Summary
Noise induced hearing loss (NIHL) is a debilitating sensory impairment affecting 10–15% of the population caused primarily through damage to the sensory hair cells or to the auditory neurons. Once lost, these never regenerate [1] and no effective drugs are available [2, 3]. Emerging evidence point towards an important contribution of synaptic ribbons in the long-term coupling of the inner hair cell and afferent neuron synapse to maintain hearing [4]. Here we show in mice (nocturnal) that night noise overexposure triggers permanent hearing loss whereas mice overexposed during the day recover to normal auditory thresholds. In view of this time-dependent sensitivity, we identified a self-sustained circadian rhythm in the isolated cochlea, as evidenced by circadian expression of clock genes and ample PERIOD2::LUCIFERASE oscillations, originating mainly from the primary auditory neurons and hair cells. The transcripts of the otoprotecting brain-derived neurotrophic factor (BDNF) showed higher levels in response to day noise versus night noise, suggesting that BDNF-mediated signaling regulates noise sensitivity throughout the day. Administration of an agonist of the selective BDNF receptor TrkB in the night protected the inner hair cell’s synaptic ribbons and subsequent full recovery of hearing thresholds after night noise overexposure. The TrkB agonist shifted the phase and boosted the amplitude of circadian rhythms in the isolated cochlea. These findings highlight the coupling of circadian rhythmicity and TrkB receptor for the successful prevention and treatment of NIHL.
Keywords: acoustic trauma, circadian rhythms, clock genes, cochlea, hearing loss, neurotrophins, noise, Period 2, TrkB
RESULTS
Diurnal sensitivity in noise overexposure and its effects on the auditory clock
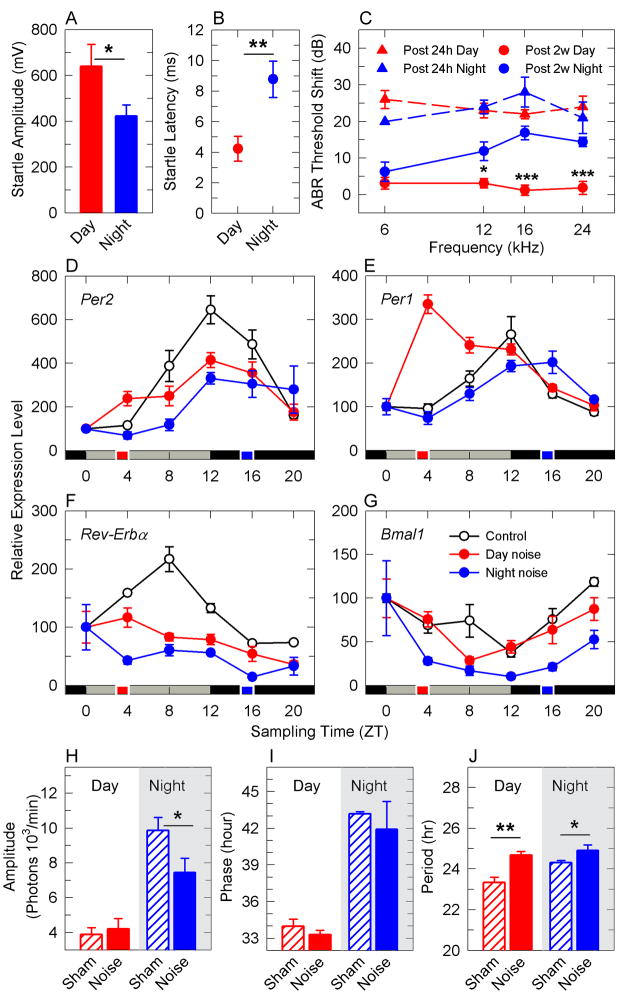
To evaluate whether the auditory clock would play a role in hearing functions in the non-anesthetized animal, CBA male mice were exposed to an acoustic startle paradigm during the inactive phase (9 am, ZT 3) or the active phase (9 pm, ZT 15). Acoustic startle amplitude was lower (p = 0.044, Figure 1A) and latency longer (p = 0.001, Figure 1B) in animals tested during the active phase than during the inactive phase. We next challenged awake mice to a noise trauma (6–12 kHz broadband noise of 100 dB SPL for 1 hour) during the inactive or active phase. This noise trauma is known to cause a temporary damage characterized by the synaptic swelling of the auditory neuron dendrites [5]. We estimated auditory brainstem response threshold shifts (ABRs) either at 24 h or 2 weeks post-exposure. Similar threshold shifts were found 24 h post noise exposure for both day and night groups. While the recovery was complete for the day group at 2 weeks post-trauma (Figure 1C), the night group showed permanent threshold shifts (higher sound levels required to elicit a response) (p<0.001) in absence of hair cell loss (data not shown). These findings illustrate differences in recovery rate depending on the time of the day the noise trauma is delivered.
Figure 1. Noise trauma affects the cochlear clock machinery.
Startle amplitudes (a) and latencies (b) were measured at the inactive phase (“day”, ZT 3–6) or the active phase (“night”, ZT 14–16). Auditory brainstem threshold shifts (c) from mice after noise overexposure at ZT 3–5 (day, red) or ZT 14–16 (night, blue), measured 24 h post (triangles and dashed lines) and 2 weeks post (filled circles). Temporal expression of Per2 (d), Per1 (e), and Reverb-α (f) and Bmal1 (g) mRNAs in the cochlea from non-exposed animals (white circles) or exposed at ZT 3 (red circles) or ZT 15 (blue circles). The time at which noise overexposure was delivered is illustrated by the red or blue squares on the time scale. Mean values ± SEM (n = 3–4) and the Zeitgeber times (ZT) at which the animals were sacrificed are indicated. Average amplitude (h), phase (i), period (j) of PER2::LUC oscillations in cochleae extracted 1 h after noise trauma (filled bars) or sham treatment (hatched bars) during day (red) or night (blue). Results are mean values ± SEM, n= 5–12. * = p<0.05, ** = p<0.001, *** = p<0.001; paired Student’s t-test (a, b), unpaired Student’s t-test (h, i, j), 2-way ANOVA with post-hoc analysis (c, d, e, f, g).
Biological rhythms that are controlled throughout the day, namely circadian rhythms, are important for many bodily functions (e.g. metabolism, cell-cycle, sleep-wake cycle, reproduction, immune function and hormonal regulation). The master clock, the suprachiasmatic nucleus (SCN) of the hypothalamus, synchronizes and coordinates rhythms to regulate these various physiological functions [6, 7]. The central and peripheral clockwork circuitry contains a self-regulated feedback loop consisting of four transcriptional repressor-encoding genes Per1, Per2, Cry1, Cry2, which are regulated by BMAL1-CLOCK complexes, which in turn will be repressed by PER-CRY complexes resulting in a 24 hour cycle. A secondary loop involves REVERB-α, which represses Bmal1 transcription in a circadian manner. These core clock elements are necessary for the generation and maintenance of circadian rhythms [8]. We examined the temporal expression patterns of mRNAs encoding central clock components from CBA mouse cochleae. As depicted in Figure S1A–D, SybrGreen RT-PCR assays show smaller amplitudes of Per1, Bmal1, and Reverb-α transcript amplitudes in the cochlea (2 fold) than in the well-characterized liver (15 to 30 fold). However, Per2 mRNA transcripts were as ample in the cochlea as in the liver (6 fold), which prompted us to evaluate whether the clock is expressed autonomously in the cochlea. For this purpose, we evaluated by means of real-time bioluminescence the expression of PER2 in adult cochlear explants using PERIOD2::LUCIFERASE (PER2::LUC) mice, in which a Luc gene has been fused in frame to the 3′end of the endogenous mouse Per2 gene generating a PER2 protein fused with luciferase [9]. We found that isolated cochleae demonstrated robust self-sustained molecular rhythmicity of PER2::LUC expression (Figure S1E–G), which damping could be reset by the addition of the synchronizing agent dexamethasone (Figure S1H). PER2 was localized mainly in inner and outer hair cells and in spiral ganglion neurons from the cochlea (Figure S1L–O). Immunoreactivity was not expressed in the hair cells and spiral ganglion neurons in mPer2Brdm1 mutants.
We next found that night noise overexposure affected molecular cochlear rhythms more than day noise. Night noise (p=0.001) but not day noise (p=0.078) overexposure decreased the amplitude of Per2 mRNA transcripts by 30% (Figure 1D). Night noise overexposure suppressed Reverb-α mRNA transcript oscillations to a greater extent than day noise overexposure (p<0.007, day noise vs night noise, Figure 1F). The known anti-phase oscillations of Bmal1 were more affected after night noise (p<0.001, Figure 1G). In control cochlea, the oscillations of Bmal1 mRNA transcript showed a 3 fold change in amplitude, but after night noise there was a 16-fold change. We reason that this increase in oscillatory amplitude is due to lesser inhibition by Reverb-alpha after night noise overexposure. Day noise overexposure caused a phase shift in Per1 transcripts by 8 hours with an amplitude peak immediately after day noise overexposure (p<0.001, Figure 1E). To assess the effects of noise overexposure in higher resolution and on a larger time-scale, we exposed PER2::LUC mice to a day or night noise trauma and then recorded the PER2::LUC bioluminescence of the cochleae in vitro for a duration of 6 days. Confirming our in vivo qRT-PCR data, night noise decreased the rhythm amplitude by 27% (p=0.036) while day noise did not (Figure 1H). As previously reported in some tissues [10], the time of the cochlear dissection largely affected amplitude, period and phases in basal conditions (in absence of noise trauma, Figure 1H-J). As compared to control, the phase was not altered after either day or night noise (Figure 1I). The period of oscillations increased slightly after day (p=0.0014) and night (p=0.032) noise overexposure (Figure 1J). Overall, the effect on the cochlear molecular clock largely depends on the circadian time at which the animals are exposed to noise.
TrkB-mediated regulation of the auditory clock
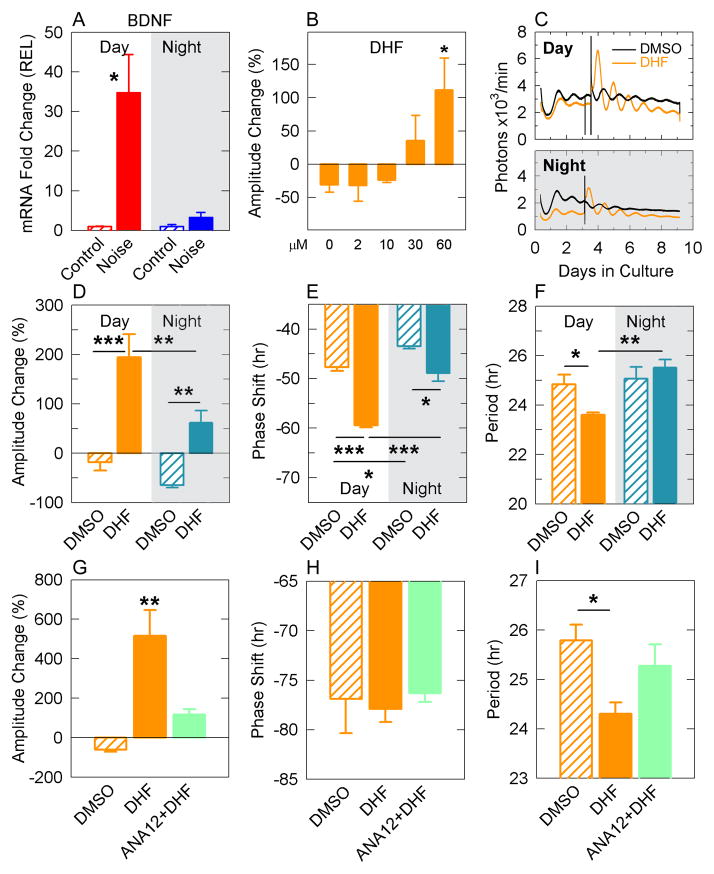
Neurotrophin-3 (NT-3) and Brain derived neurotrophic factor (BDNF) are important neurotrophins implicated in cochlear synaptogenesis [11]. NT-3 null mice lose 84% of cochlear neurons, whereas BDNF null mice mainly lose vestibular neurons [12]. Although NT-3 appears as an important regulator of auditory neuron survival, BDNF overexpression in NT-3 mutant mice almost completely rescues NT-3 deficiency in the developing cochlea, highlighting the importance of BDNF in auditory neuron maintenance [13]. When using standard SyBR Green PCR on whole cochlear material we hardly detected Bdnf amplicons, and therefore relied on Taqman assays from pools of microdissected cochleas (free of bone) to increase detection sensitivity. Only day noise overexposure increased Bdnf mRNA transcripts by 35-fold (p<0.05, Figure 2A), suggesting that the lack of Bdnf induction after night noise could contribute to the increased sensitivity (Figure 1C). Mature BDNF has preferential affinity for the tropomyosin receptor kinase type B (TrkB), a member of the tyrosine kinase family implicated in neuronal development and plasticity [14]. The effects of TrkB activation were evaluated using a selective agonist, namely 7,8-dihydroxyflavone (DHF) to assess PER2::LUC oscillations in cochlear explants. DHF caused a dose-dependent increase in PER2::LUC rhythm amplitude (Figure 2B), with 60 μM yielding the greatest changes. Cochlear explants isolated at daytime were treated with DHF at either ZT 3 (9 am) or ZT 12 (6 pm, Figure 2C). DHF effects were stronger during the day than in the night, with greater amplitude (p = 0.006; Figure 2D) and greater phase delay (p < 0,001; Figure 2E). The period was also differentially altered between day and night treatments (p = 0.012; Figure 2F). To assess the specificity of DHF, we pre-treated the cochlear explants two times with either DMSO or ANA12, a specific TrkB receptor antagonist, prior the treatment with DHF during the day. Using this protocol, we recapitulated the amplitude and period changes observed in Figure 2D and F but not the phase delay. ANA12 completely blocked the changes in amplitude (p = 0.307, ANA12 + DHF vs DMSO, Figure 2G) and in period (p = 0.444, ANA12 + DHF vs DMSO, Figure 2I) induced by DHF. These results support the notion that DHF modulates PER2 oscillations in the cochlea via TrkB.
Figure 2. Regulation of the auditory clock by TrkB.
Bdnf mRNA expression analysis (a) from cochleae isolated after day or night noise overexposure in comparison to sham- exposed groups. Results are mean values ± SEM (n = 3). Amplitude changes in PER2::LUC cochlear explants in response to increasing concentrations of DHF (b). (c) Representative bioluminescence records of circadian PER2::LUC expression from cochlear explants isolated during day time and treated with DHF (red) or DMSO (black) at ZT 3 (day) or ZT 12 (night), which quantification is shown by the average amplitude change (d), phase (e) and period (f). Day treatment (orange), night treatment (blue), DMSO (hatched bars), DHF (filled bars). Effects of TrkB blockade by ANA12 prior DHF treatment on PER2::LUC amplitude changes (g), phase shifts (h) or period (i). DMSO (orange hatched bars), DHF (orange filled bars), and ANA12 + DHF (green filled bars). Results for the PER::LUC experiments are mean values ± SEM (n = 3–7). * = p<0.05, ** = p<0.001, *** = p<0.001; two tailed Student’s t-test (a), one-way ANOVA with post-hoc Bonferroni test (b), two-way ANOVA with post-hoc Tukey test (d, e, f), one-way ANOVA, with post-hoc Tukey test (g, h, i).
DHF preserves cochlear synaptic integrity after noise overexposure
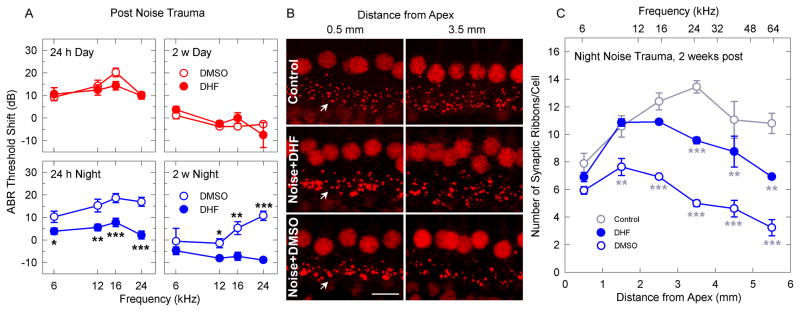
We hypothesized that the hearing loss induced by noise overexposure during the active phase could be due to the lack of Bdnf induction. To test this, we pre-treated CBA mice with DHF (5 mg/kg intraperitoneally, two hours before day or night noise overexposure) in order to activate TrkB. DHF had no effects on thresholds shifts after day exposure (Figure 3A) while DHF protected against night noise trauma 24 h and 2 weeks post exposure. These results imply that the protective effects of DHF differ during day and night. After temporary noise trauma excitotoxicity induces swelling of the dendrites that can recover through their regrowth to restore hearing. Night noise-induced permanent hearing loss, in absence of hair cell loss, suggests that a residual uncoupling of the inner hair cell - afferent neuron synapse could persist due to the loss of synaptic ribbons [4]. We evaluated synaptic integrity by assessing synaptic ribbons in the cochlea at 2 weeks post-exposure (Figure 3B). DHF treatment protected synaptic ribbons from their loss caused by night noise exposure (p<0.001, DHF vs DMSO, Figure 3C).
Figure 3. Maintenance of synaptic integrity by TrkB activation after night noise injury.
Auditory brainstem threshold shifts (a) from mice exposed to noise at ZT 3–5 (day, red) or ZT 14–16 (night, blue), measured 24 h post (left panel) and 2 weeks post (right panel). DMSO-treated animals (white circles) and DHF-treated animals (filled circles). Results are mean values ± SEM, n= 4–9, two-way ANOVA post-hoc Holm-Sidac. Representative images of synaptic ribbons (arrows) (b) beneath inner hair cell nuclei from the 0.5 mm (low frequency region) and 3.5 mm (high frequency region) distance from the apex of controls (upper panel), noise + DMSO (middle panel) and noise + DHF (lower panel). (c) Quantification of the synaptic ribbons along the length of the cochlea from controls (grey empty circles) and 2 weeks post DMSO-treated night noise (blue empty circles) and DHF-treated night noise (filled blue circles). Scale bar is 10 μm. Corresponding frequency map is provided. Results are mean values ± SEM, n= 4; * = p<0.05, ** = p<0.001, *** = p<0.001; two-way ANOVA with post-hoc Tukey test.
DISCUSSION
TrkB activation protects from noise induced hearing loss
Cochlear innervation and auditory neurite growth is highly dependent on the neurotrophins BDNF and NT-3 [12, 15–17]. Their respective receptors, TrkB and TrkC, are expressed in auditory neurons [18–20]. Loss of TrkC function but not TrkB function results in a loss of 50% of auditory neurons, however, dual knock-out of TrkB and TrkC causes a complete absence of auditory neurons, highlighting the complementary support of both signaling pathways on auditory neuronal maintenance [12]. In the cultured post-natal cochlea, TrkC but not TrkB played a role in axon growth and synaptogenesis, although both neurotrophins were equally efficient in regenerating synapses after excitotoxicity [21]. Our study provides evidence that TrkB activation may prove useful for maintaining synaptic integrity after noise injury in the adult mouse cochlea in vivo.
BDNF has been used by different means to protect from hearing loss or to restore hearing [17, 22, 23] but its size and short life-span preempt its ease of use. A selective TrkB agonist, namely dihydrozyflavone (DHF), has recently been identified in a neuronal cell-based apoptotic screen [24]. A structural activity relationship study from the same group generated 7,8,3′-trihydroxyflavone (7,8,3′-THF) with 3 times greater potency on TrkB than DHF [25]. Local delivery of DHF and THF in the ear has been shown to rescue auditory neuronal loss and function in a mouse model of auditory neuropathy (cCx26 null mice) [26, 27]. Our results are in line with these findings and suggest that DHF may i) protect immediately, presumably by decreasing glutamate release during noise overexposure and subsequent excitotoxicity, ii) act on the recovery processes after noise overexposure by facilitating synaptogenesis or iii) a combination of the two. Although we do not exclude worsen recovery or potential damage on outer hair cells after night noise overexposure, the decreased temporary threshold shifts observed 24 hours post-trauma in the DHF group (Figure 3a) suggests that the excitotoxic damage does not occur to the same extent as in the control group.
A circadian machinery responding to noise
Although numerous molecular clocks have been found in different tissues [28, 29], this is the first demonstration that the adult cochlea, contains rhythmically expressed clock genes, with robust and self-sustained oscillations. We have found, for the first time to our knowledge, that the auditory sensitivity to noise trauma depends on the time of day and that noise overexposure alters circadian oscillations of cochlear Per1, Per2, Reverb-α and Bmal1 mRNA transcripts. Correlating with the increased sensitivity to noise overexposure at night, greater effects on the circadian machinery was observed after night noise overexposure (suppression of Per2 and Reverb-α oscillations, and greater oscillations of Bmal1, Figure 1D–G). Since REVERB-α inhibits Bmal1 transcription, we propose that the suppression of Reverb-α oscillations is linked to the increased oscillations of Bmal1 circadian mRNA transcripts occurring after night noise overexposure.
TrkB may play a critical role in gating the circadian auditory clock to sound. TrkB could modulate circadian rhythms through downstream connections with the clockwork circuitry, including that of the SCN. Because TrkB mutant mice are embryonic lethal [30], targeted deletion of TrkB specifically in the SCN or the use of SCN-specific dominant-negative TrkB expressing mice should provide interesting insights into the contribution of TrkB on the auditory clock machinery.
Conclusion
Sensitivity to noise trauma is greater at certain times of the day, both at the molecular and behavioral level with consequences on hearing. It is likely that the mouse auditory-clock described here will have similar properties in humans since the auditory and circadian systems in mammalian species are highly homologous. For this reason, our findings could have important implications with reference to the circadian variation in the potential for recovery from noise trauma that may play a role for people working in noisy environments, shift workers, flight crew that frequently travel across time zones and for those who recurrently visit night clubs.
EXPERIMENTAL PROCEDURES
Ethics Statement and animal handling
All experimental procedures on animals were performed in accordance with the guidelines and regulations set forth by Karolinska Institutet and “Stockholm’s Norra Djurforsoksetiska Namnd”. Recordings of circadian oscillations of the PER2 protein were performed using tissues obtained from knock-in PERIOD2::LUCIFERASE (PER2::LUC) transgenic mice with a C57BL/6 background [9], generously provided by Prof. J. Takahashi. CBA/J and PER2::LUC male mice aged between 2 and 4 months mice were used for audiological, morphological and molecular experiments. Temperature was maintained between 19° and 21°C. Because lights were on at 6 a.m. and off by 6 p.m., we set 6 a.m. as the Zeitgeber time ZT 0. Handling at ZT 14–16 (darkness) was performed in red light.
Organotypic cultures
Adult cochleae, suprachiasmatic nucleus (SCN) and livers were dissected from PER2::LUC mice and cultured organotypically on a membrane (Millipore, PICMORG50). The isolated tissues were cultured as described [31, 32]. Cochleae, dissected free of bone and stria vascularis, were kept in culture for minimum 6 days.
Drug treatment
Mice were given a 4 ml/kg intraperitoneal injection of DHF (5 mg/kg) dissolved in the vehicle (18 % DMSO in PBS solution) 2 h prior to noise trauma. For in vitro treatments, two protocols were used for the treatment of cochlear explants: 1) DHF day-night experiments (Figure 3D–F): Cochlear explants were exposed to DMSO vehicle or DHF 60 μM either at ZT 3 or ZT12. Dexamethasone 21 phosphate disodium salt (Sigma Aldrich, D1159) was applied on day 4. 2) DHF/ANA12 experiments (Figure 3G–I): Cochlear explants were pre-exposed on day 0 and day 2 with DMSO vehicle or ANA12 (Sigma Aldrich, SML0209) at ZT 4. On day 3 the explants were co-treated with DHF (Tocris, 3826) and vehicle or ANA12 at ZT 4.
Acoustic trauma and auditory brainstem response (ABR)
To generate hearing loss, awake and unanesthetized animals were treated with free field broadband noise at 6 – 12 kHz at intensity of 100 dB SPL for 1 h, similar to previously described noise trauma paradigm [33]. Post-trauma measurements where performed either 24 h after the day or night exposures. However, the final measurements made at 2 weeks post-trauma were made only during the day because i) we found no differences in basal ABR levels between day and night and ii) after 2 weeks post-trauma, the ABR levels are considered stable.
Acoustic startle response (ASR)
CBA male mice were tested for the ASR at sleep (ZT 3–6) and awake (ZT 14–16) phases. Same animals were used for the experiment with 7 days interval between day and night sessions. The ASR paradigm was previously described [34]. The startle magnitude (Vmax, V - the mean maximal startle response to the “pulse-alone” trials) and startle latency (Tmax, msec - the mean latency to the maximal response to the “pulse-alone” trials) were calculated.
Immunocytochemistry and quantification of synaptic ribbons
PER2, wild type or mPer2Brdm1 mutants (having a frame deletion that produces an unstable PER2 protein) [35] underwent transcardiac perfusion (4% paraformaldehyde) and decalcified in EDTA 2% for 4 days. Cryosections were immunostained with a rabbit-antibody to PER2 (PER21-A, Alpha Diagnostic, Texas USA; 1:100). For the quantification of synaptic ribbons, surface preparations were stained for C-terminal binding protein 2 (mouse anti-CtBP2, 612044 from BD-Biosciences, used at 1:200) and secondary FITC-conjugated goat anti-rabbit and TRIC-conjugated goat anti-mouse antibodies (Jackson ImmunoResearch; Pennsylvania USA; 1:100). Confocal image stacks were analyzed using Image J software. Quantification was performed using an automated particle counting after converting the image to grayscale and thresholding the image. This technique was manually validated before collecting the data.
Quantitative Real Time-PCR
SybrGreen qRT-PCR assays were performed as previously described [36, 37] with minor modifications. CBA male mice were put into darkness for 72 hours prior their exposure to either silence or sound (100 dB, 6–12 kHz narrow band white noise, for 1 hour). To allow comparisons, the cochleas (controls, day and night noise exposure) were collected at fixed ZT time points. Total RNAs were extracted from whole cochleae using the Direct-zol RNA MiniPrep kit from ZymoResearch (Nordic Biolabs AB, Sweden) and immediately followed by DNAse I treatment (Invitrogen). RNA integrity was assessed using RNA 6000 nanochips with an Agilent 2100 bioanalyzer (Agilent Technologies, Inc., Palo Alto, CA) and quantity was evaluated with Nanodrop. RT-PCR assays were performed with 0.5 μg total RNA from 3–4 adult cochleae, or livers, collected every 4 h around the clock. Total RNAs were reverse transcribed with the Superscript II RT-kit from Invitrogen (Life Sciences, Sweden). A mean quantity was calculated from triplicate PCR for each sample, and this quantity was normalized with the geometric mean of two to four most stable genes out of six reference genes (tubulin β, Tubb; glyceraldehyde-3-phosphate dehydrogenase, G3pdh; transferring receptor 1, Trf1R; Tubulin α2, Tuba2; hypoxanthine phosphoribosyltransferase, HPRT; and Cyclophilin B) selected using the geNorm algorithm as described [38]. Normalized quantities were averaged for three technical replicates for each data point and represented as the mean ± SD. The highest normalized relative quantity was arbitrarily designated as a value of 1.0. Fold changes were calculated from the quotient of means of these normalized quantities and reported as ± SEM. The primers used for quantitative RT-PCR (qRT-PCR) are listed in Supplemental Table 1.
For the evaluation of Bdnf mRNA transcript levels, we performed Taqman qRT-PCR assays (Applied Biosystems, CA, USA). Bdnf (Mm01334042_m1) gene expression was quantified as described above using Cyclophilin E (Mm00450929_m1) and Hprt (Mm00446968_m1) as the most stable normalization genes.
Statistics
Data are presented as a mean ± SEM. Statistical analysis of PER2::LUC rhythms and the qRT-PCR was performed with GraphPad Prism 5.04 (GraphPad Software Inc., CA, USA). Analysis of other experiments was performed using SigmaStat v 3.5.
Supplementary Material
Highlights.
The cochlea possesses a self-sustained circadian clock
Sensitivity to noise is greater during the active phase than during the inactive phase
The BDNF receptor TrkB modulates the auditory clock
Pharmacological activation of TrkB during the active phase prevents noise-induced hearing loss
Acknowledgments
We are most grateful to Urs Albrecht for generous help with sharing the PER2 mutant mice (mPer2Brdm1) and wild-type mice samples. We thank Didier Chollet from the Genomics facility of the NCCR program “Frontiers in Genetics” at the University of Geneva for his dedicated support. The authors thank Agneta Viberg and Anne-Sofie Johansson for technical support.
Footnotes
AUTHOR CONTRIBUTIONS
BC, IM, CRC, and GSL designed research; BC, IM, CRC, GSL, SS and VB performed research; BC, IM, CRC, GSL, SS and VB analyzed data; BC, IM, CRC, and GSL wrote the paper.
COMPETING INTEREST
The authors disclose that there are no competing financial interests.
FINANCIAL DISCLOSURE
This work was supported by AFA Insurance Company (project and postdoctorate grant for IM), Swedish Medical Research Council K2010-62X-09476-20-3 and 2008-3197 (BC), National Institute on Deafness and other Communication Disorders of the National Institutes of Health R21DC013172 (BC), Swedish Society of Medicine SLS-95151 (GSL), Knut and Alice Wallenberg KAW2008.0149 (BC), Karolinska Institutet (BC), Tysta Skolan (BC, IM, CRC), Lars Hiertas Minne (CRC), Magnus Bergvalls (CRC) and Wenner Gren Stiftelse (CRC). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rubel EW, Furrer SA, Stone JS. A brief history of hair cell regeneration research and speculations on the future. Hearing research. 2013 doi: 10.1016/j.heares.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cederroth CR, Canlon B, Langguth B. Hearing loss and tinnitus-are funders and industry listening? Nature biotechnology. 2013;31:972–974. doi: 10.1038/nbt.2736. [DOI] [PubMed] [Google Scholar]
- 3.Sheridan C. Investors start backing hearing loss treatments. Nature biotechnology. 2013;31:575–576. doi: 10.1038/nbt0713-575. [DOI] [PubMed] [Google Scholar]
- 4.Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:14077–14085. doi: 10.1523/JNEUROSCI.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Puel JL, Ruel J, Gervais d’Aldin C, Pujol R. Excitotoxicity and repair of cochlear synapses after noise-trauma induced hearing loss. Neuroreport. 1998;9:2109–2114. doi: 10.1097/00001756-199806220-00037. [DOI] [PubMed] [Google Scholar]
- 6.Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330:1349–1354. doi: 10.1126/science.1195027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annual review of physiology. 2010;72:517–549. doi: 10.1146/annurev-physiol-021909-135821. [DOI] [PubMed] [Google Scholar]
- 8.Schibler U. The daily timing of gene expression and physiology in mammals. Dialogues in clinical neuroscience. 2007;9:257–272. doi: 10.31887/DCNS.2007.9.3/uschibler. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, et al. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoshikawa T, Yamazaki S, Menaker M. Effects of preparation time on phase of cultured tissues reveal complexity of circadian organization. Journal of biological rhythms. 2005;20:500–512. doi: 10.1177/0748730405280775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang T, Kersigo J, Jahan I, Pan N, Fritzsch B. The molecular basis of making spiral ganglion neurons and connecting them to hair cells of the organ of Corti. Hearing research. 2011;278:21–33. doi: 10.1016/j.heares.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fritzsch B, Tessarollo L, Coppola E, Reichardt LF. Neurotrophins in the ear: their roles in sensory neuron survival and fiber guidance. Progress in brain research. 2004;146:265–278. doi: 10.1016/S0079-6123(03)46017-2. [DOI] [PubMed] [Google Scholar]
- 13.Farinas I, Jones KR, Tessarollo L, Vigers AJ, Huang E, Kirstein M, de Caprona DC, Coppola V, Backus C, Reichardt LF, et al. Spatial shaping of cochlear innervation by temporally regulated neurotrophin expression. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:6170–6180. doi: 10.1523/JNEUROSCI.21-16-06170.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen K, Cowan CW. Guidance molecules in synapse formation and plasticity. Cold Spring Harbor perspectives in biology. 2010;2:a001842. doi: 10.1101/cshperspect.a001842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hegarty JL, Kay AR, Green SH. Trophic support of cultured spiral ganglion neurons by depolarization exceeds and is additive with that by neurotrophins or cAMP and requires elevation of [Ca2+]i within a set range. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1997;17:1959–1970. doi: 10.1523/JNEUROSCI.17-06-01959.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malgrange B, Lefebvre P, Van de Water TR, Staecker H, Moonen G. Effects of neurotrophins on early auditory neurones in cell culture. Neuroreport. 1996;7:913–917. doi: 10.1097/00001756-199603220-00016. [DOI] [PubMed] [Google Scholar]
- 17.Wise AK, Richardson R, Hardman J, Clark G, O’Leary S. Resprouting and survival of guinea pig cochlear neurons in response to the administration of the neurotrophins brain-derived neurotrophic factor and neurotrophin-3. The Journal of comparative neurology. 2005;487:147–165. doi: 10.1002/cne.20563. [DOI] [PubMed] [Google Scholar]
- 18.Ylikoski J, Pirvola U, Moshnyakov M, Palgi J, Arumae U, Saarma M. Expression patterns of neurotrophin and their receptor mRNAs in the rat inner ear. Hearing research. 1993;65:69–78. doi: 10.1016/0378-5955(93)90202-c. [DOI] [PubMed] [Google Scholar]
- 19.Pirvola U, Arumae U, Moshnyakov M, Palgi J, Saarma M, Ylikoski J. Coordinated expression and function of neurotrophins and their receptors in the rat inner ear during target innervation. Hearing research. 1994;75:131–144. doi: 10.1016/0378-5955(94)90064-7. [DOI] [PubMed] [Google Scholar]
- 20.Pirvola U, Hallbook F, Xing-Qun L, Virkkala J, Saarma M, Ylikoski J. Expression of neurotrophins and Trk receptors in the developing, adult, and regenerating avian cochlea. Journal of neurobiology. 1997;33:1019–1033. doi: 10.1002/(sici)1097-4695(199712)33:7<1019::aid-neu11>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 21.Wang Q, Green SH. Functional role of neurotrophin-3 in synapse regeneration by spiral ganglion neurons on inner hair cells after excitotoxic trauma in vitro. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:7938–7949. doi: 10.1523/JNEUROSCI.1434-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rejali D, Lee VA, Abrashkin KA, Humayun N, Swiderski DL, Raphael Y. Cochlear implants and ex vivo BDNF gene therapy protect spiral ganglion neurons. Hearing research. 2007;228:180–187. doi: 10.1016/j.heares.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pettingill LN, Wise AK, Geaney MS, Shepherd RK. Enhanced auditory neuron survival following cell-based BDNF treatment in the deaf guinea pig. PloS one. 2011;6:e18733. doi: 10.1371/journal.pone.0018733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jang SW, Liu X, Yepes M, Shepherd KR, Miller GW, Liu Y, Wilson WD, Xiao G, Blanchi B, Sun YE, et al. A selective TrkB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:2687–2692. doi: 10.1073/pnas.0913572107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu X, Chan CB, Jang SW, Pradoldej S, Huang J, He K, Phun LH, France S, Xiao G, Jia Y, et al. A Synthetic 7, 8-Dihydroxyflavone Derivative Promotes Neurogenesis and Exhibits Potent Antidepressant Effect. Journal of medicinal chemistry. 2010 doi: 10.1021/jm101206p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu Q, Chang Q, Liu X, Gong S, Ye K, Lin X. 7,8,3′-Trihydroxyflavone, a potent small molecule TrkB receptor agonist, protects spiral ganglion neurons from degeneration both in vitro and in vivo. Biochemical and biophysical research communications. 2012;422:387–392. doi: 10.1016/j.bbrc.2012.04.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu Q, Chang Q, Liu X, Wang Y, Li H, Gong S, Ye K, Lin X. Protection of spiral ganglion neurons from degeneration using small-molecule TrkB receptor agonists. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:13042–13052. doi: 10.1523/JNEUROSCI.0854-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bell-Pedersen D, Cassone VM, Earnest DJ, Golden SS, Hardin PE, Thomas TL, Zoran MJ. Circadian rhythms from multiple oscillators: lessons from diverse organisms. Nature reviews Genetics. 2005;6:544–556. doi: 10.1038/nrg1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bass J. Circadian topology of metabolism. Nature. 2012;491:348–356. doi: 10.1038/nature11704. [DOI] [PubMed] [Google Scholar]
- 30.Klein R, Smeyne RJ, Wurst W, Long LK, Auerbach BA, Joyner AL, Barbacid M. Targeted disruption of the trkB neurotrophin receptor gene results in nervous system lesions and neonatal death. Cell. 1993;75:113–122. [PubMed] [Google Scholar]
- 31.Savelyev SA, Larsson KC, Johansson AS, Lundkvist GB. Slice preparation, organotypic tissue culturing and luciferase recording of clock gene activity in the suprachiasmatic nucleus. Journal of visualized experiments : JoVE. 2011 doi: 10.3791/2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, Block GD, Sakaki Y, Menaker M, Tei H. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288:682–685. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- 33.Tahera Y, Meltser I, Johansson P, Hansson AC, Canlon B. Glucocorticoid receptor and nuclear factor-kappa B interactions in restraint stress-mediated protection against acoustic trauma. Endocrinology. 2006;147:4430–4437. doi: 10.1210/en.2006-0260. [DOI] [PubMed] [Google Scholar]
- 34.Fisahn A, Lavebratt C, Canlon B. Acoustic startle hypersensitivity in Mceph mice and its effect on hippocampal excitability. The European journal of neuroscience. 2011;34:1121–1130. doi: 10.1111/j.1460-9568.2011.07834.x. [DOI] [PubMed] [Google Scholar]
- 35.Zheng B, Larkin DW, Albrecht U, Sun ZS, Sage M, Eichele G, Lee CC, Bradley A. The mPer2 gene encodes a functional component of the mammalian circadian clock. Nature. 1999;400:169–173. doi: 10.1038/22118. [DOI] [PubMed] [Google Scholar]
- 36.Cederroth CR, Schaad O, Descombes P, Chambon P, Vassalli JD, Nef S. Estrogen receptor alpha is a major contributor to estrogen-mediated fetal testis dysgenesis and cryptorchidism. Endocrinology. 2007;148:5507–5519. doi: 10.1210/en.2007-0689. [DOI] [PubMed] [Google Scholar]
- 37.Cederroth CR, Vinciguerra M, Gjinovci A, Kuhne F, Klein M, Cederroth M, Caille D, Suter M, Neumann D, James RW, et al. Dietary phytoestrogens activate AMP-activated protein kinase with improvement in lipid and glucose metabolism. Diabetes. 2008;57:1176–1185. doi: 10.2337/db07-0630. [DOI] [PubMed] [Google Scholar]
- 38.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:RESEARCH0034. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.