Abstract
Combustion emissions from diesel engines emit particulate matter which deposits within the lungs. Alveolar macrophages (AM) encounter the particles and attempt to engulf the particles. Emissions particles from diesel combustion engines have been found to contain diverse biologically active components including metals and polyaromatic hydrocarbons which cause adverse health effects. However little is known about AM response to particles from the incorporation of biodiesel. The objective of this study was to examine the toxicity in Wistar Kyoto rat AM of biodiesel blend (B20) and low sulfur petroleum diesel (PDEP) exhaust particles. Particles were independently suspended in media at a range of 1–500µg/mL. Results indicated B20 and PDEP initiated a dose dependent increase of inflammatory signals from AM after exposure. After 24hr exposure to B20 and PDEP gene expression of cyclooxygenase-2 (COX-2) and macrophage inflammatory protein 2 (MIP-2) increased. B20 exposure resulted in elevated prostaglandin E2 (PGE2) release at lower particle concentrations compared to PDEP. B20 and PDEP demonstrated similar affinity for sequesteration of PGE2 at high concentrations, suggesting detection is not imparied. Our data suggests PGE2 release from AM is dependent on the chemical composition of the particles. Particle analysis including measurments of metals and ions indicate B20 contains more of select metals than PDEP. Other particle components generally reduced by 20% with 20% incoporation of biodiesel into original diesel. This study shows AM exposure to B20 results in increased production of PGE2 in vitro relative to diesel.
Keywords: prostaglandin E2, alveolar macrophages, biodiesel exhaust
Introduction
Inhaled diesel exhaust particles deposit in the lungs where individual alveolar macrophages (AM) engulf particles via phagocytosis. Phagocytosis initiates a response from AM to trigger an inflammatory response which includes release of cytokines, lipid mediators and other signals to recruit neutrophils to deposit site. In vivo exposures to petroleum diesel exhaust particles (PDEP) with guinea pigs and rats revealed phagocytosis by AM and increased inflammation response [1, 2]. Previous studies indicate human macrophages release cytokines IL-6 and TNFα after exposure to coarse and ultrafine particles of diesel exhaust indicating a heightened inflammatory response [3]. Exposure to filtered diesel exhaust and unfiltered resulted in both types causing similar inflammation responses from human AM from bronchoalveolar lavage fluid (BALF), suggesting the particle and its composition plays a leading role in AM response [4]. The composition of the particle and potentially extractable components vary due to incomplete combustion which directly affects inflammation. Inflammation from PDEP can be measured with release of arachidonic acid metabolites such as prostaglandins. PGE2 release indicates an inflammatory response to recruit neutrophils and may also signal helper T cells [5]. Diesel particle exposure interferes with immune responses including AM suppressed phagocytic response to bacterial challenge [6]. In vitro diesel exhaust particle exposure has also lead to increased PGE2 release from monocytes [7]. PDEP phagocytosis and inflammation response is well studied however not much is known about B20 effect on AM.
Recent toxicity studies found biodiesel appears to induce similar responses as seen with diesel. The exposure of biodiesel exhaust to rats indicates an increased number of AMs in rat lungs and many had engulfed particles [8]. A rodent study involving intratracheal instillation of both PDEP and biodiesel in mice found significantly elevated protein levels in BALF after 24h, indicating an increase of protein inflammation signaling molecules and recruited polymorphonuclear neutrophils [9]. Previous studies indicate increased release of IL-6 and IL-8 with human bronchial epithelial cells exposure to a methylene chloride extract of biodiesel blend in vitro [10]. Most studies of cellular responses to biodiesel thus far, are a reflection of the particle not necessarily a reflection of the composition of the biodiesel particle. If we compare the biodiesel particle to the petroleum diesel particle we find there are fewer numbers of polyaromatic aromatic hydrocarbons (PAHs) and aldehydes present in the emissions [11–13]. The biodiesel blend emissions emits fewer acrolein and nitro-PAH’s [12, 14]. The particle composition of PDEP consists of an inert carbon core bound with hydrophobic components whereas B100 contains hydrophilic compounds bound to the core [11]. Additionally incomplete soy biodiesel combustion was identified to emit unique chemical species (ie, methylacrylate & methyl butonate) believed to be fragments of methylated fatty acids esters [12]. The gradual incorporation of biodiesel into petroleum, identified by Brito et al [15], indicates CO2 and PAH decrease but not all emissions decrease. This non-linear emissions data can imply an irregularity in efficiency of combustion. A study by Tsai et al indicates the physiochemical difference in biodiesel and petroleum diesel exhaust can be due to incomplete combustion of soy methylated esters [14]. In this study we include composition analysis and cellular responses to petroleum and soy biodiesel blend exhaust particles (B20).
Previous observations of AM initiating an inflammatory response to inhaled PDEP particles lead us to hypothesize similar inflammation from our B20 and PDEP. We have designed an approach to study freshly collected and cultured WKY rat AM to particle exposure in vitro. At non-cytotoxic particle concentrations AM release cytokines and prostaglandins indicating inflammation. The enzymes COX-1 and COX-2, produce PGE2 relative to the intensity of the inflammation response. Our objective of this study was to examine macrophage response and the release of arachidonic acid metabolites as a result of in vitro exposure to 20% B20 in comparison to PDEP.
2. Experimental
2.1. Particle collection
The B20 and low sulfur PDEP fuels were combusted with a 300hp, 5.9L 2002 Cummins ISB engine. The engine was 2004 EPA heavy duty emission regulations certified. The system was equipped with exhaust gas recirculation (EGR) and high pressure common rail fuel injection. The engine was also fitted with a Johnson Matthey CCRT and consists of a diesel oxidation catalyst (DOC) and diesel particulate filter (DPF). Soot samples were collected by operating the engine at 2000rpm and 61N-m. These operating conditions created low DPF temperature (approximately 190C) which allowed the PM to accumulate on the DPF without oxidizing. The soot was then collected by back flush with compressed air off the DPF into a collection canister. Soot samples were stored under nitrogen in glass jars until the time of analysis [16].
2.2. Particle chemical composition
The study samples and a standard reference material: NIST SRM1649a – urban dust from Washington D.C. were analyzed for acid-soluble inorganic elements, water-soluble inorganic elements, ions and carbon content. Acid digestions were performed with 1.0 mL of 3:1 hydrochloric acid: nitric acid (Optima grades, Thermo Fisher Scientific, Fair Lawn, NJ) for 48 h at 60°C. Ultrapure deionized water (Millipore Milli-Q, Bedford, MA) extractions were performed with 10mL at room temperature. SRM 1649a was used as an internal control; our averaged values of metals from acid digestion were compared to NIST published values. The vortexing/agitation cycle was repeated twice, and finished with 1 min vortexing. Supernatants from acid digestions and water extractions were centrifuged at 17,000g, filtered through 0.2µm syringe filters. Elemental analysis was performed using inductively coupled plasma-optical emission spectrometry (ICP-OES), following U.S. Environmental Protection Agency (EPA) Method 200.7 rev4.4 (U.S. EPA 1994), using an axially-viewed, simultaneously-measured instrument (PerkinElmer 4300DV ICP-OES, Bridgeport, CT). Independently-sourced, matrix-matched calibration (VHG Labs, Manchester, NH) and quality control (SPEX Certiprep, Metuchen, NJ) standards were used as specified by the previously mentioned analytical protocol. For carbon analysis, homogeneous suspensions of study samples in 10mL of HPLC-grade methanol were sonicated (Thermo Fisher Scientific, Fair Lawn, NJ) in an ultrasonic bath at 220W for 30 min, then 150 µL was collected onto pre-fired 1.45-cm2 quartz filters and dried in a class 100 clean bench prior to analysis. Carbon fraction analysis was sent to Sunset Laboratories (Hillsborough, NC) which used a thermo-optical method based on sequential pyrolytic vaporization with detection by transmittance (Model 107A, Sunset Laboratory Inc., Tigard, OR) [17].
2.3. Endotoxin analysis
Although aseptic technique was followed for both collection and storage of particles, as a precaution a bacterial endotoxin characterization test was conducted by Cape Cod Inc (East Falmouth, Massachusetts). A gel clot method was conducted following Cape Cod standard operating procedures. 5mg of collected PM was re-suspended with 5mL of Pyrotell® for a concentration of 1mg/mL, the particle suspensions were incubated on an orbital shaker for 1 h before analysis. Endotoxin gram positive contamination was evident if a gel had formed. The assay has sensitivity up to 0.03EU/mL. Both B20 and PDEP samples were tested against the positive control the Escherichia coli 0113:H10.
2.4. Redox cycling activity of particles
A cell free assay was conducted to assess if particles alone could initiate direct redox cycling. The PDEP and B20 were suspended at 1.0mg/mL in DI water. Thiobarbituric acid reactive substances assay was used for (TBARS) quantification reactive species using a previously established protocol [18]. Protocol was modified to include one additional centrifugation at 10,000g for 30 min after initial incubation. Absorbance was detected at 532nm. The positive control used in this experiment was residual oil fly ash (ROFA; a vanadium rich particle) at a 1mg/mL concentration.
2.5. Animals
Healthy male Wistar Kyoto (WKY) between 11–17 weeks old, were purchased from Charles River Laboratories, Inc. (Raleigh, NC). All of the animals were placed in an isolated animal room in an animal facility approved by the Association for Assessment and Accreditation of Laboratory Animal Care (AALAC). The animal facility maintained an environment of 21°C (± 1°C), 50% (± 5%) relative humidity and a 12 hour light- dark cycle. The rats were housed two to a cage. Rats were allowed to adjust to their environment for at least a week before AMs were collected. All animals were provided, standard (5100) Purina rat chow (Brentwood, MO) and water ad libitum. The protocol for this study was approved by the U.S Environmental Protection Agency (EPA) Animal Care and Use Committee.
2.6. Collection of alveolar macrophages
All animals were anesthetized with euthasol in a 1:1 dilution with saline measured according to body weight, given by intra-peritoneal injection and exsanguinated via abdominal aorta. The trachea was cannulated and the entire lung was lavaged in and out three times and all aliquots were combined for each animal as conducted in a previously established protocol [19]. For H/E (Fisher Scientific Pittsburg, PA) cell staining a portion of each samples was diluted to 2 × 104 cells per 100µL and fixed with Cytospin (Shandon, Pittsburgh, PA) and were photographed with light microscope fitted with a digital camera. The total AMs were diluted at 5 × 105 cells in 1mL of RPMI media (Gibco, Grand Island, NY) with 2.5% FBS (Gibco, Grand Island, NY) and 50µg/mL gentamycin (Gibco, Grand Island, NY).
2.7. In vitro exposure
In vitro exposures of both B20 and PDEP particle suspensions were conducted on freshly isolated rat AM within polypropylene culture tubes. Particles were weighed out and diluted in RPMI media with 2.5% FBS and 50µg/mL gentamycin (at room temperature). In polypropylene culture tubes the cells were exposed to 100- 500µg/mL of either PDEP or B20 and incubated at 37°C with 5% CO2. After 24 h of exposure, the AMs were centrifuged (500× g) for 10 min at 25°C and 800µL of supernatant was collected. In experiments where macrophages were challenged post PM exposure, fresh media (800µL) containing 12.5ng/mL LPS (Sigma Aldrich, St. Louis MO) was added for a subsequent 4 h incubation. In a few experiment particles (100µg/mL) were co-incubated for 30 min with polymyxin B concentration (10µg/mL) prior to adding the modified particles onto AMs. In some experiments the supernatant was collected (stored at −20°C), cells were then lysed and stored at −280°C until mRNA isolated.
2.8. mRNA isolation & quantitative real-time PCR
Cytoplasmic RNA was collected using Qiagen’s RNAeasy Micro Column kit (Valencia, CA) manufactures protocol was followed and amended with additional column washes. The mRNA collection includes n=10 animals per dose, quantification, cDNA synthesis and RT-PCR conditions were followed from previously published method [22]. Rattus norvegicus primers PTGS2 (COX-2, cyclooxygenase 2) and Cxcl2 (MIP-2 – macrophage inflammatory protein 2) (ABI; Carlsbad, CA) were measured and data was normalized to the house keeping gene 18S.
2.9. PGE2 ELISA
PGE2 released into culture media was measured by ELISA (R & D systems Minneapolis, MN) following the manufacturer’s high sensitivity instructions. The ELISA kit uses a competitive binding method to assess the sample specific PGE2 released. An average of three animals were used for media and n=12 for all exposures. Standards were created using culture media and multiple dilutions were studied before reaching optimal absorbance.
2.10. Cell viability
AM cells were spun down after 24 h of exposure and 50µL aliquots of supernatant were assayed for cytotoxicity using LDH assay (Promega; Madison, WI). 50µL of post exposure media collected and assayed with equal parts substrate, after 30 min reaction was stopped and absorbance was measured at 490nm. Trypan blue dye incorporation was used to verify cell death.
2.11. 3H- PGE2 binding activity assay
A cell free assay was conducted to estimate the competitive binding affinity of PGE2 to either B20 or PDEP particle. Radioactively labeled 3H- PGE2 (Perkin Elmer, Boston MA) with specific activity of 200Ci/mmol was incubated in media containing 1–500µg/mL B20 or PDEP, for 24h with non-radioactive PGE2. After 24h tubes were centrifuged at (500×g) and the radioactivity in the supernatant (not bound to particle) was measured by standard scintillation counts (Liquid Scintillation Analyzer TriCarb 1500, Packard). The media contained 2.5%FBS and 50µg/mL gentamycin.
2.12. Statistical Analysis
Pair-wise group comparisons were made using Holm-Sidak method and Bonferroni (SigmaStat Software Inc., version 11.0). All graphs and figures are graphed with means and with standard error bars. A value of P< 0.05 was considered significant.
3. Results
3.1. B20 and PDEP combustion emissions analysis indicates chemical composition varies
The elemental composition of B20 and PDEP greatly differs with a few transition metals and elements (Table 1). Our data indicates Co, Cr, Cu, Fe, Mn, Mo, Ni, and Zn are at least 10-fold greater in B20 than in PDEP the acid soluble fraction analysis. Other metals in the acid soluble fraction such as Mg, Sb, and V are only about 15%- 25% more in B20 compared to PDEP. Analysis of the water soluble fraction identified 1,623µg/g Zn was present in B20 whereas PDEP had only 11µg/g. The toxic metals Mg and Pb were found, in a previous study, elevated in B20 relative to PDEP [20]. Measurement of ions from particles dissolved in water indicate increases in PO3+, Na+, Ca2+ and SO42− in B20 relative to PDEP. Also in our study, PDEP and B20 were analyzed for elemental and organic carbon content. B20 appears to have a higher EC/OC ratio than the PDEP (10.7 and 4.8, respectively). B20 contained more total carbon and elemental carbon/g PM than PDEP which had more organic carbon/g PM (Table 1).
Table 1.
Characterization of B20 and PDEP by ICP- Plasma OES. Particles were suspended in distilled water or acid (3:1::HCL:HNO3) for measurement as described in methods. Elemental components, ions and carbon type are measured in µg/gram of PM. There are measureable decreases in both metals and carbon when comparing B20 to PDEP. We used NIST SRM 1649a particles to verify our particle extraction was effective. Using our acid-soluble method we had over 93% recovery for Mn, Pb and Zn compared to the NIST published data. Elements such as Fe with our method indicated only 81% recovery relative to NIST published standards.
| PDEP | B20 | |||
|---|---|---|---|---|
| Element ug/g PM | H2O-soluble | acid-soluble | H2O-soluble | acid-soluble |
| Al | 25 | 189 | 14 | 277 |
| As | <6.9 | <11.8 | <5.9 | <5.9 |
| B | 2 | 5 | 36 | 38 |
| Ba | <1 | 7 | <1 | 9 |
| Ca | <1 | 367 | 3,833 | 6,916 |
| Cd | <0.4 | 3 | <0.4 | 2 |
| Co | <0.7 | 2 | 18 | 121 |
| Cr | <0.4 | 2 | 4 | 419 |
| Cu | <1.2 | 33 | <1 | 517 |
| Fe | <0.4 | 90 | 5 | 4,332 |
| K | 3 | 10 | 25 | 33 |
| Li | <1 | 0 | 1 | 1 |
| Mg | <1 | 49 | 60 | 102 |
| Mn | 0 | 4 | 71 | 145 |
| Mo | 4 | 8 | 17 | 88 |
| Ni | <1.7 | 3 | 25 | 762 |
| P | 85 | 142 | 1,581 | 1,659 |
| Pb | <6.6 | <11.3 | <5.6 | 32 |
| S (as SO42−) | 365 | 3,313 | 16,649 | 16,632 |
| Sb | <4.7 | <8.1 | 3 | 9 |
| Se | <6.4 | <11.0 | <5.5 | <5.5 |
| SiO2 | 29 | 86 | 29 | 134 |
| Sr | <1 | 0 | 2 | 4 |
| Ti | <0.5 | 10 | <0.4 | 17 |
| V | <0.4 | 2 | <0.3 | 3 |
| Zn | 11 | 174 | 1,623 | 2,863 |
| lons ug/g PM | DEP | B20 | ||
| H2O-soluble | H2O-soluble | |||
| F− | <41 | <32 | ||
| Cl− | <204 | <175 | ||
| NO2− | <204 | <175 | ||
| Br− | <203 | <175 | ||
| SO42− | <203 | 18,690 | ||
| NO3− | 220 | 178 | ||
| PO43+ | <408 | 4,795 | ||
| Li+ | <102 | <88 | ||
| Na+ | <410 | 382 | ||
| NH4+ | <815 | <703 | ||
| K+ | <408 | <351 | ||
| Mg2+ | <405 | <349 | ||
| Ca2+ | <2035 | 5,606 | ||
| Carbon type | ug/g by mass | ug/g by mass | ||
| organic carbon | 60,600 | 36,300 | ||
| elemental carbon | 292,500 | 388,000 | ||
| total carbon | 353,100 | 424,300 | ||
| EC/OC ratio | 4.8 | 10.7 | ||
The 20% introduction of soy based fuel appears to only slightly increase the ability of the particle to generate radicals and thereby producing malonaldehyde (the end product) measured by TBARS absorbance. The TBARS assay using B20, ROFA and PDEP relative to water control did not show statistical significance (n=3). The relative fold change of B20 and PDEP indicate 3.30 and 3.46 increase from vehicle control. ROFA was about 5.2 fold increased from water control.
PDEP and B20 were tested for endotoxin contamination which can occur in the original fuel or from improper storage conditions, which can stimulate AM to induce increased PGE2 release. Endotoxin testing resulted with no detectable endotoxins present in both B20 and PDEP particle suspensions.
3.2. In vitro particle exposure of B20 and PDEP can lead to cytotoxicity in rat AMs
Cellular cytotoxicity induced by exposure to B20 and PDEP is equivalent in rat AMs. Results of lactate dehydrogenase (LDH) released into the media after 24h in vitro exposure indicated dose dependent increase of PDEP and B20. However, no statistical significance was detected with PDEP and B20 relative to vehicle (Figure 2). Trypan blue measure of cell death indicated similar cell death results. Trypan blue dye indicated dose dependent response but not particle type dependent.
Figure 2.
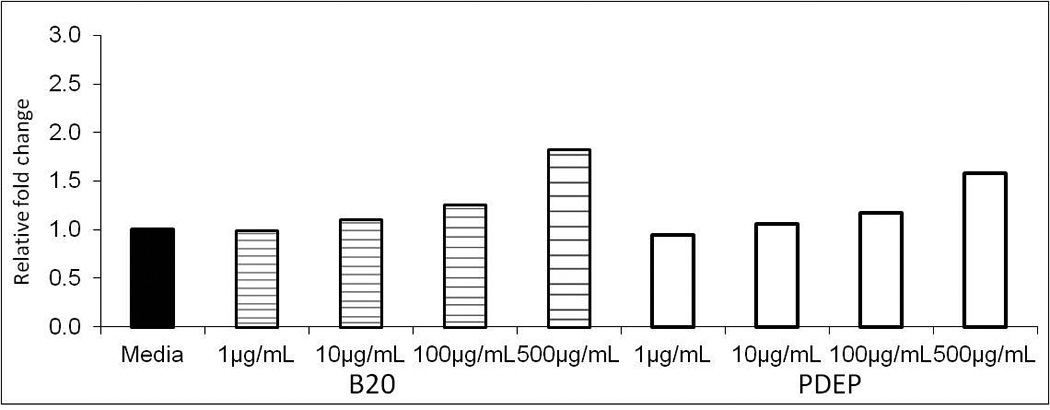
Cell cytotoxicity of AM after 24h exposure to 1–500µg/mL of B20 or PDEP measured by LDH release. Each group represents mean ± SE (n=6). Exposure to B20 or PDEP shows no significant cell toxicity as fold change relative to media control.
3.3. AM respond similarly to B20 and PDEP particles in suspension in regards to cell cytotoxicity
Half a million cells were exposed to particles with final concentrations ranging from 1–500µg/mL in RPMI media. An aliquot of AM were removed after 30 min & 24 h incubation and the sample was diluted for optimal visibility. Cells and particles were fixed and stained for image capture using a light microscope. The particle/cell suspension was photographed in which we found particles located externally on the macrophages. Fragments of B20 particles were located within the AM (Figure 1c). At 1µg/mL (Figure1b) the presence of B20 particle had attracted four macrophages to the particle site however the stain indicates AMs remain intact and alive indicating there was no cytotoxicity due to particle. The LDH assay found no statistically significant difference from all concentrations of B20, PDEP and vehicle control, indicating both particle types have similar cytotoxic response.
Figure 1.
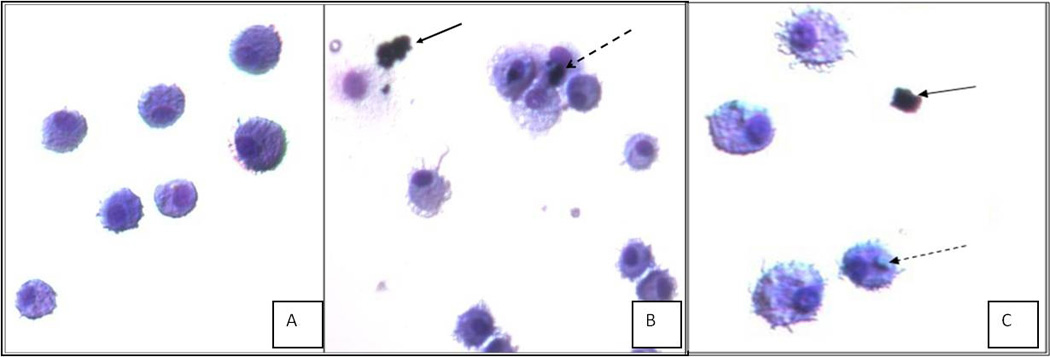
Density of 2×104 WKY AM incubated 24h with (A) vehicle, (B) B20 at 10µg/mL and (C) B20 at 1µg/mL. All images are of fixed and H&E stained cells. Images taken with Nikon eclipse E600 at 20× magnification. Solid arrow (←) points to unbound B20 (◂----) indicates B20 within or attached to the surface of the AM.
3.4. Gene expression studies indicate similar initiation of inflammatory response
Evidence of AM initiating an inflammatory response after particle exposure was collected with elevated gene expression levels of MIP-2 and COX-2 (Figure 3). The results are an average of n=10 per treatment. The mRNA levels of COX-2 after exposure to 1,10 & 100µg/mL of B20 and PDEP show a dose dependent increase. The highest dose 100µg/mL is significantly increased from media in both B20 (p>0.001) and PDEP (p>0.05). Only B20 exposure appears to increase COX-2 mRNA levels gradually with particle concentration, suggesting COX-2 expression is particle type dependent. MIP-2 expression increased in a dose dependent manner in both B20 and PDEP, suggesting MIP-2 expression is concentration dependent and identical with both particle types. PDEP at 100µg/mL increased MIP-2 expression significantly, with over four fold increase from media control. However, not even the highest concentration of B20 was able to increase MIP-2 significantly.
Figure 3.
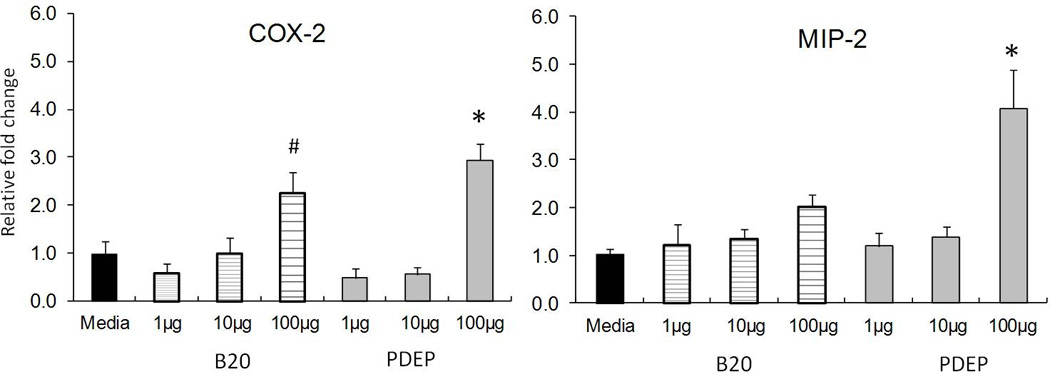
Gene expression of COX-2 and MIP-2 in AM exposed for 24h to B20 and PDEP. COX-2 expression is significantly increased in both B20 and PDEP at 100µg/mL; B20 *P< 0.05 and PDEP #P<0.001. MIP-2 expression with B20 increases with dose but not significant and PDEP at 100µg/mL is statistically significant *P<0.05. Each group represents mean ± SE (n=10).
3.5. PGE2 release depends on particle type and dose
PGE2 release from AM was measured after exposure to B20 and PDEP. The PGE2 release is elevated at low particle concentrations (1 and 10 µg/mL) and decreases with high particle suspension (100 and 500 µg/mL). At 10µg/mL of B20 PGE2 release is significantly increased from media (p≥0.01). B20 exposure increased PGE2 release by 40pg/mL from media control. While PDEP exposure also increases PGE2 release, the values are not statistically significant. Data indicates B20 particle exposure to AMs induced a unique interference which leads to increase PGE2 release. We also observed the PGE2 release by B20 at 10µg/mL is independent of the gene expression levels of COX-2 at 10µg/mL.
3.6. Particle concentration doesn’t interfere with the detection of PGE2
As we previously noted PGE2 levels increased initially at low particle concentrations and then decreased with higher particle concentrations. This observation indicates PGE2 release could be undetected due to particle sequestering i.e. PGE2 was adsorbed onto the carbon core. We tested if B20 particle is comparatively less likely than PDEP to sequester extracellular PGE2 and limit detection. Using radio-labeled PGE2 we conducted a competitive binding assay and identified the two particle types had similar affinity to bind PGE2 at low concentrations (Figure 5). Figure 5 shows disintegration counts per min (DPM) of 3H- PGE2 for each concentration and particle type. As particle concentration increases there is a decrease in the amount of detectable 3H-PGE2 available in the culture media indicating particle sequestering does occur. We initially compared particles suspended in 0% FBS and then with 2.5% (Figure 5). Without 2.5% FBS the 3H-PGE2 values remaining in supernatant were five-fold lower than with 2.5% FBS. At 1µg/mL of B20 with 17,728DPM counts B20 was statistically significant from media control. B20 with 100µg/mL had decreased slightly to 16,759 DMP. PDEP at each particle concentration shows greater counts than B20 (1µg/mL: 19,204DPM; 100µg/mL:17,855DPM; 500µg/mL: 13,080DPM). Additionally at 500µg/mL both PDEP and B20 indicate a statistically significant decrease in 3H- PGE2 from culture media indicating dose dependent sequestering. These findings demonstrate there was particle sequestering of the PGE2 and the results show a difference between B20 and PDEP in their ability to sequester.
Figure 5.
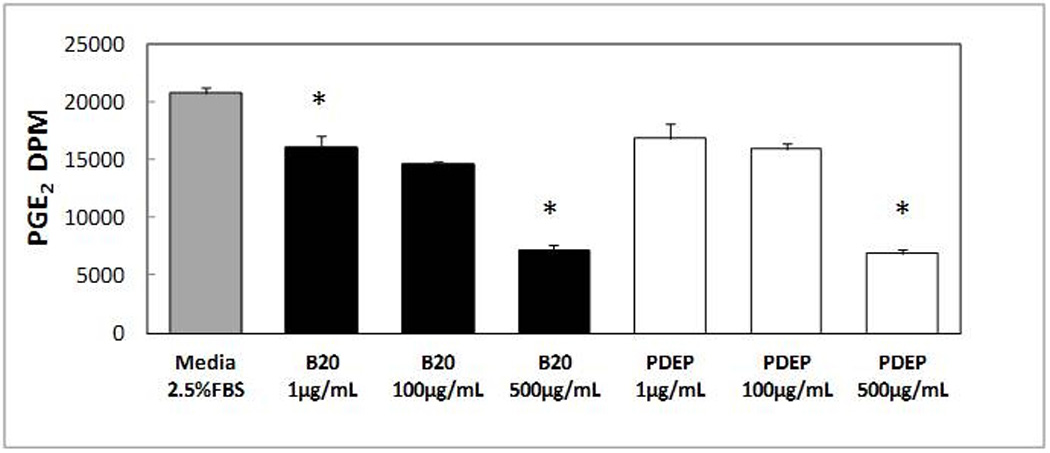
B20 and PDEP binding of hot (3H-PGE2) and cold (PGE2) in a cell free system. Particles were suspended in RPMI media with 2.5% FBS for 24h, particles were centrifuged and supernatant was collected for standard scintillation counting. B20 at both 100µg/mL and 500µg/mL and PDEP at 500µg/mL are statistically significant *P < .001 from media control.
4. Discussion
In our present study we demonstrated for the first time B20 may induce similar cellular disruptions to normal AM function as found previously with petroleum diesel exhaust particles [21–23]. Our experiments with freshly harvested rat AM suggests B20 and PDEP exposure initiate different cellular responses related to inflammatory cytokine (MIP-2) and lipid mediator production (PGE2). Our particle characterization deduced B20 and PDEP vary by only 20% in regards to a few particle bound species however there are still many species not yet studied which can vary greatly therefore it is difficult to speculate a direct effect by a single component. However, we have identified a few characteristics specific for B20 which can aid in deducing particle type dependent PGE2 release and inflammatory response from mature alveolar macrophages with in vitro exposure. Data appears to suggest there are components in exhaust which may interfere with PGE2 release and the data also suggests there
Our observation that Zn, a transition metal, was one component uniquely increased in B20 points to possible oxidative stress led mechanism of toxicity. The oxidative stress may induce the elevated inflammatory signal molecules as observed previously with petroleum diesel combustion particles [24, 25]. Previous research indicates metal content in particles especially Zn, Cu and Fe can cause acute inflammatory response [26]. Brito et al [15] indicate in their study, metal emissions released follow a linear increased directly linked to the amount of petroleum diesel in the original fuel. However our study used low sulfur petroleum diesel which contains very little Zn, suggesting, in our study the Zn source in B20 emissions is not dependent on petroleum fuel. A study recently found B100 contains high levels of Zn prior to combustion [27]. Other studies have found increased levels of transition metals from biodiesel combustion of and karanja oil [20] indicating original fuel type may not be the only source of the transition metals found in emissions. We believe in our study however the soluble fraction of these transition metals alone in B20 are not sufficient to extrapolate oxidative stress signaling responses in AM. Other studies suggest that AM are capable of responding with induction of oxidative stress [28] (measured as chemiluminescence) to very high concentrations of solubilized metals from residual oil fly ash (ROFA); our employed dose and our transition metal content was not sufficient to potentiate the robust inflammatory response seen in B20 exposed AMs. Our study found B20 and PDEP have similar cytokine (MIP-2) and COX-2 expression levels therefore ROS mediated inflammatory downstream signaling isn’t transition metal dependent.
Our study also found elemental carbon varies between the two particle types. Particle composition, specifically, carbon type along with cell type affects the uptake of particles into cells. A previous study compared PDEP to carbon black (primarily elemental carbon) uptake in mouse AM and a lung epithelial cell line [29]. The study found a fivefold increase in uptake of carbon black by the mouse AM compared to lung epithelial cells. Essentially a mouse AM had greater uptake of PDEP vs. carbon black particles because of the lack diversity of particle as opposed to elemental carbon [29]. We can deduce AM phagocytosis was more efficient with organic carbon or with particle diversity. In reference to our study by replacing 20% of PDEP with soy we elevated the EC content of particles and may have decreased particle uptake. Surmising potentially smaller cellular uptake was more potent in the induction of PGE2 release.
Results found in our in vitro exposure study are similar to studies conducted with in vivo particle exposure that show inflammatory potential with biodiesel exhaust exposure. Finch et al [8] indicate in a biodiesel inhalation exposure study, AM contain particles demonstrating this cell type as a ideal target. The study also found increased cell density and protein in lung lavage fluid after exposure [8]. Brito et al [30] also conducted an in vivo study exposure via intratracheal instillation of biodiesel and found excess protein in the BALF as well as increased cell density. Increased phagocytosis can initiate increased inflammatory signals and recruitment of other inflammatory cells mediated by release of cytokines. Elevated MIP-2 levels are produced by macrophages and can suggest alveolitis in rats [31]. Our in vitro studies MIP-2 gene expression was elevated in a dose dependent manner after 24h exposure to B20 and PDEP. Based on our similar findings with our in vitro study we can further predict biodiesel may cause protein influx and recruit neutrophils in a whole animal model.
The results of the present study illustrate in vitro exposure to B20 and PDEP can alter PGE2 release by AM. Possible causes for B20 exposed cells to have more PGE2 detected in the supernatant can include 1) less prostaglandin binding to particle; 2) increased COX-2 gene transcription; and/or 3) alterations in the protein and activity level of COX-2 as well as other enzymes involved in prostaglandin production. Previous research with supernatants from petroleum diesel particle exposed to cells indicates cytokines released from the cell can be absorbed by particles disrupting detection techniques [32]. In our study PGE2 detection was not biased solely on particle binding (adsorption). We found both B20 and PDEP at 100µg/mL have a similar affinity for PGE2 (although B20 was significantly decreased from Media Fig 5). PGE2 loss (for detection) via binding to PM becomes a significant issue at higher concentrations. A lack of an increased release (relative to control cultures) of PGE2 from PDEP-exposed AM can be due to the specificity and activity level of COX-2. Previous research identified PAHs in petroleum diesel extract, specifically phenanthrene, inhibit COX-2 activity in macrophages [33]. Both diesel extract and phenanthrene alone can inhibit PGE2 production in macrophages but the exact mechanism(s) is still unclear. Rudra-Ganguly [33] found PDEP inhibits endotoxin-stimulated PGE2 production in murine macrophages and a cell free (COX-2) enzyme system. These observations are consistent with PDEP and low PGE2 levels in our study suggesting cyclooxygenase activity was disrupted and lead to lower production and release of prostaglandin E2. Similar to our B20 soy combustion particles Ratcliff et al show B20 soy combustion has 20% lower total PAHs including phenanthrene. The data from our study suggests 20% soy incorporation into diesel fuel can likely lead to less PAH emissions and thereby stimulate PGE2 production at the lower B20 concentrations used in our studies.
We conducted experiments to identify if the particle exposure had disrupted the arachidonic acid and cyclooxygenase pathways irreversibly. After particle exposure AM were washed and challenge with LPS at 12.5ng/mL for 4hr. The supernatant was collected and PGE2 release was measured. Results indicated B20 particle-exposed AM after LPS challenge release more PGE2 with higher particle concentrations. The opposite occurred with PDEP exposure, following challenge less PGE2 was measured with cells exposed to higher particle concentrations. The results of LPS challenge indicated COX-2 remains efficient after B20 exposure but not with PDEP. These experiments informed us particle type is as important as particle concentration when challenging cells and measuring the subsequent PGE2 release. Previous results from Mundandhara et al [22] indicated macrophages with LPS challenge would bounce back to normal PGE2 release after diesel exposure. However the Mundandhara paper used different combustion particles and conducted experiments with human AMs. We believe in addition to differ particles, our rat AM size, age and previous environment might have altered the reserve of PGE2 precursors leading to different responses from previous paper. The results suggest rat WKY are more sensitive to particle exposure and sustain irreversible injury to the cyclooxygenase enzymes or to the free arachidonic acid reserve.
Many components have been found in diesel emissions to have biological significance in cells. Analysis of diesel exhaust particles indicates presence of quinones [34], specifically phenanthraquinone which has been identified in generation of ROS and oxidation of sulfhydryl groups. Other diesel exhaust components which have yet been identified to cause a biological consequence are fatty acids. Fatty acid methyl esters (FAMEs) are the building blocks for biodiesel and are suspected to be present in exhaust emissions along with other unique incomplete combustion derived from FAMEs [12]. We believe these two and possibly many other components can disrupt production of PGE2, via initiation of signaling pathways or suppression of normal functions. Free radicals generated by, e.g. quinones, are required for the second step in creating PGG2 a precursor of PGH2. ROS generation is necessary for PGE2 production however with too much ROS lipid peroxide species can inactivate prostaglandins from extracellular release. Additionally cyclooxygenase −2 has a greater affinity for unsaturated fatty acids than arachidonic acid suggesting less arachidonic acid metabolite production in the presence of unsaturated free fatty acids [35]. Additionally B20 appears to have more diverse species in reference to detected levels of ions. We believe there are maybe other components of diesel and biodiesel which can generate still unknown interactions within the cell and modulate prostaglandin production or release.
Macrophages along with other cell types can generate differential inflammatory responses to B20 and PDEP. Jalava et al [36] conducted studies in which they found AM increased apoptosis, genotoxicity, and ROS production with the use of rapeseed methylated ester (RME) particles in a dose dependent manner in vitro [36]. Although cytokines, TNFα and chemokine MIP-2 were elevated from control with RME there was significantly more detected with petroleum diesel fuel combustion particle extract exposure [36]. Rat macrophages were tested for ROS generation and found to increase in RME samples as well as diesel however the response was greater in diesel than RME [36]. Hemmingsen et al [37] conducted in vitro exposures with HUVECs and A549 cells to evaluate the strength of gene expression, ROS and DNA damage responses from PDEP and B50 (as 50% soy and 100% soy) exposures. A direct comparison between PDEP (100%) and B50 generated more than two fold increase in both ICAM-1 and VCAM-1 with PDEP but not with B50. Additionally ROS generation was substantially greater (up to four fold higher) with the PDEP than B50. The level of response by macrophages after B20 and PDEP exposure was similar in both endothelial cells and epithelial cells. The data comprehensively indicates fuel type and particle composition can produce different responses in several cell types.
In conclusion our results indicate exposure to B20 will cause a similar inflammatory response as mediated by PDEP with reference to gene expression of MIP-2. COX-2 the enzyme which produces prostaglandins increases with both PDEP and B20 however PGE2 release is significantly increased with only B20 and at low particle concentrations. Our data suggests particle composition is crucial to the AM PGE2 release. In order to better identify the exact component of exhaust which causes cellular inflammatory signals we need more detailed chemical composition analysis. Independent composition analysis of B20 products and their consequential interactions with rat AM need to be conducted as a means to explain the difference in the response.
Figure 4.
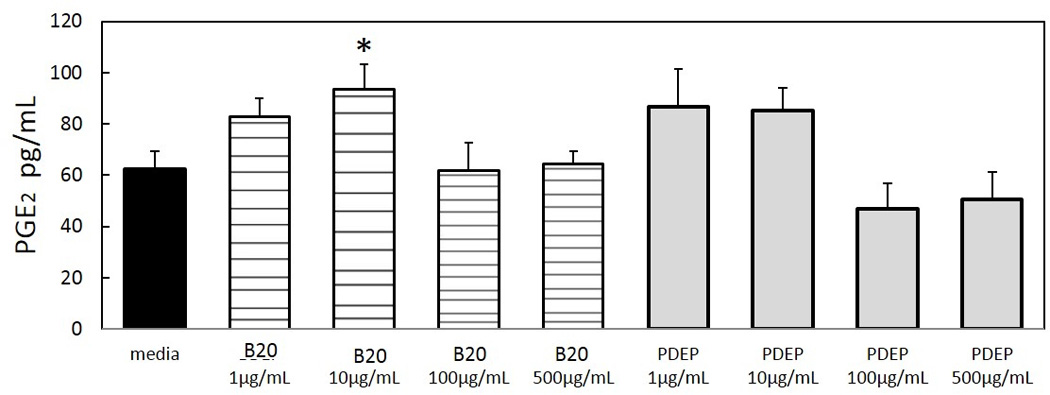
AM PGE2 release following 24h exposure to PDEP and B20 was quantified by ELISA. Each group represents mean ± SE of 3–12 animals. B20 concentration of 10µg/mL is statistically significant with reference to media control *P<0.05.
Highlights.
Petroleum diesel and biodiesel exhaust particle composition varies by species
Macrophage exposure to exhaust particles results in prostaglandin production/ release changes
Biodiesel exposure induced increased prostaglandin release compared to same dose of petroleum
Detection of prostaglandin release not inhibited by particle sequestering
Macrophage inflammation initiating pathways correlate in response to dose not particle type
Acknowledgments
We would like to thank Drs. Haiyan Tong and Weidong Wu for discussions regarding the manuscript.
Funding Support
Supported in part by NIEHS Toxicology Training Grant, T32 ES007126 and UNC-EPA Training Agreement CR833237.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Publisher's Disclaimer: Disclaimer
The research described in this article has been reviewed by the National Health and Environmental Effects Research Laboratory, US Environmental Protection Agency and approved for publication. Approval does not signify that the contents necessarily reflect the views and policies of the Agency nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
References
- 1.Yang H-M, et al. Effects of Diesel Exhaust Particles on the Release of Interleukin-1 and Tumor Necrosis Factor-Alpha from Rat Alveolar Macrophages. Experimental Lung Research. 1997;23(3):269–284. doi: 10.3109/01902149709087372. [DOI] [PubMed] [Google Scholar]
- 2.Chen S, Weller MA, Barnhart MI. Effects of Diesel engine exhaust on pulmonary alveolar macrophages. Scanning Electron Microscopy. 1980;(3):327–338. [PubMed] [Google Scholar]
- 3.Becker S, et al. Response of Human alveolar macrophages to ultrafine, fine, and coarse urban air pollution particles. Experimental Lung Research. 2003;29:29–44. doi: 10.1080/01902140303762. [DOI] [PubMed] [Google Scholar]
- 4.Rudell B, et al. Bronchoalveolar inflammation after exposure to diesel exhaust: comparison between unfiltered and particle trap filtered exhaust. Occup Envrion Med. 1999;56:527–534. doi: 10.1136/oem.56.8.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schneider JC, et al. Air Pollution Particulate SRM 1648 Causes Oxidative Stress in RAW 264.7 Macrophages Leading to Production of Prostaglandin E2, a Potential Th2 Mediator. Inhalation Toxicology. 2005;17(14):871–877. doi: 10.1080/08958370500244498. [DOI] [PubMed] [Google Scholar]
- 6.Thomas P, et al. Altered human monocyte/macrophage function after exposure to diesel exhaust particles. Environ Sci & Pollut Res. 1995;2(2):69–72. doi: 10.1007/BF02986719. [DOI] [PubMed] [Google Scholar]
- 7.Hofer TPJ, et al. Diesel exhaust particles increase LPS-stimulated COX-2 expression and PGE2 production in human monocytes. Journal of Leukocyte Biology. 2004;75(5):856–864. doi: 10.1189/jlb.0803387. [DOI] [PubMed] [Google Scholar]
- 8.Finch GL, et al. Effects of subchronic inhalation exposure of rats to emissions from a diesel engine burning soybean oil-derived biodiesel fuel. Inhal Toxicol. 2002;14(10):1017–1048. doi: 10.1080/08958370290084764. [DOI] [PubMed] [Google Scholar]
- 9.Tzamkiozis T, et al. Monitoring the inflammatory potential of exhaust particles from passenger cars in mice. Inhalation Toxicology. 2010;22(S2):59–69. doi: 10.3109/08958378.2010.519408. [DOI] [PubMed] [Google Scholar]
- 10.Swanson KJ, et al. Release of the pro-inflammatory markers by BEAS-2B cells following in vitro exposure to biodiesel extracts. Open Toxicology Journal. 2009;3:8–15. [Google Scholar]
- 11.McCormick RL. The Impact of biodiesel on pollutant emissions and public health. Inhal Toxicol. 2007;19(12):1033–1039. doi: 10.1080/08958370701533509. [DOI] [PubMed] [Google Scholar]
- 12.Ratcliff M, et al. Diesel particle filter and fuel effects on heavy duty diesel engine emissions. Environmental Science and Technology. 2010;44(21):8343–8349. doi: 10.1021/es1008032. [DOI] [PubMed] [Google Scholar]
- 13.Jalava PI, et al. Toxicological properties of emission particles from heavy duty engines powered by conventional and bio-based diesel fuels and compressed natural gas. Particle and fibre toxicology. 2012;9(1):37. doi: 10.1186/1743-8977-9-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsai J, et al. PM, carbon and PAH emissions from a diesel generator fuelled with soy-biodiesel blend. Journal of Hazardous Materials. 2010;179:237–243. doi: 10.1016/j.jhazmat.2010.02.085. [DOI] [PubMed] [Google Scholar]
- 15.Brito JM, et al. Acute Cardiovascular and Inflammatory Toxicity Induced by Inhalation of Diesel and Biodiesel Exhaust Particles. Toxicological Sciences. 2010;116(1):67–78. doi: 10.1093/toxsci/kfq107. [DOI] [PubMed] [Google Scholar]
- 16.Williams A, et al. Effect of biodiesel blends on diesel particulate filter performance. SAE. 2006 (Technical Paper No. 2006-01-3280) [Google Scholar]
- 17.Birch M, Cary R. Elemental Carbon-Based Method for Monitoring Occupational Exposures to Particulate Diesel Exhaust. Aerosol Science and Technology. 1996;25:221–241. doi: 10.1039/an9962101183. [DOI] [PubMed] [Google Scholar]
- 18.Molinelli AR, et al. Effect of metal removal on the toxicity of airborne particulate matter from the Utah Valley. Inhal Toxicol. 2002;14(10):1069–1089. doi: 10.1080/08958370290084737. [DOI] [PubMed] [Google Scholar]
- 19.Shannahan J, et al. Pulmonary oxidative stress, inflammation and dysregulated iron homeostasis in rat models of cardiovascular disease. journal of toxicology and environmental health part A. 2010;73(10):641–656. doi: 10.1080/15287390903578208. [DOI] [PubMed] [Google Scholar]
- 20.Gangwar J, et al. Emissions from diesel versus biodiesel fuel used in a CRDI SUV engine: PM mass and chemical composition. Inhal Toxicol. 2011;23(8):449–458. doi: 10.3109/08958378.2011.582189. [DOI] [PubMed] [Google Scholar]
- 21.Ahn E, et al. Cox-2 expression and inflammatory effectrs by diesel exhaust particles in vitro and in vivo. Toxicology letters. 2008;176:178–187. doi: 10.1016/j.toxlet.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 22.Mundandhara SD, Becker S, Madden MC. Effects of diesel exhaust particles on human alveolar macrophage ability to secrete inflammatory mediators in response to lipopolysaccharide. Toxicol In Vitro. 2006;20(5):614–624. doi: 10.1016/j.tiv.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 23.Hofer TP, et al. Diesel exhaust particles increase LPS-stimulated COX-2 expression and PGE2 production in human monocytes. J Leukoc Biol. 2004;75(5):856–864. doi: 10.1189/jlb.0803387. [DOI] [PubMed] [Google Scholar]
- 24.DiStefano E, et al. Determination of metal-based hydroxyl radical generating capacity of ambient and diesel exhaust particles. Inhalation Toxicology. 2009;21(9):731–738. doi: 10.1080/08958370802491433. [DOI] [PubMed] [Google Scholar]
- 25.Ning Li, et al. Ultrafine Particulate Pollutants Induce Oxidative Stress and Mitochondrial Damage. Environ Health Perspect. 2003;111(4):455–460. doi: 10.1289/ehp.6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wallenborn JG, et al. Differential pulmonary and cardiac effects of pulmonary exposure to a panel of particulate matter -assocated metals. Toxicology and Applied Pharmacology. 2009;241:71–80. doi: 10.1016/j.taap.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 27.Betha R, Balasubramanian R. Emissions of particulate -bound elements from biodiesel and ultra low sulfur diesel: size distribution and risk assessment. Chemosphere. 2013;90:1005–1015. doi: 10.1016/j.chemosphere.2012.07.052. [DOI] [PubMed] [Google Scholar]
- 28.Becker S, Soukup J, Gallagher J. Differential particulate air pollution induced oxidant stress in human granulocytes, monocytes and alveolar macrophages. Toxicology In Vitro. 2002;16(3):209–218. doi: 10.1016/s0887-2333(02)00015-2. [DOI] [PubMed] [Google Scholar]
- 29.Saxena RK, Gilmour IM, Hays MD. Isolation and quantitative estimation of diesel exhaust and carbon black particles ingested by lung eptheilal cells and alveolar macrophages in vitro. BioTechniques. 2008;44:799–805. doi: 10.2144/000112754. [DOI] [PubMed] [Google Scholar]
- 30.Samet JM, et al. Fine particulate air pollution and mortality in 20 U.S. cities, 1987–1994. The new England Journal of Medicine. 2000;343(24):1742–1749. doi: 10.1056/NEJM200012143432401. [DOI] [PubMed] [Google Scholar]
- 31.Kawajiri T, et al. Pathology and Mechanism of Lung Toxicity Following Inhalation of Hair Spray in Rats. Inhalation Toxicology. 2004;16(2):147–153. doi: 10.1080/08958370490270954. [DOI] [PubMed] [Google Scholar]
- 32.Akhtar US, et al. Cytotoxic and proinflammatory effects of ambient and source-related particulate matter (PM) in relation to the production of reactive oxygen species (ROS) and cytokine adsorption by particles. Inhal Toxicol. 2010;22(Suppl 2):37–47. doi: 10.3109/08958378.2010.518377. [DOI] [PubMed] [Google Scholar]
- 33.Ganguly NR, et al. Diesel exhaust particle extracts and associated polycyclic aromatic hydrocarbons inhibit Cox-2 dependent prostaglandin synthesis in murine macrophages and fibroblasts. The Journal of Biological Chemistry. 2002;227(42):39259–39265. doi: 10.1074/jbc.M110215200. [DOI] [PubMed] [Google Scholar]
- 34.Kumagaia Y, et al. Oxidation of proximal protein sulfhydryls by phenanthraquinone, a component of diesel exhaust particles. Chem Res Toxicol. 2002;15:483–489. doi: 10.1021/tx0100993. [DOI] [PubMed] [Google Scholar]
- 35.Hwang D. Modulation of the expression of cyclooxygenase −2 by fatty acids mediated through Toll-llike receptor 4 derived signaling pathways. FASEB J. 2001;15:2556–2564. doi: 10.1096/fj.01-0432com. [DOI] [PubMed] [Google Scholar]
- 36.Jalava PI, et al. Toxicological effects of emission particles from fossil- and biodiese-fueled diesel engine with and without DOC/POC catalytic converter. Inhalation Toxicology. 2010;22(S2):48–58. doi: 10.3109/08958378.2010.519009. [DOI] [PubMed] [Google Scholar]
- 37.Hemmingsen JG, et al. Oxidative stress, genotoxicity, and vascular cell adhesion molecule expression in cells exposed to particulate matter from combustion of conventional diesel and methyl ester biodiesel blends. Environmental Science and Technology. 2011;45(19):8545–8551. doi: 10.1021/es200956p. [DOI] [PubMed] [Google Scholar]


