Abstract
While the immune microenvironment has been investigated in breast cancers, little is known about its role in non-malignant breast tissues. Here we quantify and localize cellular immune components in normal breast tissue lobules, with and without visible immune infiltrates (lobulitis). Up to ten representative lobules each in eleven normal breast tissue samples were assessed for B cells (CD20), cytotoxic T cells (CD8), helper T cells (CD4), dendritic cells (CD11c), leukocytes (CD45), and monocytes/macrophages (CD68). Using digital image analysis, immune cell densities were measured and compared between lobules with/without lobulitis. 109 lobules in 11 normal breast tissue samples were evaluated; 31 with lobulitis and 78 without. Immune cells showed consistent patterns in all normal samples, predominantly localized to lobules rather than stroma. Regardless of lobulitis status, most lobules demonstrated CD8+, CD11c+, CD45+, and CD68+ cells, with lower densities of CD4+ and CD20+ cells. Both CD11c+ and CD8+ cells were consistently and intimately associated with the basal aspect of lobule epithelium. Significantly higher densities of CD4+, CD8+, CD20+, and CD45+ cells were observed in lobules with lobulitis. In contrast, densities of monocytes/macrophages and dendritic cells did not vary with lobulitis. In normal breast tissue, myeloid and lymphoid cells are present and localized to lobules, with cytotoxic T and dendritic cells directly integrated with epithelium. Lobules with lobulitis have significantly more adaptive immune (B and T) cells, but no increase in dendritic cells or monocytes/macrophages. These findings indicate an active and dynamic mucosal immune system in normal breast tissue.
Keywords: Immune cell, Breast lobules, Lobulitis, Mucosal immunity, Cancer immunosuppression
Introduction
The role of interactions between epithelial cells, fibroblasts, and adipocytes in normal mammary gland function has been extensively investigated; however, the role of the immune system in maintaining the mammary gland is less understood. The immune system is known to play key roles during mammary gland branching morphogenesis, lactation, post-lactational involution, and breast cancer progression [1–3]. During lactation, secretory IgA targeted against infectious agents is produced in breast milk, providing a surrogate immune system during the first few weeks of life of the infant [4, 5] and mucosal immunity protects the mammary gland from microbial infection (mastitis) [6]. During post-lactational involution, both mast cells and macrophages are key mediators of glandular regression [7, 8]. Immune cell function has also been extensively linked to progression of breast cancer [9–11]. While much of our knowledge about the specific roles of immune cells in the mammary gland have been derived from studies using experimental animals, these have glandular organization distinct from the human breast [12], and very little is known about baseline levels of immune cells in normal, non-malignant human breast tissue in the quiescent, non-lactating state from which most breast cancers develop.
Mucosal immunity develops at interfaces between the external environment and body tissues. In the gut, antigenic exposures from food and microorganisms shape the mucosal immune system [13], and the interaction of diet, intestinal microbiota, and mucosal immunity play a key role in the development of colorectal cancer [14] and extraintestinal neoplasms [15]. There are corollaries between intestinal mucosal immunity and that of the breast. For example, just as there are commensal organisms in the gut, lactobacilli species have been identified in lactating breasts [16], and may have a role in recovery from mastitis [17]. Given the role of aberrant gut mucosal immunity on carcinogenesis, it is possible that mucosal immunity of the breast can also affect the development of breast neoplasms.
We have previously identified histologic features of premalignant and normal breast tissue that are associated with increased breast cancer risk [18, 19]. We have found that age-related lobular involution of breast lobules (the natural regression of breast epithelium over time, distinct from post-lactational involution) is associated with decreased breast cancer risk [20, 21]. In a recent comparison of normal breast tissues versus those showing benign breast disease [22], we observed that immune infiltrates were common in lobules of normal breast tissue. Here, we examined the immune microenvironment in normal human breast tissues to define the baseline state of immune cell presence in the non-lactational adult state.
Methods
Tissue samples
Approval was obtained from the Institutional Review Board to conduct this research. Normal breast tissue samples were obtained from the Komen Tissue Bank at the Indiana University Simon Cancer Center [23]. From a large sample of 455 normal breast tissues previously characterized histologically for epithelial abnormalities and age-related involution [22], we selected a small number of samples for an intensive study to quantitate immune cells of various types within breast tissue lobules. In order to evaluate differences between samples with and without lobulitis, we selected samples to represent both strata of lobulitis categories (present versus absent). Age-related involution of lobules is a histologic feature associated with breast cancer risk; with lower risk seen in samples with smaller, more completely involuted lobules [20, 21]. Therefore, in order to evaluate lobules representing different states of age-related involution, we also stratified the sample selection by involution categories [minimal involution (1–24 %), partial involution (25–74 %), and complete involution (≥75 %)]. From prior review of these normal specimens [22], we had data on the number of lobules within each specimen, and we selected samples with an adequate number of lobules to evaluate (8 or more). Thus, among 107 samples meeting these criteria, we randomly selected two samples from each of 6 categories defined by involution status and lobulitis. One category (lobulitis absent and minimal involution) had only 1 sample, resulting in a total of 11 samples selected for the final study group. These eleven samples underwent multiple immunostains and comprised our analysis sample for this descriptive study. Tissue sections from each sample underwent one H&E stain and the following immunostains: CD45 (leukocytes), CD20 (B cells), CD4 (helper T cells), CD8 (cytotoxic T cells), CD11c (dendritic cells), and CD68 (monocytes/macrophages). Two positive control tissues representing non-malignant states with immune infiltrates underwent the same immunostains (resolving lactation [24, 25] and diabetic mastopathy [26, 27]).
Histologic review
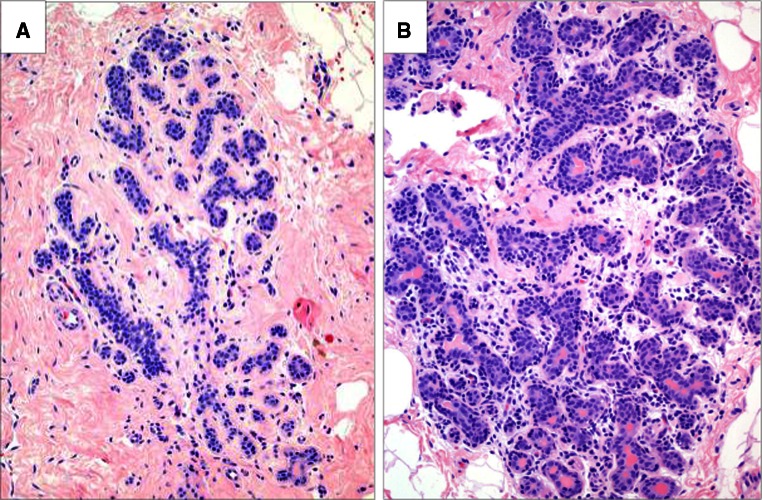
H&E slides were reviewed by the study pathologist. Up to 10 representative lobules from each tissue sample were selected for individual analysis. Lobulitis was defined on a per lobule basis as an immune cell infiltrate involving a lobule in which the intralobular stroma showed readily identifiable lymphocyte nuclei by H&E staining at low magnification (40×), and at least 4 lymphocytes between the adjacent acini at higher magnification (400×) (Fig. 1). The study pathologist, with other members of the team, reviewed immunostains at low and high power for localization of immune cells. Selected lobules were digitally annotated (see “Slide digitization and lobule annotation” section below) for quantification of immune cell densities. For illustrative purposes (see Figures), photomicrographs of representative lobules at 400× magnification were obtained using an Olympus 400 camera attached to a microscope.
Fig. 1.
The presence of immune infiltrates that define lobulitis. a Normal lobule, without lobulitis; b normal lobule, with lobulitis. H&E stain, ×200 magnification
Immunohistochemical staining
Immunohistochemical staining procedures were described previously [28]. For immunostains [CD4 (Leica Novocastra NCL-CD4-368-L-CE at 1/50), CD8 (DAKO M7103 at 1/20), CD11c (Leica Novocastra NCL-L-CD11c-563 at 1/25), CD20 (DAKO M0755at 1/60), CD45(DAKO M0710 at 1/1500), and CD68 (DAKO, M0876 at 1/100)], antibodies were prepared at stated dilutions and slides were incubated for 30 min at room temperature.
Slide digitization and lobule annotation
Whole slide digital images of each breast biopsy sample (H&E and immunostains) were captured with the Aperio Scanscope XT slide scanner (Aperio Technologies) using the 20× objective lens. The digital images were analyzed using Aperio ImageScope software (http://www.aperio.com/pathology-services/imagescope-slide-viewing-software.asp). Quantitative image analysis was performed using Spectrum version 11, based on the FDA-approved algorithms supplied by the manufacturer with modifications as described below. Using the digitized images, lobules were selected by the pathologist from the H&E slide, circled, and numerically labeled. For each immunostain on a given sample, the corresponding (same) lobules identified on the H&E slide were identified on the successive immunostain sections, similarly circled, and assigned the corresponding lobule number. The area of each circled lobule was calculated by Spectrum.
Digital image analysis and cell counts
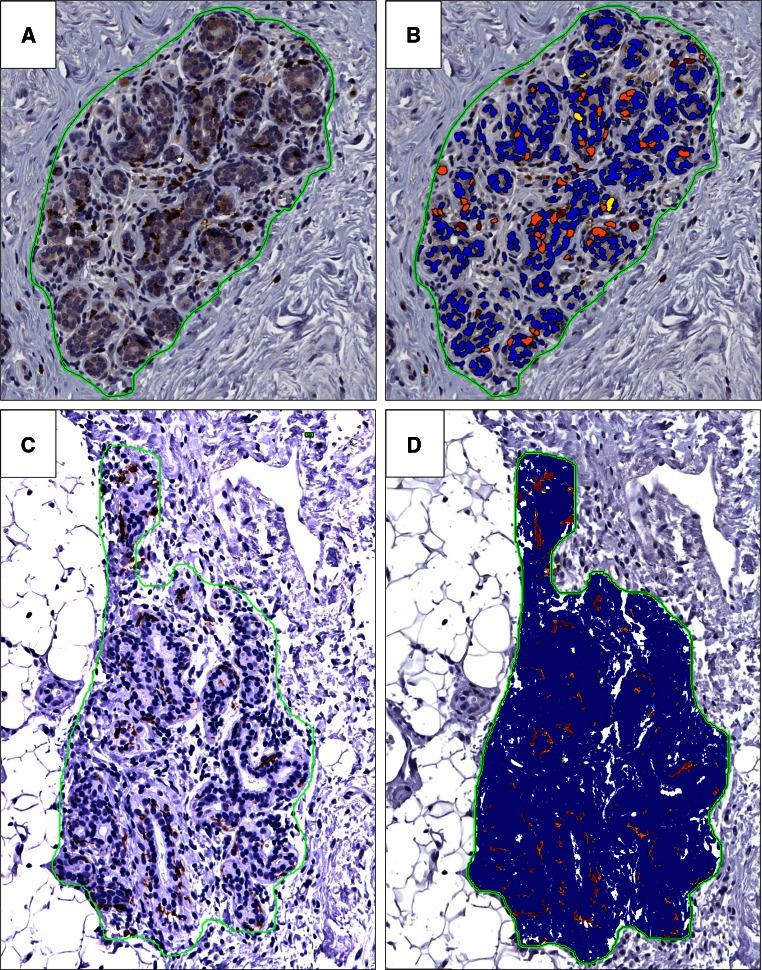
After lobule annotation, positively stained cells were counted using Spectrum software (Aperio Technologies). The manufacturer’s FDA-approved algorithms were used with customization of the parameters as follows: for each immunostain, a lobule was selected that had distinct positive and negative cells. Initially, the nuclear algorithm was used to distinguish stained from unstained cells. A digital color overlay was reviewed with the pathologist to determine concordance between algorithm and breast pathologist. The algorithm settings were then adjusted iteratively until the capture of positive cells was optimized. The algorithm was then applied to another lobule from a different sample to confirm the optimization. Once optimized, the algorithm for each immunostain was then applied uniformly to all circled lobules for that immunostain on all samples, and positively stained cells were recorded on a per lobule basis for each sample. For the dendritic cell immunostain, CD11c was measured as a ratio of positive to total pixels due to a more diffuse pattern of particle staining (Fig. 2). For the CD45 immunostain, more extensive cell counting analysis was performed to determine the density of positively stained cells within lobules versus in the remaining breast tissue. Therefore, for CD45 sections, every lobule was circled and the entire tissue section also was outlined. The CD45 cell count algorithm was then applied separately to the entire tissue section and all of the circled lobules; cell counts and areas for all lobules were then subtracted from cell counts and areas of the entire section to obtain cell density for the extralobular breast tissue.
Fig. 2.
Quantification of immune cells in normal lobules. a, b CD8+ cell quantitation in a normal lobule without lobulitis. a immunostain alone, b color overlay demonstrating positively stained cells within circled lobule; positive cells are identified by red and orange color, yellow cells are below staining threshold for positivity, and blue cells are non-staining cells. c, d CD11c quantitation by pixel count ratio in a normal lobule without lobulitis, ×200 magnification. c Immunostain alone, d color overlay demonstrating pixels with positive staining (red and orange) compared to non-stained pixels (blue). ×200 magnification
Statistical analysis
Cell densities were calculated as the number of positively stained cells per mm2 for all immunostains except CD11c for which positive:total pixel ratio was calculated. Immunostain results were compared between groups of lobules with and without lobulitis using linear mixed effects regression to account for multiple lobules from each patient and to evaluate potential confounding by age. Square root transformation was used for cell density and pixel ratio data where appropriate. An a priori two-tailed alpha level of 0.05 was used for statistical significance.
The within-sample variance of cell densities and pixel ratio was evaluated for each immunostain using intraclass correlation coefficients (ICC). ICC values >0.50 would indicate that the variance between subjects is greater than the variance within subjects while values <0.50 would indicate that the variance between subjects is less than the variance within subjects. The within-sample coefficients of variation (100 × (SD/Mean)) were also calculated and summarized across samples for each immunostain.
Results
Characteristics of normal tissue donors and samples
Normal tissue samples were obtained from women with median age 45 (range 24–63) at the time of tissue donation. Of 11 normal breast tissue samples studied, one had fewer than 10 lobules present (9 lobules). In total, per lobule data comprised 109 lobules: 96 (88 %) normal lobules, 11 non-proliferative fibrocystic lobules, and 2 proliferative fibrocystic lobules. Lobules represented the spectrum of age-related lobular involution, with 10 (10 %) having no age-related lobular involution, 49 (51 %) with partial involution, and 37 (39 %) with complete lobular involution; involution status is not applicable to fibrocystic lobules and is therefore missing for the 13 fibrocystic lobules.
Lobulitis
For the 109 lobules evaluated, 31 lobules showed lobulitis and 78 did not. Two samples had no lobulitis present in any lobules, six had lobulitis in less than half of the studied lobules, and three had lobulitis in half or more of the lobules. The presence of lobulitis varied by age-related lobular involution status of the lobules at 70, 43, and 3 % in the none, partial, and complete lobular involution categories, respectively. In accordance with these findings, there was a moderate negative correlation (r = −0.49) between age and percent of lobules with lobulitis.
Immune cells in normal breast tissues are primarily confined to breast lobules
Immune cells were present in consistent patterns in all samples and were predominantly localized to breast lobules rather than stroma and fat. This was confirmed quantitatively by CD45 (leukocyte) counts. Across all samples, median CD45 cell density was 261.3 cells/mm2 among lobules with lobulitis and 122.9 cells/mm2 among lobules without lobulitis (p < 0.0001), compared to only 15.4 cells/mm2 in extralobular breast tissue.
Immune cell subgroups in lobules and association with lobulitis
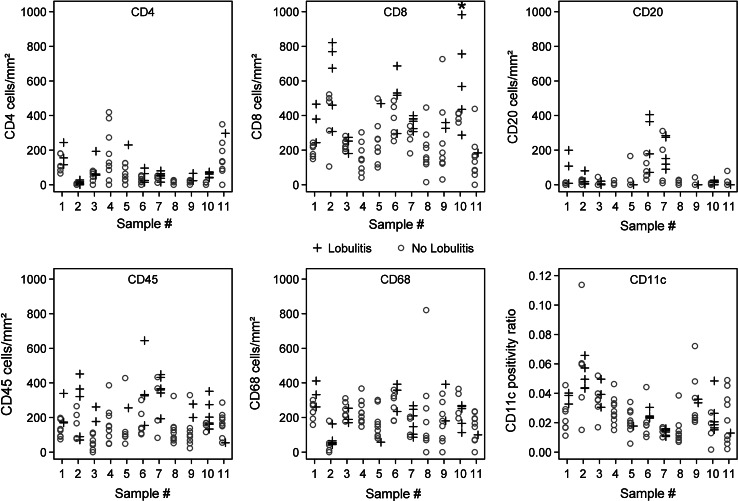
Immune cells of various types were present in the vast majority of lobules, regardless of lobulitis status (Fig. 3). Among the immune cell subgroups, CD8+ and CD68+ cells were the most numerous across lobules; comparatively, densities of CD4+ cells and CD20+ cells were lower. Significantly higher cell densities of CD4, CD8, CD20, and CD45 cell types were observed in lobules with lobulitis compared to lobules without lobulitis (Table 1). In contrast, the densities of monocytes/macrophages and dendritic cells did not vary significantly with the presence of lobulitis. Positive controls had higher median densities of all immune cell types compared to normal samples, except CD8+ cell density which was fairly similar in both groups (Table 1). Very few lobules had no immune cells (i.e., immune cell density value of 0) for CD8, CD45, and CD68 cell types (Table 2). Virtually all lobules demonstrated CD11c+ cells (100 % of lobules) and CD8+ cells (99 % of lobules). In contrast, CD4+ cells and CD20+ cells were more likely to be completely absent from lobules (and this was more frequent in lobules without lobulitis compared to those with lobulitis).
Fig. 3.
Quantitative immune cell densities for individual lobules in each of the 11 subjects studied; open circles indicate lobules without lobulitis, and crosses indicate lobules with lobulitis. Asterisk in CD8 density plot denotes one point outside the plotting region for subject #10 (value was 1336 CD8 cells/mm2)
Table 1.
Density of immune cells in normal breast tissues and benign positive controls
| Cell type | Normal samples (11 subjects) | Positive controls (2 subjects) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Lobules with lobulitis N = 31 lobules |
Lobules without lobulitis N = 78 lobules |
Age-adjusted p-value |
Resolving lactation N = 10 lobules |
Diabetic mastopathy N = 10 lobules |
|||||
| Median | (IQR) | Median | (IQR) | Median | (IQR) | Median | (IQR) | ||
| CD4 (cells/mm2) | 61.9 | (25.0, 80.2) | 27.3 | (0, 101.2) | 0.0001 | 185 | (120, 1,057) | 750 | (160, 2,336) |
| CD8 (cells/mm2) | 381.5 | (307.2, 567.7) | 216.3 | (145.8, 303.0) | <0.0001 | 395 | (177, 697) | 465 | (196, 551) |
| CD20 (cells/mm2) | 20.0 | (3.8, 151.2) | 0 | (0, 19.1) | 0.003 | 60 | (33, 262) | 2,510 | (269, 3,664) |
| CD45 (cells/mm2) | 261.3 | (169.4, 351.2) | 122.9 | (81.4, 179.9) | <0.0001 | 615 | (511, 1,762) | 3,872 | (1,171, 4,719) |
| CD68 (cells/mm2) | 210.1 | (103.1, 268.3) | 192.8 | (113.1, 263.8) | 0.41 | 1,822 | (1,412, 2,100) | 2,003 | (1,573, 2,308) |
| CD11c pixel ratio (positive/total) | 0.03 | (0.02, 0.04) | 0.02 | (0.01, 0.04) | 0.11 | 0.20 | (0.14, 0.29) | 0.19 | (0.14, 0.27) |
Table 2.
Number and percentage of lobules with a value of 0 for various immune cell types
| Cell type | All lobules N = 109 |
Lobules without lobulitis N = 78 |
Lobules with lobulitis N = 31 |
|||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| CD4 | 24 | 22.0 | 23 | 29.5 | 1 | 3.2 |
| CD8 | 1 | 0.9 | 1 | 1.3 | 0 | 0 |
| CD20 | 54 | 49.5 | 47 | 60.3 | 7 | 22.6 |
| CD45 | 3 | 2.8 | 3 | 3.8 | 0 | 0 |
| CD68 | 5 | 4.6 | 5 | 6.4 | 0 | 0 |
| CD11c | 0 | 0 | 0 | 0 | 0 | 0 |
Intraepithelial immune cells
Regardless of whether immune infiltrates were observed on H&E stain, immunostains confirmed that in all samples at high magnification power, both dendritic cells and CD8+ cells are consistently observed in intimate association with the epithelium of lobular acini and are primarily located at the basal aspect of the epithelium (Fig. 4).
Fig. 4.
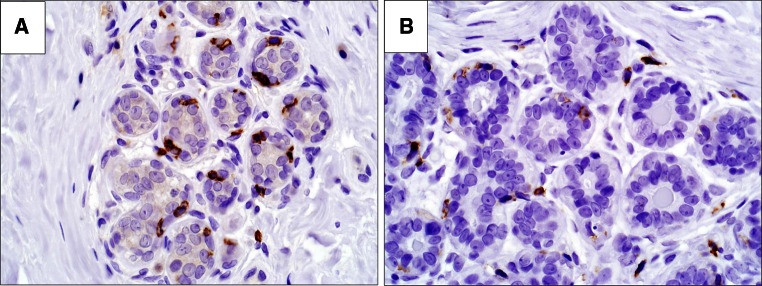
Direct association of dendritic cells and CD8 T cells within epithelium. a CD8 positive cells located at the basal aspect of the epithelium, b CD11c positive staining extending more diffusely around epithelium. ×400 magnification
Variation in immune cell densities between and within subjects
Intraclass correlation coefficients demonstrated values <0.50 for all immunostains (other than CD20 which was 0.51), indicating that the variance in immune cell densities between subjects was less than the variance within subjects, indicating heterogeneity across lobules within a patient (Table 3). The within-sample coefficients of variation also demonstrate substantial within-sample variability with respect to immune cell densities, with medians ranging from 42 to 182 %.
Table 3.
Variation in immune cell densities between and within subjects
| Cell type | Between-subject variance | Within-subject variance | ICC | CV median (range) |
|---|---|---|---|---|
| CD4 (cells/mm2) | 3,001.17 | 4,440.53 | 0.403 | 84 % (40–139 %) |
| CD8 (cells/mm2) | 17,468.78 | 26,572.44 | 0.397 | 51 % (15–78 %) |
| CD20 (cells/mm2) | 3,736.08 | 3,517.39 | 0.515 | 182 % (65–316 %) |
| CD45 (cells/mm2) | 2,696.35 | 11,552.14 | 0.189 | 65 % (37–83 %) |
| CD68 (cells/mm2) | 3,252.43 | 10,558.04 | 0.236 | 42 % (21–116 %) |
| CD11c pixel ratio (positive/total) | 0.0001179 | 0.0001736 | 0.404 | 43 % (18–76 %) |
Intraclass correlation coefficient (ICC) is the ratio of the between-subject variance to the total variance (i.e., the proportion of the total variance representing between-subject variance); values >0.50 would indicate that the variance between subjects was greater than the variance within subjects while values <0.50 would indicate that the variance between subjects was less than the variance within subjects
Coefficient of variation (CV) is the ratio of the standard deviation divided by the mean. Within-sample CVs were calculated and then summarized over all samples
Discussion
In this study, we found that immune cells are (1) a consistent part of normal breast tissue, (2) primarily localized to breast lobules, (3) closely associated with the breast epithelium, and (4) vary substantially across lobules within a woman and between different women. Lobules with immune infiltrates visualized on H&E stain do have quantitatively higher densities of adaptive immune cells (helper T cells, cytotoxic T cells, and B cells) compared to lobules without lobulitis, while innate immune components (dendritic cells and monocytes/macrophages) do not appear to vary between lobules with and without lobulitis. Even among lobules without visible immune infiltrates, dendritic cells and cytotoxic T cells are uniformly present and are located in close association with the epithelium. Taken together, these findings indicate that immune cells are present in the normal condition and that immune infiltrates detected on H&E stain are not necessarily pathologic but may represent the higher range of immune cell density that is present to some degree in almost all normal lobules.
This is the first study to evaluate normal human breast tissue samples and to quantify basic populations of immune cells in the breast epithelium in the normal non-lactating state. Strengths of the study include the use of breast tissues from normal donors, evaluation of multiple immune cell types, and quantitative data on immune cell densities. Our finding of a nearly universal presence of CD8+ and CD11c+ immune cells in breast lobules provides insight and raises questions regarding the role of the immune system in its intimate association with the breast epithelium. A mucosal immune system in breast tissue may exist primarily as a defense against microbes [16, 17], although active tumor immunosurveillance is another possible function [29, 30]. Therefore, our finding of an active mucosal immune system in breast tissue has possible far-reaching implications for breast carcinogenesis and prevention.
In the gut, another mucosal tissue for which the role of immune cell function has been more extensively studied, T and B cells are abundant and are intimately associated with the epithelial cells. In the lamina propria, immediately below the epithelial basement membrane, T cells predominate and differ from peripheral T cells by showing clonality for local antigen exposure [31, 32]. In addition to the lamina propria T cells, intraepithelial lymphocytes (IELs) accumulate directly in the epithelial plane [33, 34]. Gut IELs are more frequently CD8+ T cells with cytolytic properties [35], which may play important roles in tissue repair as well as pathogen defense [36, 37]. We observed heterogeneity in immune cells across lobules within individual subjects. Heterogeneity of the mucosal immune system in the gut is highly compartmentalized across different sites and also within sites [38, 39], thought to reflect prior exposure to microbes [40] and unique immunological needs of the tissue based on function. It has been proposed that a primary responsibility of CD8 IELs is to survey for stress in the epithelium and to control immune responses [41]. We found CD8+ T cells and CD11c+ dendritic cells directly associated with mammary epithelium in all lobules, suggesting a similar potential function in the mammary gland.
This consistent presence of CD8+ cells and dendritic cells, interspersed within the breast epithelium, strongly suggests a role for antigen presentation and immune effector function, as well as stress response and maintenance of epithelial integrity. An even more intriguing possibility is that the mucosal immune system in the breast may serve a critical role in tumor immunosurveillance, supported by the previously established role for both CD8 T cells and dendritic cells in this process [42]. The concept of immunity against cancer was first suggested by Ehrlich in 1909 [43], with dramatic increases in supporting evidence in recent years [29, 30]. In the realm of breast cancer, there is some epidemiologic evidence for spontaneous regression of human breast cancers, supporting the possibility of an immune presence for the purpose of immunosurveillance [44]. If functional breast mucosal immunity exists, then quantitative profiles of immune cells in breast tissue may be predictive of breast cancer risk. Furthermore, the presence of a breast mucosal immune system supports the possibility of immune modulation for cancer prevention, including vaccines for breast cancer prevention.
Prior studies have evaluated immune cells in breast tissue but most have focused on specific disrupted tissue states, including cancer, post-lactational involution [24, 25], and diabetic mastopathy [26]. Reports on the prognostic significance of immune infiltrates in breast cancers have varied results, with some studies showing that leukocyte infiltration is associated with improved survival [45, 46] and others showing worse survival [47]. Immune cell infiltrates and types have also been implicated in breast cancer progression, metastasis, and response to chemotherapy [9–11]. Fewer studies have evaluated immune cells in non-cancerous breast tissue, usually as a comparison group for malignant tumor tissue [48–51]. Immunohistochemical comparisons of immune infiltrates in breast cancer tissues versus tissues from women with benign breast disease or reduction mammoplasty have consistently demonstrated that CD8+ lymphocytes represent the predominant immune cell infiltrate in breast cancers and are less frequent in non-malignant breast tissue [48, 49]. One group reported a depletion of immune cells from the malignant epithelium, with localization to stroma and more than doubling of the CD4:CD8 ratio in malignant versus benign tissue [49]. In contrast, benign breast tissues demonstrated greater accumulation of immune cells in the epithelium, also with a CD8+ predominance [48, 49]. A more recent study demonstrated a greater accumulation of leukocytes in breast cancer tissue compared to non-adjacent breast tissue from the same woman [51], finding no difference in CD8+ cells in tumor tissue versus surrounding non-neoplastic breast tissue. A weakness of these prior studies is the use of samples with benign breast disease or reduction mammoplasty tissues, which have significant histologic differences from the normal state [22]. Breast tissues from women with benign breast disease differ significantly from normal breast tissues in histologic features of both epithelial proliferation and lobular involution [22], two features strongly related to breast cancer risk [18, 20]. Therefore, benign breast disease tissues may not provide the optimal source of tissues for understanding the immune microenvironment in the normal state.
Limitations of our study include a small number of samples, a consequence of our approach to obtain an intensive and detailed quantitation of multiple immune cell types. Therefore, our study cannot evaluate associations of immune cell profiles with other established breast cancer risk factors, including BMI, family history, and menopausal status [52–55]. Work is currently underway to evaluate more qualitative measures of immune cell profiles with BMI and other features of breast cancer risk. Another possible limitation is our definition of lobulitis, based upon the assessment of the breast pathologist to denote the minimum threshold of whether an immune cell infiltrate was present. We are aware of two published studies that define lobulitis using a cutoff number of lymphocytes per lobule (on H&E stain). One study used a criterion of >100 lymphocytes per lobule [56], and another used a criterion of >50 lymphocytes (moderate) or >100 lymphocytes (marked) [57]. As per the methods sections in these studies, it appears that they did not actually individually count the cells in each lobule, suggesting a more qualitative judgement of the number of lymphocytes present. This made sense in those situations because individual counting would be impractical to implement on the larger sample sets in those studies. We believe our definition is advantageous, especially since it is supported by our quantitative findings confirming higher leukocyte density (CD45) in lobules with lobulitis versus those without. Furthermore, the simple cutoff used in earlier studies does not account for the size of the lobule; smaller lobules with equal percentage immune infiltrate will have fewer lymphocytes, which could lead to incorrect assessment of lobulitis in smaller lobules and create bias in lobule selection by eliminating evaluation of small involuted lobules. Our definition allows for assignment of lobulitis, effective for any lobule size.
Conclusion
In summary, we find that in normal breast tissues, myeloid and lymphoid immune cells are present and predominantly localized to breast lobules rather than the interlobular stroma. Furthermore, CD8+ cells and dendritic cells are directly integrated with breast epithelium, indicating the presence of a mucosal immune system in human non-lactating female breast tissue. These findings suggest that there is a role for antigen presentation and orchestrated cytotoxic functionality within the epithelium. Additional studies are necessary to define specific functional roles of immune cells in the breast epithelium; however, the identification of these immune effectors indicates that immune infrastructure is present within breast epithelium to make a breast cancer prevention vaccine possible. If unique antigens could be identified for epithelial cells with early malignant change or fusion proteins unique to breast cancer, T cell recognition could be induced via a vaccine approach. Work is currently underway to improve our understanding of how lobulitis relates to age and lobular involution (factors proven to be associated with breast cancer risk), as well as whether quantitative immune cell densities are associated with breast cancer risk.
Acknowledgments
This research was supported by a Grant from Susan G. Komen for the Cure®. Samples from the Susan G. Komen for the Cure® Tissue Bank at the IU Simon Cancer Center were used in this study. We thank contributors, including Indiana University, who collected samples used in this study, as well as donors and their families, whose help and participation made this work possible. Sincere thanks to Marilyn Churchward for assistance with manuscript preparation.
Conflict of interest
The authors have no conflict of interest.
References
- 1.Ghajar CM. On leukocytes in mammary development and cancer. Cold Spring Harbor Perspect Biol. 2012 doi: 10.1101/cshperspect.a013276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghajar CM, Bissell MJ. Extracellular matrix control of mammary gland morphogenesis and tumorigenesis: insights from imaging. Histochem Cell Biol. 2008;130(6):1105–1118. doi: 10.1007/s00418-008-0537-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reed JR, Schwertfeger KL. Immune cell location and function during post-natal mammary gland development. J Mammary Gland Biol Neoplasia. 2010;15(3):329–339. doi: 10.1007/s10911-010-9188-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brandtzaeg P. The mucosal immune system and its integration with the mammary glands. J Pediatr. 2010;156(2 Suppl):S8–S15. doi: 10.1016/j.jpeds.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 5.Goldman AS. The immune system of human milk: antimicrobial, antiinflammatory and immunomodulating properties. Pediatr Infect Dis J. 1993;12(8):664–671. doi: 10.1097/00006454-199308000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Spencer JP. Management of mastitis in breastfeeding women. Am Fam Physician. 2008;78(6):727–731. [PubMed] [Google Scholar]
- 7.O’Brien J, Martinson H, Durand-Rougely C, Schedin P. Macrophages are crucial for epithelial cell death and adipocyte repopulation during mammary gland involution. Development. 2012;139(2):269–275. doi: 10.1242/dev.071696. [DOI] [PubMed] [Google Scholar]
- 8.Ramirez RA, Lee A, Schedin P, Russell JS, Masso-Welch PA. Alterations in mast cell frequency and relationship to angiogenesis in the rat mammary gland during windows of physiologic tissue remodeling. Dev Dyn. 2012;241(5):890–900. doi: 10.1002/dvdy.23778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, Coussens LM. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16(2):91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bingle L, Lewis CE, Corke KP, Reed MW, Brown NJ. Macrophages promote angiogenesis in human breast tumour spheroids in vivo. Br J Cancer. 2006;94(1):101–107. doi: 10.1038/sj.bjc.6602901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, Gallagher WM, Wadhwani N, Keil SD, Junaid SA, Rugo HS, Hwang ES, Jirstrom K, West BL, Coussens LM. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011;1(1):54–67. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cardiff RD, Wellings SR. The comparative pathology of human and mouse mammary glands. J Mammary Gland Biol Neoplasia. 1999;4(1):105–122. doi: 10.1023/A:1018712905244. [DOI] [PubMed] [Google Scholar]
- 13.Macpherson AJ, Harris NL. Interactions between commensal intestinal bacteria and the immune system. Nat Rev Immunol. 2004;4(6):478–485. doi: 10.1038/nri1373. [DOI] [PubMed] [Google Scholar]
- 14.Moore WE, Moore LH. Intestinal floras of populations that have a high risk of colon cancer. Appl Environ Microbiol. 1995;61(9):3202–3207. doi: 10.1128/aem.61.9.3202-3207.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rogers AB. Distance burning: how gut microbes promote extraintestinal cancers. Gut Microbes. 2011;2(1):52–57. doi: 10.4161/gmic.2.1.14761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hunt KM, Foster JA, Forney LJ, Schutte UM, Beck DL, Abdo Z, Fox LK, Williams JE, McGuire MK, McGuire MA. Characterization of the diversity and temporal stability of bacterial communities in human milk. PLoS One. 2011;6(6):e21313. doi: 10.1371/journal.pone.0021313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arroyo R, Martin V, Maldonado A, Jimenez E, Fernandez L, Rodriguez JM. Treatment of infectious mastitis during lactation: antibiotics versus oral administration of Lactobacilli isolated from breast milk. Clin Infect Dis. 2010;50(12):1551–1558. doi: 10.1086/652763. [DOI] [PubMed] [Google Scholar]
- 18.Hartmann LC, Sellers TA, Frost MH, Lingle WL, Degnim AC, Ghosh K, Vierkant RA, Maloney SD, Pankratz VS, Hillman DW, Suman VJ, Johnson J, Blake C, Tlsty T, Vachon CM, Melton LJ, III, Visscher DW. Benign breast disease and the risk of breast cancer. N Engl J Med. 2005;353(3):229–237. doi: 10.1056/NEJMoa044383. [DOI] [PubMed] [Google Scholar]
- 19.Visscher DW, Pankratz VS, Santisteban M, Reynolds C, Ristimaki A, Vierkant RA, Lingle WL, Frost MH, Hartmann LC. Association between cyclooxygenase-2 expression in atypical hyperplasia and risk of breast cancer. J Natl Cancer Inst. 2008;100(6):421–427. doi: 10.1093/jnci/djn036. [DOI] [PubMed] [Google Scholar]
- 20.Milanese TR, Hartmann LC, Sellers TA, Frost MH, Vierkant RA, Maloney SD, Pankratz VS, Degnim AC, Vachon CM, Reynolds CA, Thompson RA, Melton LJ, III, Goode EL, Visscher DW. Age-related lobular involution and risk of breast cancer. J Natl Cancer Inst. 2006;98(22):1600–1607. doi: 10.1093/jnci/djj439. [DOI] [PubMed] [Google Scholar]
- 21.McKian KP, Reynolds CA, Visscher DW, Nassar A, Radisky DC, Vierkant RA, Degnim AC, Boughey JC, Ghosh K, Anderson SS, Minot D, Caudill JL, Vachon CM, Frost MH, Pankratz VS, Hartmann LC. Novel breast tissue feature strongly associated with risk of breast cancer. J Clin Oncol. 2009;27(35):5893–5898. doi: 10.1200/JCO.2008.21.5079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Degnim AC, Visscher DW, Hoskin TL, Frost MH, Vierkant RA, Vachon CM, Shane Pankratz V, Radisky DC, Hartmann LC. Histologic findings in normal breast tissues: comparison to reduction mammaplasty and benign breast disease tissues. Breast Cancer Res Treat. 2012;133(1):169–177. doi: 10.1007/s10549-011-1746-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Susan G (2013) Komen for the Cure Tissue Bank at the IU SImon Cancer Center in Indianapolis http://komentissuebank.iu.edu/. Accessed 18 Sept 2013
- 24.Watson CJ. Post-lactational mammary gland regression: molecular basis and implications for breast cancer. Exp Rev Mol Med. 2006;8(32):1–15. doi: 10.1017/S1462399406000196. [DOI] [PubMed] [Google Scholar]
- 25.Watson CJ, Kreuzaler PA. Remodeling mechanisms of the mammary gland during involution. Int J Dev Biol. 2011;55(7–9):757–762. doi: 10.1387/ijdb.113414cw. [DOI] [PubMed] [Google Scholar]
- 26.Valdez R, Thorson J, Finn WG, Schnitzer B, Kleer CG. Lymphocytic mastitis and diabetic mastopathy: a molecular, immunophenotypic, and clinicopathologic evaluation of 11 cases. Mod Pathol. 2003;16(3):223–228. doi: 10.1097/01.MP.0000056627.21128.74. [DOI] [PubMed] [Google Scholar]
- 27.Haj M, Weiss M, Herskovits T. Diabetic sclerosing lymphocytic lobulitis of the breast. J Diabetes Complic. 2004;18(3):187–191. doi: 10.1016/S1056-8727(03)00034-5. [DOI] [PubMed] [Google Scholar]
- 28.Radisky DC, Santisteban M, Berman HK, Gauthier ML, Frost MH, Reynolds CA, Vierkant RA, Pankratz VS, Visscher DW, Tlsty TD, Hartmann LC. p16(INK4a) expression and breast cancer risk in women with atypical hyperplasia. Cancer Prev Res (Phila) 2011;4(12):1953–1960. doi: 10.1158/1940-6207.CAPR-11-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smyth MJ, Godfrey DI, Trapani JA. A fresh look at tumor immunosurveillance and immunotherapy. Nat Immunol. 2001;4:293–299. doi: 10.1038/86297. [DOI] [PubMed] [Google Scholar]
- 30.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21(2):137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 31.Schieferdecker HL, Ullrich R, Hirseland H, Zeitz M. T cell differentiation antigens on lymphocytes in the human intestinal lamina propria. J Immunol. 1992;149(8):2816–2822. [PubMed] [Google Scholar]
- 32.Zeitz M, Schieferdecker HL, Ullrich R, Jahn HU, James SP, Riecken EO. Phenotype and function of lamina propria T lymphocytes. Immunol Resusc. 1991;10(3–4):199–206. doi: 10.1007/BF02919693. [DOI] [PubMed] [Google Scholar]
- 33.Blumberg RS, Yockey CE, Gross GG, Ebert EC, Balk SP. Human intestinal intraepithelial lymphocytes are derived from a limited number of T cell clones that utilize multiple V beta T cell receptor genes. J Immunol. 1993;150(11):5144–5153. [PubMed] [Google Scholar]
- 34.Regnault A, Kourilsky P, Cumano A. The TCR-beta chain repertoire of gut-derived T lymphocytes. Semin Immunol. 1995;7(5):307–319. doi: 10.1016/1044-5323(95)90012-8. [DOI] [PubMed] [Google Scholar]
- 35.Montufar-Solis D, Garza T, Klein JR. T-cell activation in the intestinal mucosa. Immunol Rev. 2007;215:189–201. doi: 10.1111/j.1600-065X.2006.00471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leishman AJ, Gapin L, Capone M, Palmer E, MacDonald HR, Kronenberg M, Cheroutre H. Precursors of functional MHC class I- or class II-restricted CD8alphaalpha(+) T cells are positively selected in the thymus by agonist self-peptides. Immunity. 2002;16(3):355–364. doi: 10.1016/S1074-7613(02)00284-4. [DOI] [PubMed] [Google Scholar]
- 37.Rocha B, Vassalli P, Guy-Grand D. The V beta repertoire of mouse gut homodimeric alpha CD8+ intraepithelial T cell receptor alpha/beta+ lymphocytes reveals a major extrathymic pathway of T cell differentiation. J Exp Med. 1991;173(2):483–486. doi: 10.1084/jem.173.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beagley KW, Fujihashi K, Lagoo AS, Lagoo-Deenadaylan S, Black CA, Murray AM, Sharmanov AT, Yamamoto M, McGhee JR, Elson CO, et al. Differences in intraepithelial lymphocyte T cell subsets isolated from murine small versus large intestine. J Immunol. 1995;154(11):5611–5619. [PubMed] [Google Scholar]
- 39.Kunkel EJ, Butcher EC. Plasma-cell homing. Nat Rev Immunol. 2003;3(10):822–829. doi: 10.1038/nri1203. [DOI] [PubMed] [Google Scholar]
- 40.Cebra JJ. Influences of microbiota on intestinal immune system development. Am J Clin Nutr. 1999;69(5):1046S–1051S. doi: 10.1093/ajcn/69.5.1046s. [DOI] [PubMed] [Google Scholar]
- 41.Taylor BC, Dellinger JD, Cullor JS, Stott JL. Bovine milk lymphocytes display the phenotype of memory T cells and are predominantly CD8+ Cell Immunol. 1994;156(1):245–253. doi: 10.1006/cimm.1994.1169. [DOI] [PubMed] [Google Scholar]
- 42.Zitvogel L, Tesniere A, Kroemer G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat Rev Immunol. 2006;6(10):715–727. doi: 10.1038/nri1936. [DOI] [PubMed] [Google Scholar]
- 43.Ehrlich P. Beiträge zur experimentellen Pathologie und Chemotherapie. Leipzig: Akademische Verlagsgesellschaft; 1909. [Google Scholar]
- 44.Zahl PH, Andersen JM, Maehlen J. Spontaneous regression of cancerous tumors detected by mammography screening. JAMA J Am Med Assoc. 2004;292(21):2579–2580. doi: 10.1001/jama.292.21.2579. [DOI] [PubMed] [Google Scholar]
- 45.Lee AH, Gillett CE, Ryder K, Fentiman IS, Miles DW, Millis RR. Different patterns of inflammation and prognosis in invasive carcinoma of the breast. Histopathology. 2006;48(6):692–701. doi: 10.1111/j.1365-2559.2006.02410.x. [DOI] [PubMed] [Google Scholar]
- 46.Aaltomaa S, Lipponen P, Eskelinen M, Kosma VM, Marin S, Alhava E, Syrjanen K. Lymphocyte infiltrates as a prognostic variable in female breast cancer. Eur J Cancer. 1992;28A(4–5):859–864. doi: 10.1016/0959-8049(92)90134-N. [DOI] [PubMed] [Google Scholar]
- 47.Matkowski R, Gisterek I, Halon A, Lacko A, Szewczyk K, Staszek U, Pudelko M, Szynglarewicz B, Szelachowska J, Zolnierek A, Kornafel J. The prognostic role of tumor-infiltrating CD4 and CD8 T lymphocytes in breast cancer. Anticancer Res. 2009;29(7):2445–2451. [PubMed] [Google Scholar]
- 48.Giorno R. Mononuclear cells in malignant and benign human breast tissue. Arch Pathol Lab Med. 1983;107(8):415–417. [PubMed] [Google Scholar]
- 49.Lwin KY, Zuccarini O, Sloane JP, Beverley PC. An immunohistological study of leukocyte localization in benign and malignant breast tissue. Int J Cancer. 1985;36(4):433–438. doi: 10.1002/ijc.2910360404. [DOI] [PubMed] [Google Scholar]
- 50.Ben-Hur H, Cohen O, Schneider D, Gurevich P, Halperin R, Bala U, Mozes M, Zusman I. The role of lymphocytes and macrophages in human breast tumorigenesis: an immunohistochemical and morphometric study. Anticancer Res. 2002;22(2B):1231–1238. [PubMed] [Google Scholar]
- 51.Ruffell B, Au A, Rugo HS, Esserman LJ, Hwang ES, Coussens LM. Leukocyte composition of human breast cancer. Proc Natl Acad Sci USA. 2012;109(8):2796–2801. doi: 10.1073/pnas.1104303108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Krishnan K, Bassett JK, MacInnis RJ, English DR, Hopper JL, McLean C, Giles GG, Baglietto L. Associations between weight in early adulthood, change in weight, and breast cancer risk in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2013;22(8):1409–1416. doi: 10.1158/1055-9965.EPI-13-0136. [DOI] [PubMed] [Google Scholar]
- 53.Brisson J, Morrison AS, Kopans DB, Sadowsky NL, Kalisher L, Twaddle JA, Meyer JE, Henschke CI, Cole P. Height and weight, mammographic features of breast tissue, and breast cancer risk. Am J Epidemiol. 1984;119(3):371–381. doi: 10.1093/oxfordjournals.aje.a113755. [DOI] [PubMed] [Google Scholar]
- 54.Gail MH, Brinton LA, Byar DP, Corle DK, Green SB, Schairer C, Mulvihill JJ. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81(24):1879–1886. doi: 10.1093/jnci/81.24.1879. [DOI] [PubMed] [Google Scholar]
- 55.Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med. 2004;23(7):1111–1130. doi: 10.1002/sim.1668. [DOI] [PubMed] [Google Scholar]
- 56.Hermsen BB, von Mensdorff-Pouilly S, Fabry HF, Winters HA, Kenemans P, Verheijen RH, van Diest PJ. Lobulitis is a frequent finding in prophylactically removed breast tissue from women at hereditary high risk of breast cancer. J Pathol. 2005;206(2):220–223. doi: 10.1002/path.1774. [DOI] [PubMed] [Google Scholar]
- 57.Gulbahce HE, Vanderwerf S, Blair C, Sweeney C. Lobulitis in nonneoplastic breast tissue from breast cancer patients: association with phenotypes that are common in hereditary breast cancer. Hum Pathol. 2014;45(1):78–84. doi: 10.1016/j.humpath.2013.08.008. [DOI] [PubMed] [Google Scholar]