Abstract
Background
The strength of race as an independent predictor of long-term outcomes in a contemporary chronic heart failure (HF) population and its association with exercise training response have not been well established. We aimed to investigate the association between race and outcomes and to explore interactions with exercise training in patients with ambulatory HF.
Methods
We performed an analysis of HF-ACTION, which randomized 2331 patients with HF having an ejection fraction ≤35% to usual care with or without exercise training. We examined characteristics and outcomes (mortality/hospitalization, mortality, and cardiovascular mortality/HF hospitalization) by race using adjusted Cox models and explored an interaction with exercise training.
Results
There were 749 self-identified black patients (33%). Blacks were younger with significantly more hypertension and diabetes, less ischemic etiology, and lower socioeconomic status versus whites. Blacks had shorter 6-minute walk distance and lower peak VO2 at baseline. Over a median follow-up of 2.5 years, black race was associated with increased risk for all outcomes except mortality. After multivariable adjustment, black race was associated with increased mortality/hospitalization (hazard ratio [HR] 1.16, 95% CI 1.01–1.33) and cardiovascular mortality/HF hospitalization (HR 1.46, 95% CI 1.20–1.77). The hazard associated with black race was largely caused by increased HF hospitalization (HR 1.58, 95% CI 1.27–1.96), given similar cardiovascular mortality. There was no interaction between race and exercise training on outcomes (P >.5).
Conclusions
Black race in patients with chronic HF was associated with increased prevalence of modifiable risk factors, lower exercise performance, and increased HF hospitalization, but not increased mortality or a differential response to exercise training.
African American or black populations are at an increased risk for developing heart failure (HF), which occurs at an earlier age and may be associated with increased morbidity and mortality compared with whites.1–4 Elderly black Medicare patients were recently shown to have increased 30-day readmission rates for HF compared with whites.5 However, several studies during the 1990s in the Veterans Affairs health care system6,7 and in Medicare patients8 demonstrated better survival in black patients with HF compared with white patients. Recent registry data from patients hospitalized with acute HF have also suggested that blacks may have comparatively lower in hospital mortality and similar short-term outcomes.9–11
Importantly, none of these studies investigated the strength of race as an independent predictor of long-term outcomes in a diverse, contemporary chronic HF population, and the association between race and exercise training response has not been well established. Although there was no evidence of a significant race and treatment interaction for all-cause mortality/hospitalization in the HF-ACTION study,12 further investigation is warranted of the disease-specific outcomes of cardiovascular morbidity and mortality. We investigated the association between race and outcomes following multivariable adjustment and explored interactions with exercise training in patients with ambulatory HF enrolled in the HF-ACTION study.
Methods
The design and results of the HF-ACTION study have been published (ClinicalTrials.gov, NCT00047437).12–14 HF-ACTION was a trial of exercise training versus usual care in patients with an ejection fraction (EF) ≤35% and New York Heart Association (NYHA) class II to IV symptoms despite optimal HF therapy for at least 6 weeks. Race was documented by self-report (ie, white, black/African American, American Indian/Alaska native, Asian, and/or native Hawaiian/Pacific Islander). The protocol was approved by the institutional review boards/ethics committees for each of the sites and the coordinating center. All patients voluntarily provided written informed consent with randomization between April 2003 and February 2007.
Patients were scheduled to complete a cardiopulmonary exercise (CPX) test, 6-minute walk, and health status surveys at baseline and were subsequently randomized to aerobic exercise training + usual care or usual care alone. Patients randomized to aerobic exercise were scheduled to participate in 3 supervised exercise sessions/wk for 3 months. Patients exercised using a treadmill or stationary cycle ergometer as their primary training mode. Patients were encouraged to begin home-based exercise after 18 supervised sessions and to fully transition to home exercise after 36 supervised sessions. The primary index of adherence was weekly volume of self-reported exercise. After the trial started, it was decided that full adherence was to be defined as ≥90 min/wk of exercise during months 1 to 3 and ≥120 min/wk during subsequent months. Patients were instructed to continue home-based exercise training, along with one supervised session every 3 months, throughout follow-up. The primary end point was all-cause mortality/hospitalization. An independent clinical events committee adjudicated deaths and cardiovascular hospitalizations until the first HF hospitalization. Exercise and health status measures were repeated 3 months after baseline. Median follow-up was 2.5 years.
Statistical methods
Patients were grouped as white, black, or other. Baseline characteristics including health status (eg, Kansas City Cardiomyopathy Questionnaire [KCCQ]) and exercise parameters (eg, peak oxygen consumption [VO2] and CPX duration) were described. Continuous variables were summarized with the median and 25th and 75th percentiles and compared for black vs. white using the Wilcoxon rank sum statistic. Categorical variables are presented as percentages and compared for black vs. white with a Pearson χ2 statistic or exact test when appropriate.
Given the small sample size of nonblack minorities (n = 121), results are restricted to white and black subgroups; “other race” was included in statistical models. The primary outcome was time to mortality/hospitalization in black versus white patients. We evaluated the secondary outcomes of time to cardiovascular mortality/HF hospitalization and all-cause mortality as well as the components of the composite outcome. Results for the composite endpoint of cardiovascular mortality/cardiovascular hospitalization were similar to the results for cardiovascular mortality/HF hospitalization and thus not presented here. We investigated the relationship between race and outcomes using Cox proportional hazards models, including adjustment for a comprehensive set of predictors. The adjustment variables were developed for the data set and have been used in the post hoc HF-ACTION analyses.15 In brief, baseline predictors (Figure footnote) were selected using a step wise variable selection based on a bootstrapped-backward selection process. As a secondary analysis, we further independently adjusted for socioeconomic status (income, education, marital status, and employment status) based on previous work.16 Proportional hazards assumptions were checked and verified for race with all outcomes; no violation was suggested. Adjusted event curves for black vs. white race were plotted for mortality/hospitalization and CV mortality/HF hospitalization using the group prognosis method.
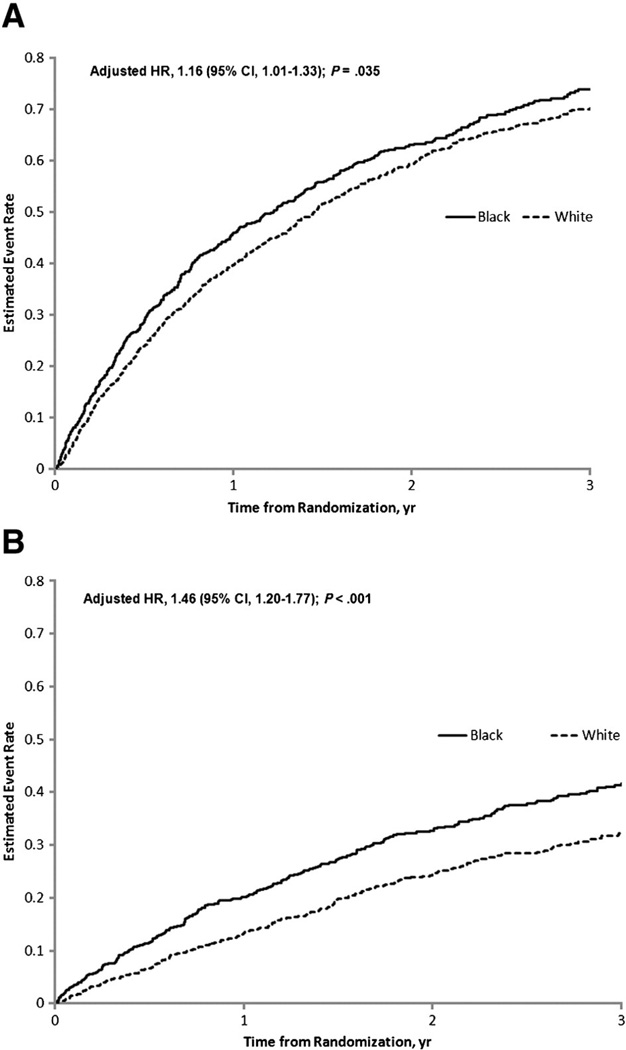
Figure.
Adjusted event curves for all-cause mortality/hospitalization* (A) and cardiovascular mortality/HF hospitalization† (B). *Adjusted for peak VO2 by Weber class, KCCQ symptom stability score, blood urea nitrogen, country, EF, sex, β-blocker dosage, mitral regurgitation (MR) grade, and ventricular conduction.†Adjusted for loop diuretic dose, EF, MR grade, ventricular conduction, KCCQ symptom stability score, blood urea nitrogen, sex, age, peak VO2 by Weber class, and VE/VCO2.
An adjusted Cox proportional hazards model was used to assess the relationship of the interaction of race and exercise treatment with time to the clinical end points. In addition, the change in exercise and health status variables (baseline to 3 months) was examined in the study arms stratified by race. Linear regression modeling, adjusted for baseline covariates selected using backward selection, was used to explore an interaction between race and treatment as a predictor of the change in exercise and health status variables. To account for missing data at 3 months, subjects in the linear regression model were inversely weighted by their estimated probability of having a missing response conditional on their baseline characteristics. The analysis plan specified that the P value would be reported for the interaction of black race and exercise therapy for clinical outcomes and the change in exercise and health status variables. If these P values for interaction were significant, then the hazard ratio (HR) for the clinical end point (or the estimate of the change at 3 months for the health status or exercise parameter) and 95% CI for treatment within each racial category would be reported. We also explored the relationships between exercise volume17 and adherence with race. A P value <.05 was considered statistically significant for all analyses. All analyses were performed by the Duke Clinical Research Institute using SAS (Cary, NC) system version 9.2.
The HF-ACTION study was funded by the National Heart, Lung, and Blood Institute, but no extramural funding was used to support the current analysis. The authors are solely responsible for the design and conduct of this study, all analyses, the drafting and editing of the manuscript, and its final contents.
Results
Of patients who self-reported race (n = 2,296), 33% (n = 749) were black. Table I presents baseline characteristics. Blacks were younger, were more often female, and had less ischemic etiology and lower socioeconomic status versus whites. Blacks also tended to have higher body mass index (BMI) and more hypertension and diabetes but less atrial fibrillation. Blacks had shorter 6-minutewalk distance and lower peak VO2 at baseline; (Table II). Differences in these exercise-testing parameters remained significant after adjustment for sex differences (both P < .01). More than 90% of black and white patients were receiving angiotensin-converting enzyme (ACE) inhibitors/angiotensin receptor blockers, with approximately half as many receiving aldosterone antagonists. Combination hydralazine/nitrate use was greater in blacks than in whites. Blacks were less likely to have an implanted cardioverter/defibrillator (ICD) or cardiac resynchronization therapy (CRT) device; however, there were significant differences in conduction pattern by race. Specifically, more blacks had normal ventricular conduction, and fewer blacks had bundle-branch block morphology (14.7% vs 22.9%).
Table I.
Baseline characteristics of the study cohort based on race
| Race | |||
|---|---|---|---|
| Variable | Black (n = 749) | White (n = 1426) | P |
| Age (y) | 55 (47–64) | 62 (54–70) | <.001 |
| Female sex | 41 | 22 | <.001 |
| Income | <.001* | ||
| <25,000 | 49 | 30 | |
| $25,000–$49,999 | 26 | 27 | |
| $50,000–$99,999 | 10 | 25 | |
| $100,000 or more | 3 | 8 | |
| Decline | 12 | 10 | |
| Education | <.001* | ||
| <High school | 14 | 12 | |
| High school graduate/equivalent | 35 | 25 | |
| Some college/no degree | 27 | 26 | |
| Completed associate degree | 8 | 9 | |
| College graduate | 11 | 18 | |
| Completed graduate school | 5 | 10 | |
| Marital status | <.001* | ||
| Married | 41 | 66 | |
| Widowed | 12 | 9 | |
| Divorced | 17 | 14 | |
| Separated | 6 | 2 | |
| Single/Never married | 20 | 7 | |
| Living with partner | 4 | 3 | |
| Employment status | <.001* | ||
| Employed full-time | 17 | 19 | |
| Employed part-time | 5 | 6 | |
| Disabled | 41 | 25 | |
| Unemployed | 8 | 4 | |
| Retired | 27 | 43 | |
| Other | 2 | 3 | |
| Ischemic etiology | 32 | 61 | <.001 |
| NYHA III–IV | 39 | 36 | .23 |
| EF (%) | 25 (20–31) | 25 (20–30) | .63 |
| Ventricular conduction | <.001 | ||
| Normal | 54.3 | 32.3 | |
| LBBB | 11.8 | 19.1 | |
| RBBB | 2.9 | 3.8 | |
| IVCD | 14.4 | 11.5 | |
| Paced | 14.8 | 27.4 | |
| Unknown | 1.7 | 3.0 | |
| History of myocardial infarction | 24 | 51 | <.001 |
| Hypertension history | 77 | 51 | <.001 |
| Diabetes history | 36 | 30 | .003 |
| Atrial fibrillation/flutter | 14 | 25 | <.001 |
| BMI (kg/m2) | 32 (27–38) | 29 (26–34) | <.001 |
| Systolic blood pressure (mm Hg) | 114 (102–130) | 110 (100–124) | <.001 |
| Heart rate (beats/min) | 72 (64–78) | 69 (62–76) | .003 |
| Sodium (mmol/L) | 139 (138–141) | 139 (137–141) | .02 |
| Creatinine (mg/dL) | 1.2 (1.0–1.5) | 1.2 (1.0–1.5) | .61 |
| blood urea nitrogen (mg/dL) | 18 (13–25) | 22 (17–30) | <.001 |
| ACE inhibitor/angiotensin receptor blocker | 95 | 94 | .22 |
| β-Blocker | 96 | 94 | .15 |
| β-Blocker type | <.001 | ||
| Atenolol | 2.7 | 2.5 | |
| Bisoprolol | 1.0 | 4.9 | |
| Carvedilol | 60.1 | 60.3 | |
| Metoprolol XL | 29.9 | 23.1 | |
| Metoprolol immediate release | 5.6 | 8.4 | |
| Other | 0.7 | 0.8 | |
| Dose (mg/d), carvedilol equivalent | 50 (25–50) | 31 (19–50) | <.001 |
| Aldosterone antagonist | 49 | 43 | .007 |
| Hydralazine† | 34 | 7 | <.001 |
| Nitrates‡ | 26 | 23 | .059 |
| Hydralazine + nitrates | 15 | 3 | <.001 |
| Loop diuretic | 84 | 74 | <.001 |
| Digoxin | 46 | 44 | .28 |
| Implantable cardioverter/defibrillator | 31 | 45 | <.001 |
| Biventricular pacemaker | 12 | 22 | <.001 |
Expressed as median (interquartile range) or %.
P values for the dichotomized comparison of each variable as follows: income: <$25,000 vs ≥$25,000; education: <high school vs ≥high school; marital status: current or prior partner (married, living with partner, widowed) vs no partner (single, divorced, separated); employment status: employed, volunteer, student, homemaker, or retired vs unemployed or disabled.
Includes combination therapy with nitrates.
Includes combination therapy with hydralazine.
Table II.
Baseline health status and exercise parameters by race
| Variable | Black (n = 749) | White (n = 1426) | P |
|---|---|---|---|
| Beck Depression Inventory II score | 8 (5–16) | 8 (4–14) | .059 |
| Kansas City Cardiomyopathy Questionnaire Overall Score | 66 (48–81) | 69 (53–84) | <.001 |
| 6-min walk distance (m) | 348 (277–416) | 383 (309–445) | <.001 |
| CPX test duration (min) | 8.7 (6.1–11.0) | 10.0 (7.3–12.5) | <.001 |
| Peak VO2 (mL kg−1 min−1) | 13.2 (10.6–16.5) | 15.0 (12.3–18.2) | <.001 |
| VE/VCO2 slope | 32 (28–38) | 33 (29–39) | <.001 |
| % with respiratory exchange ratio >1.10 | 34 | 48 | <.001 |
| Heart rate at peak exercise (beats/min) | 122 (107–138) | 118 (103–132) | <.001 |
Expressed as median (interquartile range) unless noted.
Adherence to exercise training by exercise minutes per week during the first 3 months and months 10 to 12 was 63 (18–106) in blacks versus 86 (50–125) in non blacks (P < .001) and 68 (6–167) versus 108 (26– 208) (P < .001), respectively. Median exercise volume in the exercise training arm was lower in blacks than in whites (2.9 [1.0–5.0] vs 4.4 [2.4–6.6] MET-h/wk, respectively, during months 1–3).
Overall adverse events were similar in patients of different race (Supplemental Table I), with the exception of more worsening HF in blacks than in whites (36% vs 24%).
Mode-specific event rates for death and hospitalization are presented in Supplemental Table II. Over a median follow-up of 2.5 years, all-cause mortality was 17.9% in blacks and 15.8% in whites. Hospitalization occurred in 67.0% of blacks versus 62.1% of whites. Nearly half of all hospitalizations were for HF in blacks (48.4%) compared with approximately a third in whites (33.9%). Black race was associated with increased mortality/hospitalization and cardiovascular mortality/HF hospitalization (Table III). All-cause mortality was similar in black and white patients. Cardiovascular mortality was similar between groups (P = .21), whereas black race was associated with increased hazard for HF hospitalization (HR 1.62, 95% CI 1.37–1.92) (Supplemental Table III).
Table III.
Association between black race and outcomes in chronic HF (reference = white race)
| HR (95% CI) | P | |
|---|---|---|
| All-cause mortality or all-cause hospitalization | ||
| Unadjusted | 1.15 (1.03–1.28) | .013 |
| Adjusted for HF-ACTION model covariates* (n = 1727) | 1.16 (1.01–1.33) | .035 |
| Adjusted for HF-ACTION model + income, employment, marital status, and education covariates (n = 1496) | 1.14 (0.98–1.33) | .09 |
| All-cause mortality | ||
| Unadjusted | 1.10 (0.88–1.36) | .40 |
| Adjusted for HF-ACTION model covariates† (n = 1954) | 1.01 (0.80–1.29) | .91 |
| Adjusted for HF-ACTION model + income, employment, marital status, and education covariates (n = 1670) | 1.09 (0.84–1.43) | .51 |
| Cardiovascular mortality or HF hospitalization | ||
| Unadjusted | 1.46 (1.25–1.70) | <.001 |
| Adjusted for HF-ACTION model covariates* (n = 1705) | 1.46 (1.20–1.77) | <.001 |
| Adjusted for HF-ACTION model + income, employment, marital status, and education covariates (n = 1482) | 1.41 (1.13–1.75) | .002 |
Sample size for the unadjusted analysis was n = 2296 for all end points, as 35 of the 2331 in HF-ACTION were not classified as black, white or other.
Adjusted for variables listed in Figure 1 footnote.
Adjusted for exercise duration on baseline CPX, serum creatinine level, BMI, sex, loop diuretic dose, left ventricular ejection fraction, CCS angina classification, and ventricular conduction before baseline CPX.
After multivariable adjustment for the adjustment model covariates, black race remained associated with increased mortality/hospitalization and cardiovascular mortality/HF hospitalization (Table III). Figure 1 displays adjusted event curves for the endpoints of mortality/hospitalization, and cardiovascular mortality/HF hospitalization. Exploring the individual components of these end points suggested increased risk, associated with black race for HF hospitalization (adjusted HR 1.58, 95% CI 1.27–1.96), but similar risk for cardiovascular mortality (adjusted P = .18).
After adjustment for additional socioeconomic covariates, black race was no longer associated with a significant increase in mortality/hospitalization (P = .09). The increased risk of cardiovascular mortality/HF hospitalization (Table III) and the individual component of HF hospitalization associated with black race remained statistically significant (HR 1.55, 95% CI 1.22–1.97) (Supplemental Table III).
There was no interaction between race and assignment to exercise training on clinical outcomes (Table IV). However, there was evidence for an interaction between black race and exercise training for change in 6-minute walk distance (adjusted P = .02). The estimated improvement in 6-minute walk distance with exercise training versus usual care at 3 months was +26 m (95% CI, +18 to +34) in whites versus +11 m (95% CI, 0 to +21) in blacks. No other exercise or health status variable demonstrated a statistically significant interaction with race and exercise training.
Table IV.
Interaction between race, exercise training, and outcomes
| Outcome |
P value for interaction between black vs white race and treatment assignment* |
|---|---|
| Clinical outcomes | |
| All-cause mortality or all-cause hospitalization | .66 |
| All-cause mortality | .68 |
| Cardiovascular mortality or HF hospitalization | .75 |
| Change in exercise and health status variables from baseline to 3 mo | |
| Kansas City Cardiomyopathy Questionnaire Overall Score | .82 |
| 6-min walk distance | .02 |
| CPX test duration | .10 |
| Peak VO2 | .53 |
KCCQ score: adjusted for baseline KCCQ overall summary score, Beck Depression score, and peak VO2; 6-minute walk: adjusted for baseline 6-minute walk distance, blood urea nitrogen, CPX duration, and peak VO2; CPX duration: adjusted for baseline CPX duration, number of hospitalizations in the previous 6 months, chronic obstruction pulmonary disease, blood urea nitrogen, peak respiratory exchange ratio, heart rate, and peak VO2; peak VO2: adjusted for baseline peak VO2, age, sex, number of HF hospitalizations in the previous 6 months, ischemic etiology, pacemaker, blood urea nitrogen, and CPX duration.
Clinical end points were adjusted as indicated in the footnote for Table III or in the legend for this table.
Discussion
The high enrollment of blacks, robust data collection on socioeconomic status, and long-term follow-up in HF-ACTION make this a unique data set to investigate the association between race, exercise training response, and outcomes. We demonstrated that black race was associated with more comorbidities, shorter 6-minute walk distance and lower peak VO2 at baseline, and increased mortality/hospitalization and cardiovascular morbidity/mortality driven by increased HF hospitalization compared with white race. We did not observe increased mortality or a differential response to exercise training in black patients. These findings confirm and extend previous research by demonstrating the distinct characteristics of blacks with HF and by documenting the prognostic significance of race in contemporary patients with HF.
Black patients were more likely to be female and were significantly younger than white patients. Black patients also had an increased burden of hypertension and diabetes. These risk factors coupled with their higher BMI likely contributed to more frequent nonischemic etiology as the cause of HF among black patients. As others have shown, the high prevalence of cardiovascular risk factors among black patients1,11 suggests that efforts to reduce HF incidence,18 and subsequent morbidity and mortality should include control of obesity and the prevention and treatment of diabetes. Because the risk for adverse outcomes for patients with HF substantially increases with the number of chronic conditions,19 the diagnosis and management of comorbidities should be a priority in patients with HF irrespective of race.
!HF-ACTION is among the largest studies to characterize exercise capacity in black patients with HF. Functional limitation as quantified by 6-minute walk distance and CPX testing has been associated with poor quality of life and higher morbidity/mortality.20–23 However, racial differences in baseline functional capacity of patients with HF have not been well characterized. We demonstrated that compared with white patients, black patients had a median 6-minute walk distance 35 m shorter and a median peak VO2 1.8 mL kg−1 min−1 lower. These observed Table IV. Interaction between race, exercise training, and outcomes differences in baseline exercise capacity should be considered when interpreting functional evaluations for clinical risk stratification and in the design/analysis of future trials.
Patients in the HF-ACTION study had high ACE inhibitor and β-blocker prescription rates regardless of race. This finding may have been related to the protocol, which encouraged the use of an optimal HF regimen for 6 weeks before enrollment. Based on the African American Heart Failure Trial study (A-HeFT),24 guidelines give the adjunctive use of isosorbide dinitrate/hydralazine the highest-tier recommendation in black patients with NYHA class II to IV symptoms.25 Only 15% of blacks in HF-ACTION were treated with isosorbide dinitrate/hydral-azine. Notably, A-HeFT was published during the time of HF-ACTION patient enrollment. Although the use of hydralazine/nitrates was higher than in previous registries,10 the overall modest usage indicates that further opportunities remain to improve guideline-based care for black patients with HF. Similarly, despite recent evidence suggesting that racial disparities in ICD use have narrowed over time,26 we observed a significantly lower use of ICDs in blacks. The lower prevalence of bundle-branch block in blacks likely influenced the lower use of CRT.
Previous studies have provided conflicting results with respect to outcomes in black patients.1,10,11 After adjustment for the HF-ACTION model covariates, black race was associated with increased mortality/hospitalization and cardiovascular morbidity/mortality. The secondary analysis adjusting for socioeconomic covariates attenuated the association between black race and the primary end point. The nonsignificant trend between race and mortality/hospitalization may have been related to the reduced sample size owing to exclusion of those with unavailable socioeconomic data. Another possible explanation is related to “overadjustment,” where variables in the causal pathway for poor prognosis in blacks are added to the model.
Adjustment for socioeconomic status did not markedly change the association between black race and ~40% higher cardiovascular morbidity/mortality. Previous studies have demonstrated racial disparities in hospitalization rates.5 Potential mechanisms for worse outcomes in black patients with HF include the more malignant pathophysiology of hypertension in blacks, the higher prevalence and severity of left ventricular hypertrophy, an impaired vasodilatory response, and genetic polymorphisms.3,4,27 Additional unrecognized differences in the pathophysiology of HF in blacks remain an alternative explanation. Disparities in health care access, along with a greater burden of socioeconomic stressors, may be associated with increased adverse events in black patients.
The lack of independent association between black race and mortality may be explained, in part, by the observation that factors such as BMI, gender, and renal dysfunction28,29 play a more prominent role than race on fatal events. Alternatively, similar mortality between groups may be related to improved medical follow-up in the controlled setting of a trial of exercise training.
Despite lower exercise adherence and volume in blacks and recent data suggesting that exercise volume mediates the impact of exercise training on outcomes,17 there was insufficient evidence to suggest that exercise training had a differential association with outcomes based on race. The specific reason for these findings is unclear, but potential explanations include racial differences in the association between exercise volume and outcomes or insufficient power to detect a between-group difference.
There are several additional incongruencies in this study that warrant discussion. First, clinical characteristics seen in black patients, which have been associated with a reduction in HF hospitalizations (eg, aldosterone antagonist use, higher β-blocker dose), did not translate into comparatively lower risk. One potential explanation for these findings is the higher comorbidity burden and lower CRT use in blacks. Another explanation is that despite statistically significant differences for several characteristics, the between-group differences may have not been large enough to result in clinically apparent differences in outcome (eg, impact of 43% aldosterone antagonist use in blacks vs 49% in whites). Alternatively, in some circumstances, there may be a differential association between therapies and outcomes based on race. For instance, the Beta Blocker Evaluation of Survival Trial demonstrated a blunted effect of bucindolol in African Americans.30 Future research is needed to explore racial differences in clinical risk predictors and responses to therapy in patients with HF. Furthermore, despite black patients having a higher risk for HF readmission, which has previously been associated with increased mortality, we did not observe increased mortality in black patients. Potential explanations for this finding include a differential mortality risk related to HF hospitalization depending on the severity of underlying disease31 or differences in outpatient follow-up.
Limitations
This was a retrospective analysis from a clinical trial of exercise training. The study population had strict inclusion and exclusion criteria, such that these findings may not apply to those with different baseline characteristics. The cohort was substantially younger, with higher baseline HF medication use than the general HF community, and the treatment group participated in exercise training such that these results may not be generalizable to other populations. Despite covariate adjustment, other measured and unmeasured factors may have influenced these findings. For instance, the adjustment variables for the present analysis are those that were initially developed for the HF-ACTION data set and include variables such as ventricular conduction, but not device use.
Conclusion
In patients with chronic systolic HF, black race was associated with younger age, increased prevalence of modifiable risk factors, and reduced peak VO2 at baseline. Despite a similar use of evidence-based HF pharmacologic therapies, blacks experienced increased HF hospitalization, but not increased mortality or a differential response to exercise training. Given the findings of the primary HF-ACTION trial, that regular exercise confers a modest reduction in the adjusted risk for all-cause mortality or hospitalization, these data support efforts to improve conditioning through exercise training in patients with HF regardless of race. These results highlight strategies to improve outcomes in the black population including a proactive approach to diet, exercise, and weight management and increased use of therapies, including hydralazine/nitrates and ICDs.
Supplementary Material
Footnotes
Disclosures
None.
References
- 1.Dries DL, Exner DV, Gersh BJ, et al. Racial differences in the outcome of left ventricular dysfunction. N Engl J Med. 1999;340(8):609–616. doi: 10.1056/NEJM199902253400804. [DOI] [PubMed] [Google Scholar]
- 2.Bibbins-Domingo K, Pletcher MJ, Lin F, et al. Racial differences in incident heart failure among young adults. N Engl J Med. 2009;360(12):1179–1190. doi: 10.1056/NEJMoa0807265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yancy CW. Heart failure in African Americans. J Card Fail. 2000;6(3):183–186. doi: 10.1054/jcaf.2000.17610. [DOI] [PubMed] [Google Scholar]
- 4.Yancy CW, Strong M. The natural history, epidemiology, and prognosis of heart failure in African Americans. Congest Heart Fail. 2004;10(1):15–18. doi: 10.1111/j.1527-5299.2004.02026.x. [DOI] [PubMed] [Google Scholar]
- 5.Joynt KE, Orav EJ, Jha AK. Thirty-day readmission rates for Medicare beneficiaries by race and site of care. JAMA. 2011;305(7):675–681. doi: 10.1001/jama.2011.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deswal A, Petersen NJ, Souchek J, et al. Impact of race on health care utilization and outcomes in veterans with congestive heart failure. J Am Coll Cardiol. 2004;43(5):778–784. doi: 10.1016/j.jacc.2003.10.033. [DOI] [PubMed] [Google Scholar]
- 7.Jha AK, Shlipak MG, Hosmer W, et al. Racial differences in mortality among men hospitalized in the Veterans Affairs health care system. JAMA. 2001;285(3):297–303. doi: 10.1001/jama.285.3.297. [DOI] [PubMed] [Google Scholar]
- 8.Rathore SS, Foody JM, Wang Y, et al. Race, quality of care, and outcomes of elderly patients hospitalized with heart failure. JAMA. 2003;289(19):2517–2524. doi: 10.1001/jama.289.19.2517. [DOI] [PubMed] [Google Scholar]
- 9.Kamath SA, Drazner MH, Wynne J, et al. Characteristics and outcomes in African American patients with decompensated heart failure. Arch Intern Med. 2008;168(11):1152–1158. doi: 10.1001/archinte.168.11.1152. [DOI] [PubMed] [Google Scholar]
- 10.Yancy CW, Abraham WT, Albert NM, et al. Quality of care of and outcomes for African Americans hospitalized with heart failure: findings from the OPTIMIZE-HF (Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients With Heart Failure) registry. J Am Coll Cardiol. 2008;51(17):1675–1684. doi: 10.1016/j.jacc.2008.01.028. [DOI] [PubMed] [Google Scholar]
- 11.Thomas KL, Hernandez AF, Dai D, et al. Association of race/ethnicity with clinical risk factors, quality of care, and acute outcomes in patients hospitalized with heart failure. Am Heart J. 2011;161(4):746–754. doi: 10.1016/j.ahj.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 12.O'Connor CM, Whellan DJ, Lee KL, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301(14):1439–1450. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whellan DJ, O'Connor CM, Lee KL, et al. Heart Failure and a Controlled Trial Investigating Outcomes of Exercise Training (HF-ACTION): design and rationale. Am Heart J. 2007;153(2):201–211. doi: 10.1016/j.ahj.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 14.Flynn KE, Pina IL, Whellan DJ, et al. Effects of exercise training on health status in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301(14):1451–1459. doi: 10.1001/jama.2009.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mentz RJ, Schulte PJ, Fleg JL, et al. Clinical characteristics, response to exercise training, and outcomes in patients with heart failure and chronic obstructive pulmonary disease: findings from Heart Failure and A Controlled Trial Investigating Outcomes of Exercise TraiNing (HF-ACTION) Am Heart J. 2013;165(2):193–199. doi: 10.1016/j.ahj.2012.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fiscella K, Tancredi D. Socioeconomic status and coronary heart disease risk prediction. JAMA. 2008;300(22):2666–2668. doi: 10.1001/jama.2008.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keteyian SJ, Leifer ES, Houston-Miller N, et al. Relation between volume of exercise and clinical outcomes in patients with heart failure. J Am Coll Cardiol. 2012;60(19):1899–1905. doi: 10.1016/j.jacc.2012.08.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Avery CL, Loehr LR, Baggett C, et al. The population burden of heart failure attributable to modifiable risk factors: theARIC (Atherosclerosis Risk in Communities) study. J Am Coll Cardiol. 2012;60(17):1640–1646. doi: 10.1016/j.jacc.2012.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Braunstein JB, Anderson GF, Gerstenblith G, et al. Noncardiac comorbidity increases preventable hospitalizations and mortality among Medicare beneficiaries with chronic heart failure. J Am Coll Cardiol. 2003;42(7):1226–1233. doi: 10.1016/s0735-1097(03)00947-1. [DOI] [PubMed] [Google Scholar]
- 20.Myers J, Arena R, Dewey F, et al. A cardiopulmonary exercise testing score for predicting outcomes in patients with heart failure. Am Heart J. 2008;156(6):1177–1183. doi: 10.1016/j.ahj.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 21.Francis DP, Shamim W, Davies LC, et al. Cardiopulmonary exercise testing for prognosis in chronic heart failure: continuous and independent prognostic value from VE/VCO(2)slope and peak VO(2) Eur Heart J. 2000;21(2):154–161. doi: 10.1053/euhj.1999.1863. [DOI] [PubMed] [Google Scholar]
- 22.Curtis JP, Rathore SS, Wang Y, et al. The association of 6-minute walk performance and outcomes in stable outpatients with heart failure. J Card Fail. 2004;10(1):9–14. [PubMed] [Google Scholar]
- 23.Arslan S, Erol MK, Gundogdu F, et al. Prognostic value of 6-minute walk test in stable outpatients with heart failure. Tex Heart Inst J. 2007;34(2):166–169. [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor AL, Ziesche S, Yancy C, et al. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med. 2004;351(20):2049–2057. doi: 10.1056/NEJMoa042934. [DOI] [PubMed] [Google Scholar]
- 25.Lindenfeld J, Albert NM, Boehmer JP, et al. HFSA 2010 comprehensive heart failure practice guideline. J Card Fail. 2010;16(6):e1–e194. doi: 10.1016/j.cardfail.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 26.Al-Khatib SM, Hellkamp AS, Hernandez AF, et al. Trends in use of implantable cardioverter-defibrillator therapy among patients hospitalized for heart failure: have the previously observed sex and racial disparities changed over time? Circulation. 2012;125(9):1094–1101. doi: 10.1161/CIRCULATIONAHA.111.066605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McNamara DM, Tam SW, Sabolinski ML, et al. Aldosterone synthase promoter polymorphism predicts outcome in African Americans with heart failure: results from the A-HeFT Trial. J Am Coll Cardiol. 2006;48(6):1277–1282. doi: 10.1016/j.jacc.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 28.O'Connor CM, Whellan DJ, Wojdyla D, et al. Factors related to morbidity and mortality in patients with chronic heart failure with systolic dysfunction: the HF-ACTION predictive risk score model. Circ Heart Fail. 2012;5(1):63–71. doi: 10.1161/CIRCHEARTFAILURE.111.963462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Connor CM, Hasselblad V, Mehta RH, et al. Triage after hospitalization with advanced heart failure: the ESCAPE (Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness) risk model and discharge score. J Am Coll Cardiol. 2010;55(9):872–878. doi: 10.1016/j.jacc.2009.08.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Domanski MJ, Krause-Steinrauf H, Massie BM, et al. A comparative analysis of the results from 4 trials of beta-blocker therapy for heart failure: BEST, CIBIS-II, MERIT-HF, and COPERNICUS. J Cardiac Fail. 2003;9(5):354–363. doi: 10.1054/s1071-9164(03)00133-7. [DOI] [PubMed] [Google Scholar]
- 31.Kommuri NV, Koelling TM, Hummel SL. The impact of prior heart failure hospitalizations on long-term mortality differs by baseline risk of death. Am J Med. 2012;125(2):209e9–209e15. doi: 10.1016/j.amjmed.2011.06.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.