Abstract
Objectives
To determine whether a less invasive approach to aortic valve replacement (AVR) improves clinical outcomes in diabetic patients with aortic stenosis (AS).
Background
Diabetes is associated with increased morbidity and mortality after surgical AVR for AS.
Methods
Among treated patients with severe symptomatic AS at high-risk for surgery in the PARTNER trial, we examined outcomes stratified by diabetes status of patients randomly assigned to transcatheter or surgical AVR. The primary outcome was all-cause mortality at 1 year.
Results
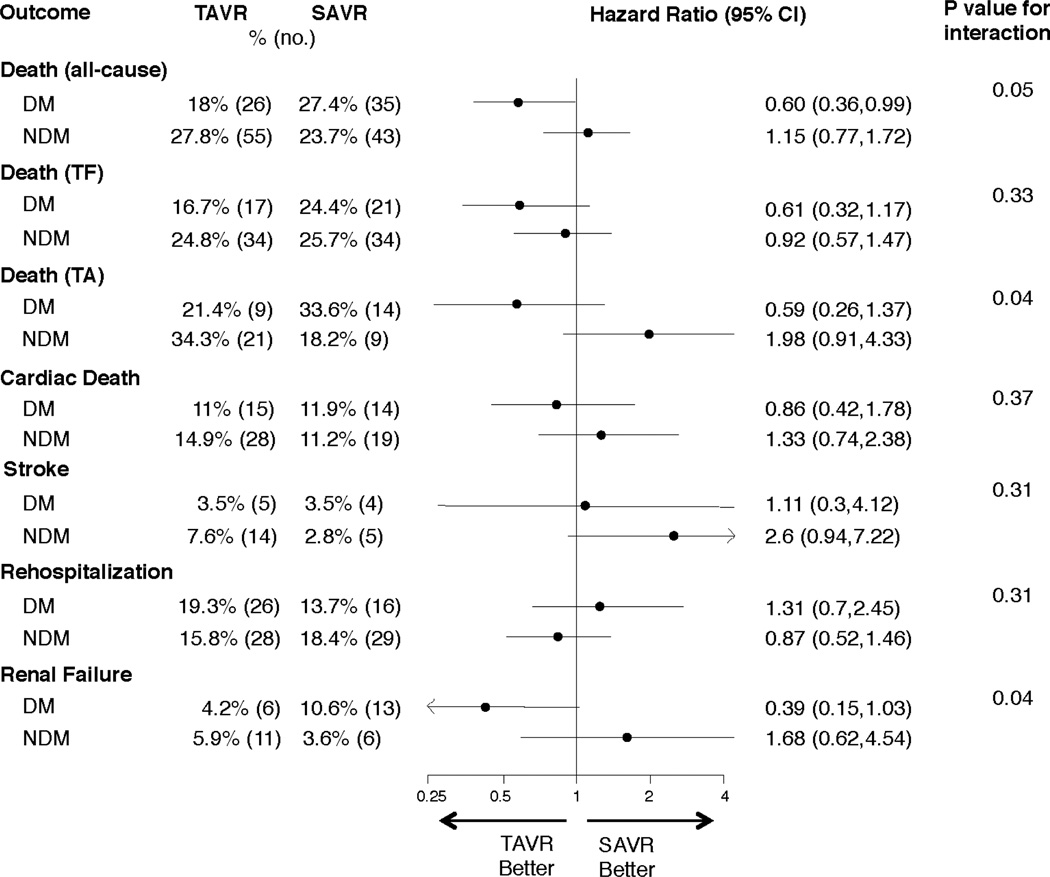
Among 657 patients enrolled in PARTNER who underwent treatment, there were 275 patients with diabetes (145 transcatheter, 130 surgical). There was a significant interaction between diabetes and treatment group for 1-year all-cause mortality (p=0.048). Among diabetic patients, all-cause mortality at 1 year was 18.0% in the transcatheter group and 27.4% in the surgical group (HR 0.60; 95% CI, 0.36–0.99; p=0.04). Results were consistent among patients treated via transfemoral or transapical routes. In contrast, among non-diabetic patients, there was no significant difference in all-cause mortality at 1 year (p=0.48). Among diabetic patients, the 1-year rates of stroke were similar between treatment groups (3.5% transcatheter vs. 3.5% surgery, p=0.88), but the rates of renal failure requiring dialysis >30 days were lower in the transcatheter group (0% vs. 6.1%, p=0.003).
Conclusions
Among patients with diabetes and severe symptomatic AS at high-risk for surgery, this post-hoc stratified analysis of the PARTNER trial suggests there is a survival benefit, no increase in stroke, and less renal failure from treatment with transcatheter compared to surgical AVR.
Keywords: aortic stenosis, transcatheter aortic valve replacement, diabetes
Introduction
Diabetes mellitus adversely affects morbidity and mortality for all types of cardiovascular diseases (1,2). In patients with aortic stenosis (AS), diabetes is associated with increased hypertrophic remodeling, worse left ventricular function, and worse heart failure symptoms (3,4). Diabetes has also been associated with increased morbidity and mortality after surgical aortic valve replacement, even after adjustment for co-morbidities such as vascular disease and renal dysfunction (5,6). The mechanisms for this additional surgical risk are not completely known, although it is hypothesized that the inflammation, oxidative stress, and reperfusion injury induced by cardioplegia and cardiopulmonary bypass are particularly harmful in the setting of diabetes and hypertrophic ventricular remodeling from chronic pressure overload due to AS, thereby causing adverse short and long-term consequences (7–13). As such, a less invasive method of valve replacement that avoids the injurious effects of cardiopulmonary bypass may lead to improved clinical outcomes among these high-risk patients with diabetes. Accordingly, we examined the clinical outcomes of patients at high risk for surgery enrolled in the PARTNER (Placement of Aortic Transcatheter Valves) trial to evaluate whether outcomes varied according to diabetes status after treatment with transcatheter versus surgical aortic valve replacement (14).
Methods
Study population
The design, inclusion and exclusion criteria, and primary results of the high-risk cohort (Cohort A) of the PARTNER trial have been reported (14). These patients were at high surgical risk as defined by a predicted risk of death of 15% or higher by 30 days after surgery. After evaluation of vascular anatomy, patients were included in either the transfemoral-placement cohort or transapical-placement cohort and randomized to transcatheter therapy with the Edwards-Sapien heart valve system (Edwards Lifesciences, Irvine, California) or surgical aortic valve replacement. Some patients did not undergo their assigned procedure due to death, refusal, study withdrawal, and/or pretreatment clinical deterioration (14). For the current analysis, we included only patients who were randomized to and received the assigned treatment (as-treated population). The diagnosis of diabetes and other clinical characteristics were determined by the enrolling sites. The study protocol was approved by the institutional review board at each enrolling site and all patients provided written informed consent.
Clinical endpoints
Clinical events including death (all-cause), death (cardiac), repeat hospitalizations, stroke, renal failure, major bleeding, myocardial infarction, and vascular complications were adjudicated by a clinical events committee. The primary end point of the PARTNER trial and our analysis was all-cause death at 1 year. A detailed report of the classification of deaths among the diabetic and non-diabetic patients treated with transcatheter or surgical aortic valve replacement in the transfemoral and transapical placement cohorts is provided in Supplemental Table 1. Repeat hospitalizations were defined as hospitalization resulting from symptoms of aortic stenosis (valve-related deterioration, including heart failure, angina, or syncope) or complications of the valve procedure. Stroke was defined as a focal neurologic deficit lasting ≥24 hours or a focal neurologic deficit lasting <24 hours with imaging findings of acute infarction or hemorrhage. Renal failure events were defined as the need for dialysis of any sort (hemodialysis, CVVHD, peritoneal). Further details on clinical events definitions are provided in Supplemental Table 2. Many of these clinical event definitions are consistent with the VARC-2 definitions (e.g., cardiac death, stroke, and myocardial infarction), but others differ substantially (e.g. renal failure and major bleeding) (15). An independent core laboratory analyzed all echocardiograms (16). The presence and severity of post-procedural prosthesis-patient mismatch and aortic regurgitation were determined according to VARC-2 criteria (15). The Kansas City Cardiomyopathy Questionnaire (KCCQ), a heart failure disease-specific health status measure, was used to assess health status (17,18).
Statistical analysis
Continuous variables are summarized as mean ± SD or medians and quartiles, and were compared using the Student’s t-test or Mann-Whitney rank sum test as appropriate. Categorical variables were compared with the chi-square or Fisher exact test. Survival curves for time-to-event variables, based on all available follow-up data, were performed with the use of Kaplan-Meier estimates and were compared between groups with the use of the log-rank test. Cox proportional hazards models were used to calculate hazard ratios and to test for interactions. KCCQ overall summary scores were compared using analysis of covariance to adjust for baseline differences in KCCQ scores between groups. All statistical analyses were performed with SAS software, version 9.2.
Results
Patient population
Among the 699 patients enrolled in the PARTNER trial Cohort A, 657 patients were randomized to and received transcatheter or surgical therapy; 313 patients were treated with surgery and 344 patients were treated with transcatheter therapy. Among the as-treated population, 275 (42%) subjects had diabetes, 145 in the transcatheter group (103 transfemoral, 42 transapical) and 130 in the surgery group (88 transfemoral cohort, 42 transapical cohort). Among the 382 patients without diabetes, 199 were treated with transcatheter valve replacement (137 transfemoral, 62 transapical) and 183 were treated with surgery (133 transfemoral cohort, 50 transapical cohort).
The clinical characteristics and medication usage of patients in the trial with and without diabetes differed in ways that would be expected based on diabetes status (Supplemental Table 3). Within each sub-group of patients (diabetic and non-diabetic patients), the clinical characteristics were generally well-matched between those who received transcatheter versus surgical valve replacement (Table 1 and Supplemental Table 4).
Table 1.
Clinical Characteristics of the Diabetic Subjects in the High Risk Cohort of the PARTNER Trial
| TAVR-DM n=145 |
SAVR-DM n=130 |
p-value | |
|---|---|---|---|
| Demographic and Clinical Data | |||
| Age | 81.8 ± 7.5 | 82.4 ± 6.8 | 0.47 |
| Female (%) | 35% | 39% | 0.57 |
| Body mass index | 30.1 ± 7.7 | 28.8 ± 6.6 | 0.13 |
| Body surface area | 1.93 ± 0.24 | 1.89 ± 0.23 | 0.20 |
| STS Score | 12.2 ± 3.4 | 11.8 ± 3.1 | 0.31 |
| STS >10 | 80% | 80% | 1.0 |
| Logistic EuroSCORE | 28.3 ± 16 | 28.3 ± 16 | 0.99 |
| Hyperlipidemia | 86% | 86% | 0.99 |
| Smoking | 55% | 55% | 0.88 |
| Hypertension | 95% | 95% | 0.73 |
| NYHA class 4 | 55% | 57% | 0.68 |
| Angina | 29% | 22% | 0.21 |
| Coronary disease | 82% | 84% | 0.70 |
| Prior myocardial infarction | 29% | 30% | 0.88 |
| Prior percutaneous coronary intervention | 35% | 35% | 0.95 |
| Prior coronary artery bypass surgery | 51% | 54% | 0.64 |
| Stroke or TIA (last 6–12 months) | 30% | 32% | 0.70 |
| Carotid disease | 32% | 27% | 0.40 |
| Peripheral vascular disease | 48% | 43% | 0.41 |
| Porcelain aorta | 0.7% | 0.0% | 1.0 |
| Pulmonary hypertension | 44% | 45% | 0.89 |
| Major arrhythmia | 40% | 49% | 0.16 |
| Permanent pacemaker | 26% | 28% | 0.68 |
| Renal disease (creatinine ³2) | 23% | 26% | 0.60 |
| Liver disease | 3.4% | 3.1% | 0.43 |
| Chronic obstructive lung disease | 48% | 42% | 0.26 |
| Oxygen dependent | 9% | 9% | 0.94 |
| Anemia | 74% | 64% | 0.10 |
| Transfemoral cohort | 71% | 68% | 0.55 |
| Baseline Cardiac Medications | |||
| βblockers | 68% | 67% | 0.91 |
| ACE-inhibitors | 40% | 39% | 0.79 |
| Angiotensin II receptor blockers (ARBs) | 16% | 22% | 0.17 |
| ACE-inhibitors or ARBs | 52% | 57% | 0.45 |
| Calcium channel blockers | 26% | 22% | 0.44 |
| Statins | 73% | 65% | 0.13 |
| Diuretics | 76% | 68% | 0.13 |
| Nitrates | 15% | 10% | 0.26 |
| Anti-arrhythmics | 26% | 30% | 0.41 |
| Aspirin | 77% | 61% | 0.003 |
| Anti-platelet (other than aspirin) | 26% | 23% | 0.64 |
Abbreviations: TAVR, transcatheter aortic valve replacement; DM, diabetes mellitus; SAVR, surgical aortic valve replacement; STS, Society of Thoracic Surgery; NYHA, New York Heart Association; ACE, angiotensin-converting enzyme.
Diabetic patients
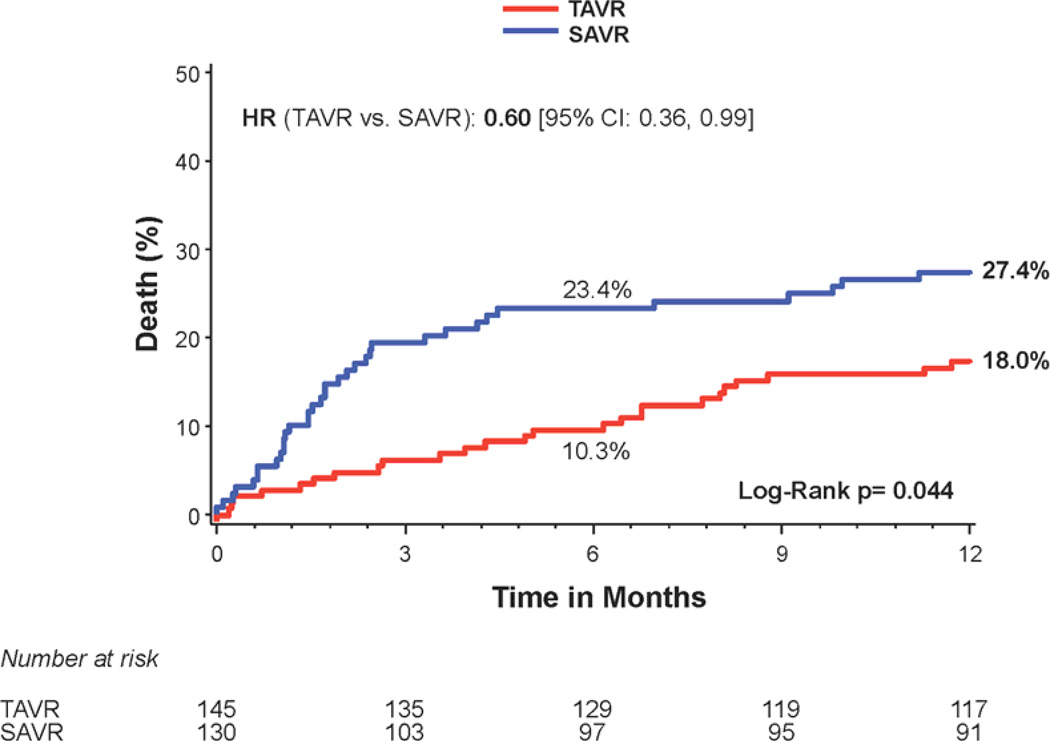
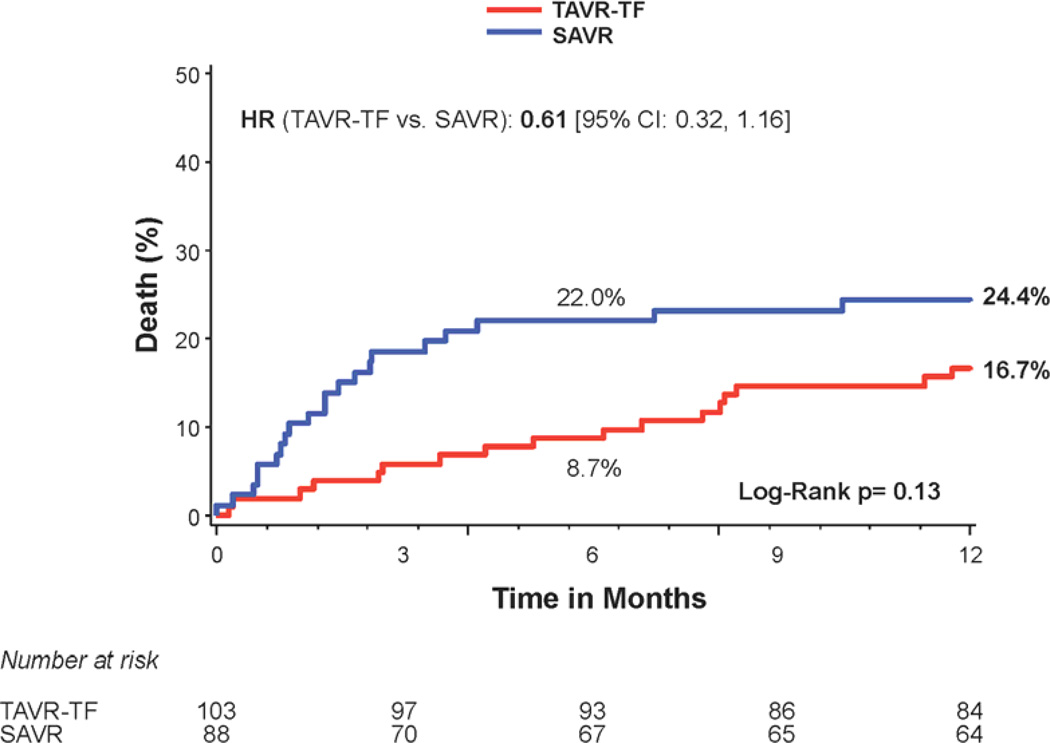
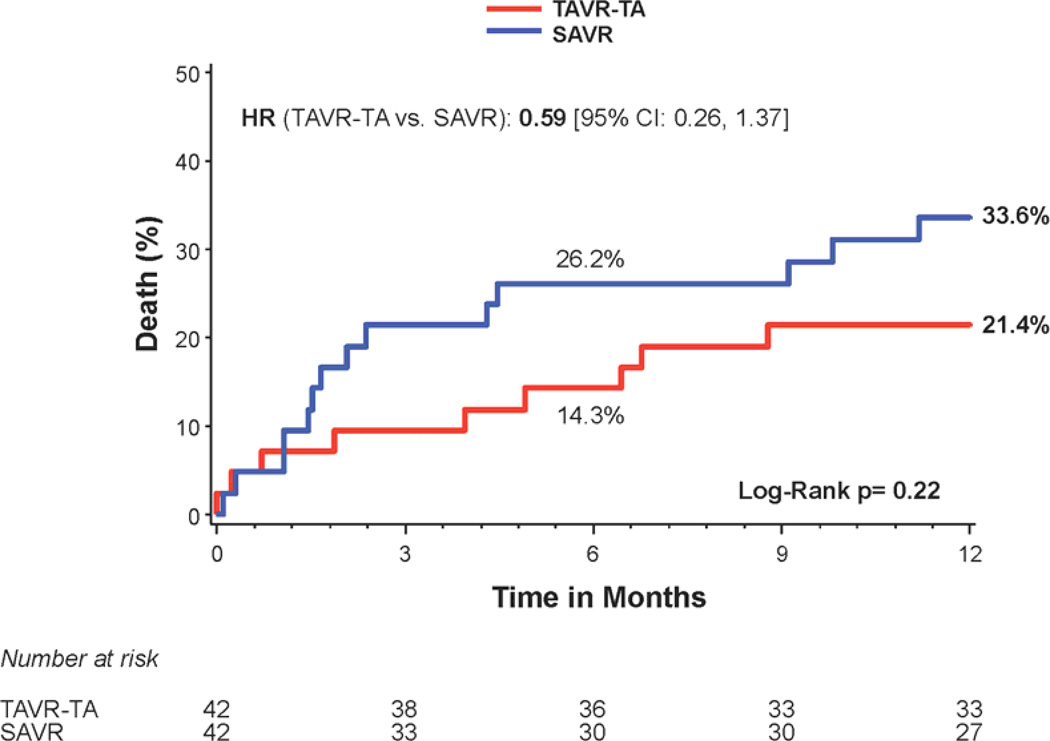
Stratified analyses based on diabetes status were performed for several important clinical outcomes at 1 year. There was a significant interaction between diabetes status and all-cause mortality (interaction p=0.048) (Figure 1). Among the patients with diabetes, 1 year all-cause mortality was 18.0% in transcatheter-treated patients versus 27.4% in the surgically-treated patients (HR 0.60, 95% CI, 0.36 to 0.99; p=0.044) (Figures 1 and 2a). The Kaplan Meier survival curves for the transfemoral-placement cohort (Figure 2b) and transapical-placement cohort (Figure 2c) demonstrate a consistent relationship of lower all-cause mortality for transcatheter-treated vs. surgically-treated diabetic patients compared with the overall population of diabetic patients (Figure 2a).
Figure 1. Clinical outcomes stratified by diabetes in the high risk cohort of the PARTNER trial.
Cox proportional hazards models were used to evaluate the hazard ratios for patients with (DM) and without (NDM) diabetes for the clinical outcomes shown and the interaction between diabetes status and treatment for each clinical outcome. Abbreviations: TAVR, transcatheter aortic valve replacement; SAVR, surgical aortic valve replacement; DM, diabetes mellitus; NDM, non-diabetes mellitus; TF, transfemoral; TA, transapical.
Figure 2. Time-to-event curves for diabetic patients for 1-year death from any cause.
One-year time-to-event curves are shown for diabetic patients for death from any cause in the as-treated population of the PARTNER trial (treated with either transcatheter aortic valve replacement (TAVR) or surgical aortic valve replacement (SAVR)). The curves are shown for all diabetic patients (A), those in the transfemoral (TF) cohort (B), and those in the transapical (TA) cohort (C). The event rates were calculated with the use of Kaplan-Meier methods and compared with the use of the log-rank test.
At 6 months, all-cause mortality was lower in transcatheter-treated diabetic patients compared to surgically-treated diabetic patients (10.3% vs. 23.4%; HR 0.41, 95% CI, 0.22 to 0.76; p=0.003) (Figure 2a). At 2 years, the survival benefit observed at 6 months and 1 year from transcatheter compared to surgical treatment in diabetic patients was no longer significant (HR 0.76, 95% CI, 0.49 to 1.19; p=0.23) (Supplemental Figure 1a).
The rates of stroke were similar between transcatheter-treated and surgically-treated diabetic patients at 30 days (3.5% vs. 2.4%, p=0.58) and 1 year (3.5% vs. 3.5%, p=0.88) (Table 2). At 1 year, there was a decreased rate of renal failure requiring dialysis with transcatheter compared to surgical therapy (4.2% vs. 10.6%, p=0.05), particularly dialysis lasting greater than 30 days (0.0% vs. 6.1%, p=0.003) (Table 2). Similar to the main trial results, among diabetic subjects there was an increased risk of major bleeding with surgery but an increased risk of major vascular complications with transcatheter therapy at 30 days and 1 year (p<0.05 for all relationships) (Table 2).
Table 2.
Clinical Outcomes in Diabetic Patients in the High Risk Cohort of the PARTNER Trial
| Clinical Outcome | TAVR-DM n=145 % (no.) |
SAVR-DM n=130 % (no.) |
Hazard Ratio (95% CI) | p-value |
|---|---|---|---|---|
| 30 days | ||||
| Death (all-cause) | 3.4% (5) | 6.2% (8) | 0.56 [0.18,1.70] | 0.29 |
| Death (all-cause), TF cohort |
1.9% (2) | 6.9% (6) | 0.28 [0.06,1.39] | 0.09 |
| Death (all-cause), TA cohort |
7.1% (3) | 4.8% (2) | 1.51 [0.25,9.07] | 0.65 |
| Death (cardiac) | 1.4% (2) | 3.2% (4) | 0.44 [0.08,2.42] | 0.33 |
| Repeat hospitalizations | 5.0% (7) | 8.0% (10) | 0.61 [0.23,1.60] | 0.31 |
| Stroke (any) | 3.5% (5) | 2.4% (3) | 1.50 [0.36,6.27] | 0.58 |
| Major bleeding | 11.1% (16) | 22.3% (29) | 0.48 [0.26,0.88] | 0.01 |
| Vascular complications (major) |
11.7% (17) | 2.3% (3) | 5.10 [1.50,17.4] | 0.003 |
| Myocardial infarction | 0.0% (0) | 0.8% (1) | --- | 0.29 |
| Renal failure (dialysis required) | 3.5% (5) | 7.8% (10) | 0.44 [0.15,1.30] | 0.12 |
| Dialysis lasting >30 days | 0.0% (0) | 3.2% (4) | --- | 0.03 |
| 1 year | ||||
| Death (all-cause) | 18.0% (26) | 27.4% (35) | 0.60 [0.36,0.99] | 0.04 |
| Death (all-cause), TF cohort |
16.7% (17) | 24.4% (21) | 0.61 [0.32,1.16] | 0.13 |
| Death (all-cause), TA cohort |
21.4% (9) | 33.6% (14) | 0.59 [0.26,1.37] | 0.22 |
| Death (cardiac) | 8.0% (11) | 8.3% (10) | 0.89 [0.38,2.11] | 0.80 |
| Repeat hospitalizations | 19.3% (26) | 13.7% (16) | 1.32 [0.71,2.45] | 0.39 |
| Stroke (any) | 3.5% (5) | 3.5% (4) | 1.11 [0.30,4.12] | 0.88 |
| Major bleeding | 15.1% (21) | 26.9% (34) | 0.52 [0.30,0.89] | 0.01 |
| Vascular complications (major) |
11.7% (17) | 2.3% (3) | 5.10 [1.50,17.4] | 0.003 |
| Myocardial infarction | 0.0% (0) | 0.8% (1) | --- | 0.29 |
| Renal failure (dialysis required) | 4.2% (6) | 10.6% (13) | 0.39 [0.15,1.03] | 0.05 |
| Dialysis lasting >30 days | 0.0% (0) | 6.1% (7) | --- | 0.003 |
The event rates were calculated with the use of Kaplan-Meier methods.
Abbreviations: TF, transfemoral; TA, transapical; others as in Table 1.
Echocardiography, symptoms, and laboratory findings
The incidence of post-operative mild and moderate or severe total aortic regurgitation was higher in diabetic patients treated with transcatheter therapy compared to surgery (Table 3). There was a trend toward a lower incidence of moderate or severe prosthesis-patient mismatch at 30 days with transcatheter therapy, whereas left ventricular mass was lower at 30 days in surgically-treated patients (Table 3). A lower incidence of NYHA class III or IV heart failure symptoms, better quality of life, and longer 6 minute walk distance were observed at 30 days in diabetic patients treated with transcatheter therapy compared to surgery, but there were no significant between-group differences at 6 months or 1 year (Table 4). Post-procedural troponin level and white blood cell count were higher in diabetic subjects treated with surgery compared to transcatheter therapy (Table 3).
Table 3.
Echocardiographic and Laboratory Data in Diabetic Patients in the High Risk Cohort of the PARTNER Trial
| TAVR-DM | SAVR-DM | p-value | |
|---|---|---|---|
| Echocardiography | |||
| Ejection fraction | |||
| Baseline | 51.7 ± 14.0 | 52.7 ± 11.7 | 0.53 |
| 30 days | 54.1 ± 11.1 | 53.7 ± 10.8 | 0.78 |
| LV Mass | |||
| Baseline | 304 ± 86 | 289 ± 88 | 0.18 |
| 30 days | 294 ± 86 | 256 ± 78 | 0.002 |
| Prosthesis-patient mismatch (moderate or severe) | |||
| 30 days | 46.9% | 60.7% | 0.07 |
| Moderate/severe mitral regurgitation | |||
| Baseline (%) | 14.2% | 15.6% | 0.75 |
| 30 days | 15.4% | 14.6% | 0.86 |
| Mild total aortic regurgitation | |||
| Baseline (%) | 48.9% | 35.2% | 0.02 |
| 30 days | 52.8% | 9.3% | <0.0001 |
| 6 months (%) | 54.1% | 5.5% | <0.0001 |
| Moderate/severe total aortic regurgitation | |||
| Baseline (%) | 6.4% | 13.6% | 0.05 |
| 30 days | 9.6% | 1.0% | 0.007 |
| 6 months (%) | 9.0% | 1.4% | 0.052 |
| Laboratory Values | |||
| Troponin I | |||
| Baseline | 0.04 (0.02, 0.08) | 0.05 (0.03, 0.10) | 0.12 |
| 24 hours post-procedure | 0.78 (0.20, 3.62) | 4.47 (2.04, 10.40) | <0.001 |
| Creatinine | |||
| Baseline | 1.30 (1.00, 1.60) | 1.25 (1.00, 1.60) | 0.85 |
| 30 days | 1.21 (1.00, 1.59) | 1.29 (0.94, 1.84) | 0.80 |
| White blood cells | |||
| Baseline | 6.9 (5.9, 8.2) | 6.7 (5.7, 8.1) | 0.35 |
| 24 hours post-procedure | 10.2 (8.6, 12.4) | 11.7 (9.9, 15.4) | 0.056 |
| 30 days | 7.0 (6.0, 8.0) | 7.3 (6.2, 9.8) | 0.04 |
| Hemoglobin | |||
| Baseline | 11.6 (10.7, 12.8) | 11.9 (10.6, 13.0) | 0.40 |
| 24 hours post-procedure | 10.0 (9.2, 11.1) | 9.9 (8.8, 11.4) | 0.89 |
| 30 days | 11.2 (10.5, 12.1) | 11.0 (10.0, 11.9) | 0.13 |
Data reported as mean ± SD or median (25th, 75th percentiles) and includes all subjects with data at the specified time.
Abbreviations: LV, left ventricle; ULN, upper limit of normal; others as in Table 1.
Patient-prosthesis mismatch (moderate or severe) = Effective orifice area index ≤0.85 cm2/m2
Table 4.
Symptoms, Quality of Life, and 6 Minute Walk in Diabetic Patients in the High Risk Cohort of the PARTNER Trial.
| TAVR-DM | SAVR-DM | p-value | |
|---|---|---|---|
| NYHA class III/IV | |||
| Baseline | 95% | 94% | 0.63 |
| Discharge / 7 days | 40% | 60% | 0.003 |
| 30 days | 21% | 40% | 0.002 |
| 6 months | 18% | 11% | 0.18 |
| 1 year | 13% | 10% | 0.58 |
| KCCQ | |||
| Baseline | |||
| Number of subjects with KCCQ data | n=139 | n=120 | |
| Overall summary score | 39.5 ± 23.1 | 44.3 ± 20.3 | 0.08 |
| 30 days | |||
| Number of subjects with KCCQ data | n=123 | n=100 | |
| Overall summary score adjusted for baseline score |
64.9 (60.6, 69.2) | 55.3 (50.4, 60.1) | 0.004 |
| 6 months | |||
| Number of subjects with KCCQ data | n=118 | n=87 | |
| Overall summary score adjusted for baseline score |
68.9 (64.7, 73.0) | 72.3 (67.5, 77.1) | 0.29 |
| 1 year | |||
| Number of subjects with KCCQ data | n=108 | n=83 | |
| Overall summary score adjusted for baseline score |
68.2 (64.0, 72.3) | 72.7 (67.8, 77.6) | 0.17 |
| 6 minute walk | |||
| Baseline | |||
| Could not perform | 41% | 39% | 0.81 |
| Distance walked (m)* | 175 ± 116 | 180 ± 103 | 0.77 |
| 30 days | |||
| Could not perform | 42% | 50% | 0.19 |
| Distance walked (m) | 208 ± 111 | 159 ± 97 | 0.01 |
| 6 months | |||
| Could not perform | 35% | 32% | 0.60 |
| Distance walked (m) | 238 ± 115 | 236 ± 115 | 0.92 |
| 1 year | |||
| Could not perform | 28% | 32% | 0.47 |
| Distance walked (m) | 190 ± 98 | 226 ± 113 | 0.06 |
Data reported as mean ± SD, median (25th, 75th percentiles), or %.
Excluding those who could not perform the 6 minute walk.
Abbreviations: KCCQ, Kansas City Cardiomyopathy Questionnaire; others as in Table 1.
Non-diabetic patients
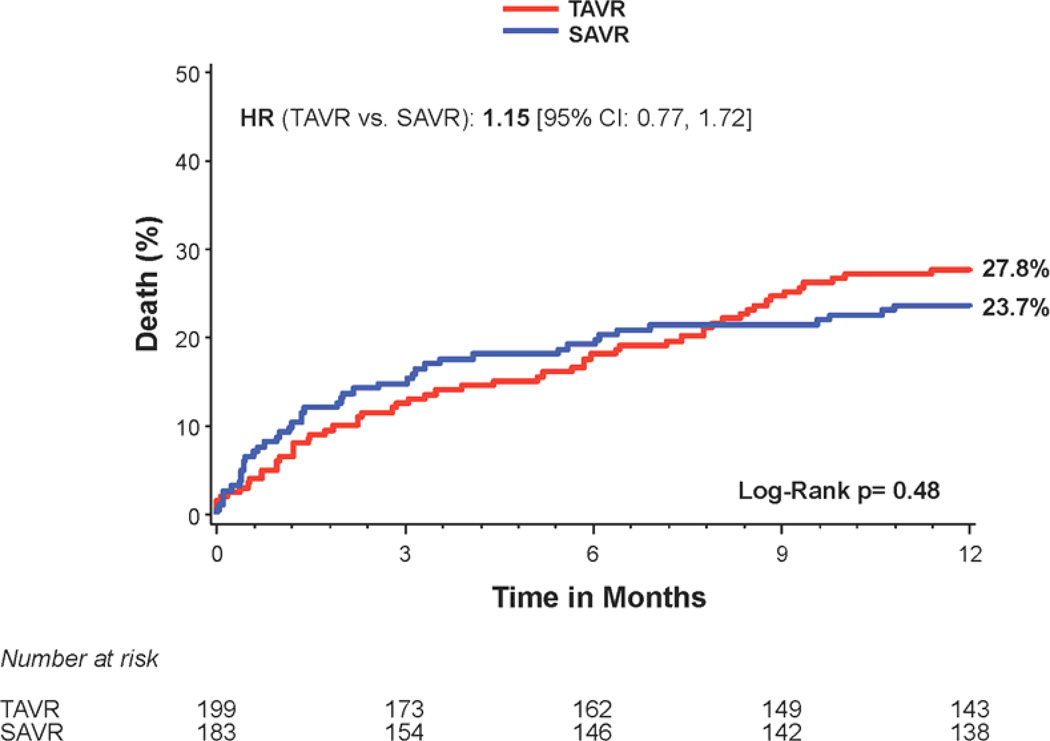
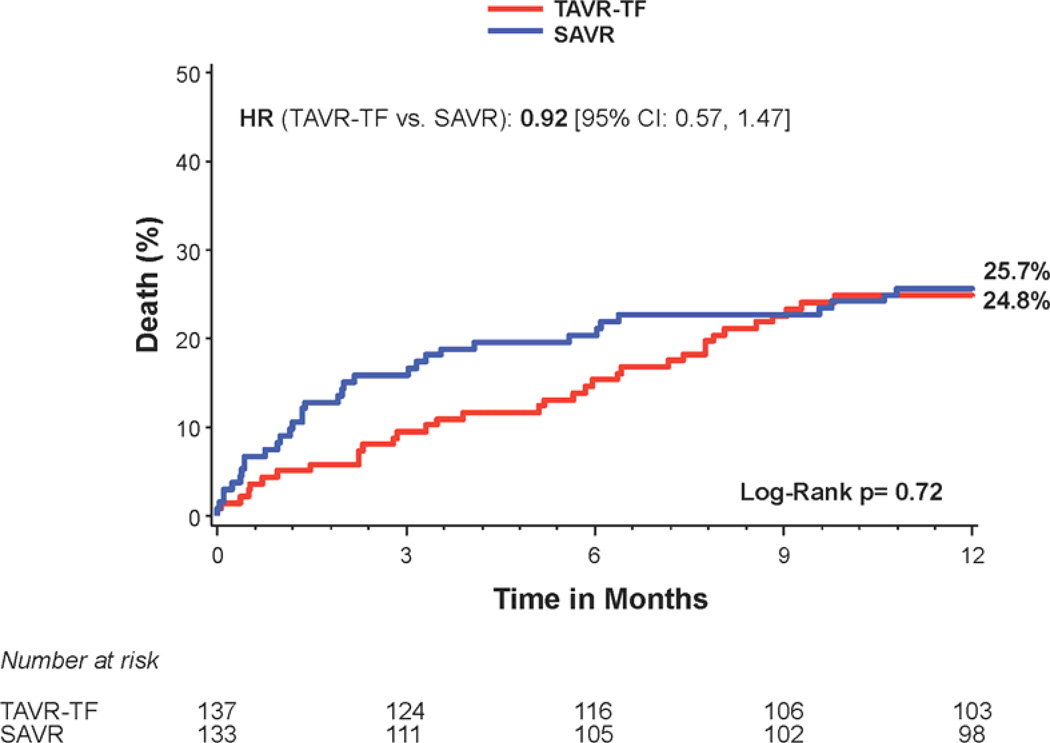
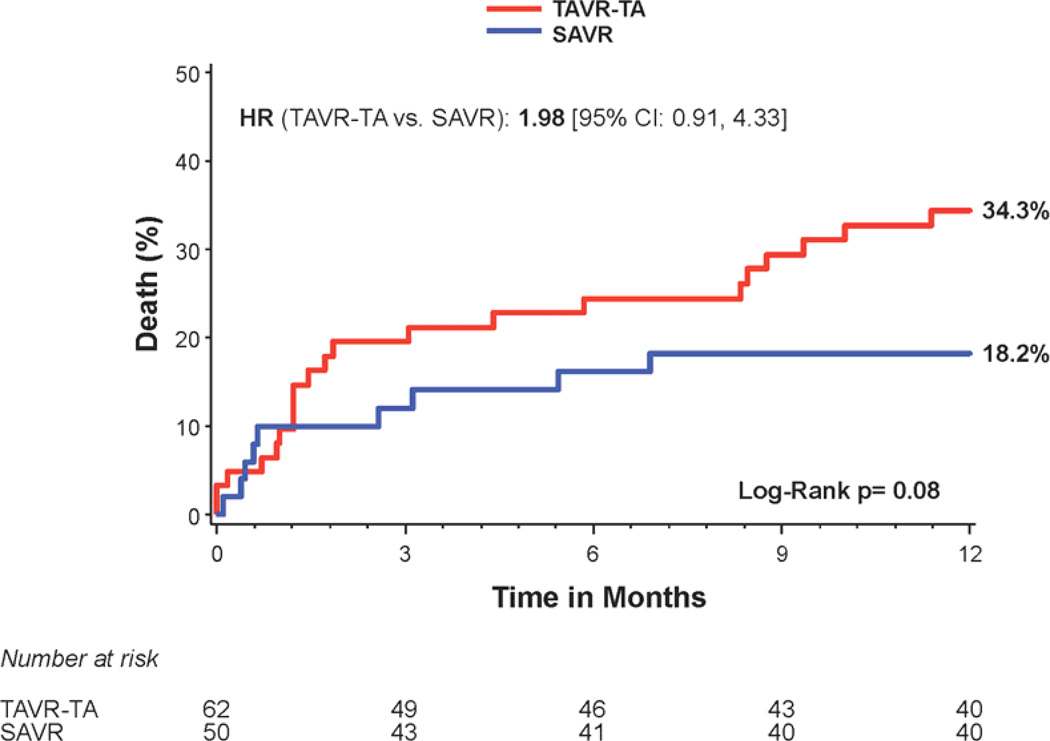
There was no difference in 1 year all-cause mortality in non-diabetic subjects treated with transcatheter versus surgical therapy (Figure 3a); however there was a trend toward increased mortality in the transapical-placement cohort from transcatheter therapy compared to surgery (Figure 3c). A trend toward a higher risk of stroke was observed in non-diabetic patients treated with transcatheter therapy compared to surgery at 1 year (7.6% vs. 2.8%; HR 2.60, 95% CI, 0.94 to 7.22, p=0.056) (Figure 1). The rates of repeat hospitalization and renal failure among non-diabetic patients were similar in the two treatment groups.
Figure 3. Time-to-event curves for non-diabetic patients for 1-year death from any cause.
One-year time-to-event curves are shown for non-diabetic patients for death from any cause in the as-treated population of the PARTNER trial (treated with either transcatheter aortic valve replacement (TAVR) or surgical aortic valve replacement (SAVR)). The curves are shown for all patients without diabetes (A), those in the transfemoral (TF) cohort (B), and those in the transapical (TA) cohort (C). The event rates were calculated with the use of Kaplan-Meier methods and compared with the use of the log-rank test.
Discussion
We report for the first time, in a post-hoc stratified analysis of the high-risk patients enrolled in the PARTNER trial, a differential response to transcatheter versus surgical treatment based on diabetes status. Although the PARTNER trial demonstrated similar rates of death at 1 year in those treated with transcatheter or surgical therapy for the overall population, we found that diabetic patients who were treated with transcatheter aortic valve replacement had a 9% lower absolute risk of 1 year all-cause mortality and a 40% lower hazard of death over the first year after the procedure compared with diabetic patients treated with surgical valve replacement. Furthermore, diabetic patients treated with transcatheter therapy had a similar rate of stroke and lower incidence of renal failure compared with those treated with surgery. These findings have important clinical implications for the treatment of patients with severe AS and diabetes at high risk for surgery.
Clinical Implications
Both transcatheter and surgical valve replacement relieve left ventricular pressure overload from AS by treating the mechanical obstruction of the valve. Among the overall population, the PARTNER trial demonstrated that survival at 1 year was similar with transcatheter and surgical valve replacement for patients with severe symptomatic AS at high risk for surgery. However, there may be sub-groups of patients that will do better with one approach than the other. As we gain more experience with these two treatment options, we will learn how to individualize treatment strategies based on a variety of potential factors to obtain the best clinical results. Our study raises the intriguing possibility that transcatheter valve replacement may be the preferred approach for diabetic patients with severe symptomatic AS who are at high surgical risk.
There is considerable interest in comparing less invasive transcatheter or percutaneous therapies to surgical therapies for a variety of cardiovascular problems including valve disease and coronary, aortic, carotid, and peripheral vascular disease, particularly in diabetic patients (19–23). These comparisons involve differences both in what therapy is provided (eg. stent vs. bypass graft) and how it is provided (eg. catheter-based vs. open surgery). When comparing transcatheter to surgical aortic valve replacement, there is relatively little difference in what therapy is provided. In both cases, the mechanical valve obstruction is treated by the placement of a new valve that relieves the pressure overload on the ventricle. Nonetheless, differences in how well the implanted valve opens the previously restricted orifice (effective orifice area) and how much it leaks could impact outcomes. In contrast, there are more obvious differences in how the therapy is provided, which we suspect underlies the difference in survival among diabetic patients between the two treatment groups. In the case of a transcatheter approach there is rapid ventricular pacing with large sheaths introduced into the major vessels and/or heart, whereas with surgery there are the injurious effects of cardiopulmonary bypass, cardioplegia, and reperfusion.
Among diabetic patients, the survival curves between the transcatheter and surgical treatment groups separate soon after valve replacement and continue to move apart until approximately 6 months, after which the curves move modestly toward each other and by 2 years there is no significant difference in survival between the two treatment groups. We hypothesize that this relationship is due to the short-term benefit of a less invasive approach to replace the valve that avoids cardiopulmonary bypass, which is mitigated over time by non-procedure related factors and the known deleterious effects of increased aortic regurgitation after transcatheter valve replacement. In the PARTNER trial, both in the whole population and the sub-group with diabetes, there was a much greater incidence of mild, moderate, and severe aortic regurgitation in the transcatheter treatment group compared to surgery, which is associated with increased all-cause mortality (24). A potential implication is that if the incidence of aortic regurgitation after transcatheter aortic valve replacement is reduced, the early substantial survival benefit of transcatheter valve replacement in diabetic patients may be sustained beyond the first year.
Other observations from this analysis merit further study. While not the focus of our analysis, the rate of all-cause mortality at 1 year was lower in diabetic patients compared to non-diabetic patients treated with transcatheter therapy. Diabetes is known to adversely affect morbidity and mortality for all types of cardiovascular disease and adversely influence post-procedural outcomes after percutaneous and surgical procedures (1,2,25,26). As such, this result was somewhat surprising. However, it should be noted that there were numerous baseline clinical differences between the diabetic and non-diabetic patients (Supplemental Table 3), which could confound this comparison. In particular and as expected, diabetic patients had a much larger body mass index than non-diabetic patients. In the PARTNER trial, higher body mass index had an independent protective effect in the transcatheter group but not surgical group. This may explain, at least in part, the unexpected observation of lower mortality in diabetic compared to non-diabetic patients in the transcatheter group. This hypothesis-generating observation of an apparent “diabetes paradox” requires further study and careful adjustment for confounders.
Possible Mechanisms
Diabetes is characterized by a milieu of hyperglycemia, insulin resistance, and increased nonesterified fatty acids, which contribute to oxidative stress, lipotoxicity, advanced glycation end products, and altered calcium handling and substrate metabolism (11). Surgical valve replacement involves cardioplegia, cardiopulmonary bypass, and reperfusion injury, which may cause more inflammation, oxidative stress, and myocardial ischemia/injury than with the rapid ventricular pacing performed during transcatheter therapy (7–10,12,13). In diabetic patients, this may intensify an already existing deleterious myocardial and systemic environment, which may have important short and long-term adverse consequences for cardiac performance and clinical outcomes after valve replacement. Recently, Sinning et al. demonstrated that the development of systemic inflammatory response syndrome during the first 48 hours after transcatheter aortic valve replacement is associated with increased 30-day and 1-year mortality (27). We speculate that surgical valve replacement may be associated with an increased incidence of systemic inflammatory response syndrome compared to transcatheter replacement. Consistent with this possibility, the 24 hour post-procedure blood analyses drawn in the PARTNER trial showed higher levels of white blood cells in patients with diabetes after surgical compared to transcatheter valve replacement. The 24 hour post-procedure cardiac enzyme levels were also higher in the diabetic patients treated with surgery, suggesting increased ischemic injury compared to a transcatheter approach. Other mechanisms whereby transcatheter therapy may confer a survival benefit in diabetic patients include less prosthesis-patient mismatch and less post-procedural renal failure requiring dialysis, both of which have a known adverse impact on clinical outcomes (16,24). However, ultimately the mechanisms underlying the survival benefit from a transcatheter valve replacement in diabetic patients require further investigation, including the impact of insulin and/or oral diabetic medical treatments and how the metabolic syndrome and diabetes separately and in combination influence outcomes in diabetic patients undergoing transcatheter or surgical valve replacement.
Limitations
Our study has several limitations to consider when interpreting the results and potential implications. Most importantly, diabetes status was not a pre-specified sub-group analysis and, as such, these results should be considered hypothesis generating and need to be confirmed in future studies. However, given the relatively low power to demonstrate superiority of transcatheter replacement over surgical replacement in a sub-group analysis, the statistically significant survival benefit is noteworthy and should encourage further evaluation. Second, the diagnosis of diabetes was determined by enrolling sites and was not verified by other mechanisms. However, the differences observed between diabetic and non-diabetic patients in the PARTNER trial with respect to baseline clinical characteristics and medication usage are consistent with those that would be expected based on the presence or absence of diabetes. Furthermore, we do not have reliable information on diabetic medication usage (insulin and/or oral medications) nor access to data on the severity or duration of diabetes, microvascular complications, or glucose control. How each of these factors contributes to the treatment effect of transcatheter versus surgical aortic valve replacement will require further study. However, by including patients with mild (recent onset, diet controlled or oral medications only) as well as severe (long-standing, requiring insulin) diabetes, we were less likely to disprove the null hypothesis that survival would be similar between the transcatheter and surgical treatment groups.
Conclusion
Diabetes is associated with increased morbidity and mortality in patients with AS undergoing surgical valve replacement. In a post-hoc stratified analysis of the PARTNER trial in which high-risk patients were randomized to transcatheter or surgical aortic valve replacement, we found that diabetic patients had a survival benefit at 1 year with no increased risk of stroke and less renal failure when treated with transcatheter valve replacement compared to surgery. These results suggest that transcatheter aortic valve replacement may be the preferred treatment approach for patients with AS and diabetes who are high-risk for surgery. Confirmation of these findings, particularly in lower risk populations, is needed as well as insights into the underlying mechanisms for the observed survival benefit.
Supplementary Material
Acknowledgments
Funding: The PARTNER Trial was funded by Edwards Lifesciences and the protocol was developed jointly by the sponsor and study steering committee. The present analysis was carried out by academic investigators with no additional funding from Edwards Lifesciences. Dr. Lindman is supported by K23 HL116660 and the Washington University Institute of Clinical and Translational Sciences grant (UL1 TR000448, KL2 TR000450) from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH).
Abbreviations and Acronyms
- AS
aortic stenosis
- AVR
aortic valve replacement
- DM
diabetes mellitus / diabetic
- KCCQ
Kansas City Cardiomyopathy Questionnaire
- NDM
non-diabetic
- NYHA
New York Heart Association
- PARTNER
Placement of Aortic Transcatheter Valves trial
- SAVR
surgical aortic valve replacement
- TA
transapical
- TAVR
transcatheter aortic valve replacement
- TF
transfemoral
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain
Disclosures: Dr. Lindman is a site co-investigator for the PARTNER Trial. Dr. Suri’s institution (Mayo Clinic) receives randomized aortic valve replacement trial funding to the Division of Cardiovascular Surgery from Edwards Lifesciences, St. Jude Medical and Sorin Medical, and he is a national PI for the PERCEVAL Trial (Sorin Medical), on the Steering Committee for the Portico Trial (St. Jude Medical), and co-Investigator for the PARTNER II (Edwards Lifesciences) and COAPT (Abbott) Trials. Dr. Zajarias is a member of the PARTNER Trial Steering Committee, site PI for the PARTNER Trial, and a consultant for Edwards Lifesciences. Dr. Kodali is a member of the PARTNER Trial Steering Committee and consultant for Edwards Lifesciences, a member of the steering committee for the Portico Trial (St. Jude Medical), and a member of the scientific advisory board of Thubrikar Aortic Valve. Dr. Thourani is a member of the PARTNER Trial Steering Committee and a consultant for Edwards Lifesciences, Sorin Medical, St. Jude Medical, and DirectFlow. Drs. Tuzcu, Svensson, Smith, and Leon are unpaid members of the PARTNER Executive Committee and have received travel reimbursements from Edwards Lifesciences for activities related to these positions. The other authors report no potential conflicts of interest.
References
- 1.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–269. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 2.Mak KH, Topol EJ. Emerging concepts in the management of acute myocardial infarction in patients with diabetes mellitus. J Am Coll Cardiol. 2000;35:563–588. doi: 10.1016/s0735-1097(99)00628-2. [DOI] [PubMed] [Google Scholar]
- 3.Lindman BR, Arnold SV, Madrazo JA, et al. The adverse impact of diabetes mellitus on left ventricular remodeling and function in patients with severe aortic stenosis. Circ Heart Fail. 2011;4:286–292. doi: 10.1161/CIRCHEARTFAILURE.110.960039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Falcao-Pires I, Hamdani N, Borbely A, et al. Diabetes mellitus worsens diastolic left ventricular dysfunction in aortic stenosis through altered myocardial structure and cardiomyocyte stiffness. Circulation. 2011;124:1151–1129. doi: 10.1161/CIRCULATIONAHA.111.025270. [DOI] [PubMed] [Google Scholar]
- 5.Halkos ME, Kilgo P, Lattouf OM, et al. The effect of diabetes mellitus on in-hospital and long-term outcomes after heart valve operations. Ann Thorac Surg. 2010;90:124–130. doi: 10.1016/j.athoracsur.2010.03.111. [DOI] [PubMed] [Google Scholar]
- 6.Smith RL, 2nd, Herbert MA, Dewey TM, et al. Does body mass index affect outcomes for aortic valve replacement surgery for aortic stenosis? Ann Thorac Surg. 2012;93:742–756. doi: 10.1016/j.athoracsur.2011.11.027. [DOI] [PubMed] [Google Scholar]
- 7.Ascione R, Caputo M, Gomes WJ, et al. Myocardial injury in hypertrophic hearts of patients undergoing aortic valve surgery using cold or warm blood cardioplegia. Eur J Cardiothorac Surg. 2002;21:440–456. doi: 10.1016/s1010-7940(01)01168-x. [DOI] [PubMed] [Google Scholar]
- 8.Cavalca V, Sisillo E, Veglia F, et al. Isoprostanes and oxidative stress in off-pump and on-pump coronary bypass surgery. Ann Thorac Surg. 2006;81:562–577. doi: 10.1016/j.athoracsur.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 9.Wan S, DeSmet JM, Barvais L, Goldstein M, Vincent JL, LeClerc JL. Myocardium is a major source of proinflammatory cytokines in patients undergoing cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1996;112:806–811. doi: 10.1016/S0022-5223(96)70068-5. [DOI] [PubMed] [Google Scholar]
- 10.Dybdahl B, Wahba A, Lien E, et al. Inflammatory response after open heart surgery: release of heat-shock protein 70 and signaling through toll-like receptor-4. Circulation. 2002;105:685–790. doi: 10.1161/hc0602.103617. [DOI] [PubMed] [Google Scholar]
- 11.Poornima IG, Parikh P, Shannon RP. Diabetic cardiomyopathy: the search for a unifying hypothesis. Circ Res. 2006;98:596–605. doi: 10.1161/01.RES.0000207406.94146.c2. [DOI] [PubMed] [Google Scholar]
- 12.Suleiman MS, Hancock M, Shukla R, Rajakaruna C, Angelini GD. Cardioplegic strategies to protect the hypertrophic heart during cardiac surgery. Perfusion. 2011;26(Suppl 1):48–56. doi: 10.1177/0267659111420607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wan S, LeClerc JL, Vincent JL. Inflammatory response to cardiopulmonary bypass: mechanisms involved and possible therapeutic strategies. Chest. 1997;112:676–692. doi: 10.1378/chest.112.3.676. [DOI] [PubMed] [Google Scholar]
- 14.Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187–2298. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 15.Kappetein AP, Head SJ, Genereux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. J Am Coll Cardiol. 2012;60:1438–1454. doi: 10.1016/j.jacc.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Hahn RT, Pibarot P, Stewart WJ, et al. Comparison of Transcatheter and Surgical Aortic Valve Replacement in Severe Aortic Stenosis: A Longitudinal Study of Echocardiography Parameters in Cohort A of the PARTNER Trial (Placement of Aortic Transcatheter Valves) J Am Coll Cardiol. 2013;61:2514–2521. doi: 10.1016/j.jacc.2013.02.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reynolds MR, Magnuson EA, Wang K, et al. Health-related quality of life after transcatheter or surgical aortic valve replacement in high-risk patients with severe aortic stenosis: results from the PARTNER (Placement of AoRTic TraNscathetER Valve) Trial (Cohort A) J Am Coll Cardiol. 2012;60:548–558. doi: 10.1016/j.jacc.2012.03.075. [DOI] [PubMed] [Google Scholar]
- 18.Arnold SV, Spertus JA, Lei Y, et al. Use of the Kansas City Cardiomyopathy Questionnaire for monitoring health status in patients with aortic stenosis. Circ Heart Fail. 2013;6:61–77. doi: 10.1161/CIRCHEARTFAILURE.112.970053. [DOI] [PubMed] [Google Scholar]
- 19.Feldman T, Foster E, Glower DD, et al. Percutaneous repair or surgery for mitral regurgitation. N Engl J Med. 2011;364:1395–1406. doi: 10.1056/NEJMoa1009355. [DOI] [PubMed] [Google Scholar]
- 20.Farkouh ME, Domanski M, Sleeper LA, et al. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med. 2012;367:2375–2484. doi: 10.1056/NEJMoa1211585. [DOI] [PubMed] [Google Scholar]
- 21.Chaitman BR, Rosen AD, Williams DO, et al. Myocardial infarction and cardiac mortality in the Bypass Angioplasty Revascularization Investigation (BARI) randomized trial. Circulation. 1997;96:2162–2270. doi: 10.1161/01.cir.96.7.2162. [DOI] [PubMed] [Google Scholar]
- 22.Bavaria JE, Appoo JJ, Makaroun MS, et al. Endovascular stent grafting versus open surgical repair of descending thoracic aortic aneurysms in low-risk patients: a multicenter comparative trial. J Thorac Cardiovasc Surg. 2007;133:369–377. doi: 10.1016/j.jtcvs.2006.07.040. [DOI] [PubMed] [Google Scholar]
- 23.Brott TG, Hobson RW, 2nd, Howard G, et al. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med. 2010;363:11–23. doi: 10.1056/NEJMoa0912321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kodali SK, Williams MR, Smith CR, et al. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med. 2012;366:1686–1695. doi: 10.1056/NEJMoa1200384. [DOI] [PubMed] [Google Scholar]
- 25.Kip KE, Faxon DP, Detre KM, Yeh W, Kelsey SF, Currier JW. Coronary angioplasty in diabetic patients The National Heart, Lung, and Blood Institute Percutaneous Transluminal Coronary Angioplasty Registry. Circulation. 1996;94:1818–1825. doi: 10.1161/01.cir.94.8.1818. [DOI] [PubMed] [Google Scholar]
- 26.Bucerius J, Gummert JF, Walther T, et al. Impact of diabetes mellitus on cardiac surgery outcome. Thorac Cardiovasc Surg. 2003;51:11–16. doi: 10.1055/s-2003-37280. [DOI] [PubMed] [Google Scholar]
- 27.Sinning JM, Scheer AC, Adenauer V, et al. Systemic inflammatory response syndrome predicts increased mortality in patients after transcatheter aortic valve implantation. Eur Heart J. 2012;33:1459–1568. doi: 10.1093/eurheartj/ehs002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.