, p = .59, MDD diagnosis χ2(1) = .01, p = .91, and comorbidity status χ2(3) = .30, p = .96. Symptoms of PTSD and depression were also similar between women who did and did not use antidepressants t(102) = 1.06, p = .29 and t(98) = .04, p = .97, respectively. Moreover, all measures of cortisol were similar between women using and not using antidepressants [waking cortisol: t(102) = .88, p = .97; AUCg: t(102) = .83, p = .41; AUCi: t(102) = .14, p = .89]. Use of mood stabilizers N = 3 was similar based on PTSD diagnosis χ2(1) = 1.40, p = .24, MDD diagnosis χ2(1) = .47, p = .49, and comorbidity status χ2(1) = 2.80, p = .42. Symptoms of PTSD and depression did not differ between women using and not using mood stabilizers t(102) = .15, p = .88 and t(98) = 1.17, p = .24, respectively. Measures of cortisol activity were also similar between women using and not using mood stabilizers [waking: t(102) = .72, p = .47; AUCg t(102) = 1.77, p = .08; AUCi t(102) = 1.26, p = .21]. Only use of anti-anxiety medication N = 7 showed any effect. While rates of anti-anxiety use were similar based on PTSD diagnosis χ2(1) = 1.68, p = .20 and MDD diagnosis χ2(1) = .06, p = .82, women in the MDD only group were more likely to be taking anti-anxiety medications than those in the PTSD only, Comorbid PTSD/MDD, or neither disorder groups χ2 = 8.03, p = .05. Symptoms of PTSD and depression did not differ by anti-anxiety medication use t(102) = .27, p = .79 and t(98) = .75, p = .46, respectively. Cortisol activity also did not differ by anti-anxiety use [waking: t(102) = .57, p = .57; AUCg: t(102) = .32, p = .75; AUCi: t(102) = .38, p = .71]. Given these relationships, we conservatively controlled for age, minority status, abuse chronicity, anti-anxiety medication, Bipolar Spectrum Disorder, and other Anxiety Disorders in all analyses. Time between waking and first sample was also controlled for in order to ensure that results were not spurious.
PTSD, MDD, & HPA Dynamics
Results were first examined based on PTSD diagnosis, and then by diagnosis of MDD, before considering comorbidity (Table 2). After accounting for age, minority status, abuse chronicity, anti-anxiety medication, Bipolar Spectrum Disorder, and other Anxiety Disorders, women with PTSD did not differ significantly from women without PTSD on waking cortisol F(8,95) = .74, p = .39 or AUCg F(8,95) = 1.13, p = .29. However, women with PTSD had significantly greater AUCi compared to those without PTSD F(8, 95) = 6.75, p = .01. Women with MDD had significantly lower waking cortisol levels F(8,95) = 5.11, p = .03, and greater AUCi F(8,95) = 8.68, p < .01, compared to women without MDD. AUCg did not differ significantly between MDD groups F(8,95) = .06, p = .80.
Comorbidity & HPA Dynamics
Data were next analyzed based on comorbidity status (i.e. neither PTSD nor MDD; PTSD only; MDD only; or PTSD plus comorbid MDD; Table 2). Comorbidity groups did not differ with respect to waking cortisol F(10,93) = 1.69, p = .18 and AUCg F(10,93) = .58, p = .63, after accounting for age, minority status, abuse chronicity, anti-anxiety medication, Bipolar Spectrum Disorder, and other Anxiety Disorders, but differences were significant for AUCi F(10, 93) = 3.93, p = .02. Post hoc analyses indicated that AUCi was higher in women with PTSD plus comorbid MDD compared to women with neither PTSD nor MDD F(8,58) = 12.48 p < .01. Women with either PTSD or MDD, alone, did not differ from each other F(8,28) < .01, p = .97. Women with MDD only had higher AUCi than women with neither PTSD nor MDD F(8,27) = 4.52, p = .04. Women with PTSD only did not differ from women in the comorbid group F(8,40) = .83, p = .37.
Discussion
Results of the present research highlight the importance of accounting for both comorbidity and HPA dynamics when seeking to understand the relation of HPA activity to trauma-related psychopathology such as PTSD and MDD. Specifically, both PTSD and MDD were associated with elevated waking cortisol responses prior to accounting for comorbidity. This is consistent with our prior report (Johnson et al., 2008) and was largely specific to the most dynamic measure of the cortisol waking response – AUCi. However, after accounting for comorbidity, PTSD only, did not differ significantly from other groups. On the other hand, women with MDD only, showed significantly higher cortisol waking response than women with neither disorder. Thus, we conclude that comorbid MDD was driving the elevated cortisol waking response originally observed in PTSD. Further, as predicted, women with comorbid PTSD and MDD showed the largest cortisol waking response. Given evidence that elevated cortisol response noted in this sample is due to MDD, rather than PTSD, results observed in the comorbid group are also likely the result of comorbid MDD.
Results replicate the majority of the literature examining the relation of depression to the cortisol waking response (Bhagwagar et al., 2005; Johnson et al., 2008; Pruessner, et al., 2003; Ulrike et al., 2013; see Huber et al., 2006 and Stetler et al., 2005 for exceptions). However, results contradict consistent previous reports indicating that PTSD is associated with blunted cortisol response to waking (Lauc et al., 2004; Neylan et al., 2005; Rohleder et al., 2004; Wessa et al., 2006). A recent meta-analysis by Morris et al. (2012) suggests that present results may be specific to samples with similar recency and/or chronicity of trauma. Specifically, the present sample experienced IPV for an average of more than 5 years, with the most recent incident being within the past month. The recency of trauma and/or the stressful nature of shelter living, including constant reminders of IPV experienced by women in the present sample, may also be responsible for apparent differences in the relation of PTSD to the waking cortisol response between present results and those reported in prior literature.
In addition to the noted relation of MDD to HPA activity, an unexpectedly flat profile of waking cortisol response was noted in women who did not meet criteria for either MDD or PTSD. While this is the group that most closely represents a control group, their history of IPV and/or the stress of the shelter environment may have contributed to the lack of expected waking cortisol increase (Yehuda et al., 2004; Savic et al., 2012). Although a non-traumatized control group is not available against which these results may be compared, these results importantly extend the only other known report of the waking cortisol response in IPV victims by examining comorbidity.
Further exploration of the relationship between PTSD, MDD, and HPA dynamics may help guide intervention efforts in the aftermath of IPV. For example, while present results suggest that MDD may drive physiological response as measured herein, results also clearly demonstrate that MDD presented as comorbid with PTSD far more often than alone. Efficacious trauma-focused interventions have been shown to also reduce depressive symptoms, while the opposite has not been shown (e.g. Schnurr et al., 2007). Rather, treatment-resistant depression is of ongoing concern (Kasper & Montgomery, 2013). Evidence for a common underlying physiology between PTSD and MDD in the context of trauma suggests that application of trauma-focused interventions to trauma-related MDD may be one avenue leading to a reduction in the incidence of treatment-resistant depression. Clearly, such clinical implications are merely speculative, based on current results. Present results require replication and extension to other populations such as male victims of IPV and victims of other forms of trauma (both chronic and acute). However, cautious speculation about clinical implications may benefit the translation of such findings as those presented herein to clinical practice. Such translation may be particularly effective to the extent that HPA dynamics associated with comorbid MDD and PTSD are first translated into the cognitive and behavioral aspects of emotion regulation that are relevant to intervening with both.
A number of limitations are acknowledged in the present study. First, our sample lacks a non-traumatized control group against which to compare existing results. In the absence of an adequate control group, it is unclear whether any of the groups represented in the present sample reflect normal HPA dynamics, or whether all groups represent different variations of HPA dysregulation. A second limitation is that the present sample size lacked statistical power to detect an interaction between PTSD and MDD status in predicting HPA dynamics η2 = .05. Testing for an interaction between PTSD and MDD may be a more appropriate approach to testing the hypotheses contained herein, but was not feasible given the present sample size. Moreover, some potential confounds were not assessed in the present study (e.g. menstrual status). Also, while assessment of the waking cortisol curve is most ecologically valid in the home environment (i.e. in-shelter, for the present sample), assessment in such a non-controlled environment is at risk of increased measurement error (e.g. inaccurate recordings of waking and sampling times, inadvertent switching of samples). Use of MEMS caps (devises to monitor, electronically, the timing at which sampling was completed) would help address this limitation in future research. Further, direct measurement of HPA response to psychosocial challenge such as exposure to reminders of one’s trauma would further contribute to the understanding of how cortisol relates to comorbid PTSD and MDD. Such research is in progress, and is expected to contribute to a better understanding of the relation of HPA dynamics to these two highly comorbid disorders, and improve treatment of PTSD and MDD in victims of IPV.
Conclusions
Results of the present research provide preliminary evidence that comorbid depression may drive the relationship between PTSD and cortisol response to waking in recently abused IPV victims. Given the preliminary nature of these results, replication and extension will be important in determining the extent to which such results may inform the treatment of these two highly comorbid and highly debilitating disorders.
Acknowledgments
We would like to acknowledge the Battered Women’s Shelters of Akron, OH for their critical contributions to this research. We thank you for all you do to better the lives of those victimized by intimate partner violence.
This research was supported by NIMH grant K23 MH067648 (DMJ) and pilot funds from the Summa-Kent State Center for the Treatment and Study of Traumatic Stress (DMJ & DLD).
Footnotes
Author Disclosure Statement
No competing financial interests exist.
Contributor Information
Keri L. M. Pinna, Email: sipx0006@umn.edu.
Dawn M. Johnson, Email: johnsod@uakron.edu.
Douglas L. Delahanty, Email: ddelahan@kent.edu.
References
- Ambrosini PJ, Metz C, Bianchi MD, Rabinovich H, Undie A. Concurrent validity and psychometric properties of the beck depression inventory in outpatient adolescents. American Journal of Child & Adolescent Psychiatry. 1991;30:51–57. doi: 10.1097/00004583-199101000-00008. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archive of General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Bhagwagar Z, Hafizi S, Cowen PJ. Increased salivary cortisol after waking in depression. Psychopharmacology. 2005;182:54–57. doi: 10.1007/s00213-005-0062-z. [DOI] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. A clinician rating scale for assessing current and lifetime PTSD: The CAPS-I. The Behaviour Therapist. 1990;13:187–188. [Google Scholar]
- Breslau N, Chilcoat HD, Kessler RC, Davis GC. Vulnerability to assaultive violence: further specification of the sex difference in post-traumatic stress disorder. American Journal of Psychiatry. 1999;156:902–907. doi: 10.1017/s0033291799008612. [DOI] [PubMed] [Google Scholar]
- Breslau N, Davis GC, Peterson EL, Schultz LR. A second look at comorbidity in victims of trauma: the posttraumatic stress disorder–major depression connection. Biological Psychology. 2000;48:902–909. doi: 10.1016/s0006-3223(00)00933-1. [DOI] [PubMed] [Google Scholar]
- Burghy CA, Stodola DE, Ruttle PL, Molloy EK, Armstrong JM, Oler JA, Birn RM. Nature & Neuroscience. 2012;15:1736–1741. doi: 10.1038/nn.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke HM, Davis MC, Otte C, Mohr DC. Depression and cortisol responses to psychological stress: A meta-analysis. Psychoneuroendocrinology. 2005;30:846–856. doi: 10.1016/j.psyneuen.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Buydens-Branchey L, Noumair K, Branchey M. Duration and intensity of combat exposure and posttraumatic stress disorder in Vietnam veterans. Journal of Nervous & Mental Disease. 1990;178:582–587. doi: 10.1097/00005053-199009000-00005. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Baum AS, Birmaher B, Keshavan MS, Eccard CH, Boring AM, Ryan ND. Developmental traumatology part I: Biological stress systems. Biological psychiatry. 1999;45:1259–1270. doi: 10.1016/s0006-3223(99)00044-x. [DOI] [PubMed] [Google Scholar]
- De Kloet CS, Vermetten E, Geuze E, Kavelaars A, Heijnen CJ, Westenberg HG. Assessment of HPA-axis function in posttraumatic stress disorder: Pharmacological and non-pharmacological challenge tests, a review. Journal of Psychiatric Research. 2006;40:550–567. doi: 10.1016/j.jpsychires.2005.08.002. [DOI] [PubMed] [Google Scholar]
- De Kloet C, Vermettena E, Lentjese E, Geuzea E, van Peltf J, Manuele R, Westenbergc H. Differences in the response to the combined DEX-CRH test between PTSD patients with and without co-morbid depressive disorder. Psychoneuroendocrinology. 2008;33:313–320. doi: 10.1016/j.psyneuen.2007.11.016. [DOI] [PubMed] [Google Scholar]
- Deuschle M, Weber B, Colla M, Depner M, Heuser I. Effects of major depression, aging and gender upon calculated diurnal free plasma cortisol concentrations: A reevaluation study. Stress. 1998;2:281–287. doi: 10.3109/10253899809167292. [DOI] [PubMed] [Google Scholar]
- Dressendorfer R, Kirschbaum C, Rohede W, Stahl F, Strasburger C. Synthesis of cortisol-biotin conjugate and evaluation as tracer in immunoassay for salivarycortisol measurement. Journal of Steroid Biochemistry & Molecular Biology. 1992;43:683–692. doi: 10.1016/0960-0760(92)90294-s. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JB. User’s guide for the Structured Clinical Interview for DSM-IV-TR Axis I Disorders – Research Version. Journal of Applied Social Psychology. 2002;36:2001–2026. [Google Scholar]
- Ginzburg K. Comorbidity of PTSD and depression following myocardial infarction. Journal of Affective Disorders. 2006;94:135–143. doi: 10.1016/j.jad.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Goto T, Wilson JP, Kahana B, Slane S. The miyake island volcano disaster in Japan: Loss, uncertainty, and relocation as predictors of PTSD and depression. International Journal of Emergency Mental Health. 2002;4:157–71. [PubMed] [Google Scholar]
- Green BL, Krupnick JL, Chung J, Siddique J, Krause ED, Revicki D, Miranda J. Impact of PTSD comorbidity on one-year outcomes in a depression trial. Journal of Clinical Psychology. 2006;62:815–835. doi: 10.1002/jclp.20279. [DOI] [PubMed] [Google Scholar]
- Griffin MG, Resick PA, Yehuda R. Enhanced cortisol suppression following dexamethasone administration in domestic violence survivors. American Journal of Psychiatry. 2005;162:1192–1199. doi: 10.1176/appi.ajp.162.6.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbreich U, Olympia J, Glogowski J, Carson S, Axelrod S, Yeh CM. The importance of past psychological trauma and pathophysiological process as determinants of current biological abnormalities. Archives of General Psychiatry. 1988;45:293–294. doi: 10.1001/archpsyc.1988.01800270113016. [DOI] [PubMed] [Google Scholar]
- Huber T, Issa K, Schik G, Wolf O. The cortisol awakening response is blunted in psychotherapy inpatients suffering depression. Psychoneuroendocrinolog. 2006;31:900–904. doi: 10.1016/j.psyneuen.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Humphreys J, Cooper BA, Miaskowski C. Differences in depression, posttraumatic stress disorder, and lifetime trauma exposure in formerly abused women with mild versus moderate to severe chronic pain. Journal of Interpersonal Violence. 2010;25:2316–2338. doi: 10.1177/0886260509354882. [DOI] [PubMed] [Google Scholar]
- Johnson DM, Delahanty DL, Pinna K. The cortisol awakening response as a function of PTSD severity and abuse chronicity in sheltered battered women. Journal of Anxiety Disorders. 2008;22:793–800. doi: 10.1016/j.janxdis.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DM, Delahanty DL, Pinna K. Corrigendum to “The cortisol awakening response function of PTSD severity and abuse chronicity in sheltered batteredwomen” [Journal of Anxiety Disorders 22, 793–800] . Journal of Anxiety Disorders. 2012;26:633. doi: 10.1016/j.janxdis.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper S, Montgomery S. Treatment-resistant Depression. Oxford: John Wiley & Sons; 2013. The Role of Nonpharmacological Interventions in Treatment-resistant Depression. [Google Scholar]
- Klaassens ER, Giltay EJ, Cuijpers P, van Veen T, Zitman FG. Adulthood trauma and HPA-axis functioning in healthy subjects and PTSD patients: A meta-analysis. Psychoneuroendocrinology. 2012;37:317–331. doi: 10.1016/j.psyneuen.2011.07.003. [DOI] [PubMed] [Google Scholar]
- Lauc G, Zvonar K, Vusic-Mihaljevic Z, Flogel M. Short communication: post- awakening changes in salivary cortisol in veterans with and without PTSD. Stress & Health. 2004;20:99–102. doi: 10.1002/smi.1001. [DOI] [Google Scholar]
- McEwen Allostasis and allostatic load: Implications for neuropsychopharmacology. Neuropsychopharmacology. 2000;22:102–124. doi: 10.1016/S0893-133X(99)00129-3. [DOI] [PubMed] [Google Scholar]
- McEwen Plasticity of the hipposcampus: Adaptation to chronic stress and allostatic load. Annals of the New York Academy of Sciences. 2001;933:265–277. doi: 10.1111/j.1749-6632.2001.tb05830.x. [DOI] [PubMed] [Google Scholar]
- Meewisse M, Reitsma JB, de Vries G, Gersons BP, Olff M. Cortisol and post- traumatic stress disorder in adults. British Journal of Psychiatry. 2007;191:387–392. doi: 10.1192/bjp.bp.106.024877. [DOI] [PubMed] [Google Scholar]
- Morris MC, Compas BE, Garber J. Relations among posttraumatic stress disorder, comorbid major depression, and HPA function: A systematic review and meta-analysis. Clinical Psychology Review. 2012;32:301–315. doi: 10.1016/j.cpr.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neria Y, Bromet EJ. Comorbidity of PTSD and depression: linked or separate incidence. Biological Psychiatry. 2000;48:878–880. doi: 10.1016/s0006-3223(00)01012-x. [DOI] [PubMed] [Google Scholar]
- Neylan TC, Brunet A, Pole N, Best SR, Metzler TJ, Yehuda R, Marmar CR. PTSD symptoms predict waking salivary cortisol levels in police officers. Psychoneuroendocrinology. 2005;30:373–381. doi: 10.1016/j.psyneuen.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Nixon RD, Resick PA, Nishith P. An exploration of comorbid depression among female victims of intimate partner violence with posttraumatic stress disorder. Journal of Affective Disorders. 2004;82:315–320. doi: 10.1016/j.jad.2004.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oquendo MA, Echavarria G, Galfalvy HC, Grunebaum MF, Burke A, Barrera A, Mann JJ. Lower cortisol levels in depressed patients with comorbid post-traumatic stress disorder. Neuropsychopharmacology. 2003;28:591–598. doi: 10.1038/sj.npp.1300050. [DOI] [PubMed] [Google Scholar]
- Patton GC, Coffey C, Posterino M, Carlin JB, Bowes G. Life events and early onset depression: cause or consequence? Psychological Medicine. 2003;33:1203–1210. doi: 10.1017/S0033291703008626. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/S0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Rasmusson A, Vasek J, Lipschitz DS, Vojvoka D, Mustone ME, Shi Q, Charney DS. An increased capacity for adrenal DHEA release is associated with decreased avoidance and negative mood symptoms in women with PTSD. Neuropsychopharmacology. 2004;29:1549–1557. doi: 10.1038/sj.npp.1300432. [DOI] [PubMed] [Google Scholar]
- Rohleder N, Joksimovic L, Wolf JM, Kirschbaum C. Hypocortisolism and increased glucocorticoid sensitivity of pro-inflammatory cytokine production in Bosnian war refugees with posttraumatic stress disorder. Biological Psychiatry. 2004;55:745–751. doi: 10.1016/j.biopsych.2003.11.018. [DOI] [PubMed] [Google Scholar]
- Sabri B, Bolyard R, McFadgion A, Stockman J, Lucea M, Callwood G, Campbell J. Intimate partner violence, depression, PTSD and use of mental health resources among ethnically diverse black women. Social Work in Health Care. 2013;52:351–369. doi: 10.1080/00981389.2012.745461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savic D, Knezevic G, Damjanovic S, Spiric Z, Matic G. Is there a biological difference between trauma-related depression and PTSD? DST says “NO. Psychoneuroendocrinology. 2012;37:1516–1520. doi: 10.1016/j.psyneuen.2012.02.005. [DOI] [PubMed] [Google Scholar]
- Schnurr P, Friedman M, Engel C, Foa E, Shea M, Bernardy N. Cognitive behavioral therapy for posttraumatic stress disorder in women. JAMA. 2007;297:820–830. doi: 10.1001/jama.297.8.820. [DOI] [PubMed] [Google Scholar]
- Schuhmacher A, Mossner R, Jessen F, Scheef L, Block W, Belloche AC, Zobel A. Association of amygdala volumes with cortisol secretion in unipolar depressed patients. Psychiatry Research: Neuroimaging. 2012;202:96–103. doi: 10.1016/j.pscychresns.2011.09.007. [DOI] [PubMed] [Google Scholar]
- Scott L, Dinan T. Vasopressin and regulation hypothalamic-pituitary-adrenal axis function: implications for pathophysiology of depression. Life Science. 1998;62:1985–1998. doi: 10.1016/s0024-3205(98)00027-7. [DOI] [PubMed] [Google Scholar]
- Stetler C, Miller GE. Blunted cortisol response to awakening in mild to moderate depression: regulatory influences of sleep patterns and social contacts. Journal of Abnormal Psychology. 2005;114:697–705. doi: 10.1037/0021-843X.114.4.697. [DOI] [PubMed] [Google Scholar]
- Straus MA, Hamby SL, Boney-McCoy S, Sugarman DB. The Revised Conflict Tactics Scales (CTS2): Development and preliminary psychometric data. Journal of Family Issues. 1996;17:283–316. [Google Scholar]
- Ulrike S, Reinhold L, Dirk H. Major depression in young girls is related to altered cortisol awakening response. European Child & Adolescent Psychiatry. 2013;22:379–384. doi: 10.1007/s00787-012-0371-9. [DOI] [PubMed] [Google Scholar]
- Vachon-Presseau E, Roy M, Marc-Olivier M, Caron E, Marin M, Chen J, Rainville P. The stress model of chronic pain: evidence from basal cortisol and hippocampal structure and function in humans. Brain: A Journal of Neurology. 2013;136:815–827. doi: 10.1093/brain/aws371. [DOI] [PubMed] [Google Scholar]
- Vedhara K, Miles J, Crown A, McCarthy A, Shankds N, Davies D, Ben-Shlomo Y. Relationship of early childhood illness with adult cortisol in the Barry Caerphilly Growth (BCG) cohort. Psychoneuroendocrinology. 2007;32:865–873. doi: 10.1016/j.psyneuen.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Weathers FW, Keane TM, Davidson JR. Clinician-administered PTSD scale: A review of the first ten years of research. Depression & Anxiety. 2001;13:132–156. doi: 10.1002/da.1029. [DOI] [PubMed] [Google Scholar]
- Wessa M, Rohleder N, Kirschbaum C, Flor H. Altered cortisol awakening response in posttraumatic stress disorder. Psychoneuroendocrinology. 2006;31:209–215. doi: 10.1016/j.psyneuen.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Halligan SL, Golier JA, Grossman R, Bierer LM. Effects of trauma exposure on the cortisol response to dexamethasone administration in PTSD and major depressive disorder. Psychoneuroendocrinology. 2004;29:389–404. doi: 10.1016/S0306-4530(03)00052-0. [DOI] [PubMed] [Google Scholar]
- Young EA, Breslau N. Saliva cortisol in posttraumatic stress disorder: a community epidemiologic study. Biological Psychiatry. 2004;56(3):205–209. doi: 10.1016/j.biopsych.2004.05.011. [DOI] [PubMed] [Google Scholar]