Abstract
A phase l study using intravesical Ad-IFNα/Syn3 for patients with BCG resistant superficial bladder cancer showed a complete remission (CR) of 43% at 90 days after treatment with high levels of IFNα being produced. Ad-IFNα kills bladder cancer cells by two apoptotic and one necrotic mechanism that can be measured by soluble forms of cytokeratin 18 (CK 18) using M30 and M65 ELISAs, assays for caspase –cleaved (apoptotic) and uncleaved (necrotic) cell death, respectively. Therefore we determined whether M30 and M65 levels in the urine after treatment could document all three mechanisms of cancer cell kill and also predict having a CR. High levels of both M30 and M65 were found in all patients within 24 hours after treatment with all three types of cancer cell death occuring. Moreover, the return of both M30 and M65 levels in the urine to normal levels within 5 days or more after treatment was strongly associated with obtaining a CR (p=0.003). This is the first time that such assays have been used to study response to therapy in the urine of patients with bladder cancer and in the future may prove valuable in predicting clinical outcome.
Keywords: Adenoviral-mediated interferon α treatment, urine cytokeratin 18 levels, bladder cancer, Phase l study
Introduction
Our phase l study of intravesical Ad-IFNα/Syn3 treatment in patients with non-muscle invasive bladder cancer previously failing BCG (bacillus Calmette-Guérin) therapy has been completed (1). The Syn3 is an excipient Syn3 that facilitates effective gene transfer and expression of IFNα within the urothelium and tumor with subsequent secretion into the urine (2,3). In the phase l study only a single instillation of Ad-IFNα/Syn3 was given. A complete remission (CR) as defined by no evidence of tumor and negative biopsies by cystoscopy 90 days after treatment was found in 6 of the 14 patients treated who produced IFNα in their urine (1).
Three distinct mechanisms of cancer cell death caused by Ad-IFNα have been identified by us (4–9). The IFNα produces a tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) - mediated cancer cell kill (4) whereas cancer cells which are resistant to the interferon α protein are killed by Ad-IFNα in two additional ways, one indirect (apoptotic) and the other direct (necrotic). The indirect mechanism involves a potent soluble bystander factor(s) secreted by both Ad-IFNα infected tumor and normal urothelial cells which is strongly cytotoxic to all tumor types tested to date, including bladder cancer, but is not cytotoxic to normal cells (5,6). The direct cancer cell kill occurs from the high level IFNα accumulation found perinuclearly after Ad-IFNα treatment which causes ER stress related cancer cell death (7).
Levels of cytokeratins, especially those which detect both caspase-cleaved and intact cytokeratin 18 (CK 18), have been used as biomarkers of tumor cell kill measured in the blood after chemotherapeutic treatment (10,11). The M30 antibody recognizes a CK18 fragment that is produced following caspase cleavage of the CK18 protein and is thought to be a selective biomarker for apoptotic cell death, whereas the M65 ELISA uses the M5 antibody thought to detect both the uncleaved and cleaved CK18 protein fragments. By subtracting the concentration of cleaved CK18 obtained using the M30 ELISA (M30 increase) from the level obtained in the M65 ELISA, the necrotic component of cell death is determined (M65 increase).
Both the TRAIL and bystander related cancer cell apoptosis produced by Ad-IFNα can be measured by increases in M30, whereas the endoplasmic stress related direct cancer cell death is mostly necrotic and can be measured by an increase in M65 (7).
In this report patients from the Phase l trial were studied. Changes in urine M30 and M65 levels as well as IFN and TRAIL levels were examined at various times after intravesical Ad-IFNα /Syn3 treatment. We hypothesized that increases in M30 and M65 level would reflect the mechanisms of tumor cell death produced by the Ad-IFNα and that changes in M30 and M65 levels might be predictive of the CRs obtained.
Material and Methods
Urine Samples
The urine samples were collected on ice after treatment and were stabilized by the addition of a buffer containing 10% bovine serum albumin and 50 mM HEPES (4-[2-hydroxyethyl]-1-piperazineethanesulfonic). 1ml of buffer was added to 10 ml of urine. After centrifugation urine was then stored at −80° C until it was thawed to conduct the various assays. Both early morning samples brought by the patient and a freshly voided urine was collected approximately every 24 hours after treatment.
ELISA M30, M65 and TRAIL assays
M30-Apoptosense and M65 ELISA kits were obtained from Peviva AB (Bromma, Sweden,) and used according to manufacturer protocol. Briefly, 25µl of each urine sample were tested in duplicate or triplicate. 75µl of the combined monoclonal M30 antibody (anti-CK18 Asp 396 neoepitope) conjugated with horseradish peroxidase substrate and the conjugate dilution buffer was added to each well for the M30-Apoptosense ELISA and mouse monoclonal M5 antibody for the M65 ELISA. Incubation times were 4h and 2h for the M30 and M65 ELISAs, respectively. After washing the plate, 200µl of tetramethylbenzidine substrate solution was added to each well followed by stop solution (1M sulfuric acid) after 20 minutes. Absorbance was read in a microplate reader at 450nm. Samples were diluted, as necessary, for the absorbance (450nm) to fall within the range of the standard curve. TRAIL protein levels were determined using the TRAIL ELISA kit purchased from R&D Systems Inc., Minneapolis, MN according to the manufacturer’s instructions (4). All results were given in units (U/L).
Determination of IFNα levels
IFNα levels were determined by ELISA from R&D Systems Inc., Minneapolis, MN utilizing the manufacturer protocol with the extended range standard curve. Briefly, 100µl of each urine sample and standards were tested in duplicate or triplicate. After one hour incubation and plate washing, 100µl of HRP substrate was added to each well. After incubation for 1h and washing the plate, 100µl TMP substrate solution was added to each well followed by stop solution. Absorbance was read in a microplate reader at 450nm. Samples were diluted, as necessary, for the absorbance (450nm) to fall within the range of the standard curve. Final results were given as pg/ml.
Biostatistical Analysis
Patients were analyzed to determine if M30 and /or M65 levels could be correlated with achieving a CR. Each patient was classified as having M30 levels which returned to normal levels if it reached a level below 175 U/L by day 10 or day 14 after treatment when the last urine sample was obtained for a given patient. Similarly patients were classified as having M65 return to normal levels using the same threshold. These were the levels seen in the normal control urine A Fisher’s exact test was used to compare patients with and without CR with respect to M30 and M65 returning to normal levels.
Results
Urine IFN Levels
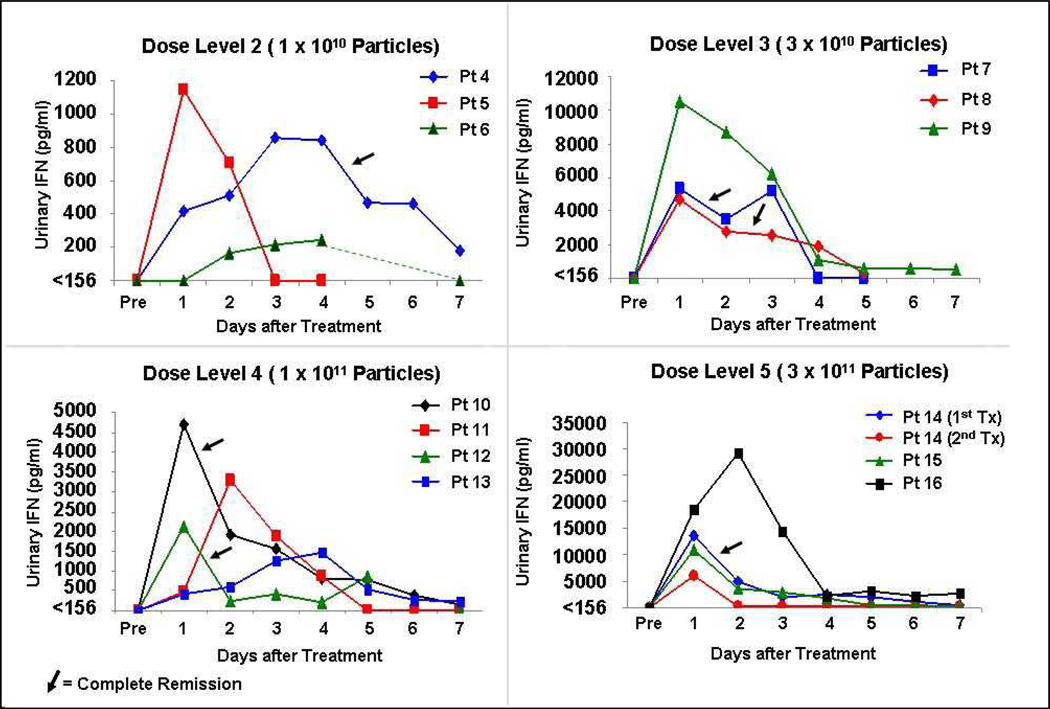
The phase l study design provided for a single intravesical instillation of Ad-IFNα (75 ml in 1mg/ml Syn3) with re-treatment if a complete remission (CR) was achieved at 90 days. High and prolonged dose-dependent urine IFN levels were observed in all dose levels of 1×1010 particles/mL and above with the top concentration of 3×1011 particles/mL producing the most increase in urine IFN levels which in certain patients were measurable up to 7 days or more (Fig.1). The patients who achieved a CR are shown by arrows. The difference in scale of IFN levels at each dose level should be noted. Additionally, a significant IFN urine level was measured in Pt.14 retreated 90 days after his initial treatment at the 24-hr time-point (6082 pg/ml). Six of 14 patients (43%) receiving doses that produced measurable IFNα in the urine achieved a CR at 3 months after treatment.
Fig.1.
Urine IFNα levels are shown in patients at various times after intravesical treatment with Ad-IFNα/Syn3 at different dose levels. Pt 14 at dose level 5 also had high urine levels of IFNα (>6000 pg/ml) after a second treatment 90 days later. The arrows indicate patients who achieved a complete remission (CR). Please note difference in scale of the Y axis in the 4 graphs. No significant urine IFNα levels were measured at dose level 1 (1×109 particles/mL).
M30, M65 and TRAIL Levels Indicate That All Mechanisms of Ad-IFNα Produced Cell Death Occur in a Clinical Setting
Increases in both M30 and M65 were found following all 14 patients in whom increases in IFN were also seen (all patients shown in Fig.1). Representative examples of the changes found in M30 an M65 levels over time after treatment as well as TRAIL and IFNα levels are shown in Table 1. Patient # 7, who achieved a CR, had high M30 and M65 levels in his urine one day after treatment which returned to normal levels by day 4. He also had high TRAIL levels indicating that the tumor was IFNα sensitive with considerable tumor cell kill occurring because of the high and prolonged IFNα levels produced in the urine. This mechanism of tumor cell kill was likely reflected by the M30 levels in which the TRAIL related tumor cell kill cannot be distinguished in this case from the bystander produced tumor cell kill since both mechanisms cause apoptotic cell death. The initial large increase in M65 levels seen in this patient is likely the result of the direct mechanism of Ad-IFNα produced cell death, namely necrotic cancer cell kill following Ad-IFNα infection and expression in a significant portion of the tumor cells.
Table 1.
The M30, M65, TRAIL, and IFN levels in the urine are shown for patients #7, #9, and #14 at various times after treatment. The M30 and M65 levels that returned to normal levels after treatment are shown in bold for patients #7 and #14 both of whom achieved a CR. M30, M65 and TRAIL were given in U/L and IFN as pg/ml.
| Pt #7 (CR) | Days After TX | M30 | M65 | TRAIL | IFN Levels |
| 1 | 553 | 7644 | 156 | 5330 | |
| 2 | 420 | 518 | 164 | 3511 | |
| 3 | 116 | 61 | 44 | 5228 | |
| 4 | 75 | 162 | 59 | <156 | |
| Pt #9 | 1 | 697 | 8637 | 336 | 10568 |
| 2 | 320 | 876 | 135 | 8699 | |
| 3 | 271 | 915 | 65 | 6248 | |
| 4 | 121 | 682 | 32 | 1122 | |
| 5 | 169 | 448 | 21 | 558 | |
| 6 | 1127 | 395 | 17 | 553 | |
| 7 | 190 | 1252 | - | 508 | |
| 10 | 1797 | 3471 | 162 | <156 | |
| 14 | 523 | 1304 | 41 | <156 | |
| Pt #14 (CR) | 1 | 155 | 8762 | 7 | 13544 |
| 2 | 93 | 119 | 3 | 4951 | |
| 3 | 429 | 344 | 0 | 3997 | |
| 4 | 340 | 804 | 2 | 2468 | |
| 5 | 587 | 516 | 0 | 2097 | |
| 6 | 228 | 323 | 1 | 998 | |
| 7 | 361 | 282 | 0 | 1543 | |
| 10 | 122 | 156 | 0 | 334 | |
| 14 | 71 | 126 | 0 | <156 |
In contrast, patient #14, who also achieved a CR, now maintained for more than 2 years, produced no TRAIL in his urine. Therefore his tumor likely was IFN resistant and the increase seen in the urine M30 levels was likely the result of bystander factor caused cancer cell kill and was substantial. This patient had never responded to any prior intravesical therapy and would have been directed to cystectomy if not for serious co-morbidities. Again both M30 and M65 returned to normal by 10 days after treatment.
In contrast Patient # 9 did not achieve a CR. This patient had very extensive disease within the bladder at the time of treatment with Ad-IFNα/Syn3. After therapy significant levels of urine IFNα, TRAIL, M30, and M65 were obtained but the M30 and M65 levels remained high for the 14 days after treatment that urine samples were obtained. These results suggest that much tumor cell kill occurred in this patient from all three mechanisms of Ad-IFNα–produced cancer cell kill and that M30 and M65 never reached a normal level because of the extensive and persisting tumor burden present.
Correlation Between M30 and M65 Returning to Normal Levels in the Urine After Ad-IFNα Treatment and Achieving a CR at 90 days
In this phase I study 6 of 14 patients (43%) achieved a CR. All 6 patients with a CR (100%) had M30 levels in their urine that returned to normal (< 175 U/L) at day 5 or later, while 2 of 8 (25.0%) patients who did not achieve a CR had M30 levels that returned to normal levels (p=0.010). In addition five of 6 patients with a CR (83%) had M65 levels which returned to normal (< 175 U/L) at day 5 or later, while none of the 8 patients without a CR (0%) had M65 levels which returned to normal (p=0.003). Similarly, 5 of 6 patients with a CR (83.3%) had both M30 and M65 levels which returned to normal levels at day 5 or later, while none of the 8 patients without a CR (0%) had both M65 and M30 levels return to normal (p=0.003).
Discussion
Our phase l study using intravesical Ad-IFNα /Syn3 for the treatment of patients with BCG refractory nonmuscle invasive bladder cancer resulted in high and prolonged urine IFNα levels. In addition 43% of the patients with measurable urine IFNα levels achieved a CR at 90 days after treatment (1). Using ELISA assays to examine the levels of both caspase-cleaved (M30) and intact (M65) CK18 levels in the strong evidence was provided that significant apoptosis and necrosis occurred in each of the patient’s tumors. We believe that this represented all three mechanisms of Ad-IFNα - produced cancer cell death (9). The increases in M65 levels were likely from the direct tumor cell kill caused by the significant gene transfer and expression of the Ad-IFNα in many cancer cells resulting in necrotic cancer cell death, whereas the high levels of M30 observed were the result of apoptosis from both TRAIL and bystander - mediated mechanisms of Ad-IFNα - produced cancer cell death (9). In those patients in which significant TRAIL urine levels were seen, much of the increase in M30 levels likely was indicative of TRAIL-mediated cancer cell death caused by the high and prolonged IFNα levels produced in IFNα sensitive tumors such as in patient #7 (Table 1). In contrast the tumors in patients showing little or no TRAIL levels in their urine after Ad-IFNα treatment but high M30 levels most likely were IFNα resistant and the M30 increase seen reflected primarily bystander tumor cell death such as that seen in patient #14 (Table 1).
Since the urine high M30 and M65 levels determined within 24 hours after treatment appeared to indicate that significant tumor kill occurred in all patients we thought it would be prudent to examine whether return to normal levels in the urine over time might correlate with a better prognosis. This was indeed the case. All six patients with a CR at day 90 the M30 levels returned to normal by day 5 or later whereas this occurred in only two of eight patients who did not achieve a CR (p=0.010). Moreover five of 6 patients with a CR had M65 levels which returned to normal while none of the eight patients without a CR had M65 levels which returned to normal (p=0.003). To our knowledge this study is the first to suggest that examining M30 and M65 levels in the urine might be used as a potential surrogate biomarker of tumor cell kill and prognosis after treatment of non-muscle invasive bladder cancer with any therapeutic agent.
Although this analysis was done retrospectively it indicates that measurement of M30 and M65 levels in urine after intravesical Ad-IFNα /Syn3 treatment, particularly return to normal levels might be useful as a prognostic indicator of attaining a CR at 90 days. Such studies may therefore have clinical value in future trials. Consequently these determinations will be done in a phase lB trial planned in which intravesical Ad-IFNα /Syn3 treatment will be given on day 1 and again on day 4 to examine whether or not even higher and more prolonged urine IFNα levels can be achieved as well as whether an increase in the number of CRs can be obtained. Finally measurement of M30 and M65 levels in urine might be useful to follow tumor cell kill for other clinical studies in which tumor in the bladder is present.
Acknowledgments
Supported by a GU SPORE in Bladder Cancer (P50 CA91846).
References
- 1.Dinney CP, Fisher MB, Navai N, O'Donnell MA, Cutler D, Abraham A, et al. Phase I trial of intravesical recombinant adenovirus-mediated interferon-α2b formulated in syn3 for BCG failures in non-muscle-invasive bladder cancer. J Urol. 2013 Mar 15; doi: 10.1016/j.juro.2013.03.030. e-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benedict WF, Tao Z, Kim CS, Zhang X, Zhou JH, Adam L, et al. Intravesical Ad-IFN alpha causes marked regression of human bladder cancer growing orthotopically in nude mice and overcomes resistance to IFN-alpha protein. Mol Ther. 2004;10(3):525–532. doi: 10.1016/j.ymthe.2004.05.027. [DOI] [PubMed] [Google Scholar]
- 3.Tao Z, Connor RJ, Ashoori F, Dinney CP, Munsell M, Philopena JA, Benedict WF. Efficacy of a single intravesical treatment with Ad-IFN/Syn 3 is dependent on dose and urine IFN concentration obtained:implications for clinical investigation. Cancer Gene Therapy. 2006;13(2):125–130. doi: 10.1038/sj.cgt.7700865. [DOI] [PubMed] [Google Scholar]
- 4.Papageorgiou A, Lashinger L, Millikan R, Grossman HB, Benedict WF, Dinney CP, McConkey DJ. Role of tumor necrosis factor-related apoptosis-inducing ligand in interferon-induced apoptosis in human bladder cancer cells. Cancer Res. 2004;64(24):8973–8979. doi: 10.1158/0008-5472.CAN-04-1909. [DOI] [PubMed] [Google Scholar]
- 5.Zhang X-Q, Yang Z, Dong L, Papageorgiou A, McConkey D, Benedict WF. Adenoviral- mediated interferon α overcomes resistance to the interferon protein in various cancer types and has marked bystander effects. Cancer Gene Therapy. 2007;14:241–250. doi: 10.1038/sj.cgt.7701011. [DOI] [PubMed] [Google Scholar]
- 6.Zhang X-Q, Dong L, Chapman E, Benedict WF. Conditioned medium from Ad-IFN-alpha-infected bladder cancer and normal urothelial cells is cytotoxic to cancer cells but not normal cells: further evidence for a strong bystander effect. Cancer Gene Therapy. 2008;12:817–822. doi: 10.1038/cgt.2008.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang X-Q, Yang Z, Benedict WF. Direct gene transfer of adenoviral-mediated interferon α into human bladder cancer cells but not the bystander factors produced induces endoplasmic reticulum stress-related related cytotoxicity. Cancer Gene Therapy. 2011;18(4):260–264. doi: 10.1038/cgt.2010.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhibo Y, Zhang XQ, Dinney CNP, Benedict WF. Direct cytotoxicity produced by adenoviral-mediated interferon α infection in interferon resistant cancer cells Involves ER stress and caspase 4 activation. Cancer Gene Therapy. 2011;18(9):609–616. doi: 10.1038/cgt.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fisher MB, Zhang X-Q, McConkey DJ, Benedict WF. Measuring soluble forms of extracellular cytokeratin 18 identifies both apoptotic and necrotic mechanisms of cell death produced by adenoviral-mediated interferon alpha: possible use as a surrogate marker. Cancer Gene Therapy. 2009;16(7):567–572. doi: 10.1038/cgt.2009.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Linder S. Cytokeratin Markers come of age. Tumor Biol. 2007;28:189–195. doi: 10.1159/000107582. [DOI] [PubMed] [Google Scholar]
- 11.de Hass EC, di Pietro A, Simpson KL, Meijer C, Suurmeijer AJH, Lancashire LJ, et al. Clinical evaluation of M30 and M65 ELISA cell death assays as circulating biomarkers in a drug – sensitive tumor, testicular cancer. Neoplasia. 2008;10:1041–1048. doi: 10.1593/neo.08620. [DOI] [PMC free article] [PubMed] [Google Scholar]