Abstract
Appetitive and defensive motivation account for a good deal of variance in personality and mental health, but whether individual differences in these systems are correlated or orthogonal has not been conclusively established. Previous investigations have generally relied on self-report and have yielded conflicting results. We therefore assessed the relation between psychophysiological indices of appetitive and defensive motivation during elicitation of these motivational states: specifically, frontal EEG asymmetry during reward anticipation and startle response during anticipation of predictable or unpredictable threat of shock. Results in a sample of psychopathology-free community members (n=63), an independent sample of undergraduates with a range of internalizing symptoms (n=64), and the combination of these samples (n=127) revealed that differences in responding to the two tasks were not significantly correlated. Average coefficients approached zero in all three samples (community: .04, undergraduate: −.01, combined: .06). Implications of these findings for research on normal and abnormal personality are discussed.
Several prominent models posit that two broad neurobehavioral systems underlie individual differences in motivation (Davidson, 1998; Depue & Collins, 1999; Elliot & Thrash, 2002; Gray, 1994; Lang, Bradley, & Cuthbert, 1997). The appetitive or approach system underlies pursuit of reward or other positive stimuli and leads to positive emotions such as excitement, enjoyment, and happiness. The defensive, withdrawal, or avoidance system underlies avoidance or behavioral inhibition in response to threatening stimuli, and leads to negative emotions such as fear and anxiety. The constructs of appetitive and defensive motivation have been examined in nearly all areas of psychology for decades.
However, whether and how individual differences in these two systems are related is not fully understood. That is, is the tendency to approach positive stimuli correlated with the tendency to act defensively in the face of threatening stimuli, or are the two tendencies independent? This question is not simply academic, but has important public health implications. Positive and negative affect are disturbed in a wide range of mental illnesses (e.g., depression, anxiety, substance use), and as such have been proposed as core transdiagnostic domains of psychopathology in the National Institute of Mental Health's Research Domain Criteria initiative (RDoC; Insel et al., 2010). Establishing whether the systems are independent or correlated may help to elucidate why these disorders are so highly comorbid (Kessler et al., 2005).
Measuring Motivational Tendencies: Challenges and a Possible Solution
Although there is a long-standing debate regarding whether appetitive and defensive motivation are orthogonal or correlated (DeYoung, 2006; Markon, Krueger, & Watson, 2005), most studies on the question have relied on trait self-report measures. Some of these have reported a small but significant negative correlation between the two systems (Elliot & Thrash, 2002; DeYoung, 2006), while others have shown them to be orthogonal (Carver & White, 1994; Torrubia, Avila, Molto, & Caseras, 2001). These mixed findings are not surprising given that self-report of motivational tendencies is subject to limitations including demand characteristics (Faith, Wong, & Allison, 1998), social desirability bias (Barrett, 1996), recall bias (Sato & Kawahara, 2011), and random responding (Holden, Wheeler, Sarah, & Marjanovic, 2012).
Some investigators have turned to psychophysiological measures to avoid these limitations. For instance, degree of left relative to right activity in frontal cortical regions (i.e., “frontal asymmetry”) is a putative measure of individual differences in appetitive motivation (Allen, Coan, & Nazarian, 2004; Davidson, 1998). Eyeblink acoustic startle response is thought to be sensitive to differences in defensive motivation (Lang, 1995).
However, psychophysiological measures have a significant limitation of their own–poor specificity. For example, resting frontal EEG asymmetry is sensitive to individual differences in not only appetitive motivation (Coan & Allen, 2003), but also behavioral inhibition and negative affect (Wacker, Chayanon, & Stemmler, 2010). Frontal EEG asymmetry changes in response to anger manipulations (Carver & Harmon-Jones, 2009) and even factors like experimenter gender (Kline, Blackhart, & Joiner, 2002). Likewise, startle is attenuated by pleasant stimuli (Giargiari, Mahaffey, Craighead, & Hutchison, 2005) and potentiated during non-emotional vigilance (Böhmelta, Schellb, & Dawsona, 1999).
The problem of poor specificity may be addressed by recording physiological measures in contexts that elicit the motivational state of interest. This approach is based on a capability model of personality and other traits (Coan, Allen, & McKnight, 2006; Mischel, Shoda, & Mendoza-Denton, 2002; Wallace, 1966). The capability model asserts that individual differences are best thought of as interactions between the emotional demands of specific situations and the abilities individuals bring to those situations. This is analogous to a cardiac stress test, where electrocardiogram during a physiologically relevant state (physical exertion) is more informative regarding cardiovascular function than resting electrocardiogram (Gibbons et al., 2002). Coan and colleagues (2006) presented evidence that a capability approach to psychophysiological measurement decreases associations with extraneous variables (such as EEG reference scheme) while strengthening relationships with variables of interest (e.g., positive affectivity), thus improving both specificity and sensitivity. Physiological indicators recorded during laboratory emotion inductions therefore provide good measures of individuals’ motivational tendencies while avoiding pitfalls of self-report. For this reason, we employed two validated psychophysiological emotion induction paradigms to assess individual differences in appetitive and defensive motivation in the present study.
Appetitive motivation paradigm
Several electroencephalogram (EEG) studies have found associations between resting frontal asymmetry and self-reported sensitivity to appetitive stimuli (e.g., Coan & Allen, 2003) or behavioral performance on reward-related tasks (e.g., Pizzagalli, Sherwood, Henriques, & Davidson, 2005). However, fewer studies have taken a capability approach and measured EEG asymmetry during an appetitive motivational state.
We recently developed a capability-based paradigm wherein changes in EEG asymmetry are recorded as participants anticipate monetary reward during a slot machine game. Several findings indicate that this task provides a valid means of assessing individual differences in appetitive motivation. First, this paradigm effectively manipulates EEG asymmetry: individuals exhibited greater activity in left (relative to right) frontal regions while anticipating winning money than during the control condition (Shankman, Klein, Tenke, & Bruder, 2007; Shankman et al., 2013). Second, the paradigm effectively indexes differences in appetitive motivation: individuals with major depressive disorder (MDD) exhibited less of an increase in left-sided asymmetry during reward anticipation compared to healthy participants (Shankman et al., 2007, 2013). Third, the task is more sensitive to individual differences than self-report: individuals with MDD differed from controls on EEG asymmetry during the task, but not on self-reported anticipation of winning (Shankman et al., 2013). The task is therefore well suited for assessing appetitive motivation in the present study.
Defensive motivation paradigm
Unlike EEG, startle research has long employed a capability-like approach (Lang, 1995), and a number of studies have found associations between fear-potentiated startle and individual differences in defensive motivation (e.g., Corr, Kumari, Wilson, Checkley, & Gray, 1997; Vaidyanathan, Patrick, & Bernat, 2009). As mentioned above, recent conceptualizations have delineated two separable aspects of defensive responding–a phasic “fear” response to predictable or imminent danger and a sustained “anxiety” response to unpredictable or contextual danger (Gray & McNaughton, 2000; Grillon, 2002). Grillon and colleagues designed a novel startle paradigm that differentiates these two types of responses. In this paradigm, an aversive stimulus (e.g., an electric shock) is presented either only when a cue is present (predictable condition) or at any time (unpredictable condition; Grillon, Baas, Lissek, Smith, & Milstein, 2004; Schmitz & Grillon, 2012).
Several lines of evidence support the validity of this paradigm for assessing individual differences in defensive motivation. First, numerous startle, neuroimaging, and pharmacological challenge studies have shown not only that this paradigm effectively modulates startle responding, but that it discriminates between responses to predictable and unpredictable threat. For instance, alcohol and benzodiazepines suppress responding during the unpredictable, but not predictable, condition (Grillon et al., 2006; Moberg & Curtin, 2009), and the two conditions activate different brain regions (Alvarez, Chen, Bodurka, Kaplan, & Grillon, 2011). Responses to the task therefore map on to distinct neurobiological systems for fear and anxiety. Second, the paradigm is sensitive to differences in defensive motivation: responses to the task discriminate individuals with panic disorder (Grillon et al., 2008; Shankman et al., 2013) and posttraumatic stress disorder (Grillon et al., 2009) from healthy controls. Third, the task is more sensitive to individual differences than self-report: although individuals with panic disorder in one study differed from controls on startle potentiation, they did not differ on self-reported anxiety in response to the task (Shankman et al., 2013). This paradigm is therefore well suited for assessing defensive motivation in the present study.
Present Study
The aim of this study is to examine whether appetitive motivation (as measured by frontal cortical asymmetry during monetary reward anticipation) and defensive motivation (as measured by startle response during predictable and unpredictable threat of shock) are correlated or orthogonal. The two tasks complement each other well—each is a capability-based physiological paradigm, and each assesses affect during anticipation (of reward and threat, respectively).
Although it is relatively straightforward to show that appetitive and defensive motivation are correlated, demonstrating that they are orthogonal presents special challenges, as it essentially requires one to argue for the null hypothesis. Simply demonstrating that the two types of motivation are not significantly correlated is hardly convincing evidence of orthogonality. Our design therefore included several features that allowed a more conclusive test of orthogonality:
Multiple indices of appetitive and defensive responding (i.e., multiple EEG electrode pairs, response to predictable and unpredictable threat, startle amplitude and magnitude).
Attention to statistical issues that may confound interpretation of associations (e.g., analyses both with and without covariates that may act as suppressors).
Replication in two samples with somewhat different participant characteristics, as well as in a combined sample, and an analysis of achieved power / chance of Type II error.
Study One
Method
Participants
Sixty-three individuals without a history of Axis I psychopathology were recruited from the community via print and online advertisements as part of a larger study on the relation between psychopathology and motivation (Shankman et al., 2013). Participants were right handed (as determined by the Edinburgh Handedness Inventory, Oldfield, 1971; range of laterality quotient +42.9 to +100.0; M = +88.0, SD = 15.4), between ages 18 and 70, had no prior head trauma, and no lifetime diagnosis of a psychotic, mood, or anxiety disorder; alcohol or drug dependence (with the exception of cannabis); or anorexia or bulimia nervosa according to the Structured Clinical Interview for DSM-IV Axis I Disorders–Non-Patient Edition (SCID-NP; First et al., 2002). Sample characteristics are presented in Table 1. All participants gave informed consent and all procedures were approved by the university Institutional Review Board.
Table 1.
Sample Characteristics
| Variable | Study 1 (n = 63) | Study 2 (n = 64) | Study 1 vs. Study 2 |
|---|---|---|---|
| Female | 63.5% | 65.6% | χ2(1) = 0.06, ns |
| Age (SD) | 30.8 (12.7) | 19.2 (1.9) | t(125) = 7.25, p < .001 |
| Race | |||
| Caucasian | 47.6% | 34.4% | |
| Asian | 12.7% | 23.4% | |
| χ2(4) = 19.53, p < .001 | |||
| Latino | 4.8% | 28.1% | |
| African-American | 23.8% | 9.4% | |
| Other | 11.1% | 4.7% | |
| IDAS dysphoria (SD)a | 13.9 (5.1) | 21.6 (7.9) | t(113) = −6.07, p < .001 |
| HRSD total (SD)b | 2.7 (3.6) | - | - |
| BAI total (SD)b | 3.1 (4.7) | - | - |
Note. IDAS = Inventory of Depression and Anxiety Symptoms; HDRS = Hamilton Rating Scale for Depression; BAI = Beck Anxiety Inventory.
The first 12 participants in Study 1 did not complete the IDAS.
The HRSD and BAI were not administered in Study 2.
SCIDs were conducted by S.A.S. and advanced clinical psychology doctoral students. Diagnosticians were trained to criterion by viewing the SCID-101 training videos (Biometrics Research Department, New York, NY), observing 2-3 joint SCID interviews with S.A.S., and completing 3 SCID interviews (observed by S.A.S. or an advanced interviewer) where diagnoses were in agreement with the observer. In addition, interviewers received ongoing supervision by S.A.S. throughout the course of data collection to ensure reliable administration. Our group has obtained excellent inter-rater reliability using this approach (Shankman et al., 2013).
Symptoms of depression and anxiety from the previous week were assessed with the Hamilton Rating Scale for Depression (HRSD; Hamilton, 1960), a 24-item interviewer-rated measure of depression severity, and the Beck Anxiety Inventory (BAI; Beck, Brown, Epstein, & Steer, 1988), a 23-item self-report measure of anxiety severity. The HRSD and BAI have demonstrated excellent psychometric properties in past studies (Beck & Steer, 1990; Hedlund & Vieweg, 1979) and in the present sample had Cronbach's alphas of .92 and .95, respectively.
Procedure
Two computerized tasks designed to assess appetitive and defensive motivation were administered in counterbalanced order. Participants were seated in an electrically-shielded, sound-attenuated booth approximately 3.5 feet from a 19-inch computer monitor that displayed both tasks.
Approximately 9 days (M = 9.46; SD = 3.71; range 5-17 days) after initial task administration, a subset of participants (n = 32 for slot machine and n = 33 for startle task) completed both tasks a second time. This provided a measure of reliability for the slot machine and startle paradigms.
Appetitive motivation task
A computerized slot machine paradigm previously used by Shankman et al. (2007) was used to assess appetitive motivation. The task consisted of three reels of numbers and fruit which “spun” simultaneously for 11s and then “landed” on a result. To start the reels spinning, participants pressed a button with both thumbs that pulled a lever on the computer screen. The task included 60 “spins” which were divided into two pay-off conditions of 30 trials each–a reward condition in which participants won money if the reels landed on three fruits and a no incentive condition in which participants were ineligible to win money no matter the outcome. Thus, the reward condition was designed to elicit reward anticipation while the no incentive condition served as a control for several aspects of the reward condition (e.g., visual input, anticipating an outcome). The amount of money that could be won during each reward trial ranged from $0.50 to $3.00. In both conditions, participants did not lose money if the reels did not land on three pieces of fruit.1
Trials were presented in a pseudo-random order and there were never more than two consecutive trials of similar type or outcome. Participants began the game with $2.00 and were told the specific pay-off conditions (reward or no incentive) prior to each trial, but not the potential dollar amount in each reward condition. Unbeknownst to the participant, half of the two pay-off situations “landed” on three fruits. Trials were divided into three blocks. Participants completed ratings of their emotional state during each condition after the first and second blocks (see below). At the end of the task, all participants were given their winnings (~$12.00) in cash.
EEG data were recorded from Ag/AgCl electrodes in a 64-channel stretch-lycra electrode cap (Compumedics NeuroScan 4.4, Charlotte, NC). The ground electrode was at the frontal pole (AFZ) and the online reference was near the vertex (between CZ and CPZ). Vertical and horizontal eye movements were monitored using electrodes placed at right supra- and infra-orbital sites (VEOG) and right and left outer canthi (HEOG). Electrode impedances were under 5,000 ohms, and homologous sites (e.g., F3/F4) were within 1,500 ohms of each other. Data were recorded through a NeuroScan Synamp2 data acquisition system at a gain of 10K (5K for eye channels) with a bandpass of DC-200 Hz. Data were acquired and digitized continuously at a rate of 1,000 Hz. For consistency with prior studies based on this dataset (Shankman et al., 2013; Nelson et al., in press), EEG data were re-referenced offline by computing a digitally derived linked mastoids reference using data from the left and right mastoid.
Defensive motivation task
Participants first completed a 2.5-min habituation task in which 9 acoustic startle probes were presented to prevent early exaggerated startle responding (data not presented). Next, a shock work-up procedure was completed in which participants received shocks of increasing intensity until reaching a level that they described as being “highly annoying but not painful.” Ideographic shock levels were used to ensure equality in perceived shock aversiveness (Rollman & Harris, 1987) and for consistency with prior studies (Grillon et al., 2004). The maximum shock level a participant could achieve was 5 mA; within our sample, the mean shock level was 1.91 mA (SD = 0.30 mA).
The defensive motivation task was modeled after the NPU task used by Grillon and colleagues (Grillon et al., 2004, 2008, 2009; Schmitz & Grillon, 2012). The task included three conditions - no shock (N), predictable shock (P), and unpredictable shock (U). During each condition, participants were intermittently presented with 8 s geometric cues (blue circle for N, red square for P, and green star for U). Interstimulus intervals (ISIs) ranged from 8 to 15 s (M = 11.6 s). No shocks were delivered during the N condition, while shocks were delivered only during cues in the P condition, and at any time during the U condition (i.e., the shocks were predictable in the P, but not U, condition). Blocks of each condition were 90 s in duration and were administered twice, in one of the following orders (counterbalanced): PNUPNU or UNPUNP. Between blocks, participants were given a break and reported on their emotional state during the task. All participants received 12 shocks (6 each during P and U) and 72 startle probes (24 each during N, P, and U). To ensure that startle responses were not affected by an immediately preceding shock, startle probes never followed shocks by fewer than 10s.
All stimuli for the task were administered using PSYLAB (Contact Precision Instruments, London, UK) and psychophysiological data were acquired using NeuroScan 4.4. Acoustic startle probes were 40 ms duration, 103 dB bursts of white noise with near-instantaneous rise time presented binaurally through headphones. Electric shocks lasted 400 ms and were administered to the wrist of the participants’ left (non-dominant) hand.
Startle response was recorded from two 4mm Ag/AgCl electrodes placed over the orbicularis oculi muscle below the right eye. As per published guidelines (Blumenthal et al., 2005), one electrode was 1 cm below the pupil and the other was 1 cm lateral. Data were collected using a bandpass filter of DC-200 Hz at a sampling rate of 1000 Hz. Although the upper end of this frequency band is below the Blumenthal et al. recommendation of 500 Hz, the missing bandwidth (200-500 Hz) was not likely to affect the results (T. Blumenthal, personal communication, December 14, 2009; A. Van Boxtel, personal communication, December 14, 2009). The ground electrode was the same as that used for the appetitive motivation task.
Emotion ratings
After each of the first two blocks of the appetitive motivation task, participants rated how much they “looked forward to three pieces of fruit” during both the reward and no incentive conditions. Similarly, after each of the two blocks of the defensive motivation task, participants rated their level of “nervousness/anxiety” during the cues and ISIs for each condition. Both measures were 7-point Likert scales ranging from 1 (Not at all) to 7 (Extremely). For each questionnaire, we used the average rating across the two administrations in analyses. Analyzing the two administrations separately yielded nearly identical results.
Physiological data processing
EEG data from the 11 s period while the slot machine reels were spinning were segmented into consecutive 1.024 s epochs every 0.512 s (50% overlap). After referencing to a linked mastoid reference offline and then applying a baseline correction, epochs contaminated by blinks, eye movements, and other artifacts were excluded from analyses manually, by direct visual inspection of the data. The EEG was tapered over the entire 1.024 s epoch by a Hanning window to suppress spectral side lobes. After artifact exclusion, the number of epochs per condition ranged from 134 to 548 (Win: M = 381.6, SD = 110.6; NI: M = 383.7, SD = 103.5). Artifact-free data were recovered in adjacent (overlapping) epochs and power spectra were computed offline by using a fast Fourier transform. Subsequently, the average absolute alpha power was computed for each electrode site and then natural log transformed in order to normalize the data. Consistent with previous studies (e.g., Bruder et al., 1997), the alpha band was defined as 7.81–12.70 Hz and used as an inverse measure of regional brain activity (Klimesch, Sauseng, & Hanslmayr, 2007). We computed frontal asymmetry scores for the reward and no incentive conditions by subtracting power at left frontal electrodes from power at homologous right electrodes (e.g., F8 - F7), so that the higher values reflected greater activity in left relative to right frontal regions. Fifteen participants were excluded due to excessive artifacts (e.g., muscle related artifact, excessive blinking or eye movement) in electrodes of interest in the no incentive or reward condition. The final sample size of 63 reflects these exclusions.
Startle blinks were scored according to published guidelines (Blumenthal et al., 2005). Data were first rectified and then smoothed using a FIR filter with a band pass of 28-40 Hz. Blink response was defined as the peak amplitude of EMG within the 20-150 ms following startle probe onset relative to baseline (average baseline EMG level for the 50 ms preceding the startle probe onset). Each peak was identified by software but visually inspected to ensure acceptability (e.g., not a double blink). Blinks were scored as missing if the baseline period was contaminated with noise, movement artifact, or if a spontaneous blink began before minimal onset latency and thus interfered with the startle probe-elicited blink response. Blinks were scored as non-responses if EMG activity during the 20-150ms post-stimulus time frame did not produce a blink peak that was visually differentiated from baseline activity. After exclusion of missing blinks, each condition included from 2 to 12 blinks (M =10.3, SD = 1.8). We conducted analyses using both blink magnitude (i.e., condition averages include values of 0 for non-response trials), and amplitude (i.e., condition averages do not include non-response trials). Results between the two indices were comparable but we only present full results of analyses including magnitude, as it is a more conservative estimate of blink response (Blumenthal et al., 2005). All participants provided usable startle data.
Data analysis plan
First, we examined the validity of the two tasks and the stability of the physiological and self-report assessments. For the appetitive motivation task, we conducted three one-way repeated measures ANOVAs comparing participants’ frontal asymmetry during the reward and no incentive conditions for each electrode pair (F7/F8, F5/F6, and F3/F4) as well as how much they reported looking forward to the outcome (i.e. three fruits) in the two conditions. For the defensive motivation task, we conducted a 3 (Condition: N, P, U) X 2 (Cue: ISI, cue) repeated measures ANOVA on startle magnitude as well as nervousness/anxiety ratings. To assess stability, we conducted Pearson's correlations between visit one and visit two psychophysiological indices and self-report ratings.
Second, to test the primary aim of the study, we examined correlations between responses to induced appetitive and defensive motivation. Specifically, we computed partial correlations between left-sided frontal EEG asymmetry potentiation during reward anticipation and startle potentiation during predictable or unpredictable threat of electric shock, adjusted for task order (i.e., which task the participant received first). EEG asymmetry potentiation was defined as the difference in EEG asymmetry between reward and no incentive conditions. We report results for F7/F8, F5/F6, and F3/F4 potentiation because these are the sites most commonly examined in the literature (Allen et al., 2004). Startle potentiation was defined as the difference in startle response between Pcue and Ncue (i.e., potentiation to predictable threat) and the difference between the average of Ucue/UISI and average of Ncue/NISI (i.e., potentiation to unpredictable threat). When separate analyses were conducted for potentiation to Ucue and UISI, similar results were found. A parallel series of partial correlations was conducted for self-report ratings.
Results
Validity of tasks
Appetitive motivation
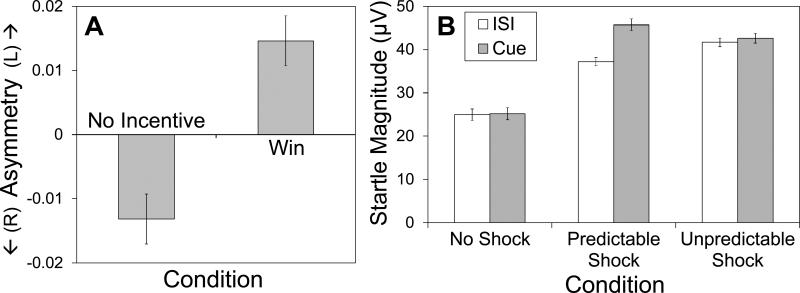
Consistent with hypotheses, participants showed greater left-sided frontal asymmetry during the reward compared to no incentive condition across all three electrode pairs [F7/F8, F(1, 63) = 7.63, p < .01; F5/F6, F(1, 62) = 4.77, p < .05; F3/F4, F(1, 62) = 3.30, p < .10; Figure 1A]. Participants also reported looking forward to the outcome more during the reward than the no incentive condition, F(1, 57) = 178.52, p < .001.
Figure 1.
Frontal EEG asymmetry during reward anticipation versus no incentive condition (A) and eye blink startle magnitude while anticipating temporally predictable or unpredictable shocks versus no-shock condition (B) in Study One. Values for Panel A reflect log-transformed alpha band power at F8 minus log transformed power at F7. Error bars represent normalized within-subject standard errors (as described by Cousineau, 2005).
Defensive motivation
Results revealed main effects for Condition, F(2, 124) = 56.58, p < .001, and Cue, F(1, 62) = 17.58, p < .001. More importantly, a Condition by Cue interaction emerged, F(2, 124) = 15.85, p < .001 (Figure 1B). Follow-up analyses indicated that startle magnitude during UISI was higher than magnitude during PISI, F(1, 62) = 8.74, p < .01, which was higher than NISI, F(1, 63) = 44.26, p < .001. Startle magnitude during Pcue was higher than magnitude during Ucue, F(1, 62) = 5.48, p < .05, which was higher than during the N cue, F(1, 62) = 55.83, p < .001. In other words, the threat-of-shock task manipulated startle responding as expected: startle magnitude was higher during the Pcue, and U condition (when shocks were possible) than during the N condition and PISI (when participants were safe from shock).
Analyses for self-reported anxiety revealed main effects of Condition, F(2, 124) = 133.24, p < .001, and Cue, F(1, 62) = 35.45, p < .001, as well as a Condition by Cue interaction, F(1, 124) = 76.01, p < .001. Follow-up analyses revealed an identical pattern to that observed for startle magnitude, except that self-reported anxiety did not differ between the P and U cues.
Stability of measures
Data from individuals who completed the task approximately 9 days later indicated high test-retest reliability for potentiation of frontal alpha asymmetry during reward anticipation (F7/F8: r(31) = .68; F5/F6: r(31) = .71; F3/F4: r(31) = .75; all ps < .001). In addition, results indicated high test-retest reliability for potentiation of startle magnitude during both P, r(32) = .66, p < .001, and U conditions, r(32) = .80, p < .001. Emotion ratings also exhibited adequate test-retest reliability (rs > .71).
Correlations between appetitive and defensive motivation
A total of six partial correlations (asymmetry potentiation at three electrode pairs X startle potentiation during two threat conditions, controlling for task order) did not reveal any significant relationships, suggesting that appetitive motivation was orthogonal with defensive motivation (Table 2). The average correlation was .04 (range 0 to |.13|). An identical pattern emerged when task order was not included as a covariate.
Table 2.
Partial Correlations Between Reward-Related Frontal EEG Asymmetry and Threat-Related Startle Response
| Electrode pair |
||||||
|---|---|---|---|---|---|---|
| F7/F8 |
F5/F6 |
F3/F4 |
||||
| Threat condition | pr | p | pr | p | pr | p |
| Study One | ||||||
| Predictable shock | .04 | .78 | .13 | .31 | .05 | .72 |
| Unpredictable shock | < .01 | .95 | .04 | .76 | −.02 | .88 |
| Study Two | ||||||
| Predictable shock | −.17 | .19 | −.11 | .39 | −.09 | .50 |
| Unpredictable shock | .09 | .46 | .14 | .29 | .10 | .44 |
| Combined Sample | ||||||
| Predictable shock | .02 | .80 | .07 | .44 | .05 | .59 |
| Unpredictable shock | .05 | .54 | .10 | .29 | .05 | .56 |
Note. Partial correlations are adjusted for task order.
Unlike psychophysiological indices, self-report ratings indicated that changes in reward anticipation were positively correlated with changes in self-reported anxiety during both predictable threat, pr(55) = .41, p < .01, and unpredictable threat, pr(55) = .36, p < .01. This pattern remained when not adjusted for task order. However, changes in self-reported anticipation and anxiety were not correlated with asymmetry potentiation or startle potentiation, respectively (Supplementary Tables 1 and 2).
Discussion
Primary analyses of psychophysiological measures suggested that appetitive and defensive motivation are uncorrelated, consistent with some studies of self-reported traits (e.g., Carver & White, 1994). However, self-reported responses to the tasks were positively correlated. This latter finding is surprising, as previous studies have found either orthogonality or negative correlations for self-reported appetitive and defensive motivation (e.g., DeYoung, 2006).
Although independence between EEG and startle was observed across several metrics, their apparent orthogonality may nonetheless have been unique to the Study One sample. We therefore attempted to replicate these findings in an independent sample that differed in several ways. First, the Study One sample was selected to be free of current or lifetime psychopathology. This may have led to somewhat restricted range of motivational tendencies, as depression and anxiety predict abnormal EEG asymmetry and startle response (Henriques & Davidson, 1991; Grillon et al., 2008). Thus, Study Two employed an unselected sample in which internalizing symptoms were free to vary and included as a moderator in analyses. Second, the Study Two sample was younger and had a different racial composition. Third, Study Two employed a slightly different version of the defensive motivation task, as described below.
After examining the relationship between appetitive and defensive motivation in the Study Two sample, we investigated whether the constructs were correlated or orthogonal in a more highly-powered sample by combining the samples from the two studies. We also examined whether these results held when using an alternative (average) reference scheme.
Study Two
Method
Participants
Sixty-four undergraduates consented to participate. Inclusion criteria were right-handedness (range of laterality quotient +57.9 to +100.0, M = +86.9, SD = 14.1) and no history of head trauma. Sample characteristics are presented in Table 1.
Procedure and data processing
The general procedures (e.g., counterbalancing of tasks, use of booth), processing of physiological data, and appetitive motivation task were identical to those used in Study One. The defensive motivation task was also very similar with the exceptions that (a) it consisted of just one block; (b) it therefore included 8 (rather than 12) startle probes per condition; and more importantly, (c) 6 s countdowns (CDs) were used instead of geometric cues. During the P condition, participants always and only received shocks when the countdown reached 1. In contrast, in Study One, shocks during the “predictable” condition were not entirely predictable–participants knew that they could only receive a shock when the 8 s geometric cue was present, but did not know exactly when shocks would occur while the cue was on the screen. Thus, the use of countdowns in Study Two allowed the shocks to be completely predictable during P and completely unpredictable during U. As in Study One, text at the bottom of the screen always indicated the current condition (i.e., “no shock,” “shock at 1,” or “shock at any time”).
After artifact exclusion, the number of EEG epochs for each slot machine game condition ranged from 94 to 564 (Win: M = 370.0, SD = 110.3; NI: M = 349.4, SD = 104.4). After exclusion of missing blinks, each startle task condition included from 2 to 8 blinks (M = 7.27, SD = 0.6). In addition to the linked mastoids reference, we re-referenced EEG from both Study One and Study Two using a reference derived from the average of all scalp electrodes.
Emotions ratings similar to those used in Study One were administered after completion of the first two blocks of the appetitive motivation task and after the defensive motivation task.
Data analysis plan
The data analytic strategies were generally the same as those used in Study One. However, because the sample had a broader range of internalizing symptoms, which may relate to individual differences in appetitive and defensive motivation, we included these symptoms as a continuous between-subjects factor in analyses assessing the effects of the tasks. We measured internalizing symptoms using the Dysphoria scale of the Inventory of Depression and Anxiety Symptoms (IDAS; Watson et al., 2007). The IDAS is a 64-item self-report measure of symptoms of anxiety and depression during the previous two weeks. Participants are asked to respond to each item using a 5-point Likert scale ranging from 1 (not at all) to 5 (extremely). The Dysphoria subscale was designed to represent the symptoms that are common to depression and anxiety disorders (e.g., feeling inadequate, discouraged, trouble concentrating, worrying frequently). The subscale has been shown to have excellent construct validity (Watson et al., 2007) and Cronbach's alpha in the current sample was excellent (α = .87).
Results
Sample characteristics
As shown in Table 1, the Study Two sample was younger and had a different racial composition than the Study One sample: Study One had a greater proportion of Caucasians and African-Americans, while Study Two had a greater proportion of Asians and Latinos. As expected, the Study Two sample had higher internalizing symptoms than the Study One sample.
Validity of tasks
Appetitive motivation
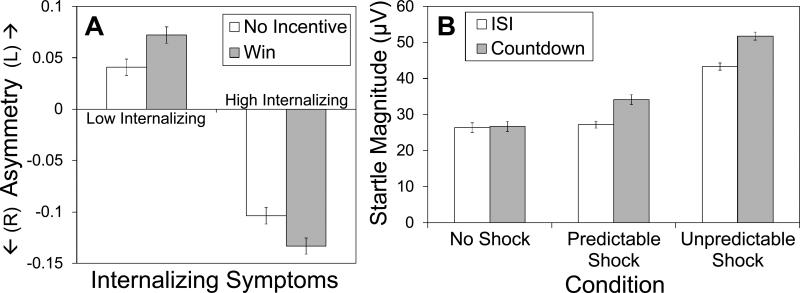
Analyses did not reveal a main effect of Condition (reward vs. no incentive) on frontal asymmetry for any electrode pair (all ps > .37). However, there were main effects of internalizing symptoms on asymmetry at F7/F8, F(1, 62) = 11.59, p < .01, and F3/F4, F(1, 62) = 6.08, p < .05, as well as a trend for F5/F6, F(1, 62) = 3.16, p < .10. Individuals low in internalizing symptoms showed greater activity at left compared to right frontal sites, whereas those high in internalizing symptoms showed greater activity at right than at left frontal sites (Figure 2A). Moreover, internalizing symptoms moderated the effect of condition for all three electrode pairs [F7/F8, F(1, 62) = 7.31, p < .01; F5/F6, F(1, 62) = 7.61, p < .01; F3/F4, F(1, 62) = 6.26, p < .05; Figure 2A]. We therefore examined simple slopes of Condition at high and low levels of internalizing symptoms (Aiken & West, 1991). These analyses indicated decreased left-sided asymmetry during the win condition at high levels of internalizing [F7/F8, F(1, 62) = 3.45, p < .10; F5/F6, F(1, 62) = 4.74, p < .05; F3/F4, F(1, 62) = 5.79, p < .05], but generally increased left-sided asymmetry during the win condition at low levels of internalizing [F7/F8, F(1, 62) = 3.92, p = .05; F5/F6, F(1, 62) = 3.02, p < .10; F3/F4, F(1, 62) = 1.31, ns]. Thus, in most electrode pairs, low-symptom individuals differentially activated left hemisphere during the reward condition, while high-symptom individuals differentially activated right hemisphere.
Figure 2.
Frontal EEG asymmetry during reward anticipation versus no incentive condition (A) and eye blink startle magnitude while anticipating temporally predictable or unpredictable shocks versus no-shock condition (B) in Study Two. Values for Panel A reflect log-transformed alpha band power at F8 minus log transformed power at F7, among individuals at one standard deviation below or above the mean for internalizing symptoms (IDAS Dysphoria). Error bars represent normalized within-subject standard errors.
Participants reported looking forward to the outcome more during the reward than during the no incentive condition, F(1, 62) = 132.84, p < .001, and this result was nearly identical when internalizing symptoms were not included in the model.
Defensive motivation
As in Study One, main effects emerged for both Condition, F(2, 124) = 49.34, p < .001, and Cue, F(1, 62) = 23.06, p < .001, as well as a Condition by Cue interaction, F(2, 124) = 11.89, p < .001 (Figure 2B). As expected, follow-up analyses indicated that startle magnitude during the UISI was higher than that during the PISI, F(1, 62) = 42.03, p < .001, which did not differ from magnitude during the NISI, F < 1. In contrast, magnitude during the UCD was higher than that during the PCD, F(1, 62) = 35.86, p < .001, and magnitude during the PCD was higher than during the NCD, F(1, 62) = 14.86, p < .001. In contrast to the appetitive motivation task, internalizing symptoms did not moderate these results (Cue X Condition X internalizing: F < 1), and the pattern of results was identical when internalizing symptoms were not included as a covariate. In sum, the threat-of-shock task manipulated startle responding as expected: startle was potentiated during the countdown, but not during the ISI during the P condition (when the countdown reliably signaled shock), but startle was potentiated during both the countdown and the ISI during the U condition (when shocks could be delivered at any time).
A parallel analysis of self-reported anxiety during the task also revealed main effects for Condition, F(2, 120) = 58.34, p < .001, and Cue, F(1, 60) = 5.81, p < .05, as well as a Condition by Cue interaction, F(2, 120) = 3.86, p < .05. Follow-up analyses at each level of Cue indicated a pattern of results similar to that found for startle magnitude, with the exception that participants reported more anxiety during the PISI than during the NISI. The pattern of results was identical when internalizing symptoms were not included in the model.
Correlations between appetitive and defensive motivation
Table 2 displays partial correlations between frontal EEG asymmetry potentiation during the slot machine task and startle potentiation during the threat-of-shock task, adjusted for task order. None of these six correlations revealed a significant relationship. The average correlation was −.01 (range |.09| to|.17|). The pattern of results was identical when internalizing symptoms were included in the model, as well as when task order was not included in the model. Moreover, a series of moderated linear regressions indicated that internalizing symptoms did not moderate any of the six appetitive-defensive relationships tested.
Partial correlations controlling for task order did not reveal an association between changes in self-reported reward anticipation during the slot task and anxiety during the predictable condition countdown, pr(59) = .20, ns, although changes in reward anticipation and anxiety during the unpredictable condition were positively correlated at a trend level, pr(59) = .24, p < .10. The same pattern emerged when (a) internalizing symptoms were included as a covariate and (b) task order was not included as a covariate. Internalizing symptoms did not moderate these results. Changes in self-reported reward anticipation and anxiety were not correlated with asymmetry potentiation or startle potentiation, respectively (Supplementary Tables 1 and 2).
Combined Sample
We next attempted to replicate findings from Studies One and Two in a more highly powered combined sample (n = 127). Because Type II error is a particular concern when attempting to accept the null hypothesis (i.e., orthogonality), we first determined the statistical power achieved by the combined sample. We then examined whether psychophysiological indicators were correlated or orthogonal in the combined sample.
Results
Statistical power
A power analysis using G*Power 3.1 (Faul, Erdfelder, Buchner, & 2009) indicated that the sample had greater than 99.9% power to detect a large effect (r = .5) and 93.3% power to detect a medium effect (r = .3). Power to detect a small effect (r = .2) was lower (62.0%).
Correlations between appetitive and defensive motivation
As shown in Table 2, all correlations were non-significant. The average coefficient was .06 (range |.02| to |.10|). The pattern of results was identical when study was included as a covariate, as well as when task order was not included as a covariate. Again, non-significant correlations emerged when startle amplitude was analyzed instead of startle magnitude.
When EEG asymmetry data based on an average reference were used, most partial correlations remained non-significant (Supplementary Table 3). However, unpredictable startle potentiation was positively correlated with asymmetry potentiation at F3/F4. There were also trends towards a positive relationship between unpredictable startle potentiation and asymmetry potentiation at F7/8, and a negative relationship between predictable startle potentiation and asymmetry potentiation at F5/6. The average coefficient was .06 (range |.08| to |.20|).
As in Study One, partial correlations of self-reported emotion during the tasks, controlling for task order, indicated positive associations of anticipation during the win condition of the slot task with anxiety during both the predictable cue, pr(117) = .27, p < .01, and unpredictable condition, pr(117) = .29, p < .01.These correlations remained significant when controlling for study or internalizing symptoms, and when not controlling for task order. Again, however, changes in self-reported reward anticipation and anxiety were not correlated with asymmetry potentiation or startle potentiation, respectively (Supplementary Tables 1 and 2).
General Discussion
Acquisition of rewarding stimuli (e.g., food, sex) and avoidance of harmful stimuli (e.g., predation, social rejection) are two broad motivators influencing much of human behavior (Elliot & Thrash, 2002; Gray, 1994). Although individual differences in these motivational systems are highly relevant to both normal personality variation and risk for mental illness, the relationship between them has not been conclusively established.
In this study, we elicited appetitive and defensive motivation and demonstrated that physiological indicators of these two systems were generally not correlated. This finding was consistent across multiple measures, including frontal EEG asymmetry across three electrode pairs and startle during anticipation of both predictable and unpredictable threat. We replicated the independence of these measures in a separate sample, as well as in a more highly-powered combined sample. Our conclusion is based not simply on the fact that correlations failed to reach statistical significance, but also on the consistently small effect sizes of correlations. Specifically, the average correlation coefficients observed in all four sets of analyses fell in the “trivial” range, according to Cohen's (1992) conventions (Study One: .04, Study Two: −.01, combined sample: .06, combined sample, alternative reference scheme: .06). Finally, a power analysis in the combined sample indicated that there is only a 6.7% chance that we committed a Type II error and failed to detect a significant medium-sized association between appetitive and defensive motivation. There was, however, a somewhat larger (38%) chance of Type II error for a small association. The findings are also qualified by the fact that one small significant correlation emerged in an analysis based on the average reference. Nonetheless, these results indicate that individuals’ tendency to approach rewarding experiences and their tendency to act defensively in response to threats are either orthogonal or only weakly related.
This is only the second study to our knowledge to examine the association of startle and EEG asymmetry. Jackson et al. (2003) found that resting frontal EEG asymmetry was not related to startle magnitude while viewing aversive pictures in a sample of 57 to 60 year olds.2 Our findings extend those of Jackson et al. by demonstrating that EEG asymmetry and startle magnitude remain independent even when EEG is recorded during an appetitive state (reward anticipation), and when startle potentiation is measured in two different aversive contexts (predictable threat vs. unpredictable threat). These findings are also in line with those of Sandt, Sloan, and Johnson (2009), who found that eyeblink startle response during a defensive state was not significantly correlated with the post-auricular reflex during an appetitive state.
Implications for Understanding Normal and Abnormal Personality Variation
Certain structural models of personality (e.g., the five-factor model; McCrae & Costa, 1987) have excellent psychometric properties, but are largely descriptive and do not account for how personality domains operate (Cervone, 2005). The existence of two relatively independent motivational systems dedicated to responding to threat and pursuing reward raises the possibility that these systems may be core mechanisms of neuroticism/negative emotionality and extraversion/positive emotionality, or at least facets thereof. Indeed, evidence suggests that neuroticism and extraversion are associated with neurobiological mechanisms of the defensive and appetitive systems (Depue & Collins, 1999; Depue & Lenzenweger, 2005).
Although our results indicate that the between-individual structure of the appetitive and defensive motivational systems are orthogonal, the within-individual psychological structures subserving these systems may nonetheless interact and influence one another (Cervone, 2005). This would be consistent with Diener & Emmons’ (1984) suggestion that indicators of these systems are orthogonal when assessed over long periods of time, but may be reciprocally related when assessed in the same moment or state (Davidson, 1998). Consistent with this idea, these two systems share neuroanatomical bases (e.g., Morrison & Salzman, 2010; Trainor, 2011). It is also possible that appetitive and defensive tendencies interact with each other to predict particular behaviors (e.g., in approach-avoidance conflicts) or mental disorders (Gray & McNaughton, 2000; Shankman & Klein, 2003). One way to clarify within-individual relationships between the defensive and appetitive systems is to challenge one system (e.g., pharmacologically or via emotion induction) and observe changes in the other. Interestingly, one study employing this strategy found that prednisone-induced attenuation of left-sided resting frontal EEG asymmetry was not associated with changes in startle response (Schmidt, Fox, Goldberg, Smith, & Schulkin, 1999). Additional studies are needed to elucidate how the appetitive and defensive systems interact within individuals.
These results may also aid in understanding mechanisms of mental illness. The NIMH recently established the Research Domain Criteria (RDoC), an initiative that seeks to define and study transdiagnostic mechanisms of psychopathology. Appetitive and defensive responding are disturbed in mood, anxiety, and other disorders and as such have been proposed as domains of interest (Insel et al., 2010). Establishing that these are orthogonal (rather than correlated aspects of a unitary dimension) is an important first task in validation of the RDoC domains. Other tasks for future studies include not only assessing how these dimensions relate to psychopathology, but also their discriminant and convergent validity in predicting psychopathology.
Methodological Implications
The present study provides further support for the validity of the slot machine and threat-of-shock paradigms to potentiate EEG asymmetry and startle, respectively (Grillon et al., 2004; Schmitz & Grillon, 2012; Shankman et al., 2007).3 Additionally, as both tasks demonstrated high test-retest reliability over approximately 9 days, they appear to capture stable individual differences. Consistent with prior studies, individuals with higher levels of internalizing symptoms showed less left-sided asymmetry potentiation to the slot machine game than individuals with low levels of symptoms (Shankman et al., 2007, 2013). More surprisingly, high-symptom individuals showed a significant decrease in left-sided asymmetry during the win condition compared to the no incentive condition. That is, the difference between high and low internalizers was driven not by a mere lack of potentiation among high internalizers, but instead by “potentiation” in the unexpected direction. One possible explanation for this result is that although the Win condition was intended to foster anticipation of the positive possibility of winning money (if the reels land on three pieces of fruit), it may have caused depressed and anxious individuals to instead focus on the negative possibility of “missing out” on money (if the reels do not land on three pieces of fruit). These differing interpretations would presumably have led to greater left frontal activation among low internalizers, but greater right frontal activation among high internalizers, during the Win condition.
In contrast to psychophysiological measures, self-reported appetitive and defensive responding were positively correlated in Study One and in the combined sample. Consistent with these divergent results, self-reported changes in affect were not correlated with changes in physiological measures. This finding is not surprising, as physiological responses often do not correlate strongly with other response systems (Mauss et al., 2005). One possible interpretation of the divergent findings is that they reflect a dissociation between two affective response systems. That is, it may be that the physiological systems underlying appetitive and defensive motivation are uncorrelated, but higher-order cognitive systems involved in self-reported affect are correlated. It should also be noted that a small positive correlation emerged between defensive responding to unpredictable threat and asymmetry at one electrode pair based on average reference data, indicating that the results for psychophysiological and self-report indicators may not be entirely divergent.
An alternative interpretation is that one set of measures (and results) is more valid than the other. Previous research by our group and other researchers suggests that psychophysiological indicators often have greater criterion validity than self-report (Harmon-Jones & van Honk, 2012). For instance, psychophysiological responses to our slot machine and threat-of-shock tasks were associated with MDD and panic disorder diagnoses, respectively, whereas self-reported affective responses were not (Shankman et al., 2013). Similarly, several studies have found that physiological, but not self-reported, responses to trauma-related cues distinguish trauma survivors with versus without PTSD (Carson et al., 2000; Wessa, Karl, & Flor, 2005). Another study found that physiological arousal during a social interaction task, but not self- or parent-reported anxiety, was associated with selective mutism (Young, Bunnel, & Beidel, 2012).
Thus, the positive association between self-reported affective responses found in the present study may stem from limitations of self-report. Some participants, for example, may have been more sensitive to demand characteristics, or prone to making extreme self-reports regarding emotion. These individuals would have rated the slot machine task as highly rewarding and the threat-of-shock task as highly anxiogenic, producing a positive correlation between these measures. In any case, the divergent findings for physiological and self-report measures underscore the importance of further replication. If this positive correlation is indeed spurious, it nonetheless highlights the utility of psychophysiological measures in emotion research.
Strengths and Limitations
The present study had several limitations. First, EEG lacks the high spatial resolution of imaging techniques such as fMRI and is informative only about cortical activity. Future research should examine relationships among subcortical structures involved in defensive and appetitive motivation. Nonetheless, an advantage of EEG relative to fMRI is lower cost, which allows larger samples than are generally feasible with fMRI (such as the current total n of 127). A second limitation is that although EEG alpha power has been shown to be inversely correlated with brain activity, it may not be related to brain activity equally throughout the brain, particularly in frontal regions (Hagemann, 2004). Finally, our conclusions rely on acceptance of the null hypothesis, which poses interpretive challenges. Although we were able to replicate the null findings across multiple measures in two samples, further replication will be important.
A strength of the study is its examination of multiple physiological measures of appetitive and defensive motivation, assessed in situations relevant to each system. Additionally, the fact that responses to both tasks were stable over approximately 9 days suggests that the effects were not due to differential reliability. Finally, we were able to replicate our findings in an independent sample that differed in several respects from the initial sample, as well as in a more highly-powered combination of the two samples.
Conclusion
Using validated physiological measures recorded in motivationally-relevant contexts, we demonstrated that between-individual differences in appetitive and defensive motivation are independent. The findings have important implications for understanding both normal and abnormal personality and emotion, and point the way towards future research on relationships among the motivational systems that drive human behavior.
Supplementary Material
Acknowledgments
This research was supported by NIMH grants T32 MH067631 (C.S.), R21 MH080689 (S.A.S), R01 MH098093 (S.A.S), and K08 MH083888 (J.R.B.), and a UIC Chancellor's Discovery grant (S.A.S. and J.R.B.).
Footnotes
There were also 12 loss trials during which participants lost money if the reels landed on three pieces of fruit (data not presented). The reason for the these trials was that pilot testing on the task suggested that the task was uninteresting if there were only no incentive and reward trials and interspersing loss trials during the game made the reward trials feel ‘more exciting.’
It should be noted that examining the relationship between appetitive and defensive motivation was not the primary aim of Jackson et al.'s study. They did report negative correlations of asymmetry at FP1/FP2 and FC3/FC4 with startle magnitude after picture offset, which they interpret as implicating prefrontal cortical involvement in automatic emotion regulation.
Furthermore, the finding in Study Two that internalizing symptoms moderated EEG response to the slot machine task replicates that of Shankman et al. (2007), who found that early-onset depression moderated the effect of this task.
Contributor Information
Casey Sarapas, Department of Psychology, University of Illinois at Chicago.
Andrea C. Katz, Department of Psychology, University of Illinois at Chicago
Brady D. Nelson, Department of Psychology, University of Illinois at Chicago
Miranda L. Campbell, Department of Psychology, University of Illinois at Chicago
Jeffrey R. Bishop, College of Pharmacy, University of Illinois at Chicago
E. Jenna Robison-Andrew, Minneapolis VA Healthcare System.
Sarah E. Altman, Cleveland Center for Eating Disorders
Stephanie M. Gorka, Department of Psychology, University of Illinois at Chicago
Stewart A. Shankman, Departments of Psychology and Psychiatry, University of Illinois at Chicago
References
- Aiken LS, West St. G. Multiple regression: Testing and interpreting interactions. Sage; Newbury Park, CA: 1991. [Google Scholar]
- Allen JJ, Coan JA, Nazarian M. Issues and assumptions on the road from raw signals to metrics of frontal EEG asymmetry in emotion. Biological Psychology. 2004;67:183–218. doi: 10.1016/j.biopsycho.2004.03.007. doi:10.1016/j.biopsycho.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Alvarez RP, Chen G, Bodurka J, Kaplan R, Grillon C. Phasic and sustained fear in humans elicits distinct patterns of brain activity. NeuroImage. 2011;55:389–400. doi: 10.1016/j.neuroimage.2010.11.057. doi:10.1016/j.neuroimage.2010.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF. Hedonic tone, perceived arousal, and item desirability: Three components of self-reported mood. Cognition & Emotion. 1996;10:47–68. doi:10.1080/026999396380385. [Google Scholar]
- Beck A, Brown G, Epstein N, Steer R. An inventory for measuring clinical anxiety - psychometric properties. Journal of Consulting and Clinical Psychology. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. doi:10.1037/0022-006X.56.6.893. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA. Relationship between the Beck Anxiety Inventory and the Hamilton Anxiety Rating-Scale with anxious outpatients. Journal of Anxiety Disorders. 1990;5:213–223. doi:10.1016/0887-6185(91)90002-B. [Google Scholar]
- Blumenthal TD, Cuthbert BN, Filion DL, Hackley S, Ottmar VL, Boxtel AV. Committee report: Guidelines for human startle eyeblink electromyographic studies. Psychophysiology. 2005;42:1–15. doi: 10.1111/j.1469-8986.2005.00271.x. doi: 10.1111/j.1469-8986.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- Böhmelt AH, Schell AM, Dawson ME. Attentional modulation of short- and long-lead-interval modification of the acoustic startle eyeblink response: comparing auditory and visual prestimuli. International Journal of Psychophysiology. 1999;32:239–250. doi: 10.1016/s0167-8760(99)00019-7. doi:10.1016/S0167-8760(99)00019-7. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Fong R, Tenke CE, Leite P, Towey JP, Stewart JE, Quitkin FM. Regional brain asymmetries in major depression with or without an anxiety disorder: A quantitative electroencephalographic study. Biological Psychiatry. 1997;41:939–948. doi: 10.1016/S0006-3223(96)00260-0. doi:10.1016/S0006-3223(96)00260-0. [DOI] [PubMed] [Google Scholar]
- Carson MA, Paulus LA, Lasko NB, Metzger LJ, Wolfe J, Orr SP, Pitman RK. Psychophysiologic assessment of posttraumatic stress disorder in Vietnam nurse veterans who witnessed injury or death. Journal of Consulting and Clinical Psychology. 2000;68:890–897. doi:10.1037//0022-006X.68.5.890. [PubMed] [Google Scholar]
- Carver CS, Harmon-Jones E. Anger is an approach-related affect: Evidence and implications. Psychological Bulletin. 2009;135:183–204. doi: 10.1037/a0013965. doi:10.1037/a0013965. [DOI] [PubMed] [Google Scholar]
- Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS Scales. Journal of Personality and Social Psychology. 1994;67:319–333. doi:10.1037/0022-3514.67.2.319. [Google Scholar]
- Cervone D. Personality architecture: Within-person structures and processes. Annual Review of Psychology. 2005;56:423–452. doi: 10.1146/annurev.psych.56.091103.070133. doi:10.1146/annurev.psych.56.091103.070133. [DOI] [PubMed] [Google Scholar]
- Coan JA, Allen JJB. Frontal EEG asymmetry and the behavioral activation and inhibition systems. Psychophysiology. 2003;40:106–114. doi: 10.1111/1469-8986.00011. [DOI] [PubMed] [Google Scholar]
- Coan JA, Allen JJB, McKnight PE. A capability model of individual differences in frontal EEG asymmetry. Biological Psychology. 2006;72:198–207. doi: 10.1016/j.biopsycho.2005.10.003. doi: 10.1016/j.biopsycho.2005.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychological Bulletin. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. doi:10.1037/0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Corr PJ, Kumari V, Wilson GD, Checkley S, Gray JA. Harm avoidance and affective modulation of the startle reflex: A replication. Personality and Individual Differences. 1997;22:591–593. doi:10.1016/S0191-8869(96)00228-0. [Google Scholar]
- Cousineau D. Confidence intervals in within-subject designs: A simpler solution to Loftus and Masson's method. Tutorial in Quantitative Methods for Psychology. 2005;1:42–45. doi:10.3139/146.101514. [Google Scholar]
- Davidson RJ. Affective style and affective disorders: Perspectives from affective neuroscience. Cognition & Emotion. 1998;12:307–330. doi:10.1080/026999398379628. [Google Scholar]
- Depue RA, Collins PF. Neurobiology of the structure of personality: Dopamine, facilitation of incentive motivation, and extraversion. Behavioral and Brain Sciences. 1999;22:491–569. doi: 10.1017/s0140525x99002046. 10.1017/S0140525X99002046. [DOI] [PubMed] [Google Scholar]
- Depue RA, Lenzenweger MF. A neurobehavioral dimensional model of personality disturbance. In: Lenzenweger MF, Clarkin JF, editors. Major theories of personality disorder (391-453) Guilford Press; New York: 2005. [Google Scholar]
- DeYoung C. Higher-order factors of the Big Five in a multi-informant sample. Journal of Personality and Social Psychology. 2006;91:1138–1151. doi: 10.1037/0022-3514.91.6.1138. doi: 10.1037/0022-3514.91.6.1138. [DOI] [PubMed] [Google Scholar]
- Diener E, Emmons RA. The independence of positive and negative affect. Journal of Personality and Social Psychology. 1984;47:1105–1117. doi: 10.1037//0022-3514.47.5.1105. doi:10.1037//0022-3514.47.5.1105. [DOI] [PubMed] [Google Scholar]
- Dollard J, Miller NE. Personality and Psychotherapy (309-378) McGraw Hill; New York: 1950. [Google Scholar]
- Elliot AJ, Thrash TM. Approach-avoidance motivation in personality: Approach and avoidance temperaments and goals. Journal of Personality and Social Psychology. 2002;82:804–818. doi: 10.1037//0022-3514.82.5.804. doi: 10.1037/0022-3514.82.5.804. [DOI] [PubMed] [Google Scholar]
- Faith MS, Wong JY, Allison DB. Demand characteristics of the research setting can influence indexes of negative affect-induced eating in obese individuals. Obesity Research. 1998;6:134–136. doi: 10.1002/j.1550-8528.1998.tb00327.x. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Buchner A, Lang A-G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behavior Research Methods. 2009;41:1149–1160. doi: 10.3758/BRM.41.4.1149. doi:10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Non-Patient Edition (SCID-NP) American Psychiatric Press; Washington D.C.: 2002. [Google Scholar]
- Funder DC. Persons, situations and person-situation interactions. In: John OP, Robins R, Pervin L, editors. Handbook of personality. 3rd ed. Guilford; New York: 2008. pp. 568–580. [Google Scholar]
- Giargiari TD, Mahaffey AL, Craighead WE, Hutchison KE. Appetitive responses to sexual stimuli are attenuated in individuals with low levels of sexual desire. Archives of Sexual Behavior. 2005;34:547–556. doi: 10.1007/s10508-005-6280-y. doi: 10.1007/s10508-005-6280-y. [DOI] [PubMed] [Google Scholar]
- Gibbons R, Balady G, Timothybricker J, Chaitman B, Fletcher G, Froelicher V, Smith SC. ACC / AHA 2002 guideline update for exercise testing: Summary article. A report of the American College of Cardiology / American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology. 2002;48:1731. doi:0.1016/j.jacc.2006.09.003. [Google Scholar]
- Gray JA. Framework for a taxonomy of psychiatric disorder. In: van Goozen SHM, Van de Poll NE, J. A., Sergeant SH, editors. Emotions: Essays on emotion theory. Lawrence Erlbaum Associates; Mahwah, NJ: 1994. pp. 29–59. [Google Scholar]
- Gray JA, McNaughton N. Neural anxiety systems: Relevant fault-times to trace and treat disorders. European Journal of Neuroscience. 2000;12:311–311. [Google Scholar]
- Grillon C. Startle reactivity and anxiety disorders: Aversive conditioning, context, and neurobiology. Biological Psychiatry. 2002;52:958–975. doi: 10.1016/s0006-3223(02)01665-7. doi:10.1016/S0006-3223(02)01665-7. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas JM, Lissek S, Smith K, Milstein J. Anxious responses to predictable and unpredictable aversive events. Behavioral Neuroscience. 2004;118:916–924. doi: 10.1037/0735-7044.118.5.916. doi:10.1037/0735-7044.118.5.916. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas JM, Pine DS, Lissek S, Lawley M, Ellis V, Levine J. The benzodiazepine alprazolam dissociates contextual fear from cued fear in humans as assessed by fear-potentiated startle. Biological Psychiatry. 2006;60:760–766. doi: 10.1016/j.biopsych.2005.11.027. doi:10.1016/j.biopsych.2005.11.027. [DOI] [PubMed] [Google Scholar]
- Grillon C, Lissek S, Rabin S, McDowell D, Dvir S, Pine DS. Increased anxiety during anticipation of unpredictable but not predictable aversive stimuli as a psychophysiologic marker of panic disorder. American Journal of Psychiatry. 2008;165:898–904. doi: 10.1176/appi.ajp.2007.07101581. doi: 10.1176/appi.ajp.2007.07101581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Pine DS, Lissek S, Rabin S, Bonne O, Vythilingam M. Increased anxiety during anticipation of unpredictable aversive stimuli in posttraumatic stress disorder but not in generalized anxiety disorder. Biological Psychiatry. 2009;66:47–53. doi: 10.1016/j.biopsych.2008.12.028. doi: 10.1016/j.biopsych.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagemann D. Individual differences in anterior EEG asymmetry: Methodological problems and solutions. Biological Psychology. 2004;67:157–182. doi: 10.1016/j.biopsycho.2004.03.006. doi: 10.1016/j.biopsycho.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery & Psychiatry. 1960;23:56–61. doi: 10.1136/jnnp.23.1.56. doi:10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon-Jones E, van Honk J. Introduction to a special issue on the neuroscience of motivation and emotion. Motivation and Emotion. 2012;36:1–3. doi:10.1007/s11031-012-9281-x. [Google Scholar]
- Hedlund JL, Vieweg BW. The Hamilton Rating Scale for Depression: A comprehensive review. Journal of Operational Psychiatry. 1979;10:149–165. [Google Scholar]
- Henriques JB, Davidson RJ. Left frontal hypoactivation in depression. Journal of Abnormal Psychology. 1991;100:535–545. doi: 10.1037//0021-843x.100.4.535. doi: 10.1037/0021-843X.100.4.535. [DOI] [PubMed] [Google Scholar]
- Holden RR, Wheeler S, Marjanovic Z. When does random responding distort self-report personality assessment? An example with the NEO PI-R. Personality and Individual Differences. 2012;52:15–20. doi: 10.1016/j.paid.2011.08.021. [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Wang P. Research Domain Criteria (RDoC): Toward a new classification framework for research on mental disorders. American Journal of Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. doi:10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Jackson DC, Mueller CJ, Dolski I, Dalton KM, Nitschke JB, Urry HL, Davidson RJ. Now you feel it, now you don't: Frontal brain electrical asymmetry and individual differences in emotion regulation. Psychological Science. 2003;14:612–617. doi: 10.1046/j.0956-7976.2003.psci_1473.x. doi:10.1046/j.0956-7976.2003.psci_1473.x. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:617–27. doi: 10.1001/archpsyc.62.6.617. doi:10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W, Sauseng P, Hanslmayr S. EEG alpha oscillations: The inhibition-timing hypothesis. Brain Research Reviews. 2007;53:63–88. doi: 10.1016/j.brainresrev.2006.06.003. doi:10.1016/j.brainresrev.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Kline JP, Blackhart GC, Joiner TE. Sex, lie scales, and electrode caps: An interpersonal context for defensiveness and anterior electroencephalographic asymmetry. Personality and Individual Differences. 2002;33:459–478. doi:10.1016/S0191-8869(01)00167-2. [Google Scholar]
- Lang PJ. The emotion probe: Studies of motivation and attention. The American Psychologist. 1995;50:372–385. doi: 10.1037//0003-066x.50.5.372. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Emotion, attention, and the startle reflex. Psychological Review. 1990;97:377–395. [PubMed] [Google Scholar]
- Markon KE, Krueger RF, Watson D. Delineating the structure of normal and abnormal personality: An integrative hierarchical approach. Journal of Personality and Social Psychology. 2005;88:139–157. doi: 10.1037/0022-3514.88.1.139. doi: 10.1037/0022-3514.88.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrae RR, Costa PT. Validation of the five-factor model of personality across instruments and observers. Journal of Personality and Social Psychology. 1987;52:81–90. doi: 10.1037//0022-3514.52.1.81. doi: 10.1037/0022-3514.52.1.81. [DOI] [PubMed] [Google Scholar]
- Mischel W, Shoda Y, Mendoza-Denton R. Situation–behavior profiles as a locus of consistency in personality. Current Directions in Psychological Science. 2002;11:50–54. [Google Scholar]
- Moberg CA, Curtin JJ. Alcohol selectively reduces anxiety but not fear: Startle response during unpredictable versus predictable threat. Journal of Abnormal Psychology. 2009;118:335–347. doi: 10.1037/a0015636. doi: 10.1037/a0015636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SE, Salzman CD. Re-valuing the amygdala. Current Opinion in Neurobiology. 2010;20:221–230. doi: 10.1016/j.conb.2010.02.007. doi:10.1016/j.conb.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson BD, McGowan SK, Sarapas C, Robison-Andrew EJ, Altman SE, Campbell ML, Shankman SA. Biomarkers of threat and reward sensitivity demonstrate unique associations with risk for psychopathology. Journal of Abnormal Psychology. 2013;122:662–671. doi: 10.1037/a0033982. doi:10.1037/a0033982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson BD, Shankman SA. Does intolerance of uncertainty predict anticipatory startle responses to uncertain threat? International Journal of Psychophysiology. 2011;81:107–115. doi: 10.1016/j.ijpsycho.2011.05.003. doi: 10.1016/j.ijpsycho.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Sherwood RJ, Henriques JB, Davidson RJ. Frontal brain asymmetry and reward responsiveness: A source-localization study. Psychol Science. 2005;16:805–813. doi: 10.1111/j.1467-9280.2005.01618.x. doi: 10.1111/j.1467-9280.2005.01618.x. [DOI] [PubMed] [Google Scholar]
- Rollman GB, Harris G. The detectability, discriminability, and perceived magnitude of painful electrical shock. Perception & Psychophysics. 1987;42:257–268. doi: 10.3758/bf03203077. [DOI] [PubMed] [Google Scholar]
- Sandt AR, Sloan DM, Johnson KJ. Measuring appetitive responding with the postauricular reflex. Psychophysiology. 2009;46:491–497. doi: 10.1111/j.1469-8986.2009.00797.x. doi:10.1111/j.1469-8986.2009.00797.x. [DOI] [PubMed] [Google Scholar]
- Sato H, Kawahara J. Selective bias in retrospective self-reports of negative mood states. Anxiety, Stress & Coping: An International Journal. 2011;24:359–367. doi: 10.1080/10615806.2010.543132. doi: 10.1080/10615806.2010.543132. [DOI] [PubMed] [Google Scholar]
- Schmidt L, Fox NA, Goldberg MC, Smith CC, Schulkin J. Effects of acute prednisone administration on memory, attention and emotion in healthy human adults. Psychoneuroendocrinology. 1999;24:461–483. doi: 10.1016/s0306-4530(99)00007-4. doi: 10.1016/S0306-4530(99)00007-4. [DOI] [PubMed] [Google Scholar]
- Schmitz A, Grillon C. Assessing fear and anxiety in humans using the threat of predictable and unpredictable aversive events (the NPU-threat test). Nature Protocols. 2012;7:527–532. doi: 10.1038/nprot.2012.001. doi:10.1038/nprot.2012.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankman SA, Klein DN. The relation between depression and anxiety: An evaluation of the tripartite, approach-withdrawal and valence-arousal models. Clinical Psychology Review. 2003;23:605–637. doi: 10.1016/s0272-7358(03)00038-2. doi:10.1016/S0272-7358(03)00038-2. [DOI] [PubMed] [Google Scholar]
- Shankman SA, Klein DN, Tenke CE, Bruder GE. Reward sensitivity in depression: a biobehavioral study. Journal of Abnormal Psychology. 2007;116:95–104. doi: 10.1037/0021-843X.116.1.95. doi:10.1037/0021-843X.116.1.95. [DOI] [PubMed] [Google Scholar]
- Shankman SA, Nelson BD, Sarapas C, Robison-Andrew EJ, Campbell ML, Altman SE, Gorka SM. A psychophysiological investigation of threat and reward sensitivity in individuals with panic disorder and/or major depressive disorder. Journal of Abnormal Psychology. 2013;122:322–338. doi: 10.1037/a0030747. doi:10.1037/a0030747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrubia R, Ávila C, Moltó J, Caseras X. The Sensitivity to Punishment and Sensitivity to Reward Questionnaire (SPSRQ) as a measure of Gray's anxiety and impulsivity dimensions. Personality and Individual Differences. 2001;31:837–862. doi: 10.1016/S0191-8869(00)00183-5. [Google Scholar]
- Trainor BC. Stress responses and the mesolimbic dopamine system: Social contexts and sex differences. Hormones and Behavior. 2011;60:457–469. doi: 10.1016/j.yhbeh.2011.08.013. doi:10.1016/j.yhbeh.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidyanathan U, Patrick CJ, Bernat EM. Startle reflex potentiation during aversive picture viewing as an indicator of trait fear. Psychophysiology. 2009;46:75–85. doi: 10.1111/j.1469-8986.2008.00751.x. doi:10.1111/j.1469-8986.2008.00751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacker J, Chayanon M-L, Stemmler G. Resting EEG signatures of agentic extraversion: New results and meta-analytic integration. Journal of Research in Personality. 2010;44:167–179. doi: 10.1016/j.jrp.2009.12.004. [Google Scholar]
- Wallace J. An abilities conception of personality: Some implications for personality measurement. American Psychologist. 1966;21:132–138. [Google Scholar]
- Watson D, O'Hara MW, Simms LJ, Kotov R, Chmielewski M, McDade-Montez EA, Stuart S. Development and validation of the inventory of depression and anxiety symptoms (IDAS). Psychological Assessment. 2007;19:253–268. doi: 10.1037/1040-3590.19.3.253. doi:10.1037/1040-3590.19.3.253. [DOI] [PubMed] [Google Scholar]
- Wessa M, Karl A, Flor H. Central and peripheral psychophysiological responses to trauma-related cues in subclinical posttraumatic stress disorder: A pilot study. Experimental Brain Research. 2005;167:56–65. doi: 10.1007/s00221-005-0007-0. doi:10.1007/s00221-005-0007-0. [DOI] [PubMed] [Google Scholar]
- Young BJ, Bunnell BE, Beidel DC. Evaluation of children with selective mutism and social phobia: a comparison of psychological and psychophysiological arousal. Behavior Modification. 2012;36:525–544. doi: 10.1177/0145445512443980. doi:10.1177/0145445512443980. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.