Abstract
Poly(ADP-ribose) polymerase (PARP) inhibitors exploit synthetic lethality to target epithelial ovarian cancer (EOC) with hereditary BRCA mutations and defects in homologous recombination repair (HRR). However, such an approach is limited to a small subset of EOC patients and compromised by restored HRR due to secondary mutations in BRCA genes. Here, it was demonstrated that triapine, a small molecule inhibitor of ribonucleotide reductase, enhances the sensitivity of BRCA wild-type EOC cells to the PARP inhibitor olaparib and the topoisomerase II inhibitor etoposide. Triapine abolishes olaparib-induced BRCA1 and Rad51 foci, and disrupts BRCA1 interaction with the Mre11-Rad50-Nbs1 (MRN) complex in BRCA1 wild-type EOC cells. It has been shown that phosphorylation of CtIP (RBBP8) is required for interaction with BRCA1 and with MRN to promote DNA double-strand break (DSB) resection during S- and G2-phases of the cell cycle. Mechanistic studies within reveal that triapine inhibits CDK activity and blocks olaparib-induced CtIP phosphorylation through Chk1 activation. Furthermore, triapine abrogates etoposide-induced CtIP phosphorylation and DSB resection as evidenced by marked attenuation of RPA32 phosphorylation. Concurrently, triapine obliterates etoposide-induced BRCA1 foci and sensitizes BRCA1 wild-type EOC cells to etoposide. Using a GFP-based HRR assay, it was determined that triapine suppresses HRR activity induced by an I-SceI-generated DSB. These results suggest that triapine augments the sensitivity of BRCA wild-type EOC cells to drug-induced DSBs by disrupting CtIP-mediated HRR.
Keywords: Triapine, PARP inhibitor, BRCA1, CtIP, homologous recombination, cyclin-dependent kinase, ribonucleotide reductase, ovarian cancer
Introduction
Epithelial ovarian cancer (EOC) is the leading cause of death among gynecological malignancies in the United States, with more than 14,000 women dying of the disease each year. Following optimal cytoreduction surgery, combination regimens consisting of platinum and paclitaxel are used as first-line chemotherapy for EOC. Despite a high clinical response rate of 70-80% with the initial therapy, most patients relapse and eventually develop a platinum-resistant EOC with an overall response rate of 10-20% to second line therapy (1). Thus, development of mechanism-based, targeted combination therapy is paramount to improving interval free survival and overall survival in EOC.
Poly(ADP-ribose) polymerase (PARP) inhibitors belong to a class of agents that exploit synthetic lethality to target DNA repair defects in hereditary breast and ovarian cancer (2). The majority of hereditary EOC harbor germ line mutations in BRCA1 and BRCA2 genes. BRCA1 and BRCA2 proteins normally function as central components of the homologous recombination repair (HRR) pathway for the repair of DNA double-strand breaks (DSBs). PARP inhibitors block the repair of single-strand breaks (SSBs) and in turn cause the formation of DSBs (3). Cancer cells with deleterious BRCA1/2 mutations are defective in HRR and therefore are hypersensitive to PARP inhibitors (4, 5).
In clinical trials, EOC patients with BRCA mutations exhibit favorable responses to the PARP inhibitor olaparib compared to patients without BRCA mutations (6, 7). However, the use of PARP inhibitors may be limited to patients with BRCA mutations, which represent only a small subset (10-15%) of EOC cases. A considerable proportion of sporadic EOCs remain HRR-proficient and resistant to PARP inhibitors. Furthermore, secondary mutations in mutated BRCA genes have been reported to restore BRCA functions to repair DSBs in platinum-resistant cancer cells (8, 9). These cancer cells are also likely to become resistant to PARP inhibitors (10). Therefore, new therapeutic strategies and approaches are urgently needed to make broader use of PARP inhibitors for the majority of EOC cases, and to overcome the resistance developed to PARP inhibitors.
Triapine (3-aminopyridine-2-carboxaldehyde thiosemicarbazone) is the most active small molecule inhibitor of ribonucleotide reductase (RNR) identified among a large number of thiosemicarbazones designed and synthesized in our laboratory (11). It is 1000 times more potent than the clinically used RNR inhibitor hydroxyurea (12, 13). RNR is a heteromeric enzyme consisting predominantly of R2 and R1 subunits during the S phase of the cell cycle (14). RNR catalyzes the rate-limiting step in the conversion of ribonucleoside diphosphates into corresponding deoxyribonucleoside diphosphates, the immediate precursors of the deoxyribonucleoside triphosphates (dNTPs) required for DNA replication and repair (15). Triapine strongly chelates iron in cells to form a triapine-Fe complex that quenches the tyrosyl radical at the R2 subunit of RNR and leads to enzymatic inactivation (16). As a result, triapine causes rapid depletion of purine nucleotides/dNTPs concurrent with cessation of DNA synthesis (14, 17, 18). Preclinical studies from our laboratory and clinical trials by others have demonstrated that triapine sensitizes cancer cells to several DNA damaging agents, as well as to radiation (12, 19). Since the supply of dNTPs by RNR is also required for DNA repair, the sensitization to DNA damaging agents has been attributable to the inhibition of DNA repair by triapine. However, the exact mechanism by which triapine affected DNA repair processes remained poorly understood.
Given the promising results of triapine in clinical trials with cervical cancer and the ability of triapine to sensitize cancer cells to platinum and radiation-induced DNA damage (19, 20), we sought to determine the effects of triapine on the sensitivity of BRCA wild-type EOC cells to PARP and topoisomerase inhibitors. The mechanism underlying triapine-mediated impairment of HRR in EOC cells was also elucidated. We demonstrated that treatment with triapine obliterated CtIP-mediated HRR and consequently resulted in enhanced sensitization of BRCA wild-type EOC cells to PARP and topoisomerase inhibitors. Our findings provide a novel and mechanism-based therapeutic strategy by which PARP/topoisomerase inhibitors can be used in combination with triapine to target HRR-proficient sporadic EOC cells that constitute the majority of EOC cases.
Materials and Methods
Cell Lines and Chemicals
Ovarian cancer cell lines SKOV-3, BG-1, PEO1/PEO4 were maintained in logarithmic growth in McCoys 5A, DMEM/F12, and DMEM media, respectively, each supplemented with 10% FBS and penicillin-streptomycin antibiotics. SKOV-3 cells (HTB-77) were purchased from the American Type Culture Collection (ATCC, Manassas, VA). Cell line authentication has been done by short tandem repeat analysis. BG-1 cells were obtained from Dr. Joanne Weidhaas (Yale University). PEO1 and PEO4 cells (10) were provided by Dr. Peter Glazer (Yale University). BG-1 and PEO1/4 cell lines have been authenticated by ATCC using short tandem repeat analysis in November 2013. All cell lines were acquired from 2009 to 2012 and passaged fewer than 3 months after resuscitation. Non-targeted siRNA control (NTC) and BRCA1-knockdown (BRCA1-kd) SKOV-3 cell lines were established by stable transfection with non-targeted shRNA (pZBsdmU6-NT-shRNA; GCGCGCUUUGUAGGAUUCG) and BRCA1-shRNA (pBsdmU6-BRCA1-shRNA; GUCACAGUGUCCUUUAUGUA) (17, 21) expression constructs, respectively. SKOV-3-DR-GFP cells were generated by stable transfection of SKOV-3 cells with the pDR-GFP plasmid developed by Dr. Maria Jasin (Memorial Sloan-Kettering Cancer Center) (Addgene plasmid 26475, Addgene, Cambridge, MA) (22). Triapine (3-aminopyridine-2-carboxaldehyde-thiosemicarbazone) was synthesized in our laboratory. Roscovitine was purchased from EMD Biosciences (Billerica, MA). Etoposide was purchased from R&D Systems (Minneapolis, MN). Olaparib was purchased from Axon Medchem (Groningen, Netherlands). Hydroxyurea was purchased from Sigma-Aldrich (St. Louis, MO).
siRNA Transfection
Cells were plated and grown for 24 hr and then transfected with 50 nM siRNA using the Lipofectamine 2000 transfection reagent (Life Technologies, Grand Island, NY) according to the manufacturer’s protocol. Transfected cells were incubated for appropriate times before assays. The sequences of siRNAs were as follows: Control-siRNA, GCGCGCUUUGUAGGAUUCGdTdT (17); CtIP-siRNA1, GCUAAAACAGGAACGAATCdTdT; CtIP-siRNA2, GGACCUUUGGACAAAACUAdTdT (23); R2-siRNA, GAGGCUACCUAUGGUGAACdTdT (14); Rad51-siRNA, GCAGUGAUGUCCUGGAUAAdTdT; Chk1-siRNA, GCAACAGUAUUUCGGUAUAdTdT (17). siRNAs were synthesized by Thermo Fisher Scientific (Waltham, MA).
Clonogenic Survival and Cytotoxicity Assays
Cells were plated at various densities in 6-well plates in triplicate. After 24 hr of incubation, cells were treated continuously with various concentrations of olaparib, triapine, or both drugs in combination. After 9-13 days, colonies were fixed/stained with crystal violet solution and counted to determine the % survival using a GelDoc imaging system with QuantityOne software (Bio-Rad Laboratories; Hercules, CA). Colorimetric MTS cytotoxicity assay was described previously (14).
Western Blot Analyses and Protein Phosphatase Treatment
Western blotting was performed as described previously (17). For protein phosphatase treatment, cells were lysed with native lysis buffer (10 mM Tris-HCl, pH 7.4), 150 mM NaCl, 1 mM EDTA, 0.5% NP-40, 1% Triton X-100). Thirty μg of protein were incubated with λ phosphatase (New England BioLabs; Ipswich, MA) at 30°C for 30 min prior to western blot analyses for CtIP and phosphorylation-induced gel retardation of CtIP proteins (24).
Nuclear Protein Extraction and Co-immunoprecipitation
Cells were plated and subjected to drug treatment. Then cells were harvested and nuclear protein was isolated using a Nuclear Complex Co-IP Kit (Active Motif; Carlsbad, CA) according to the manufacturer’s instruction. Three hundred μg of nuclear protein was used for co-immunoprecipitation using the anti-Mre11 antibody and Protein A/G beads (Santa Cruz Biotechnology; Santa Cruz, CA). Beads were washed with low-stringency buffer. Immunoprecipitates were extracted from beads with SDS sample buffer and analyzed by western blotting as described above.
Immunofluorescent Staining and Confocal Microscopy
Cells were grown for 24 hr prior to drug treatment. Cells were then fixed with 2% formaldehyde and permeabilized in cooled 100% methanol. The slides were blocked with 3% bovine serum albumin and stained with primary antibodies followed by incubation with corresponding AlexaFluor 488- or AlexaFluor 546-conjugated secondary antibodies (Life Technologies). Nuclei were counterstained with 1 μM TO-PRO-3 (Life Technologies) and the slides were then mounted with coverslips using the ProLong Gold Anti-Fade reagent (Life Technologies). Immunofluorescence of target proteins and nuclei were viewed with a Zeiss Laser Scanning System LSM510 (Zeiss; Thornwood, NY) and confocal images were analyzed with a Zeiss LSM Image Browser.
Cyclin-Dependent Kinase (CDK) Assays
Native protein was prepared as described previously (14). Five hundred μg of native protein were incubated with anti-CDK2 antibody (Cell Signaling Technology; Danvers, MA) overnight and then incubated with Protein A/G Plus agarose beads (Santa Cruz Biotechnology) with constant agitation for an additional 3 hr. The agarose beads were washed and resuspended in kinase assay buffer (20 mM Tris-HCl, pH 7.4, 120 mM NaCl, 8 mM MgCl2, 0.8 mM dithiothreitol) containing 40 μM ATP and 50 μg/ml of CtIP peptide substrate (PTRVSSPVFGAT; a.a. 322-333) synthesized by Selleck Chem (Houston, TX). The kinase reaction was carried out at 30°C for 45 min. Phosphorylation of the peptide substrate correlated positively with ADP generated by the kinase activity of immunoprecipitated CDK2. The level of ADP was measured by the ADP-Glo Kinase Assay kit and a TD-20/20 luminometer (Promega; Madison, WI).
Antibodies
Anti-BRCA1 (D-9), anti-CtIP (T-16), anti-Cdk1/2 (AN21.2), anti-Chk1 (G-4), anti-histone H1 (N-16), anti-Mre11 (C-16), anti-Rad51 (H-92), and anti-HSC70 (K-19) antibodies were purchased from Santa Cruz Biotechnology. Anti-phospho-Chk1 (Ser-345), anti-phospho-CDK1/2 (Tyr-15), anti-Nbs1, anti-Rad50, and anti-γ-H2AX-Alexa Fluor 488 antibodies were from Cell Signaling. Anti-phospho-RPA32 (S4/S8) and anti-RPA32 antibodies were from Bethyl Laboratories (Montgomery, TX). Anti-phospho-histone H1 was from EMD Biosciences. Anti-RAP80 was from Novus Biochemicals (Littleton, CO).
HRR Assays
SKOV-3-DR-GFP cells were transiently transfected with the empty vector pcDNA/Neo (Life Technologies) or the I-SceI endonuclease expression vector pCBASceI (Addgene plasmid 26477; Addgene) (25) using the TransFast reagent (Promega) according to the manufacturer’s protocol. In addition, SKOV-3 cells were transiently transfected with the EGFP expression vector pEGFP-N1 (Clontech Laboratories; Mountain View, CA) for monitoring the levels of GFP-positive cells to ensure that the expression of I-SceI was not affected by triapine treatment. Five to 6 hr after transfection, cells were treated with 0.75 μM triapine for 24 hr followed by an additional 24 hr of incubation without triapine, or treated continuously with 0.75 μM triapine for 48 hr. For co-transfection experiments, cells were transfected with 50 nM siRNA 18 hr prior to transfection with pCDASceI. Cells were then trypsinized and analyzed for the percentage of GFP-positive cells by flow cytometry using an LSR II flow cytometer (BD Biosciences) and FlowJo software (Tree Star; Ashland, OR).
Combination Index (CI) and Data Analyses
The CI analyses for evaluating the effects of two drugs in combination were performed using CalcuSyn software (Biosoft; Cambridge, UK) based on the method of Chou and Talalay (26). CI<1, CI=1, and CI>1 represent synergism, additivity, and antagonism, respectively. For statistical analyses, data were compared using the paired Student’s t-test. A p value of < 0.05 was considered statistically significant. All data were obtained from at least three independent experiments.
Results
Deficiency in BRCAs causes defective DSB repair and confers enhanced sensitivity to the PARP inhibitor olaparib
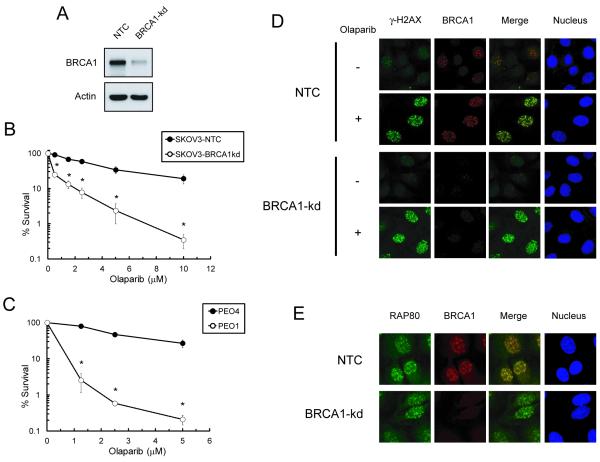
To evaluate the role of BRCAs in the response of EOC cells to PARP inhibitor-induced DSBs, clonogenic assays were also performed to determine the effects of the BRCA1 knockdown on the sensitivity of SKOV-3 cells to olaparib. SKOV-3 cells with stable BRCA1 knockdown were markedly sensitive to olaparib compared to NTC SKOV-3 cells (Fig. 1A and B). In a manner similar to BRCA1-kd SKOV3 cells, the BRCA2 mutant EOC cells PEO1 exhibited a pronounced increase in sensitivity to olaparib, compared to the isogenic BRCA2 wild-type EOC cells PEO4 (Fig. 1C). In addition, BRCA1-kd SKOV-3 and PEO1 cells exhibited increasing sensitivity to high concentrations of triapine compared to their BRCA wild-type counterparts (Fig. S1).
Fig. 1. Lack of BRCA1 foci formation and enhancement of olaparib sensitivity in BRCA deficient EOC cell lines.
A. Western blot analysis of BRCA1 levels in non-targeted siRNA control (NTC) and BRCA1-knockdown (BRCA1-kd) SKOV-3 cells. B. SKOV3 cells and C. PEO1 and PEO4 cells were exposed continuously to various concentrations of olaparib and clonogenic survival was determined. Data are means ± SD. * p<0.05 compared to NTC SKOV-3 cells at each concentration. D. Cells were untreated or treated with 5 μM olaparib for 6 hr. Immunofluorescence of γ-H2AX (green), BRCA1 (red) foci, and nuclei (blue) was visualized by confocal microscopy. E. Cells treated with 5 μM olaparib for 6 hr are shown for immunofluorescence of RAP80 (green), BRCA1 (red) foci, and nucleus (blue).
To corroborate the finding that BRCA1 knockdown caused a deficiency in localization of BRCA1 for the repair of olaparib-induced DSBs, nuclear foci of γ-H2AX, RAP80, and BRCA1 were determined by confocal microscopy. ATM/ATR-mediated phosphorylation of histone H2AX (γ-H2AX) occurs in the chromatin surrounding DSBs (27). RAP80 (receptor-associated protein 80) recruits BRCA1 to lysine 63-linked ubiquitinated H2AX at sites of DSBs (28). Olaparib induced co-localization of BRCA1 with γ-H2AX and with RAP80 in NTC SKOV-3 cells (Fig. 1D and E). In BRCA1-kd SKOV-3 cells, olaparib induced γ-H2AX and RAP80 foci but failed to induce co-localization of BRCA1 at sites of DSBs.
Triapine augments the sensitivity of BRCA wild-type EOC cells to olaparib
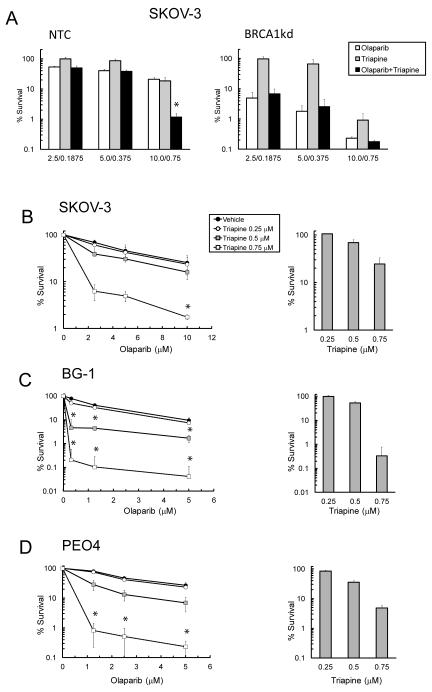
Given that triapine sensitizes cancer cells to various DNA damaging agents (12, 19), the effects of triapine on the sensitivity of EOC cells to olaparib with respect to BRCA1 status were evaluated. NTC and BRCA1-kd SKOV-3 cells were treated with the combination of olaparib and triapine in a constant ratio and clonogenic survival was determined. The combination at the highest concentrations of olaparib and triapine resulted in a synergistic sensitization of NTC SKOV-3 cells as shown by the CI analysis (Fig. 2A). In contrast, BRCA1-kd cells were sensitive to either olaparib or triapine and did not exhibit a synergistic sensitization by the combination. Similar results were also obtained using the cytotoxicity assay (Table S1).
Fig. 2. Triapine augments the sensitivities of BRCA1 wild-type EOC cells to olaparib.
A. NTC and BRCA1-kd SKOV-3 cells were treated with various concentrations of olaparib, triapine, or both agents in combination at a constant ratio (olaparib: triapine=13:1). Clonogenic survival and CI values were determined. B. SKOV-3, C. BG-1, and D. PEO4 cells were treated with various concentrations of olaparib in combination with fixed concentrations of triapine as indicated. Clonogenic survival of cells treated with triapine alone is shown in bar graphs (right). Data are means ± SD. *, CI<1.
To extend the generality of these findings, we examined the sensitivities of BRCA wild-type SKOV-3, BG-1, and PEO4 cells to a range of concentrations of olaparib in combination with various fixed levels of triapine. Triapine at 0.25 μM had minimal or no effects on the sensitivity of all EOC lines to olaparib. Triapine at 0.5 μM produced a synergistic sensitization of BG-1 cells to all concentrations of olaparib (Fig. 2C). Triapine at 0.75 μM caused synergistic sensitization of SKOV-3 cells to 10 μM olaparib, as well as of BG-1 and PEO4 cells to all concentrations of olaparib (Fig. 2B-D). All CI values for drug combinations are listed in Table S2.
Triapine abrogates olaparib-induced formation of BRCA1 and Rad51 foci in BRCA1-wild type EOC cells
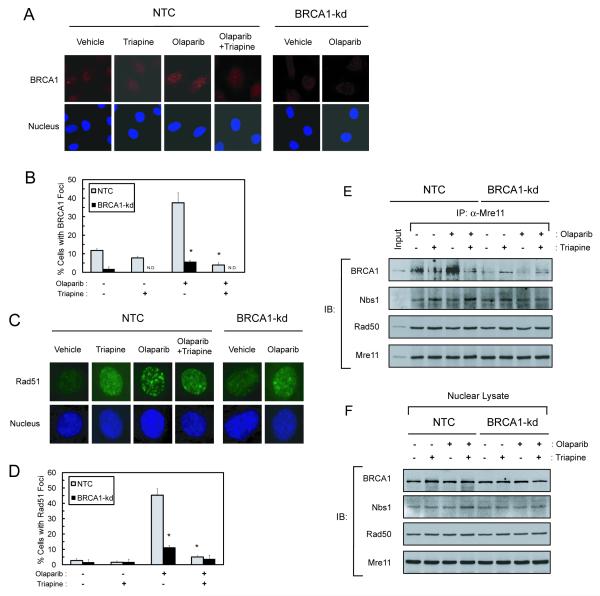
Because triapine produced pronounced sensitization of BRCA-wild type and HRR proficient EOC cells to olaparib (Fig. 2), we ascertained whether triapine had effects on BRCA1- and Rad51-mediated repair of DSBs. NTC and BRCA1-kd SKOV-3 cells were treated with triapine, olaparib, and both agents in combination. The formation of BRCA1 and Rad51 foci in nuclei were determined by confocal microscopy. Olaparib induced a marked increase in BRCA1 foci in NTC-SKOV-3 cells but not in BRCA1-kd cells. Triapine had minimal effects on the basal level of BRCA1 foci but significantly attenuated olaparib-induced BRCA1 foci in NTC cells (Fig. 3A and B). BRCA1-kd cells exhibited low levels of basal and olaparib-induced BRCA1 foci, which were obliterated by triapine (Fig. 3B).
Fig. 3. Triapine abrogates olaparib-induced nuclear repair foci and disrupts BRCA1 interaction with the MRN complex in BRCA1 wild-type SKOV-3 cells.
A, B. NTC and BRCA1-kd SKOV-3 cells were treated with 5 μM olaparib, 0.75 μM triapine, or both agents in combination for 6 hr. Cells were stained with an anti-BRCA1 antibody and nuclei were counterstained. Immunofluorescence of BRCA1 foci (red) and nuclei (blue) were visualized by confocal microscopy. Cells were scored for nuclei containing more than ten BRCA1 foci to determine the % cells with positive BRCA1 foci from 5-10 random fields. N.D. indicates no detectable foci. Data are means ± SD. * p<0.05 compared to NTC cells treated with olaparib. C, D. Cells were treated as described in A. Cells were stained with an anti-Rad51 antibody and nuclei were counterstained. Immunofluorescence of Rad51 foci (green) and nuclei (blue) were visualized by confocal microscopy. Immunofluorescence of Rad51 foci in a single cell is shown. Cells were also scored for nuclei containing more than ten distinct Rad51 foci to determine the % cells positive for Rad51 foci. Data are means ± SD. * p<0.05 compared to NTC cells treated with olaparib. E. NTC and BRCA1-kd SKOV-3 cells were treated with 5 μM olaparib, 0.75 μM triapine, or both agents in combination for 6 hr. Nuclear protein was isolated for co-immunoprecipitation with an anti-Mre11 antibody. Immunoprecipitates were analyzed for the protein levels of BRCA1, Nbs1, Rad50, and Mre11 by western blotting. Ten μg of nuclear protein from untreated NTC cells were also included as an input for positive controls in the analysis. F. Nuclear protein was analyzed by western blotting using BRCA1, Nbs1, Rad50, and Mre11 antibodies to demonstrate approximately equal amounts of protein in all lysate samples used for co-immunoprecipitation.
The formation of nuclear Rad51 foci, the key step of HRR, was also examined. At the level of single nuclei, olaparib induced punctated and distinct Rad51 foci in NTC cells but caused diffused nuclear staining in BRCA1-kd cells (Fig. 3C). Triapine abolished olaparib-induced Rad51 foci in NTC cells. Quantitative analyses of cell populations positive for distinct Rad51 foci showed a pattern (Fig. 3D) comparable to that of BRCA1 foci (Fig. 3B). Both NTC and BRCA1-kd cells exhibited similar basal levels of Rad51 foci which were not affected by triapine.
Olaparib-induced BRCA1 interaction with the MRN complex is abolished by triapine
The MRN complex comprised of Mre11, Rad50, and Nbs1 binds to the ends of DSBs and mediates end resection required for HR repair (29). BRCA1 has been shown to promote MRN-mediated end resection through interaction with the BRCA1-associated protein CtIP (24, 30). To determine whether triapine disrupts the interaction of BRCA1 with MRN, nuclear protein co-immunoprecipitation was performed. Treatment with olaparib caused a marked increase in the level of BRCA1 co-immunoprecipitated with Mre11 (Fig. 3E). Triapine attenuated the basal and olaparib-induced levels of BRCA1 in the co-immunoprecipitates of NTC-SKOV-3 cells. In BRCA1-kd cells, BRCA1 was not co-immunoprecipitated with Mre11. Both Nbs1 and Rad50, constitutive components of the MRN complex, were evenly co-immunoprecipitated with Mre11 independently of BRCA1. Treatment with olaparib and/or triapine did not affect the protein levels of BRCA1, Nbs1, Rad50, and Mre11 in nuclear lysates (Fig 3F).
Triapine activates replication checkpoints, inhibits CDK activity, and blocks CtIP phosphorylation
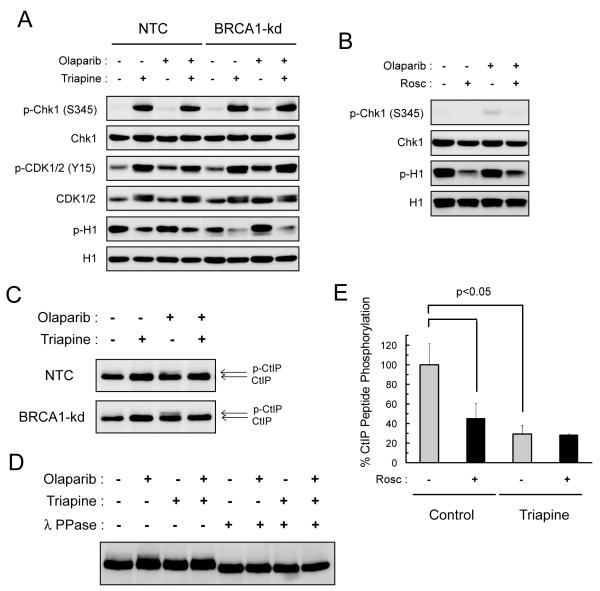
Because triapine effectively depletes dNTPs and halts DNA synthesis (14, 17, 31), the effects of triapine on replication checkpoint responses were determined. Triapine treatment caused Chk1 activation as evidenced by a marked increase in phosphorylation of Chk1 at Ser345 in both NTC and BRCA1-kd SKOV-3 cells (Fig. 4A). In contrast, olaparib caused only a minor increase in Chk1 phosphorylation, most noticeably in BRCA1-kd cells. CDK1/2 activity, which is required for S and G2/M transitions, is negatively regulated by Chk1-mediated checkpoint activation following DNA damage (32, 33). Consistent with the observation that triapine delays S phase transition (31), exposure to triapine caused an increase in phosphorylation of CDK1/2 at Tyr15, indicative of inhibition of CDK1/2 activity (34). Concurrently, a pronounced reduction in phosphorylation of histone H1, a substrate of CDK1/2 (35), occurred in both NTC and BRCA1-kd SKOV-3 cells treated with triapine. To corroborate these findings, the effects of roscovitine, a selective ATP-competitive inhibitor of CDK1/2, were also examined. Inhibition of CDK1/2 activity by roscovitine led to a reduction in phosphorylation of H1 (Fig. 4B) in a manner similar to that caused by triapine. However, roscovitine did not cause Chk1 phosphorylation because of its direct inhibitory effect on CDK1/2. In both NTC and BRCA1-kd cells, olaparib had relatively minor effects on Chk1, CDK1/2, and H1 phosphorylation.
Fig. 4. Triapine inhibits Cdk activity and blocks olaparib-induced CtIP phosphorylation.
A. NTC and BRCA1-kd SKOV-3 cells were treated with 5 μM olaparib, 0.75 μM triapine, or both agents in combination for 24 hr. Total protein was analyzed for the levels of phosphorylated and total Chk1, Cdk1/2, and histone H1 proteins by western blotting. B. NTC SKOV-3 cells were treated with 5 μM olaparib, 20 μM roscovitine (Rosc), or both agents in combination for 24 hr. Total protein was analyzed for the levels of phosphorylated and total Chk1 and histone H1 proteins. C. NTC and BRCA1-kd SKOV-3 cells were treated with 5 μM olaparib, 0.75 μM triapine, or both agents in combination for 24 hr. Total protein was analyzed for CtIP and its phosphorylation-induced band retardation. D. NTC SKOV-3 cells were treated as described in A. Total protein was treated with and without λ protein phosphatase (λ PPase), and then analyzed for CtIP by western blotting. E. NTC SKOV-3 cells were untreated or treated with 0.75 μM triapine for 24 hr. Total native protein was prepared for immunoprecipitation with an anti-CDK2 antibody. In vitro kinase reactions were performed with immunoprecipitated CDK2 and the CtIP peptide substrate in the presence or absence of 10 μM roscovitine. Levels of phosphorylation were measured by the ADP-Glo kinase assay to determine % CtIP peptide phosphorylation. Data are means ± SD.
CtIP interacts with the MRN complex and stimulates the nuclease activity of the complex that carries out end-processing/resection of DSBs necessary for HRR (24, 30, 36, 37). In addition, phosphorylation of CtIP at Ser327 is required for the interaction with BRCA1 for activation of the G2/M checkpoint and efficient HRR of DSBs (23, 30). Because CtIP is a substrate of CDKs during S and G2/M phases (23, 30) and CDK activity is inhibited by triapine (Fig 4A), the effects of triapine on CtIP phosphorylation was examined. DNA damage causes hyper-phosphorylation of CtIP manifested by retardation of band migration (36). Treatment with olaparib led to phosphorylation of CtIP in both NTC and BRCA1-kd SKOV-3 cells (Fig. 4C). Triapine abolished olaparib-induced phosphorylation of CtIP in these cells. Hyper-phosphorylated CtIP was confirmed by the treatment with the λ phosphatase that eliminated the phosphorylated form of CtIP (Fig. 4D).
To substantiate the finding that triapine inhibited CDK2, in vitro kinase activity assays were conducted using the CDK2 isolated from untreated and triapine-treated NTC SKOV-3 cells. CDK2 was immunoprecipitated and the kinase activity was assayed with the CtIP peptide substrate containing the consensus phosphorylation sequence SPVF at Ser327 (23, 38). The CDK2 isolated from triapine-treated cells exhibited a significant reduction in the activity to phosphorylate the peptide substrate compared to that from untreated cells (Fig. 4E). To confirm the specificity of CDK2, addition of roscovitine to the kinase reactions demonstrated significant suppression of substrate phosphorylation by the CDK2 from untreated cells, while producing no effect on substrate phosphorylation by the CDK2 from triapine-treated cells.
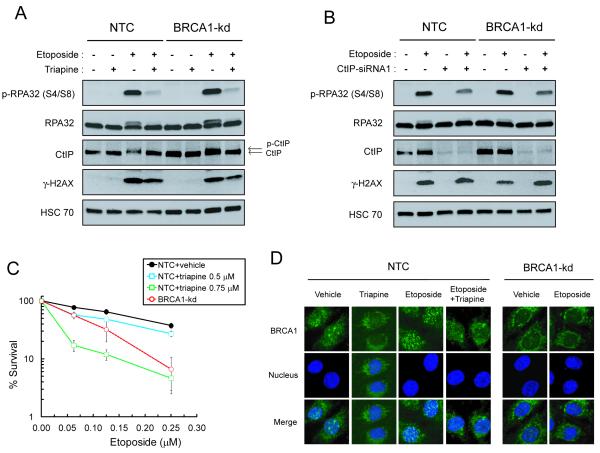
Triapine blocks CtIP-mediated RPA32 phosphorylation and sensitizes BRCA1 wild-type SKOV-3 cells to etoposide
Since olaparib-induced DSBs are indirect and possibly replication-dependent (3, 5), the inhibitory effects of triapine on DNA synthesis may potentially prevent the formation of DSBs and subsequent HRR. To rule out this possibility, we ascertained whether triapine was able to disrupt HRR of DSBs induced by etoposide, a topoisomerase II inhibitor that directly causes DSBs (36). Treatment with etoposide caused a robust induction of phospho-RPA32, which binds with high affinity to single strand DNA generated by resection of DSB ends (39) in both NTC and BRCA1-kd SKOV-3 cells (Fig. 5A). This phenomenon closely corresponded to an increase in CtIP phosphorylation. Triapine drastically attenuated etoposide-induced RPA and CtIP phosphorylation. In contrast, triapine only caused a minor reduction in the level of etoposide-induced γH2AX, confirming the finding that etoposide-induced formation of DSBs was not prevented by triapine. RPA32 phosphorylation was also induced by olaparib at much higher concentrations, which was counteracted by triapine (Fig. S2). Given that CtIP is required to promote DSB resection (36, 37), the role of CtIP in etoposide-induced RPA32 phosphorylation was determined. Depletion of CtIP attenuated etoposide-induced phosphorylation of RPA32 in both NTC and BRCA1-kd SKOV3 cells (Fig. 5B). Consistent with the findings in Fig. 5A, BRCA1-kd cells did not exhibit a decrease in RPA32 phosphorylation compared to NTC cells. Depletion of Chk1 partially reversed the inhibitory effects of triapine on etoposide-induced phosphorylation of RPA32 and CtIP (Fig. S3). This observation substantiates the finding that triapine restrains CtIP-dependent DSB end resection through activation of Chk1.
Fig. 5. Triapine blocks CtIP-dependent DSB resection in SKOV-3 cells treated with etoposide.
A. NTC and BRCA1-kd SKOV-3 cells were treated with 5 μM etoposide, 0.75 μM triapine, or both agents in combination for 4 hr. Total protein was analyzed for the levels of phospho-RPA32 (S4/S8), RPA32, CtIP, γ-H2AX, HSC70 by western blotting. The HSC70 level was used as a loading control. B. Cells were transfected with 50 nM CtIP-siRNA1. After 48 hr, cells were treated with 5 μM etoposide for 4 hr and total protein was analyzed by western blotting as described in A. C. NTC SKOV-3 cells were treated with various concentrations of etoposide in combination with fixed concentrations of triapine as indicated. BRCA1-kd cells were also treated with various concentrations of etoposide in the absence of triapine. Colonies were stained and clonogenic survival was determined. Data are means ± SD. D. NTC and BRCA1-kd SKOV-3 cells were treated with 5 μM etoposide, 0.75 μM triapine, or both agents in combination for 4 hr. Cells were stained with an anti-BRCA1 antibody and counterstained for nuclei. Immunofluorescence of BRCA1 foci (green) and nuclei (blue) were visualized by confocal microscopy.
In addition, we determined whether triapine sensitized BRCA1 wild-type EOC cells to etoposide. Treatment with triapine at 0.5 μM produced a minor increase in the sensitivity of NTC SKOV-3 cells over a range of etoposide concentrations (Fig. 5C). Triapine at 0.75 μM caused a considerable increase in sensitivity of NTC cells to etoposide. To corroborate these findings, we ascertained whether triapine had effects on etoposide-induced BRCA1 foci. In a manner comparable to olaparib (Fig. 3A), etoposide induced BRCA1 foci in NTC cells but not in BRCA1-kd cells (Fig. 5D). Furthermore, triapine effectively abolished etoposide-induced BRCA1 foci in NTC cells.
Triapine or CtIP depletion impairs the repair of I-SceI-generated DSBs in BRCA1-wild type SKOV-3 cells
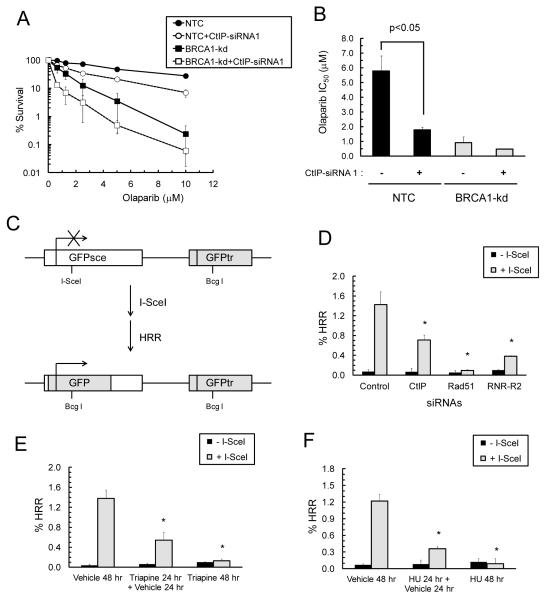
To substantiate the findings that functional ablation of CtIP by triapine caused impairment of HRR, we determined whether depletion of CtIP by siRNA mimicked the effects of triapine on the sensitivity of EOC cells to olaparib. siRNA-mediated depletion of CtIP caused a significant increase in the sensitivity of NTC cells to the entire range of olaparib (Fig. S4 and 6A). Measurement of the IC50 of olaparib for each group of transfected cells confirmed that CtIP depletion significantly increased the sensitivity of NTC cells, but not BRCA1-kd cells, to olaparib (Fig. 6B).
Fig. 6. Triapine and CtIP depletion causes impairment of HRR and sensitization of BRCA1-wild type SKOV-3 cells to olaparib.
A. NTC and BRCA1-kd SKOV-3 cells were transfected with CtIP-siRNA1 for 48 hr and treated continuously with various concentrations of olaparib. Colonies were stained and clonogenic survival was determined. Data are means ± SD. B. The IC50 for each dose response curve from B was determined. Data are means ± SD. * p<0.05 compared to cells transfected with control-siRNA. C. The GFP-based HRR reporter gene construct DR-GFP stably integrated in SKOV-3 cells. Transient expression of I-SceI introduces a DSB at the I-SceI site in the inactivated GFPsce gene. The repair of DSB by HRR using the truncated GFP gene (grey-shaded) located downstream produces functional GFP gene. D. SKOV-3-DR-GFP cells were transfected with control-, Rad51-, RNR-R2-, or CtIP-siRNA. After 18 hr, cells were transfected with an empty vector (−SceI) or the I-SceI expression vector (+SceI) and incubated for 48 hr. GFP-positive cells were quantified by flow cytometry and expressed as %HRR. * p<0.05 compared to cells transfected with control-siRNA and the I-SceI expression vector. E. Five hr after transfection with I-SceI expression vector, cells were treated with vehicle for 48 hr, with 0.75 μM triapine for 24 hr followed by a 24 hr triapine-free incubation, or with 0.75 μM triapine for 48 hr. F. Transfected cells were treated with vehicle for 48 hr, with 1 mM hydroxyurea (HU) for 24 hr followed by a 24 hr HU-free incubation, or with 1 mM HU for 48 hr. GFP-positive cells were quantified by flow cytometry and expressed as %HRR. * p<0.05 compared to cells transfected with the I-SceI expression vector and treated with vehicle. Data are means ± SD.
To further evaluate the direct impact of triapine or CtIP depletion on HRR, we conducted a GFP-based reporter assay to monitor the level of HRR activity upon introduction of a DSB by transient expression of I-SceI endonuclease (22) in SKOV-3-DR-GFP cells (Fig. 6C). At 48 hr post-transfection, I-SceI expression caused an increase in GFP-positive cells indicative of elevated HRR activity (Fig. 6D, E and F). siRNA-mediated depletion of CtIP, Rad51, or RNR-R2 significantly attenuated I-SceI-induced HRR activity (Fig. 6D). Treatment with triapine for 24 hr followed by an additional 24 hr recovery period resulted in more than a 50% decrease in I-SceI-induced HRR activity (Fig 6E). Furthermore, treatment with triapine for 48 hr without a recovery period led to nearly complete abrogation of I-SceI-induced HRR activity. Similar results were also obtained by exposure to hydroxyurea (Fig. 6F). These results corroborate the findings that RNR inhibition by triapine causes impairment of CtIP-dependent HRR and sensitizes BRCA1-wild type EOC cells to DSBs.
Discussion
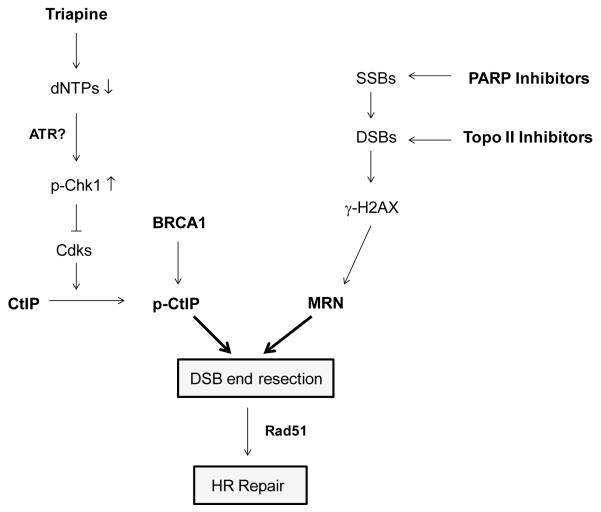
Triapine has been evaluated in both preclinical studies and clinical trials primarily as a chemo- and radiation-sensitizer for the treatment of solid tumors (12, 19). The rationale for the utility of triapine is based upon the notion that triapine causes depletion of dNTPs required for DNA repair following chemotherapy or radiation-induced DNA damage. The present studies produce further mechanistic insight into the DNA repair processes influenced by triapine. By activating the Chk1-mediated replication checkpoint pathway, triapine disrupts the function of CtIP in DSB resection, thereby impairing HRR of PARP and topisomerase II inhibitor-induced DSBs in EOC cells. Our results from GFP-based HRR assays further substantiate the findings that triapine-mediated inhibition of RNR abrogates HRR in BRCA1 wild-type EOC. We speculate that triapine activates the ATR-Chk1 axis by single-stranded DNA exposed at stalled replication forks due to depletion of dNTPs (40). The postulated model of impairment of the CtIP-dependent HRR pathway by triapine is illustrated in Fig. 7. This described mechanism provides an explanation for why triapine augments the sensitivity of BRCA wild-type EOC cells to PARP and topoisomerase inhibitors. However, we do not rule out the possibility that triapine-mediated chemosensitization in EOC is also produced by other mechanisms such as the formation of replication-associated DSBs, metal chelation, and/or reactive oxygen species. These possible pleiotropic effects of triapine await future investigation.
Fig. 7. Proposed model for impairment of HRR by triapine in EOC cells.
Triapine causes depletion of dNTPs and activation of Chk1, which in turn blocks CDK-mediated phosphorylation of CtIP. As a result, CtIP-mediated resection of DSBs caused by PARP and topoisomerase II inhibitor is abrogated, leading to impairment of HRR.
On the basis of our findings, we propose that the inhibitory effects of triapine on HRR are largely due to a reduction in CDK activity. Cumulative evidence indicates that the kinase activity of CDKs is necessary for HRR during S and G2 phases of the cell cycle. CDK activity is required to promote DSB resection and initiate the HRR pathway (41). CtIP is one of many DNA damage/repair proteins targeted by CDKs for phosphorylation (41). CDK2-mediated phosphorylation of CtIP at Ser327 is necessary for the interaction of CtIP with the BRCA1-BRCT domain to activate the G2/M checkpoint and to promote HRR (23, 24, 30). In addition, CtIP interacts with the FHA domain of Nbs1 of the MRN complex to stimulate the nuclease activity of Mre11 for DSB resection (42, 43). Our results indicate that triapine causes Chk1 activation which in turn suppresses CDK2 activity (Fig. 4). A recent study reports that CDK-mediated phosphorylation of CtIP promotes the interaction of CtIP with the FHA domain of Nbs1, which is required for subsequent ATM-mediated phosphorylation of CtIP upon DNA damage (44). These findings support our observation that triapine abolishes olaparib- and etoposide-induced CtIP phosphorylation (Figs. 4 and 5) possibly mediated by ATM. In addition, phosphorylation of CtIP at Ser327 may bridge or facilitate the interaction between BRCA1 and the MRN complex (30). Therefore, by blocking this phosphorylation event in CtIP, triapine reduces BRCA1 co-immunoprecipitation with the MRN complex (Fig. 3E) and BRCA1 foci formation induced by olaparib or etoposide at sites of DSBs (Figs. 3A and 5D). In agreement with these findings, an early report demonstrates that hydroxyurea causes BRCA1 to dissociate from the MRN complex (45). Furthermore, it has recently been shown that BRCA1 is required to stabilize hydroxyurea-induced stalled replication forks (46). We postulate that triapine may lead to redistribution of BRCA1 to stalled replication forks, thereby reducing the interaction of BRCA1 with the MRN for HRR.
Although the interaction of BRCA1 with CtIP has been shown to be important for facilitating HRR (24, 30), our results indicate that stable BRCA1 knockdown has no negative impact on etoposide-induced DSB resection (Fig. 5A and B). Evidence shows that CtIP interaction with BRCA1 is required for ubiquitination and localization of CtIP to damaged chromatin (47). Given that the basal level of CtIP is considerably elevated in BRCA1-kd SKOV-3 cells (Figs. 5A and B, S4), we speculate that the increased level of CtIP may promote its interaction with the MRN complex and compensate for the loss of BRCA1 function in facilitating DSB resection. Because of the multifaceted roles of BRCA1 in the HRR pathway, BRCA1 is also critically involved in the recruitment of PALB2 and BRCA2, and the subsequent loading of Rad51 onto resected single-stranded DSB ends (48). Therefore, even with no effects on DSB resection, stable BRCA1 knockdown still causes impairment of HRR and confers hypersensitivity to DSBs caused by PARP and topoisomerase inhibitors.
Here we have elucidated the molecular mechanism by which triapine sensitizes BRCA wild-type EOC to drug-induced DSBs. The results also provide the rationale for the use of triapine in combination with a PARP inhibitor or a DSB-inducing agent to treat EOC with functional HRR. Such an approach augments the effectiveness of DSB-inducing agents and broadens the use of PARP inhibitors to treat sporadic EOC that accounts for the majority of EOC cases. Cumulative evidence indicates that functional p53 prevents formation of replication-associated DSBs and down-regulates Rad51-dependent HRR (49, 50). With p53 mutations/dysfunction being a common feature of EOC, our proposed combination therapy with triapine should exhibit selectivity towards EOC and have minimal impact on normal tissue.
Supplementary Material
Acknowledgments
This work was supported in part by US Public Health Service Grants CA122112 and CA129186 from the National Cancer Institute and by the National Foundation for Cancer Research (A.C.S.); and by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Grant K12HD047018 (E.S.R.).
Footnotes
The authors have no conflicts of interest to disclose.
Implications
These findings provide a strong rationale for combining triapine with PARP or topoisomerase inhibitors to target HRR-proficient EOC cells.
References
- 1.Gordon AN, Fleagle JT, Guthrie D, Parkin DE, Gore ME, Lacave AJ. Recurrent epithelial ovarian carcinoma: a randomized phase III study of pegylated liposomal doxorubicin versus topotecan. J Clin Oncol. 2001;19:3312–22. doi: 10.1200/JCO.2001.19.14.3312. [DOI] [PubMed] [Google Scholar]
- 2.Ratner ES, Sartorelli AC, Lin ZP. Poly (ADP-ribose) polymerase inhibitors: on the horizon of tailored and personalized therapies for epithelial ovarian cancer. Curr Opin Oncol. 2012;24:564–71. doi: 10.1097/CCO.0b013e3283564230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnaudeau C, Lundin C, Helleday T. DNA double-strand breaks associated with replication forks are predominantly repaired by homologous recombination involving an exchange mechanism in mammalian cells. J Mol Biol. 2001;307:1235–45. doi: 10.1006/jmbi.2001.4564. [DOI] [PubMed] [Google Scholar]
- 4.Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913–7. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- 5.Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–21. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 6.Audeh MW, Carmichael J, Penson RT, Friedlander M, Powell B, Bell-McGuinn KM, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet. 2010;376:245–51. doi: 10.1016/S0140-6736(10)60893-8. [DOI] [PubMed] [Google Scholar]
- 7.Kaye SB, Lubinski J, Matulonis U, Ang JE, Gourley C, Karlan BY, et al. Phase II, open-label, randomized, multicenter study comparing the efficacy and safety of olaparib, a poly (ADP-ribose) polymerase inhibitor, and pegylated liposomal doxorubicin in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer. J Clin Oncol. 2012;30:372–9. doi: 10.1200/JCO.2011.36.9215. [DOI] [PubMed] [Google Scholar]
- 8.Norquist B, Wurz KA, Pennil CC, Garcia R, Gross J, Sakai W, et al. Secondary somatic mutations restoring BRCA1/2 predict chemotherapy resistance in hereditary ovarian carcinomas. J Clin Oncol. 2011;29:3008–15. doi: 10.1200/JCO.2010.34.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swisher EM, Sakai W, Karlan BY, Wurz K, Urban N, Taniguchi T. Secondary BRCA1 mutations in BRCA1-mutated ovarian carcinomas with platinum resistance. Cancer Res. 2008;68:2581–6. doi: 10.1158/0008-5472.CAN-08-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakai W, Swisher EM, Jacquemont C, Chandramohan KV, Couch FJ, Langdon SP, et al. Functional restoration of BRCA2 protein by secondary BRCA2 mutations in BRCA2-mutated ovarian carcinoma. Cancer Res. 2009;69:6381–6. doi: 10.1158/0008-5472.CAN-09-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu MC, Lin TS, Sartorelli AC. Synthesis and antitumor activity of amino derivatives of pyridine-2-carboxaldehyde thiosemicarbazone. J Med Chem. 1992;35:3672–7. doi: 10.1021/jm00098a012. [DOI] [PubMed] [Google Scholar]
- 12.Finch RA, Liu M, Grill SP, Rose WC, Loomis R, Vasquez KM, et al. Triapine (3-aminopyridine-2-carboxaldehyde-thiosemicarbazone): A potent inhibitor of ribonucleotide reductase activity with broad spectrum antitumor activity. Biochem Pharmacol. 2000;59:983–91. doi: 10.1016/s0006-2952(99)00419-0. [DOI] [PubMed] [Google Scholar]
- 13.Finch RA, Liu MC, Cory AH, Cory JG, Sartorelli AC. Triapine (3-aminopyridine-2-carboxaldehyde thiosemicarbazone; 3-AP): an inhibitor of ribonucleotide reductase with antineoplastic activity. Adv Enzyme Regul. 1999;39:3–12. doi: 10.1016/s0065-2571(98)00017-x. [DOI] [PubMed] [Google Scholar]
- 14.Lin ZP, Belcourt MF, Cory JG, Sartorelli AC. Stable suppression of the R2 subunit of ribonucleotide reductase by R2-targeted short interference RNA sensitizes p53(−/−) HCT-116 colon cancer cells to DNA-damaging agents and ribonucleotide reductase inhibitors. J Biol Chem. 2004;279:27030–8. doi: 10.1074/jbc.M402056200. [DOI] [PubMed] [Google Scholar]
- 15.Thelander L, Reichard P. Reduction of ribonucleotides. Annu Rev Biochem. 1979;48:133–58. doi: 10.1146/annurev.bi.48.070179.001025. [DOI] [PubMed] [Google Scholar]
- 16.Sartorelli AC, Agrawal KC, Tsiftsoglou AS, Moore EC. Characterization of the biochemical mechanism of action of alpha-(N)-heterocyclic carboxaldehyde thiosemicarbazones. Adv Enzyme Regul. 1976;15:117–39. doi: 10.1016/0065-2571(77)90012-7. [DOI] [PubMed] [Google Scholar]
- 17.Lin ZP, Lee Y, Lin F, Belcourt MF, Li P, Cory JG, et al. Reduced level of ribonucleotide reductase R2 subunits increases dependence on homologous recombination repair of cisplatin-induced DNA damage. Mol Pharmacol. 2011;80:1000–12. doi: 10.1124/mol.111.074708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cory JG, Cory AH, Rappa G, Lorico A, Liu MC, Lin TS, et al. Structure-function relationships for a new series of pyridine-2-carboxaldehyde thiosemicarbazones on ribonucleotide reductase activity and tumor cell growth in culture and in vivo. Adv Enzyme Regul. 1995;35:55–68. doi: 10.1016/0065-2571(94)00005-n. [DOI] [PubMed] [Google Scholar]
- 19.Kunos CA, Waggoner S, von Gruenigen V, Eldermire E, Pink J, Dowlati A, et al. Phase I trial of pelvic radiation, weekly cisplatin, and 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP, NSC #663249) for locally advanced cervical cancer. Clin Cancer Res. 2010;16:1298–306. doi: 10.1158/1078-0432.CCR-09-2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barker CA, Burgan WE, Carter DJ, Cerna D, Gius D, Hollingshead MG, et al. In vitro and in vivo radiosensitization induced by the ribonucleotide reductase inhibitor Triapine (3-aminopyridine-2-carboxaldehyde-thiosemicarbazone) Clin Cancer Res. 2006;12:2912–8. doi: 10.1158/1078-0432.CCR-05-2860. [DOI] [PubMed] [Google Scholar]
- 21.Kim H, Chen J, Yu X. Ubiquitin-binding protein RAP80 mediates BRCA1-dependent DNA damage response. Science. 2007;316:1202–5. doi: 10.1126/science.1139621. [DOI] [PubMed] [Google Scholar]
- 22.Pierce AJ, Johnson RD, Thompson LH, Jasin M. XRCC3 promotes homology-directed repair of DNA damage in mammalian cells. Genes Dev. 1999;13:2633–8. doi: 10.1101/gad.13.20.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu X, Chen J. DNA damage-induced cell cycle checkpoint control requires CtIP, a phosphorylation-dependent binding partner of BRCA1 C-terminal domains. Mol Cell Biol. 2004;24:9478–86. doi: 10.1128/MCB.24.21.9478-9486.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yun MH, Hiom K. CtIP-BRCA1 modulates the choice of DNA double-strand-break repair pathway throughout the cell cycle. Nature. 2009;459:460–3. doi: 10.1038/nature07955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richardson C, Moynahan ME, Jasin M. Double-strand break repair by interchromosomal recombination: suppression of chromosomal translocations. Genes Dev. 1998;12:3831–42. doi: 10.1101/gad.12.24.3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 27.Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem. 1998;273:5858–68. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- 28.Wu J, Liu C, Chen J, Yu X. RAP80 protein is important for genomic stability and is required for stabilizing BRCA1-A complex at DNA damage sites in vivo. J Biol Chem. 2012;287:22919–26. doi: 10.1074/jbc.M112.351007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bernstein KA, Rothstein R. At loose ends: resecting a double-strand break. Cell. 2009;137:807–10. doi: 10.1016/j.cell.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen L, Nievera CJ, Lee AY, Wu X. Cell cycle-dependent complex formation of BRCA1.CtIP.MRN is important for DNA double-strand break repair. J Biol Chem. 2008;283:7713–20. doi: 10.1074/jbc.M710245200. [DOI] [PubMed] [Google Scholar]
- 31.Lin ZP, Belcourt MF, Carbone R, Eaton JS, Penketh PG, Shadel GS, et al. Excess ribonucleotide reductase R2 subunits coordinate the S phase checkpoint to facilitate DNA damage repair and recovery from replication stress. Biochem Pharmacol. 2007;73:760–72. doi: 10.1016/j.bcp.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 32.Sanchez Y, Wong C, Thoma RS, Richman R, Wu Z, Piwnica-Worms H, et al. Conservation of the Chk1 checkpoint pathway in mammals: linkage of DNA damage to Cdk regulation through Cdc25. Science (New York, NY) 1997;277:1497–501. doi: 10.1126/science.277.5331.1497. [DOI] [PubMed] [Google Scholar]
- 33.Zhao H, Watkins JL, Piwnica-Worms H. Disruption of the checkpoint kinase 1/cell division cycle 25A pathway abrogates ionizing radiation-induced S and G2 checkpoints. Proc Natl Acad Sci U S A. 2002;99:14795–800. doi: 10.1073/pnas.182557299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gu Y, Rosenblatt J, Morgan DO. Cell cycle regulation of CDK2 activity by phosphorylation of Thr160 and Tyr15. EMBO J. 1992;11:3995–4005. doi: 10.1002/j.1460-2075.1992.tb05493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo CY, Wang Y, Brautigan DL, Larner JM. Histone H1 dephosphorylation is mediated through a radiation-induced signal transduction pathway dependent on ATM. J Biol Chem. 1999;274:18715–20. doi: 10.1074/jbc.274.26.18715. [DOI] [PubMed] [Google Scholar]
- 36.Sartori AA, Lukas C, Coates J, Mistrik M, Fu S, Bartek J, et al. Human CtIP promotes DNA end resection. Nature. 2007;450:509–14. doi: 10.1038/nature06337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huertas P, Jackson SP. Human CtIP mediates cell cycle control of DNA end resection and double strand break repair. J Biol Chem. 2009;284:9558–65. doi: 10.1074/jbc.M808906200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams RS, Lee MS, Hau DD, Glover JN. Structural basis of phosphopeptide recognition by the BRCT domain of BRCA1. Nat Struct Mol Biol. 2004;11:519–25. doi: 10.1038/nsmb776. [DOI] [PubMed] [Google Scholar]
- 39.Patrick SM, Oakley GG, Dixon K, Turchi JJ. DNA damage induced hyperphosphorylation of replication protein A. 2. Characterization of DNA binding activity, protein interactions, and activity in DNA replication and repair. Biochemistry. 2005;44:8438–48. doi: 10.1021/bi048057b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zou L. Single- and double-stranded DNA: building a trigger of ATR-mediated DNA damage response. Genes Dev. 2007;21:879–85. doi: 10.1101/gad.1550307. [DOI] [PubMed] [Google Scholar]
- 41.Yata K, Esashi F. Dual role of CDKs in DNA repair: to be, or not to be. DNA Repair (Amst) 2009;8:6–18. doi: 10.1016/j.dnarep.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 42.Lloyd J, Chapman JR, Clapperton JA, Haire LF, Hartsuiker E, Li J, et al. A supramodular FHA/BRCT-repeat architecture mediates Nbs1 adaptor function in response to DNA damage. Cell. 2009;139:100–11. doi: 10.1016/j.cell.2009.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Williams RS, Dodson GE, Limbo O, Yamada Y, Williams JS, Guenther G, et al. Nbs1 flexibly tethers Ctp1 and Mre11-Rad50 to coordinate DNA double-strand break processing and repair. Cell. 2009;139:87–99. doi: 10.1016/j.cell.2009.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang H, Shi LZ, Wong CC, Han X, Hwang PY, Truong LN, et al. The interaction of CtIP and Nbs1 connects CDK and ATM to regulate HR-mediated double-strand break repair. PLoS Genetics. 2013;9:e1003277. doi: 10.1371/journal.pgen.1003277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chiba N, Parvin JD. Redistribution of BRCA1 among four different protein complexes following replication blockage. J Biol Chem. 2001;276:38549–54. doi: 10.1074/jbc.M105227200. [DOI] [PubMed] [Google Scholar]
- 46.Schlacher K, Wu H, Jasin M. A distinct replication fork protection pathway connects Fanconi anemia tumor suppressors to RAD51-BRCA1/2. Cancer cell. 2012;22:106–16. doi: 10.1016/j.ccr.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu X, Fu S, Lai M, Baer R, Chen J. BRCA1 ubiquitinates its phosphorylation-dependent binding partner CtIP. Genes Dev. 2006;20:1721–6. doi: 10.1101/gad.1431006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sy SM, Huen MS, Chen J. PALB2 is an integral component of the BRCA complex required for homologous recombination repair. Proc Natl Acad Sci U S A. 2009;106:7155–60. doi: 10.1073/pnas.0811159106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Squires S, Coates JA, Goldberg M, Toji LH, Jackson SP, Clarke DJ, et al. p53 prevents the accumulation of double-strand DNA breaks at stalled-replication forks induced by UV in human cells. Cell Cycle. 2004;3:1543–57. doi: 10.4161/cc.3.12.1272. [DOI] [PubMed] [Google Scholar]
- 50.Yoon D, Wang Y, Stapleford K, Wiesmuller L, Chen J. P53 inhibits strand exchange and replication fork regression promoted by human Rad51. J Mol Biol. 2004;336:639–54. doi: 10.1016/j.jmb.2003.12.050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.