Abstract
Campylobacter jejuni is one of the predominant causes for foodborne bacterial infections worldwide. We investigated whether signaling of C. jejuni-lipoproteins and -lipooligosaccharide via Toll-like-receptor (TLR) -2 and -4, respectively, is inducing intestinal and extra-intestinal immune responses following infection of conventional IL-10−/− mice with chronic colitis. At day 3 following oral infection, IL-10−/− mice lacking TLR-2 or TLR-4 harbored comparable C. jejuni strain ATCC 43431 loads in their colon. Interestingly, infected TLR-4−/− IL-10−/− mice displayed less compromized epithelial barrier function as indicated by lower translocation rates of live gut commensals into mesenteric lymphnodes (MLNs), and exhibited less distinct B lymphocyte responses in their colonic mucosa as compared to naїve IL-10−/− controls. Furthermore, in extra-intestinal compartments such as MLNs and spleens, abundance of myeloid cells was less distinct whereas relative percentages of activated T helper cells and cytotoxic T cells were higher in spleens and dendritic cells more abundant in MLNs of infected IL-10−/− animals lacking TLR-4 as compared to IL-10−/− controls. Taken together, in conventionally colonized IL-10−/− mice, TLR-4, but not TLR-2, is involved in mediating extra-intestinal pro-inflammatory immune responses following C. jejuni infection. Thus, conventional IL-10−/− mice are well suited to further dissect mechanisms underlying Campylobacter infections in vivo.
Keywords: Campylobacter jejuni, chronic colitis, conventional intestinal microbiota, host–pathogen interaction, IL-10, innate and adaptive immune responses, lipopeptide, lipopolysaccharide, pro-inflammatory cytokines, TLR-2, TLR-4
Introduction
Campylobacter (C.) jejuni is one of the most common causes for acute diarrhea in humans worldwide. Campylobacteriosis is characterized by fever, watery to bloody diarrhea, and abdominal cramping [1, 2]. Although the course of disease is self-limiting in the vast majority of infections, post-infectious complications such as Guillain–Barré and Reiter’s syndrome may occur [3, 4]. C. jejuni infections mainly affect the colon [5, 6] and induce the recruitment of immune cells such as neutrophilic granulocytes and mononuclear cells into the lamina propria, leading to crypt abscesses, focal ulcerations, and plasma cell proliferation [7, 8]. Despite the high prevalence of C. jejuni induced disease and its distinctive socioeconomic impact, molecular and cellular events leading to campylobacteriosis are still poorly understood [9]. A very recent review article from Ó Cróinin and Backert highlighted various pathogenicity factors and host cell determinants proposed to be involved in establishing C. jejuni infection and triggering disease [10]. Mechanisms of intestinal inflammation are scarcely investigated due to a lack of suitable vertebrate models mimicking human disease. Mice harboring a conventional gut microbiota display a physiological colonization resistance against C. jejuni preventing infection by the pathogen [4, 11]. The colonization resistance, however, can be overcome by modifying or eradicating the microbiota within the gastrointestinal tract. Already two decades ago germfree mice raised in isolators have been shown to be stably colonized following C. jejuni infection [12–16]. Germfree mice, however, do not represent an appropriate experimental model due to their lack of an intact immune system and the need for an extensive infrastructure in order to accomplish breeding, housing, and performing experiments under absolute sterile conditions. In mice harboring a limited enteric flora, C. jejuni could colonize at least 5 months [17]. In addition, gnotobiotic (GB) wildtype mice generated by quintuple antibiotic treatment of conventionally colonized mice (displaying an entirely developed immune system) for 8–10 weeks, GB mice reconstituted with a human microbiota as well as GB animals fed a Western style cafeteria diet could be stably colonized by C. jejuni inducing a pro-inflammatory immune response in the colonic mucosa [18, 19]. In these animals lacking any gut bacteria or harboring a modified intestinal microbiota classical symptoms of human campylobacteriosis such as bloody diarrhea, however, were missing. Therefore, we established another infection model: three-week-old infant mice, harboring a different gastrointestinal microflora when compared to adult mice 3 months of age, were infected with C. jejuni right after weaning and developed severe ulcerative enterocolitis with bloody diarrhea until day 7 post infection (p.i.) which was self-limiting and resolved within 2 weeks following infection [20]. In the inflamed colonic mucosa and lamina propria, a significant influx of innate and adaptive immune cells could be observed [20]. Most strikingly, in conventional adult mice in which the intestinal Escherichia coli loads had been artificially elevated thereby mimicking microbiota shifts seen in conditions of acute intestinal inflammation [21–24], C. jejuni was able to overcome colonization resistance and induced enterocolitis [25].
Toll-like receptors (TLRs) are pattern-recognition receptors of the innate immune system recognizing microbial components known as PAMPs (pathogen-associated molecular patterns) which are essential for the survival of microorganisms [26]. The lipopolysaccharide (LPS) and lipooligosaccharide (LOS) of Gram-negative bacteria are recognized by TLR-4 whereas lipoproteins, lipoteichoic acid, and peptidoglycan activate TLR-2. TLRs are expressed on various immune cells including dendritic cells (DCs), B lymphocytes and macrophages as well as epithelial cells [26]. TLRs activate the NF-κB-pathway which in turn regulates the transcription of pro-inflammatory cytokines such as IL-12p40, TNF-α, and IL-8 [27]. Recent investigations from our group revealed that C. jejuni mediates immunopathology via TLR-2, -4 and -9 signaling [18, 28]. C. jejuni induced the secretion of pro-inflammatory cytokines such as IL-6, IL-8, IL-1β, IL-12p70, and TNF-α in epithelial cell lines, monocytes, and DCs in culture [29, 30]. Furthermore, co-culturing these cells with T cells led to a high level of IFN-γ production which is mainly caused by a Th1-type immune response [30, 31]. In ex vivo colon cultures taken from GB IL-10−/− mice displaying severe enterocolitis following C. jejuni infection, an increased secretion of pro-inflammatory cytokines such as TNF-α, IFN-γ, and MCP-1 could be observed [28]. These mice exhibited higher counts of T and B lymphocytes in their colonic mucosa and lamina propria [28]. Recently, our group demonstrated that the induction of pro-inflammatory cytokine production following oral C. jejuni infection of GB mice was dependent on the detection of C. jejuni-LPS- and -CpG-DNA via TLR-4 and TLR-9, respectively [18]. Furthermore, GB IL-10 deficient mice lacking TLR-4 exhibited significantly less pro-inflammatory cytokines and diminished cellular responses in their colon [28]. Finally, we observed that TLR-4, signaling is essentially involved in acute inflammatory processes in murine models of acute ileitis and colitis [22, 23].
Conventionally colonized IL-10−/− mice developing chronic colitis due to bacterial stimuli derived from their commensal intestinal microbiota within 5 to 6 months of age can be stably infected by C. jejuni at intermediate levels and develop enteritis [25, 32]. Given that TLR-2 and -4 mediate C. jejuni induced acute enterocolitis in gnotobiotic IL-10−/− mice [28], we were interested whether signaling of C. jejuni lipoproteins and lipooligosaccharide, respectively, is also essentially involved in immunopathogenesis following infection of conventional IL-10−/− mice with chronic colitis. In the study presented here we analyzed the activation of effector cells from the innate and adaptive immune system following C. jejuni infection of conventionally colonized TLR-2 IL-10 and TLR-4 IL-10 double deficient animals with chronic colitis in vivo and focused on extra-intestinal immune responses.
Results
C. jejuni infection of conventional IL-10−/− mice lacking TLR-2 or -4 exhibiting chronic colitis
We have recently shown that 5- to 6-month-old IL-10−/− mice harboring a conventional intestinal microbiota and suffering from chronic colitis, but not age-matched wildtype controls without intestinal inflammation, can be colonized by C. jejuni at intermediate levels for 2 weeks in approximately 70% of the cases [25]. Given that TLR-2 and -4 play pivotal roles in mediating acute enterocolitis in C. jejuni infected GB IL-10 deficient mice [28], we were interested whether TLR-2 and/or TLR-4 signaling were also essentially involved in C. jejuni infected conventional IL-10 deficient mice with chronic colitis. Interestingly, IL-10 deficient mice lacking TLR-2 or -4 as well as IL-10−/− controls displayed comparable clinical as well as histopathological signs of chronic colitis before and 3 days after C. jejuni (ATCC 43431 strain) infection indicating that C. jejuni infection did not deteriorate already established chronic colitis (not shown). Furthermore, irrespective of their genotype, conventionally colonized mice harbored comparable intestinal pathogen loads in their feces at d3 p.i. (not shown).
Barrier defect and bacterial translocation in conventional IL-10−/− mice lacking TLR-2 or -4
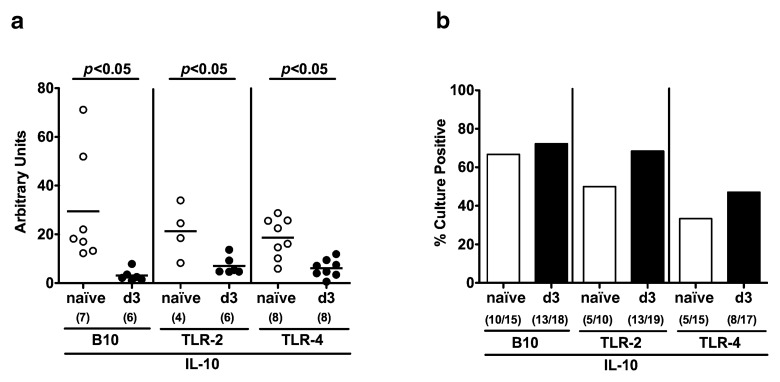
Next, we were interested whether C. jejuni infection induced translocation of commensal gut bacteria due to compromized epithelial barrier functions. Irrespective of the genotype, mRNA expression of Mucin-2 forming an integral part of the mucus significantly decreased 3 days following C. jejuni infection (Fig. 1a). Interestingly, uninfected IL-10 deficient mice lacking TLR-2 or TLR-4 exhibited lower rates of translocated live bacteria originating from the commensal gut microbiota into their mesenteric lymphnodes (MLNs; 50.0 and 33.3%, respectively) as compared to IL-10 controls (66.7%) (Fig. 1b). Three days following C. jejuni infection, however, bacterial translocation rates into MLNs of IL-10 deficient mice lacking TLR-4 (47.1%), but not TLR-2 (68.4%), were lower as compared to IL-10 deficient controls (72.2%) (Fig. 1b). Of note, live C. jejuni could not be cultured from the other examined extra-intestinal tissue sites at day 3 p.i. (not shown).
Fig. 1.
Epithelial barrier function following C. jejuni ATCC 43431 infection of conventional IL-10 deficient mice lacking TLR-2 or -4. Conventionally colonized IL-10 deficient (B10 background) as well as TLR-2 IL-10 and TLR-4 IL-10 double deficient mice were orally infected with C. jejuni ATCC 43431 (solid circles or bars) and analyzed at day 3 post infection (d3) as described in Methods. Uninfected mice served as negative controls (naïve, open circles or bars). (a) RT-PCR results of Mucin-2 expression in colonic samples of individual mice are expressed as fold changes HPRT mRNA expression (arbitrary units). (b) Translocation of live commensal gut bacteria to extra-intestinal compartments at day 3 post infection was assessed by cultivation of mesenteric lymphnodes (MLN) in thioglycolate enrichment broths for maximum 7 days. Turbid broths were sub-cultivated on solid media (refer to Materials). Bars indicate mean relative rates of positive samples (in %). Absolute numbers of positive samples out of total number analyzed are given in parentheses. Means (black bars) and significance levels (P-values) determined by the Student’s t-test are indicated. Data shown are representative for (a) or were pooled from three independent experiments (b)
Colonic B lymphocyte responses in C. jejuni-infected conventional IL-10−/− mice lacking TLR-2 or TLR-4
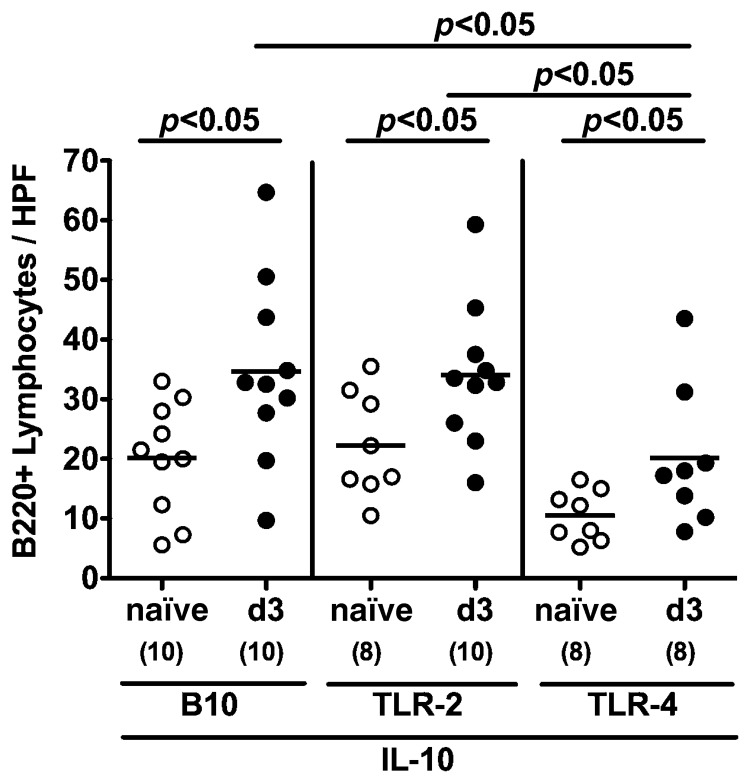
In humans, C. jejuni induces the recruitment of pro-inflammatory immune cell populations to sites of inflammation in the colon [5, 6]. To further elucidate whether C. jejuni infection of conventional IL-10−/− mice lacking TLR-2 or -4 was accompanied by cellular immune responses, we next analyzed B220+ cells in the colonic mucosa by in situ immunohistochemistry. B cell numbers increased within 3 days following C. jejuni infection irrespective of the genotype of mice. This increase in colonic B cell numbers, however, was significantly less distinct in TLR-4−/− IL-10−/− animals (Fig. 2).
Fig. 2.
Colonic B lymphocyte responses following C. jejuni ATCC 43431 infection of conventional IL-10 deficient mice lacking TLR-2 or -4. Conventionally colonized IL-10 deficient (B10 background) as well as TLR-2 IL-10 and TLR-4 IL-10 double deficient mice were orally infected with C. jejuni ATCC 43431 (solid circles) and analyzed at day 3 post infection (d3) as described in Methods. Uninfected mice served as negative controls (naïve, open circles). The average numbers of B lymphocytes (positive for B220) from at least six high power fields (HPF, ×400 magnification) per animal were determined microscopically in immunohistochemically stained colon sections. Numbers of analyzed animals are given in parentheses. Means (black bars) and levels of significance (P-values) determined by the Student’s t-test are indicated. Data shown are pooled from three independent experiments
Extra-intestinal pro-inflammatory immune responses in C. jejuni-infected conventional IL-10−/− mice lacking TLR-2 or TLR-4
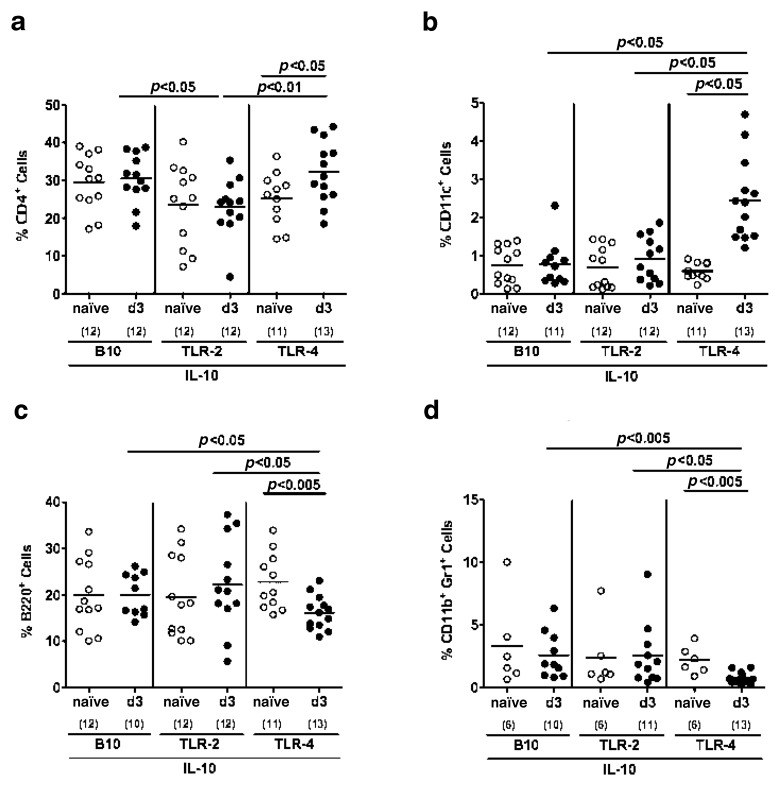
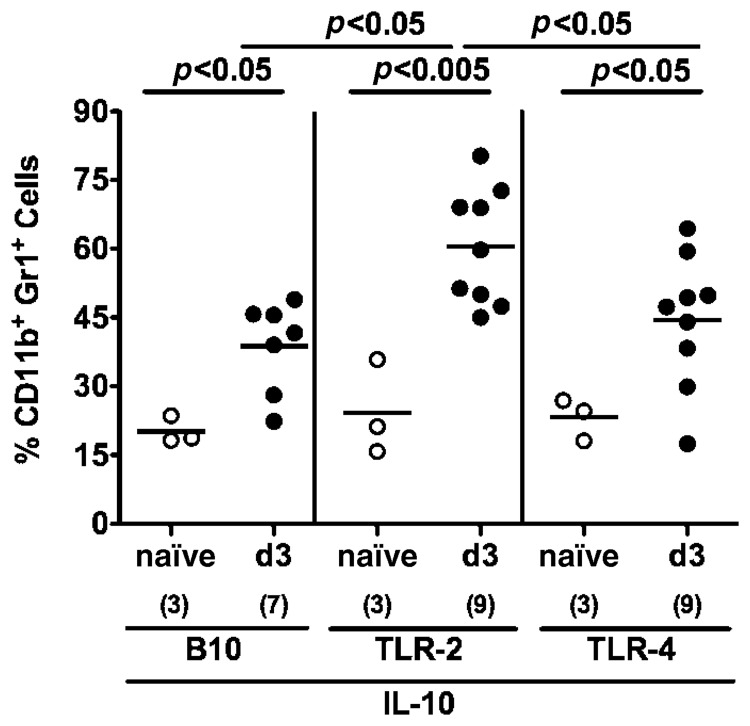
C. jejuni infection has been recently shown to be associated with extra-intestinal immune responses [20]. Therefore, we investigated leukocytes isolated from MLNs, spleens and livers stained with respective surface markers. Following C. jejuni infection, only conventional TLR-4−/− IL-10−/− mice exhibited an increase of the relative percentages of CD4+ T lymphocytes (Fig. 3a) and CD11c+ dendritic cells (Fig. 3b) in their MLNs whereas B220+ B lymphocytes (Fig. 3c) and CD11b+ Gr1+ myeloid cells such as inflammatory monocytes and neutrophilic granulocytes [33–36] (Fig. 3d) had decreased until day 3 p.i. and were significantly lower as compared to MLNs taken from conventional TLR-2−/− IL-10−/− and IL-10−/− mice. Furthermore, IL-10 deficient mice lacking TLR-2 displayed relatively lower CD4+ cells as compared to TLR-4−/− IL-10−/− and IL-10−/− animals (Fig. 3a).
Fig. 3.
Immune cell responses in mesenteric lymphnodes of C. jejuni ATCC 43431 infected conventional IL-10 deficient mice lacking TLR-2 or -4. Conventionally colonized IL-10 deficient (B10 background) as well as TLR-2 IL-10 and TLR-4 IL-10 double deficient mice were orally infected with C. jejuni ATCC 43431 (solid circles) analyzed at day 3 post infection (d3) as described in Methods. Uninfected mice served as negative controls (naïve, open circles). Leukocytes isolated from mesenteric lymphnodes (MLN) were isolated, stained with surface markers and analyzed by flow cytometry. Relative abundance of Th1 cells (CD4+; panel a), dendritic cells (CD11c+, panel b) B lymphocytes (B220+, panel c), and myeloid cells such as inflammatory monocytes and neutrophilic granulocytes (CD11b+ Gr1+, panel d) is expressed in %. Numbers of analyzed animals are given in parentheses. Medians (black bars) and significance levels (P-values) determined by Student’s t-test are indicated. Data shown are pooled from three independent experiments
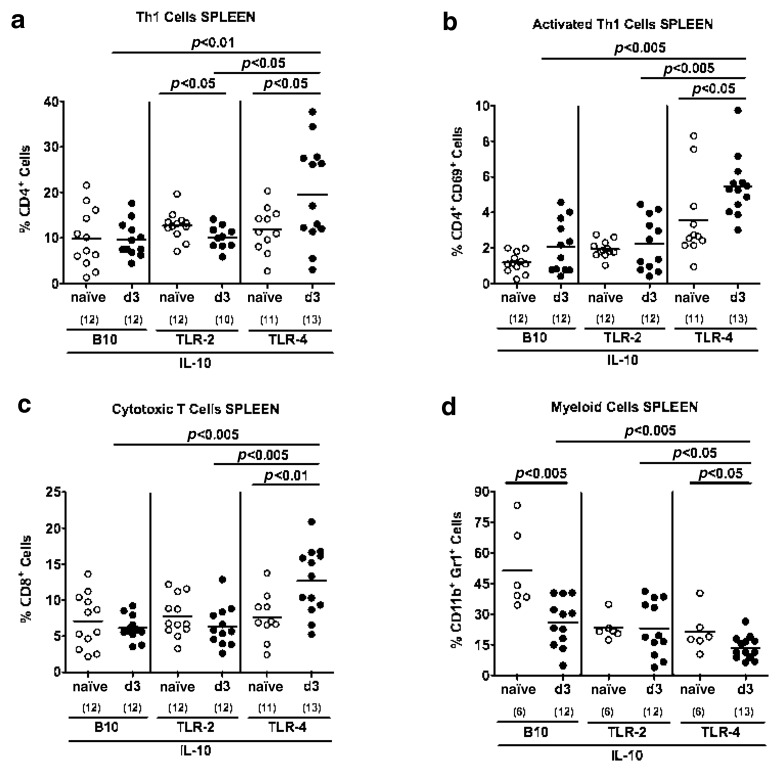
In C. jejuni infected IL-10−/− mice lacking TLR-4, but not TLR-2, or IL-10−/− control animals, an increase of CD4+ Th1 and activated CD4+ CD69+ Th1 lymphocytes as well as CD8+ cytotoxic lymphocytes could be detected in spleens at day 3 p.i. (Fig. 4a–c). Interestingly, splenic CD11b+ Gr1+ myeloid cells decreased upon C. jejuni infection in TLR-4−/− IL10−/− and IL-10−/−, but not TLR-2 IL-10 double deficient animals with lowest abundance in the former at day 3 p.i. (Fig. 4d).
Fig. 4.
Immune cell responses in spleens of C. jejuni ATCC 43431 infected conventional IL-10 deficient mice lacking TLR-2 or -4. Conventionally colonized IL-10 deficient (B10 background) as well as TLR-2 IL-10 and TLR-4 IL-10 double deficient mice were orally infected with C. jejuni ATCC 43431 (solid circles) and analyzed at day 3 post infection (d3) as described in Methods. Uninfected mice served as negative controls (naïve, open circles). Splenic leukocytes were isolated, stained with surface markers, and analyzed by flow cytometry. Relative abundance of Th1 cells (CD4+; panel a), activated Th1 cells (CD4+ CD69+, panel b), cytotoxic T cells (CD8+, panel c), and myeloid cells such as inflammatory monocytes and neutrophilic granulocytes (CD11b+ Gr1+, panel d) is expressed in %. Numbers of analyzed animals are given in parentheses. Medians (black bars) and significance levels (P-values) determined by Student’s t-test are indicated. Data shown are pooled from three independent experiments
In the liver, however, CD11b+ Gr1+ myeloid cells increased within 3 days following C. jejuni infection irrespective of the genotype with significantly higher relative rates in TLR-2−/− IL-10−/− as compared to TLR-4−/− IL-10−/− and IL-10−/− controls (Fig. 5).
Fig. 5.
Immune cell responses in livers of C. jejuni ATCC 43431 infected conventional IL-10 deficient mice lacking TLR-2 or -4. Conventionally colonized IL-10 deficient (B10 background) as well as TLR-2 IL-10 and TLR-4 IL-10 double deficient mice were orally infected with C. jejuni ATCC 43431 (solid circles) and analyzed at day 3 post infection (d3) as described in Methods. Uninfected mice served as negative controls (naïve, open circles). Liver leukocytes were isolated, stained with surface markers, and analyzed by flow cytometry. Relative abundance of myeloid cells such as inflammatory monocytes and neutrophilic granulocytes (CD11b+ Gr1+) is expressed in %. Numbers of analyzed animals are given in parentheses. Medians (black bars) and significance levels (P-values) determined by Student’s t-test are indicated. Data shown are pooled from three independent experiments
Taken together, following oral C. jejuni infection conventional TLR-4 IL-10 double deficient mice displayed a less compromized epithelial barrier function as indicated by lower translocation rates of live commensal gut bacteria into MLNs, and less distinct intestinal pro-inflammatory immune responses as mirrored by lower B lymphocyte numbers within the colonic mucosa. In extra-intestinal compartments, abundance of myeloid cells (consisting of inflammatory monocytes and neutrophilic granulocytes) was less distinct in MLNs and spleens whereas activated T helper cells and cytotoxic T cells were higher in spleens and dendritic cells more abundant in MLNs of conventional TLR-4−/− IL-10−/− animals as compared to TLR-2−/− IL-10−/− and IL-10−/− mice following C. jejuni infection.
Discussion
Campylobacter jejuni is a leading cause of bacterial foodborne enterocolitis worldwide. Despite the increasing incidence and the related socioeconomic burden, immunopathology underlying human campylobacteriosis is not well understood. Detailed investigation is hampered by the lack of appropriate vertebrate models. In the present study, we aimed at investigating TLR-dependent immune responses to C. jejuni in conventionally bred IL-10 deficient mice with a conventional gut microbiota. Therefore, IL-10−/− mice (in C57BL/10 background) lacking TLR-2 or -4 were infected with C. jejuni ATCC 43431. As shown earlier, IL-10 deficient animals in C57BL/6 background could be stably colonized by the pathogen [32]. Following C. jejuni infection, IL-10 deficient mice of either genotype exhibited no significant aggravation of colonic histopathology but displayed a reduction of Mucin-2 mRNA expression in the colon compared to uninfected controls. Mucin-2 acts as the first barrier invading microorganisms have to overcome and thus plays a pivotal role in colonic protection [37]. However, C. jejuni is able to bind to Mucin-2 [38] and to upregulate mucin-degrading enzymes [39]. The impact of mucins in contributing to resistance to C. jejuni infection is further underlined by the fact that Mucin-deficient mice experience higher intestinal pathogen burden, display more distinct epithelial damage as well as rapidly developing systemic infection upon C. jejuni infection [40].
These results are well in line with our findings that C. jejuni infected mice contained higher concentrations of commensal bacteria in their MLNs as compared to naїve mice. This can be explained by (1) the diminished mucus layer which facilitates the pathogen to get in close contact with the intestinal epithelium and to consecutively translocate to sub-epithelial tissue sites [41] and (2) by the earlier finding that C. jejuni supports translocation of commensal bacteria via lipid rafts [42]. Interestingly, in C. jejuni-infected TLR-4−/− IL10−/− mice, less frequent bacterial translocation to MLNs could be detected as compared to TLR-2−/− IL10−/− and IL-10 deficient controls.
Furthermore, a diminished count of myeloid cells was found in the MLNs of TLR-4−/− IL-10−/− mice compared to TLR-2−/− IL-10−/− and IL-10−/− control animals. Given that myeloid cells such as inflammatory monocytes and neutrophilic granulocytes mainly act on eliminating invading microorganisms one can see these TLR-4-dependent results in coherence with the lower bacterial translocation rates into the MLNs of infected TLR-4−/− IL-10−/− mice.
Toll-like receptors recognize highly conserved microbial structures such as LPS and LOS (by TLR-4) as well as lipoteichoic acid (by TLR-2), all expressed on non-immune cells (e.g. epithelial cells) and immune cells (e.g. DCs) [26]. DCs sample commensals and pathogens, get activated (e.g. by C. jejuni) which in turn leads to a Th1-type immune response [30, 31]. Bacteria-laden DCs migrate to MLNs and activate T and B lymphocytes. Activated B lymphocytes differentiate into plasma cells and produce IgA specific for the sampled bacteria [43, 44]. The detection of serum antibodies from infected patients indicates that C. jejuni induces both innate and specific (adaptive) immune responses [45]. In the colonic mucosa and lamina propria we detected an increase of B lymphocytes as early as day 3 p.i., irrespective of the genotype of C. jejuni infected mice. Like in GB TLR-4−/− animals [18], infected TLR-4−/− IL-10−/− mice harboring a conventional intestinal microbiota displayed less colonic B lymphocytes indicating that TLR-4 is essential in recruiting adaptive immune cells into the intestinal compartment. This is supported by the fact that, even in the MLNs of TLR-4−/− IL-10−/− mice, B cell numbers were lower as compared to TLR-2−/− IL-10−/− and IL-10−/− mice.
In MLNs of C. jejuni infected IL-10 deficient mice lacking TLR-4, but not TLR-2, we detected a higher DC abundance as compared to IL-10−/− controls. In vitro experiments revealed that cytokine production by DC in response to C. jejuni was shown to be predominantly TLR-4-dependent [46]. Our group recently showed that GB IL-10 deficient mice lacking TLR-4 exhibited significantly less pro-inflammatory cytokines and a diminished cellular response in the colon following oral C. jejuni infection [28] further underlining that C. jejuni-LOS is a major trigger of TLR-4 activation in vivo [47].
In contrast, Rose et al. observed that in TLR-4−/− DCs an acapsular C. jejuni mutant strain induced higher pro-inflammatory cytokine levels as compared to the wildtype control strain [48]. As a strategy of evading the host’s immune system, Gram-negative bacteria modify cell surface structures. C. jejuni can adapt to its host by modification of its LOS, flagella and capsule for instance [49]. Hereby, C. jejuni is able to impact TLR-4 activation and enhance antimicrobial resistance [50].
In the study presented here we were interested in examining C. jejuni induced immune responses not only in intestinal but also in extra-intestinal compartments. For this purpose, we focused on the spleen initiating lymphocyte differentiation, activation, and proliferation and thereby being involved in the systemic immune responses. In chickens, Li and coworkers found a more distinct systemic host response in the spleen as compared to local immune responses in the caecum following C. jejuni infection [51]. In our experiments we detected higher abundances of CD4+ Th1 cells, activated CD4+ CD69+ Th1 cells and cytotoxic CD8+ T lymphocytes in the spleen of infected TLR-4−/− IL-10−/− mice as compared to TLR-2−/− IL-10−/− and IL-10−/− controls. Both CD4+ and CD8+ T lymphocytes contribute to the protection against C. jejuni [30, 52]. Just as in MLNs of infected mice, abundance of myeloid cells, however, was less distinct in the spleen of TLR-4−/− IL-10−/− mice as compared to TLR-2−/− IL-10−/− and IL-10−/− animals supporting further evidence for a TLR-4 dependent activation of myeloid cells such as inflammatory monocytes and neutrophilic granulocytes in IL-10−/− mice following C. jejuni infection.
Besides its metabolic properties, the liver contributes to intestinal immune functions [53]. When the epithelial barrier function is compromized, e.g. due to a diminished mucus layer, bacterial compounds such as LPS reach the liver and induce pro-inflammatory responses [54]. In support of this, our experiments revealed a higher abundance of myeloid cells in the liver of infected animals compared to uninfected controls irrespective of their genotype. Interestingly, this C. jejuni-induced increase in liver myeloid cells such as inflammatory monocytes and neutrophilic granulocytes was more pronounced in TLR-2 IL-10 double deficient animals as compared to TLR-4−/− IL-10−/− and IL-10−/− controls.
Taken together, the study presented here clearly demonstrates that, in conventionally colonized IL-10−/− mice, TLR-4, but not TLR-2, is involved in mediating extra-intestinal pro-inflammatory immune responses following C. jejuni infection. Thus, conventional IL-10−/− mice with established enterocolitis are well suited to further dissect mechanisms underlying Campylobacter infections in vivo.
Materials and methods
Ethics statement
Animal experiments were conducted according to the European Guidelines for animal welfare (2010/63/EU) with approval of the commission for animal experiments headed by the “Landesamt für Gesundheit und Soziales” (LaGeSo, Berlin, Germany; Registration numbers: G0173/07 and G135/10). Animal welfare was monitored twice daily by assessment of clinical conditions.
Mice and infection
IL-10 deficient mice (in C57BL/10 background, B10) were bred and maintained in the facilities of the “Forschungsinstitut für Experimentelle Medizin” (FEM, Charité — Universitätsmedizin, Berlin, Germany), under specific pathogen-free (SPF) conditions. In order to gain double-deficient animals, TLR-2−/− and TLR-4−/− mice (in B10 background each) were crossed to IL-10−/− mice and backcrossed at least 7 generations before use.
Six-month-old female mice of either genotype exhibiting chronic colitis were infected with approximately 109 viable CFU of C. jejuni strain ATCC 43431 by gavage in a total volume of 0.3 ml PBS on two consecutive days as described earlier in detail [18].
Clinical score
To assess clinical/macroscopic signs of C. jejuni induced infection on a daily basis, a standardized cumulative clinical score (maximum 12 points; modified according to Ref. [55]) addressing the occurrence of blood in feces (0 points: no blood; 2 points: microscopic detection of blood by the Guajac method using Haemoccult™, Beckman Coulter/PCD, Krefeld, Germany; 4 points: macroscopic blood visible), diarrhea (0: formed feces; 2: pasty feces; 4: liquid feces), and the clinical aspect (0: normal; 2: ruffled fur, less locomotion; 4: isolation, severely compromized locomotion, pre-final aspect) was used.
Sampling procedures, determination of colon length, and histopathology
Mice were sacrificed by isofluran treatment (Abbott, Germany). From each mouse, samples derived from the colon were collected in parallel for histological, microbiological, and immunobiological analyses. Cardiac blood and tissue samples from mesenteric lymphnodes, spleen, liver, and the gastrointestinal tract were asserved under sterile conditions. Histopathological changes were determined in colon samples immediately fixed in 5% formalin and embedded in paraffin. Sections (5 µm) were stained with respective antibodies for immunohistochemistry.
Immunohistochemistry
In situ immunohistochemical analysis of colonic paraffin sections was performed as described previously [18, 23, 56]. Primary antibodies against B220 (eBioscience, San Diego, CA, USA, 1:200) were used. For each animal, the average number of positive stained cells within at least six independent high power fields (HPF, ×400 magnification) was determined microscopically and subjected to statistical analysis as indicated.
Real time-PCR
RNA was isolated from organs using the RNeasy Mini Kit (Qiagen, Hilden, Germany). mRNA was reverse transcribed and analyzed in triplicate assays by TaqMan PCR using the ABI Prism 7700 Sequence Detection System (Applied Biosystems) as described previously [57]. For detection of murine mucin-2 (MUC-2), assays including double-fluorescent probes in combination with assays for the murine housekeeping gene hypoxanthine phosphoribosyltransferase 1 (HPRT) were purchased from Applied Biosystems. Expression levels were calculated relative to the HPRT expression (expressed as normalized ratio and indicated as Arbitrary Units).
Isolation of lymphocytes and flow cytometry
Mesenteric lymphnodes were removed and subsequently minced through a 70-µm filter. For intracellular staining, cells were stimulated for 6 h with 50 ng/ml 12-O-tetradecanoylphorbol-13 acetate (Sigma, Missouri, USA), 750 ng/ml ionomycin (Sigma) and Golgi Stop (BD Biosciences, San Diego, USA) at 37 °C.
Stainings and cell sorting with anti-CD4 (L3T4 clone APC conjugated), anti-CD69 (FITC-conjugated), anti-CD8 (53–6.7 clone pacific blue conjugated), anti-B220 (PE conjugated), anti-CD11c (APC conjugated), anti-CD11b (FITC-conjugated), and anti-Gr1 (APC-conjugated) were performed. Cells were analyzed with a FACSCalibur Flow Cytometer (BD Biosciences; Invitrogen, Karlsruhe, Germany).
In order to isolate splenic lymphocytes, spleens were removed and homogenized through a nylon mesh sieve (70 µm). Cell suspensions were washed with PBS, centrifuged at 350 g for 10 min, and supernatants were taken. For lysis of erythrocytes, 1-ml distilled water was added. After 45 s 1.8% NaCl was added, and the cells were centrifuged again. Supernatants were resolved in PBS containing 5% FCS/0.4% EDTA 2 mM. Staining and cell sorting were performed as mentioned above.
Liver lymphocytes were isolated following perfusion of mice through the portal vein using PBS. Livers were then removed and homogenized through a nylon mesh sieve (70 µm). Cell suspensions were washed with PBS, centrifuged at 50 g for 1 min, and supernatants were taken. This procedure was repeated four times. Afterwards, supernatants were pooled, washed using RPMI 1640 containing 5% FCS and penicillin/streptomycin, and centrifuged through a 40%/70% Percoll gradient. Cells were collected from the interface, washed with RPMI 1640, and used for flow cytometry analysis. Staining and cell sorting were performed as mentioned above.
Microbiota analyses
Cultural analyses, biochemical identification and molecular detection of luminal bacterial communities from colon as well as feces were performed as previously described [18, 21–23]. For bacterial translocation experiments, mesenteric lymphnodes were transferred into thioglycollate enrichment broths under sterile conditions and cultivated for 7 days. Turbid broths were streaked onto solid culture media and cultivated under aerobic, microaerophilic, and obligate anaerobic conditions in order to detect translocated live commensal gut bacteria or C. jejuni followed by species identification via biochemical and molecular means [18]. Given that bacterial growth in enrichment broths cannot be quantitated, the relative rates of translocating bacteria are expressed in %.
Statistical analysis
Mean values, medians, standard deviations, and levels of significance were determined applying the two-tailed Student‘s t-test as indicated. Two-sided probability (P) values ≤0.05 were considered significant. All experiments were repeated at least twice.
Acknowledgments
We thank Michaela Wattrodt, Ursula Rüschendorf, Gernot Reifenberger, Uwe Lohmann, and the staff of the animal research facility for excellent technical assistance and animal breeding. We are grateful to Simone Spieckermann for immunohistochemistry staining of colon sections.
Glossary
Abbreviations
- GB:
gnotobiotic
- CR:
colonization resistance
- p.i.:
post infection
- CFU:
colony forming units
- spp.:
species
- IBD:
inflammatory bowel diseases
- TLR:
Toll-like receptor
- PAMP:
pathogen-associated molecular patterns
- LPS:
lipopolysaccharide
- LOS:
lipooligosaccharide
- LP:
lipoprotein
- MLN:
mesenteric lymph nodes
- DC:
dendritic cell
- Th1:
T helper 1 cell
- NaCl:
natrium chloride
- EDTA:
ethylene diamine tetraacetic acid
- FCS:
fetal calf serum
- NF-κB:
nuclear factor ‘kappa-light-chain-enhancer’ of activated B-cells
- MUC:
mucin
- HPRT:
hypoxanthine phosphoribosyltransferase 1
- PBS:
phosphate buffered saline
- TNF:
tumor necrosis factor
- IFN:
interferon
- MCP:
monocyte chemotactic protein
Footnotes
Author contributions:
S. Bereswill, A. Fischer, U. B. Göbel, L.-M. Haag, R. Plickert, and M. M. Heimesaat conceived and designed the experiments.
A. Fischer, L.-M. Haag, B. Otto, R. Plickert, and M. M. Heimesaat performed the experiments.
A. Fischer, L.-M. Haag, B. Otto, A. A. Kühl, and M. M. Heimesaat analyzed the data.
A. A. Kühl contributed reagents/materials/analysis tolls.
B. Otto, L.-M. Haag, S. Bereswill, A. Fischer, and M. M. Heimesaat wrote the paper.
Contributor Information
B. Otto, 1Department of Microbiology and Hygiene, Charité – University Medicine Berlin, Berlin, Germany.
L.-M. Haag, 1Department of Microbiology and Hygiene, Charité – University Medicine Berlin, Berlin, Germany.
A. Fischer, 1Department of Microbiology and Hygiene, Charité – University Medicine Berlin, Berlin, Germany.
R. Plickert, 1Department of Microbiology and Hygiene, Charité – University Medicine Berlin, Berlin, Germany.
A. A. Kühl, 2Department of Internal Medicine, Rheumatology and Clinical Immunology / Research Center Immuno-Sciences (RCIS), Charité – University Medicine Berlin, Berlin, Germany.
U. B. Göbel, 1Department of Microbiology and Hygiene, Charité – University Medicine Berlin, Berlin, Germany.
M. M. Heimesaat, 1Department of Microbiology and Hygiene, Charité – University Medicine Berlin, Berlin, Germany.
S. Bereswill, 1Department of Microbiology and Hygiene, Charité – University Medicine Berlin, Berlin, Germany.
References
- 1.Black RE, Levine MM, Clements ML, Hughes TP, Blaser MJ. Experimental Campylobacter jejuni infection in humans. J Infect Dis. 1988 Mar;157(3):472–479. doi: 10.1093/infdis/157.3.472. [DOI] [PubMed] [Google Scholar]
- 2.Young KT, Davis LM, Dirita VJ. Campylobacter jejuni: molecular biology and pathogenesis. Nat Rev Microbiol. 2007 Sep;5(9):665–679. doi: 10.1038/nrmicro1718. [DOI] [PubMed] [Google Scholar]
- 3.Tsang RS. The relationship of Campylobacter jejuni infection and the development of Guillain-Barré syndrome. Curr Opin Infect Dis. 2002 Jun;15(3):221–228. doi: 10.1097/00001432-200206000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Kist M, Bereswill S. Campylobacter jejuni. Contrib Microbiol. 2001;8:150–165. doi: 10.1159/000060405. [DOI] [PubMed] [Google Scholar]
- 5.van Spreeuwel JP, Duursma GC, Meijer CJ, Bax R, Rosekrans PC, Lindeman J. Campylobacter colitis: histological immunohistochemical and ultrastructural findings. Gut. 1985 Sep;26(9):945–951. doi: 10.1136/gut.26.9.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walker RI, Caldwell MB, Lee EC, Guerry P, Trust TJ, Ruiz-Palacios GM. Pathophysiology of Campylobacter enteritis. Microbiol Rev. 1986 Mar;50(1):81–94. doi: 10.1128/mr.50.1.81-94.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butzler JP. Campylobacter, from obscurity to celebrity. Clin Microbiol Infect. 2004 Oct;10(10):868–876. doi: 10.1111/j.1469-0691.2004.00983.x. [DOI] [PubMed] [Google Scholar]
- 8.Wassenaar TM, Blaser MJ. Pathophysiology of Campylobacter jejuni infections of humans. Microbes Infect. 1999 Oct;1(12):1023–1033. doi: 10.1016/s1286-4579(99)80520-6. [DOI] [PubMed] [Google Scholar]
- 9.Sun X, Threadgill D, Jobin C. Campylobacter jejuni induces colitis through activation of mammalian target of rapamycin signaling. Gastroenterology. 2012 Jan;142(1):86–95.e5. doi: 10.1053/j.gastro.2011.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ó Cróinin T, Backert S. Host epithelial cell invasion by Campylobacter jejuni: trigger or zipper mechanism? Front Cell Inf Microbiol. 2012;2:25. doi: 10.3389/fcimb.2012.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dorrell N, Wren BW. The second century of Campylobacter research: recent advances, new opportunities and old problems. Curr Opin Infect Dis. 2007 Oct;20(5):514–518. doi: 10.1097/QCO.0b013e3282a56b15. [DOI] [PubMed] [Google Scholar]
- 12.Yrios JW, Balish E. Immune response of athymic and euthymic germfree mice to Campylobacter spp. Infect Immun. 1986 Nov;54(2):339–346. doi: 10.1128/iai.54.2.339-346.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yrios JW, Balish E. Colonization and infection of athymic and euthymic germfree mice by Campylobacter jejuni and Campylobacter fetus subsp. fetus. Infect Immun. 1986 Aug;53(2):378–383. doi: 10.1128/iai.53.2.378-383.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yrios JW, Balish E. Pathogenesis of Campylobacter spp. in athymic and euthymic germfree mice. Infect Immun. 1986 Aug;53(2):384–392. doi: 10.1128/iai.53.2.384-392.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jesudason MV, Hentges DJ, Pongpech P. Colonization of mice by Campylobacter jejuni. Infect Immun. 1989 Aug;57(8):2279–2282. doi: 10.1128/iai.57.8.2279-2282.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Youssef M, Corthier G, Goossens H, Tancrede C, Henry-Amar M, Andremont A. Comparative translocation of enteropathogenic Campylobacter spp. and Escherichia coli from the intestinal tract of gnotobiotic mice. Infect Immun. 1987 Apr;55(4):1019–1021. doi: 10.1128/iai.55.4.1019-1021.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang C, Miller JF. Campylobacter jejuni colonization of mice with limited enteric flora. Infect Immun. 2006 Sep;74(9):5261–5271. doi: 10.1128/IAI.01094-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bereswill S, Fischer A, Plickert R, Haag LM, Otto B, Kühl AA, Dasti JI, Zautner AE, Muñoz M, Loddenkemper C, Gross U, Göbel UB, Heimesaat MM. Novel murine infection models provide deep insights into the "ménage à trois" of Campylobacter jejuni, microbiota and host innate immunity. PLoS One. 2011;6(6):e20953. doi: 10.1371/journal.pone.0020953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bereswill S, Plickert R, Fischer A, Kühl AA, Loddenkemper C, Batra A, Siegmund B, Göbel UB, Heimesaat MM. What you eat is what you get: Novel Campylobacter models in the quadrangle relationship between nutrition, obesity, microbiota and susceptibility to infection. Eur J Microbiol Immunol (Bp) 2011 Sep;1(3):237–248. doi: 10.1556/EuJMI.1.2011.3.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haag LM, Fischer A, Otto B, Grundmann U, Kühl AA, Göbel UB, Bereswill S, Heimesaat MM. Campylobacter jejuni infection of infant mice: acute enterocolitis is followed by asymptomatic intestinal and extra-intestinal immune responses. Eur J Microbiol Immunol (Bp) 2012 Mar;2(1):2–11. doi: 10.1556/EuJMI.2.2012.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heimesaat MM, Bereswill S, Fischer A, Fuchs D, Struck D, Niebergall J, Jahn HK, Dunay IR, Moter A, Gescher DM, Schumann RR, Göbel UB, Liesenfeld O. Gram-negative bacteria aggravate murine small intestinal Th1-type immunopathology following oral infection with Toxoplasma gondii. J Immunol. 2006 Dec 15;177(12):8785–8795. doi: 10.4049/jimmunol.177.12.8785. [DOI] [PubMed] [Google Scholar]
- 22.Heimesaat MM, Fischer A, Jahn HK, Niebergall J, Freudenberg M, Blaut M, Liesenfeld O, Schumann RR, Göbel UB, Bereswill S. Exacerbation of murine ileitis by Toll-like receptor 4 mediated sensing of lipopolysaccharide from commensal Escherichia coli. Gut. 2007 Jul;56(7):941–948. doi: 10.1136/gut.2006.104497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heimesaat MM, Fischer A, Siegmund B, Kupz A, Niebergall J, Fuchs D, Jahn HK, Freudenberg M, Loddenkemper C, Batra A, Lehr HA, Liesenfeld O, Blaut M, Göbel UB, Schumann RR, Bereswill S. Shift towards pro-inflammatory intestinal bacteria aggravates acute murine colitis via Toll-like receptors 2 and 4. PLoS One. 2007 Jul 25;2(7):e662. doi: 10.1371/journal.pone.0000662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Erridge C, Duncan SH, Bereswill S, Heimesaat MM. The induction of colitis and ileitis in mice is associated with marked increases in intestinal concentrations of stimulants of TLRs 2, 4, and 5. PLoS One. 2010 Feb 9;5(2):e9125. doi: 10.1371/journal.pone.0009125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haag LM, Fischer A, Otto B, Plickert R, Kühl AA, Göbel UB, Bereswill S, Heimesaat MM. Intestinal microbiota shifts towards elevated commensal Escherichia coli loads abrogate colonization resistance against Campylobacter jejuni in mice. PLoS One. 2012;7(5):e35988. doi: 10.1371/journal.pone.0035988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006 Feb 24;124(4):783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 27.Strober W, Murray PJ, Kitani A, Watanabe T. Signalling pathways and molecular interactions of NOD1 and NOD2. Nat Rev Immunol. 2006 Jan;6(1):9–20. doi: 10.1038/nri1747. [DOI] [PubMed] [Google Scholar]
- 28.Haag LM, Fischer A, Otto B, Plickert R, Kühl AA, Göbel UB, Bereswill S, Heimesaat MM. Campylobacter jejuni induces acute enterocolitis in gnotobiotic IL-10-/- mice via Toll-like-receptor-2 and -4 signaling. PLoS One. 2012;7(7):e40761. doi: 10.1371/journal.pone.0040761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Friis LM, Keelan M, Taylor DE. Campylobacter jejuni drives MyD88-independent interleukin-6 secretion via Toll-like receptor 2. Infect Immun. 2009 Apr;77(4):1553–1560. doi: 10.1128/IAI.00707-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu L, Hickey TE. Campylobacter jejuni induces secretion of proinflammatory chemokines from human intestinal epithelial cells. Infect Immun. 2005 Jul;73(7):4437–4440. doi: 10.1128/IAI.73.7.4437-4440.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rathinam VA, Hoag KA, Mansfield LS. Dendritic cells from C57BL/6 mice undergo activation and induce Th1-effector cell responses against Campylobacter jejuni. Microbes Infect. 2008 Oct;10(12-13):1316–1324. doi: 10.1016/j.micinf.2008.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mansfield LS, Bell JA, Wilson DL, Murphy AJ, Elsheikha HM, Rathinam VA, Fierro BR, Linz JE, Young VB. C57BL/6 and congenic interleukin-10-deficient mice can serve as models of Campylobacter jejuni colonization and enteritis. Infect Immun. 2007 Mar;75(3):1099–1115. doi: 10.1128/IAI.00833-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dunay IR, Damatta RA, Fux B, Presti R, Greco S, Colonna M, Sibley LD. Gr1(+) inflammatory monocytes are required for mucosal resistance to the pathogen Toxoplasma gondii. Immunity. 2008 Aug 15;29(2):306–317. doi: 10.1016/j.immuni.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dunay IR, Fuchs A, Sibley LD. Inflammatory monocytes but not neutrophils are necessary to control infection with Toxoplasma gondii in mice. Infect Immun. 2010 Apr;78(4):1564–1570. doi: 10.1128/IAI.00472-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dunay IR, Sibley LD. Monocytes mediate mucosal immunity to Toxoplasma gondii. Curr Opin Immunol. 2010 Aug;22(4):461–466. doi: 10.1016/j.coi.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karlmark KR, Tacke F, Dunay IR. Monocytes in health and disease. Eur J Microbiol Immunol (Bp) 2012;2(2):97–102. doi: 10.1556/EuJMI.2.2012.2.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van der Sluis M, De Koning BA, De Bruijn AC, Velcich A, Meijerink JP, Van Goudoever JB, Büller HA, Dekker J, Van Seuningen I, Renes IB, Einerhand AW. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 2006 Jul;131(1):117–129. doi: 10.1053/j.gastro.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 38.Hugdahl MB, Beery JT, Doyle MP. Chemotactic behavior of Campylobacter jejuni. Infect Immun. 1988 Jun;56(6):1560–1566. doi: 10.1128/iai.56.6.1560-1566.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tu QV, McGuckin MA, Mendz GL. Campylobacter jejuni response to human mucin MUC2: modulation of colonization and pathogenicity determinants. J Med Microbiol. 2008 Jul;57(Pt 7):795–802. doi: 10.1099/jmm.0.47752-0. [DOI] [PubMed] [Google Scholar]
- 40.McAuley JL, Linden SK, Png CW, King RM, Pennington HL, Gendler SJ, Florin TH, Hill GR, Korolik V, McGuckin MA. MUC1 cell surface mucin is a critical element of the mucosal barrier to infection. J Clin Invest. 2007 Aug;117(8):2313–2324. doi: 10.1172/JCI26705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McGuckin MA, Lindén SK, Sutton P, Florin TH. Mucin dynamics and enteric pathogens. Nat Rev Microbiol. 2011 Apr;9(4):265–278. doi: 10.1038/nrmicro2538. [DOI] [PubMed] [Google Scholar]
- 42.Kalischuk LD, Inglis GD, Buret AG. Campylobacter jejuni induces transcellular translocation of commensal bacteria via lipid rafts. Gut Pathog. 2009 Feb 3;1(1):2. doi: 10.1186/1757-4749-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Macpherson AJ, Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science. 2004 Mar 12;303(5664):1662–1665. doi: 10.1126/science.1091334. [DOI] [PubMed] [Google Scholar]
- 44.Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat Rev Immunol. 2010 Mar;10(3):159–169. doi: 10.1038/nri2710. [DOI] [PubMed] [Google Scholar]
- 45.Kaldor J, Pritchard H, Serpell A, Metcalf W. Serum antibodies in Campylobacter enteritis. J Clin Microbiol. 1983 Jul;18(1):1–4. doi: 10.1128/jcm.18.1.1-4.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rathinam VA, Appledorn DM, Hoag KA, Amalfitano A, Mansfield LS. Campylobacter jejuni-induced activation of dendritic cells involves cooperative signaling through Toll-like receptor 4 (TLR4)-MyD88 and TLR4-TRIF axes. Infect Immun. 2009 Jun;77(6):2499–507. doi: 10.1128/IAI.01562-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moran AP. Structure and conserved characteristics of Campylobacter jejuni lipopolysaccharides. J Infect Dis. 1997 Dec;176(Suppl 2):S115–S121. doi: 10.1086/513781. [DOI] [PubMed] [Google Scholar]
- 48.Rose A, Kay E, Wren BW, Dallman MJ. The Campylobacter jejuni NCTC11168 capsule prevents excessive cytokine production by dendritic cells. Med Microbiol Immunol. 2012 May;201(2):137–144. doi: 10.1007/s00430-011-0214-1. [DOI] [PubMed] [Google Scholar]
- 49.Wilson DL, Rathinam VA, Qi W, Wick LM, Landgraf J, Bell JA, Plovanich-Jones A, Parrish J, Finley RL, Mansfield LS, Linz JE. Genetic diversity in Campylobacter jejuni is associated with differential colonization of broiler chickens and C57BL/6J IL10-deficient mice. Microbiology. 2010 Jul;156(Pt 7):2046–2057. doi: 10.1099/mic.0.035717-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Mourik A, Steeghs L, van Laar J, Meiring HD, Hamstra HJ, van Putten JP, Wösten MM. Altered linkage of hydroxyacyl chains in lipid A of Campylobacter jejuni reduces TLR4 activation and antimicrobial resistance. J Biol Chem. 2010 May 21;285(21):15828–15836. doi: 10.1074/jbc.M110.102061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li X, Swaggerty CL, Kogut MH, Chiang HI, Wang Y, Genovese KJ, He H, McCarthy FM, Burgess SC, Pevzner IY, Zhou H. Systemic response to Campylobacter jejuni infection by profiling gene transcription in the spleens of two genetic lines of chickens. Immunogenetics. 2012 Jan;64(1):59–69. doi: 10.1007/s00251-011-0557-1. [DOI] [PubMed] [Google Scholar]
- 52.Vucković D, Abram M, Bubonja M, Wraber B, Dorić M. Host resistance to primary and secondary Campylobacter jejuni infections in C57Bl/6 mice. Microb Pathog. 2006 Jan;40(1):35–39. doi: 10.1016/j.micpath.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 53.Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol. 2009;27:147–163. doi: 10.1146/annurev.immunol.021908.132629. [DOI] [PubMed] [Google Scholar]
- 54.Hakansson A, Molin G. Gut microbiota and inflammation. Nutrients. 2011 Jun;3(6):637–682. doi: 10.3390/nu3060637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Siegmund B, Fantuzzi G, Rieder F, Gamboni-Robertson F, Lehr HA, Hartmann G, Dinarello CA, Endres S, Eigler A. Neutralization of interleukin-18 reduces severity in murine colitis and intestinal IFN-gamma and TNF-alpha production. Am J Physiol Regul Integr Comp Physiol. 2001 Oct;281(4):R1264–R1273. doi: 10.1152/ajpregu.2001.281.4.R1264. [DOI] [PubMed] [Google Scholar]
- 56.Heimesaat MM, Nogai A, Bereswill S, Plickert R, Fischer A, Loddenkemper C, Steinhoff U, Tchaptchet S, Thiel E, Freudenberg MA, Göbel UB, Uharek L. MyD88/TLR9 mediated immunopathology and gut microbiota dynamics in a novel murine model of intestinal graft-versus-host disease. Gut. 2010 Aug;59(8):1079–1087. doi: 10.1136/gut.2009.197434. [DOI] [PubMed] [Google Scholar]
- 57.Muñoz M, Heimesaat MM, Danker K, Struck D, Lohmann U, Plickert R, Bereswill S, Fischer A, Dunay IR, Wolk K, Loddenkemper C, Krell HW, Libert C, Lund LR, Frey O, Hölscher C, Iwakura Y, Ghilardi N, Ouyang W, Kamradt T, Sabat R, Liesenfeld O. Interleukin (IL)-23 mediates Toxoplasma gondii-induced immunopathology in the gut via matrixmetalloproteinase-2 and IL-22 but independent of IL-17. J Exp Med. 2009 Dec 21;206(13):3047–3059. doi: 10.1084/jem.20090900. [DOI] [PMC free article] [PubMed] [Google Scholar]