Abstract
The interface between synthetic organic chemistry and natural products was explored in order to unravel the structure of coibacin A, a metabolite isolated from the marine cyanobacterium cf. Oscillatoria sp. that exhibits selective antileishmanial activity and potent anti-inflammatory properties. Our synthetic plan focused on a convergent strategy that allows rapid access to the desired target by coupling of three key fragments involving E-selective Wittig and modified Julia olefinations. CD measurements and comparative HPLC analyses between the natural product and four synthetic stereoisomers led to determination of its absolute configuration thus correcting the original assignment at C-5 and unambiguously establishing those at C-16 and C-18. Additionally, we have synthesized coibacin B based on the assignment of configuration for coibacin A.
1. INTRODUCTION
Marine organisms are a rich source of diverse and biologically active natural products, which have inspired the development of novel pharmacologically relevant compounds.1 The α,β-unsaturated δ-lactone moiety is a privileged scaffold widely distributed among natural products that display a broad range of biological activities, such as fostriecin,2–4 leptomycins,5 and callystatin A.6,7
The unequivocal assignment of the absolute configuration of the structure of a novel compound is one of the key issues in natural products chemistry. In this regard, and despite the fact that spectroscopic and crystallographic techniques are highly advanced, chemical synthesis still makes important contributions in natural products elucidation.8,9
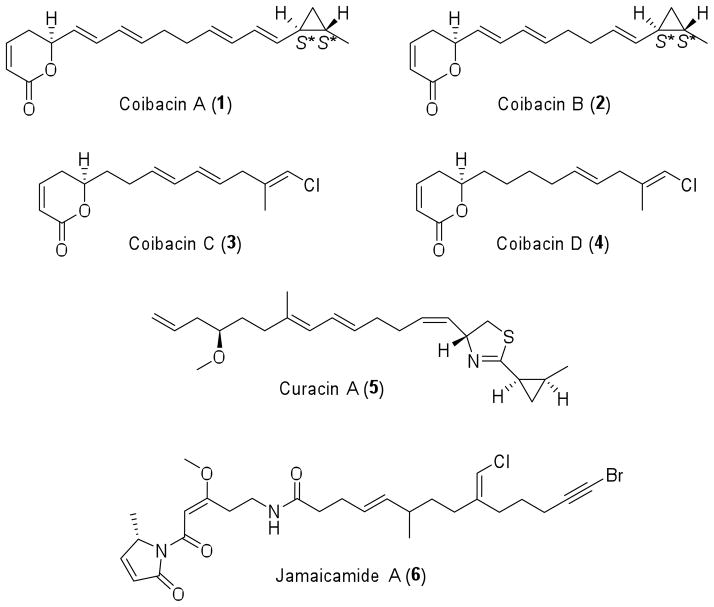
Recently, we described the isolation and structure elucidation of four unsaturated polyketides lactone derivatives, named coibacins A–D (compounds 1–4, Figure 1), from the marine cyanobacterium cf. Oscillatoria sp. which displayed the dihydropyran-2-one moiety as well as either a cyclopropyl ring (coibacins A and B) or a methyl vinyl chloride (coibacins C and D).10 The methyl cyclopropyl ring and methyl vinyl chloride are similar to those observed in other marine cyanobacterial metabolites such as curacin A (5),11 and jamaicamide A (6),12 respectively. These co-occurring metabolites in a single organism suggest an intriguing flexibility in the biosynthetic pathway.10
Figure 1.
Structures of marine cyanobacterial metabolites: coibacins A–D (1–4), curacin A (5) and jamaicamide A (6).
Among these cyanobacterial metabolites, coibacin A (1) displayed potent and selective activity against axenic amastigotes of Leishmania donovani (IC50 = 2.4 μM). Coibacin B (2) was less active as a leishmanicidal drug (IC50 = 7.2 μM); however, it exhibited higher cytotoxicity against human cancer lung cell lines (NCI-H460), with an IC50 value of 17.0 μM compared to 31.5 μM for coibacin A (1). Evaluation of anti-inflammatory activity by cell-based nitric oxide (NO) inhibition assay13 revealed that coibacin B (2) was the most active coibacin representative (IC50 = 5 μM). Because coibacin A (1) was isolated in the largest amount, it was further evaluated and shown to reduce gene transcription of several inflammatory cytokines TNF-α, IL1-β, IL-6 and iNOS (1, at 10 μg/mL).10
The absolute configuration of the dihydropyran-2-one moiety was determined to be S based on a positive Cotton effect at λ 259 nm, observed in circular dichroism (CD) measurements, while the trans relationship of the substituents in the methyl cyclopropyl ring was established using NOESY correlations and J-coupling constant analysis. However, because of the scarcity of the natural products, the absolute configuration of cyclopropyl portion could not be assigned.10
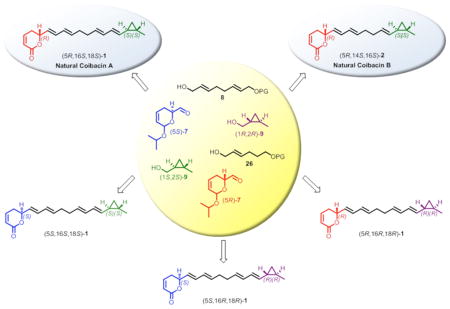
Due to our interest into the synthesis and biological properties of α,β-unsaturated δ-lactones14–18 we embarked on the total synthesis of stereoisomers of coibacin A (1) with the aim of establishing its absolute configuration and providing enough material for further exploration of its biological properties. Additionally, coibacin B (2) was synthesized based on the assignment of configuration for coibacin A (1) by a similar synthetic route.
2. RESULTS AND DISCUSSION
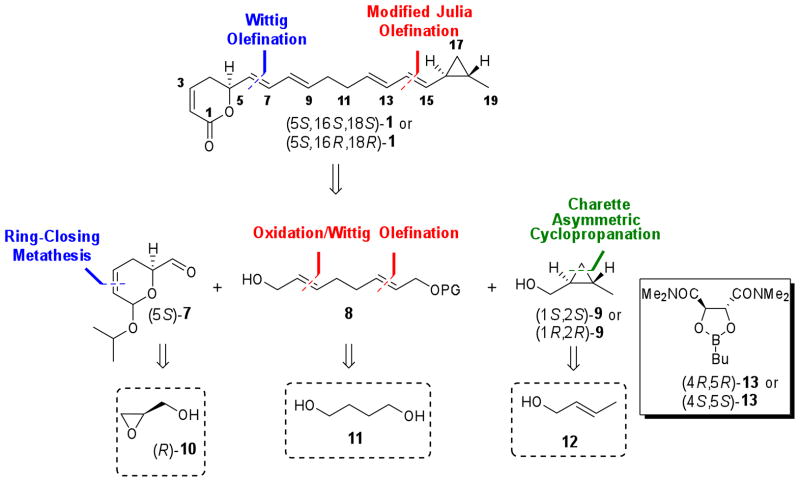
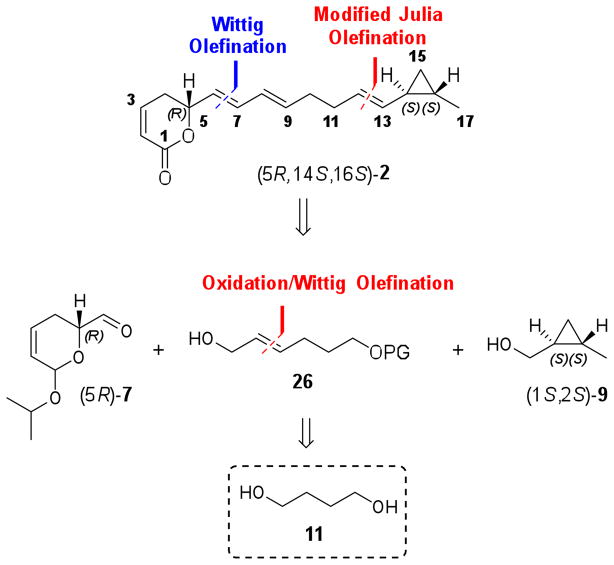
Assuming the S absolute configuration of δ-lactone moiety and the trans relationship between the substituents in the cyclopropyl ring, we envisioned a convergent strategy based on the coupling of three key fragments to quickly provide the two possible isomers (5S,16S,18S)- and (5S,16R,18R)-1 (Scheme 1). This synthetic route would be carried out by E-selective Wittig olefination of dihydropyran aldehyde (5S)-7 with a tri-n-butyl phosphorane derived from alcohol 8. The resulting tetrahydropyran would be converted to the corresponding aldehyde for the final modified Julia olefination with a sulfone derived from (1S,2S)- or (1R,2R)-9. The enantiomerically pure fragment (5S)-7 would be synthesized from commercially available (R)-glycidol, (R)-10, by similar methodology as previously reported for its enantiomer (5R)-7, involving as key steps ring opening of a chiral epoxide and ring-closing metathesis reaction of the corresponding diene.19,20 Preparation of fragment 8 could be performed in few steps from 1,4-butanediol (11) using tandem oxidation/Wittig olefination.21 trans-Cyclopropyl fragment (1S,2S)-9 has been described using Charette asymmetric cyclopropanation of trans-crotyl alcohol (12) mediated by dioxaborolane-derived chiral ligand (4R,5R)-13.22 Similarly, the enantiomer (1R,2R)-9 could be obtained employing (4S,5S)-13.
Scheme 1.
Retrosynthetic plan for coibacin A (1) isomers.
Our synthesis began with the preparation of aldehyde (5S)-7 from (R)-10 according to a modification of the procedures described by Crimmins & King20 and Boger et al.19 for (5R)-7. Protection of (R)-10 with tert-butyldiphenylsilyl chloride (TBDPSCl) and Cu(I)-mediated epoxide opening with vinylmagnesium bromide were carried out to provide allylic alcohol (S)-14 in 81% overall yield.20 The dihydropyran-2-one (S)-15 was obtained in good yield after conversion of secondary alcohol (S)-14 to the corresponding ester, followed by ring-closing metathesis using Grubbs I catalyst (71% yield, 2 steps). Reduction of (S)-15 with diisobutylaluminum hydride (DIBALH) and protection of the resulting lactol as the corresponding isopropyl ketal afforded (5S)-16 in 86% yield as a single isomer.19,23 Deprotection of silyl ether group of (5S)-16 (83% yield) and Swern oxidation furnished the desired aldehyde (5S)-7,20 which was synthesized from (R)-10 in 8 steps and 42% overall yield (Scheme 2).
Scheme 2.
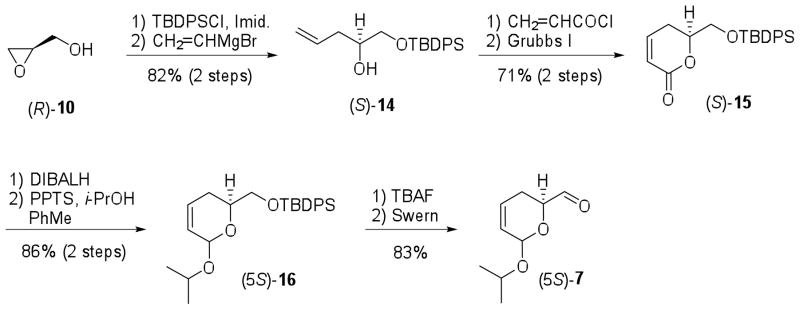
Preparation of aldehyde (5S)-7.
The synthesis of fragment 8 started with the conversion of 1,4-butanediol (11) to the diester 18. Two reaction steps were necessary to perform this transformation because treatment of 1,4-butanediol (11) with 20 equiv of magnesium dioxide (MnO2) and 2.5 equiv of [(ethoxycarbonyl)methylene]triphenylphosphorane in dichloromethane or chloroform furnished the desired bis-olefinated compound 18 in very poor yield (15–18%) even after reflux for 3 d. Thus, mild conditions were employed to conduct a desymmetrization reaction via tandem oxidation of unactivated 11 with MnO2 and in situ Wittig olefination to provide olefin (E)-17 in 75% yield.21 The scale up of this reaction (from 2 to 30 mmol of substrate) required increased reaction time from 2 to 7 d and optimization studies indicated that 50% reduction in the amount of MnO2 did not affect the yield. Byproducts of about 4% of diester 18 and 10% of (Z)-17 were obtained from this reaction. Intermediate 17 was homologated to afford 18 in good yield (77%) after one-pot oxidation with pyridinium chlorocromate (PCC) and Wittig olefination. In this reaction the byproduct (2E,6Z)-isomer of 18 was isolated in 7% yield (Scheme 3).
Scheme 3.
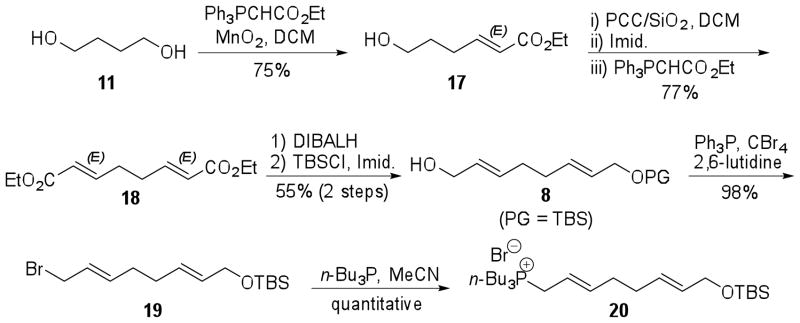
Synthesis of tri-n-butyl phosphonium salt 20.
Reduction of 18 to the corresponding diol with DIBALH and mono-protection with tert-butyldimethylsilyl chloride (TBSCl) afforded fragment 8 (PG = TBS) in 55% yield for 2 steps. Conversion to the allylic bromide 19 upon treatment with triphenylphosphine (Ph3P) and carbon tetrabromide (CBr4) in the presence of 2,6-lutidine was accomplished in high yield (98%);24 however, in the absence of base the yield decreased to 45% and the dibromide byproduct was obtained in 28% yield. Subsequently, substitution with tri-n-butylphosphine (n-Bu3P) afforded phosphonium salt 20 in quantitative yield. Therefore, 20 was prepared in 6 steps and 31% overall yield from readily available diol 11 (Scheme 3).
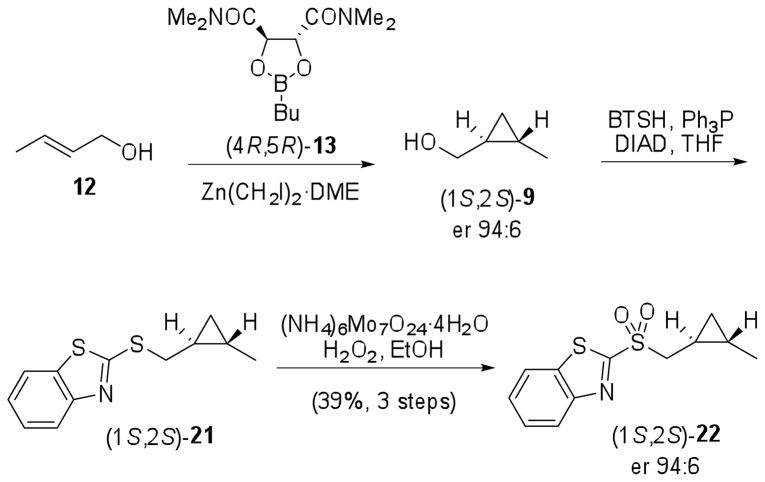
trans-Cyclopropyl fragment (1S,2S)-9 was obtained from Charette asymmetric cyclopropanation of trans-crotyl alcohol (12) mediated by dioxaborolane-derived chiral ligand (4R,5R)-13.22,25 Treatment of (1S,2S)-9 with 2-mercaptobenzothiazole (BTSH), Ph3P and diisopropyl azodicarboxylate (DIAD) afforded (1S,2S)-21 after Mitsunobu reaction.25 Oxidation with ammonium molybdate/H2O2 provided sulfone (1S,2S)-22 in 39% yield for 3 steps and enantiomeric ratio (er) of 94:6 (Scheme 4). Preparation of (1R,2R)-22 was accomplished from the same starting material 12 but using dioxaborolane (4S,5S)-13 and the overall yield was 31%. The enantiomeric ratio of (1S,2S)-22 and (1R,2R)-22 was determined by chiral HPLC analysis.26
Scheme 4.
Synthesis of sulfone (1S,2S)-22.
Synthesis of the C-1/C-14 fragment of isomers (5S,16R,18R)- and (5S,16S,18S)-1 (Scheme 1) involved coupling of aldehyde (5S)-7 with tri-n-butyl phosphonium salt 20 by E-selective Wittig olefination (Scheme 5). When this reaction was performed with potassium tert-butoxide (t-BuOK) in a mixture of toluene/THF (5:1), the desired product (5S)-23 was obtained as a 5:1 mixture of E:Z isomers. Using the DMSO derived lithium base [LiCH2S(O)CH3] in toluene a 7:1 E:Z isomeric ratio was formed. However, in both cases the yields were low (46% or less) regardless of the reaction scale and modifications in the amount of base or Wittig salt (20) employed. Therefore, we used sodium hexamethyldisilazide (NHMDS) as a more hindered base to prevent deprotonation α to the carbonyl group of (5S)-7. Fortunately, the yield was increased to about 70% (6:1 E:Z ratio) by using 2 equiv of NHMDS and 1.5 equiv of 20 in toluene. The moderate E selectivity of this reaction may be attributed to the low steric hindrance of the ylide.27 The triene (5S)-23 was converted uneventfully to aldehyde (5S)-24 upon deprotection of the TBS ether followed by MnO2-mediated allylic alcohol oxidation in 86% yield for 2 steps (Scheme 5).
Scheme 5.
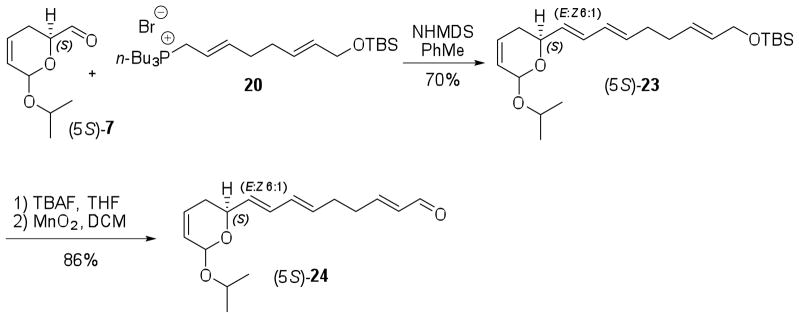
Synthesis of aldehyde (5S)-24.
The final coupling between aldehyde (5S)-24 and sulfonylbenzothiazole 22 required extensive optimization in order to improve the E:Z ratio of the newly formed double bond.28 To avoid use of expensive enantiomerically enriched substrate, racemic 22 was employed in the modified Julia olefination reaction (Table 1). When NHMDS was used, either DMF or THF afforded (5S)-25 as a 1.7:1 mixture of E:Z isomers at C-14 in moderate to low yields (entries 1–3, Table 1). Under the same condition, reduction of the reaction time led to an increase in the yield from 28% to 65%, probably due to reduced decomposition of the product in the reaction medium (entries 2 and 3). Use of DME as solvent increased the E:Z ratio at C-14 to 2.7:1 and the yield (95%) (entry 4). Addition of hexamethylphosphoramide (HMPA) did not affect the selectivity (entries 5 and 6) although studies in the literature have shown improvement of the E selectivity in the presence of this additive.29,30 While LHMDS, as expected, favored the Z configuration of the newly formed double bond (entry 7), KHMDS displayed similar results to those reported for NHMDS (entry 8).
Table 1.
Modified Julia olefination involving aldehyde (5S)-24 and racemic sulfone 22.
| |||||
|---|---|---|---|---|---|
| Entrya | Solvent | MHMDS | Time | Ratio E:Z at C-14b | Yield |
| 1 | DMF | NHMDS | 1.5 h (−78 °C), 1.5 h (rt) | 1.7:1 | 4%c |
| 2 | THF | NHMDS | 1.5 h (−78 °C), 1.5 h (rt) | 1.7:1 | 28%c |
| 3 | THF | NHMDS | 10 min | 1.7:1 | 65%d,e |
| 4 | DME | NHMDS | 20 min | 2.7:1 | 95%d |
| 5 | THF/HMPA 4:1 | NHMDS | 1.5 h | 1.7:1 | 66%d |
| 6 | DME/HMPA 4:1 | NHMDS | 1.5 h | 2:1 | 53%d |
| 7 | DME | LHMDS | 10 min | 1:10 | 54%d |
| 8 | DME | KHMDS | 10 min | 2.4:1 | 95%d |
Scale: 0.06 mmol of (5S)-24; Volume of solvent: 2.5 mL.
E:Z ratio of the double bond at C-14 of the isomers with E geometry on the C-6 double bond.
Addition order: sulfone, NHMDS and aldehyde.
Addition order: sulfone, aldehyde and NHMDS.
When the scale was duplicated the yield was 70%.
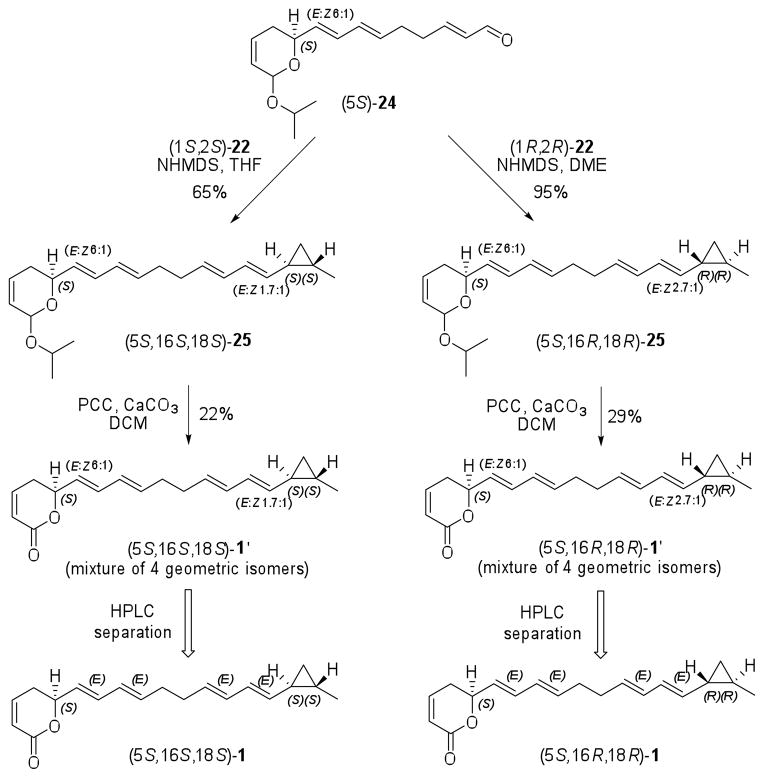
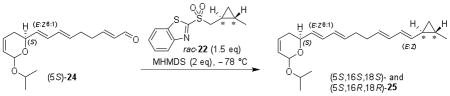
The isomers (5S,16S,18S)- and (5S,16R,18R)-1 were prepared as described in Scheme 6. Coupling of aldehyde (5S)-24 with sulfones (1S,2S)- or (1R,2R)-22 provided the tetraenes (5S,16S,18S)- and (5S,16R,18R)-25, respectively, as mixture of four geometric isomers with the major isomer displaying all E configuration.31 The mixtures of isomers (5S,16S,18S)- and (5S,16R,18R)-1′ were obtained after oxidation of (5S,16S,18S)- and (5S,16R,18R)-25 with PCC and CaCO332 in 22% and 29% yield, respectively. Separation of the major isomers by semi-preparative reverse phase HPLC33 afforded dihydropyran-2-ones (5S,16S,18S)- and (5S,16R,18R)-1 which displayed 1H- and 13C-NMR data identical to each other, as well as to that reported for natural coibacin A (1), in spite of their diastereoisomeric relationship.
Scheme 6.
Synthesis of enantiomerically pure isomers (5S,16S,18S)- and (5S,16R,18R)-1
Comparison of the specific optical rotation of the synthetic samples of (5S,16S,18S)-1 ([α]D20 − 10, c 0.1, CHCl3) and (5S,16R,18R)-1 ([α]D20 − 100, c 0.1, CHCl3) with that reported for natural coibacin A (1) ([α]D20 + 46, c 0.1, CHCl3), indicated non-identity of the compounds. Based on the opposed signal of the specific optical rotation of the synthetic and natural compounds, we suspected that the original assignment of absolute configuration of lactone moiety was in error. Therefore, we conducted the total synthesis of isomers (5R,16S,18S)- and (5R,16R,18R)-1 by a similar route as described above for their enantiomers (See Experimetal Section for further details). As expected, these compounds also displayed identical 1H- and 13C-NMR data to that reported for natural coibacin A (1). Despite their dextrorotatory values, the specific optical rotations of (5R,16S,18S)-1 ([α]D20 + 100, c 0.1, CHCl3) and (5R,16R,18R)-1 ([α]D20 + 10, c 0.1, CHCl3) proved to be significantly different from that reported for the natural coibacin A (1).
Due to the difficulty of unequivocal assignment of the absolute configuration of coibacin A (1) based only on NMR and specific optical rotation data, we carried out HPLC comparisons34 of natural coibacin A (1) with all four synthetic stereoisomers displaying a trans configuration of the cyclopropane ring (Figure 2). When a mixture of these four compounds was co-injected with natural coibacin A (1), an enhancement in the intensity of the peak corresponding to (5R,16S,18S)-1 was observed, unequivocally establishing the absolute configuration of the natural product. Additionally, the CD curve of (5R,16S,18S)-1 was identical to the one described for coibacin A (1), with both displaying a positive Cotton effect (see CD curves in Supporting Information). Therefore these results led to the revision of the configuration at C-5 in our original publication which was previously assigned to be S,10 possibly due to a misapplication of the rules described by Beecham.35
Figure 2.
Specific optical rotation of the four isomers of coibacin A (1).
It is interesting to compare the chirality established here for coibacin A (1) with that of curacin A (5, Figure 1), a methylcyclopropane-containing metabolite isolated from another marine cyanobacterium, Moorea producens (formerly Lyngbya majuscula). There, detailed biosynthetic mechanism studies have shown that S-configuration of the secondary methyl group appended to the cyclopropyl ring is established by the trajectory of hydride delivery to the β-position of an achiral enone.36 However, the adjacent cyclopropyl methine center is rendered chiral (and in the case of curacin A, cis-configured relative to the methyl substituent) after collapse of the enolate to displace chloride and form the three-membered ring. Thus, because curacin A (5) and coibacin A (1) possess secondary methyl groups of identical chirality, divergence in their biosynthetic pathways must lie within this second step. A range of possibilities exist, including the geometry of the intermediate enone formed by the enoyl CoA hydratase 1, the relative positions of the oxyanion and primary chloride substituents to the methyl group prior to cyclopropyl ring formation, and the overall orientation of the substrate in the enoyl reductase. Thus, similar to the subtle alternate functioning of β-branch forming enzymes that lead to the diverse functional groups observed in curacin A (5, Figure 1) and jamaicamide A (6, Figure 1) (cyclopropyl ring versus vinyl chloride),37 the stereo-structures of the coibacins implicate additional dimensions of flexibility in this biosynthetic manifold.
A similar synthetic approach was applied to the synthesis of coibacin B (2) displaying 5R,14S,16S configuration, the same one found for coibacin A (1) (Scheme 7). Fragment 26 was planned to be obtained after two steps from alcohol 17, already prepared from diol 11 for the synthesis of coibacin A (1).
Scheme 7.
Retrosynthetic plan for correct isomer of coibacin B (2).
Protection of alcohol 17 with TBSCl, followed by reduction of ester group with excess of DIBALH, furnished fragment 26 (PG = TBS) in high yield (94%, 2 steps). Appel reaction converted the alcohol 26 to the corresponding bromide 27 in 91% yield, which was treated with n-Bu3P to give the tri-n-butyl phosphonium salt 28 in quantitative yield (Scheme 8).
Scheme 8.
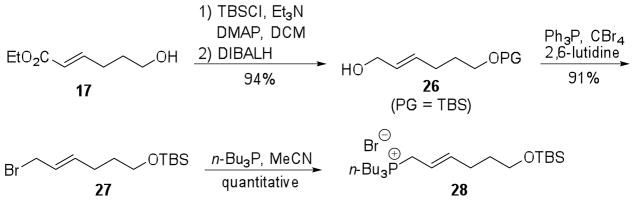
Synthesis of tri-n-butyl phosphonium salt 28.
E-selective Wittig reaction between (5R)-7 and 28 mediated by NHMDS in toluene furnished (5R)-29 as a 6:1 mixture of E:Z isomers at C-6 in 69% yield. Alcohol (5R)-30, obtained in 94% yield from deprotection of TBS ether (5R)-29, was oxidized under Swern conditions and the unstable crude product was immediately used in the modified Julia olefination with sulfone (1S,2S)-22 to afford (5R,14S,16S)-31 as a mixture of geometric isomers. While the use of NHMDS in DME provided a 1:1 mixture of geometric isomers at C-12 double bond, better E selectivity (2.2:1 E:Z ratio) was obtained by using of KHMDS. The moderate yield for the Swern oxidation and Julia olefination (41%) can be explained by the instability of aldehyde derived from alcohol (5R)-30. The mixture (5R,14S,16S)-31 was treated with PCC in the presence of CaCO3 to give a mixture of four geometric isomers (5R,14S,16S)-2′ in 46% yield. The major isomer (5R,14S,16S)-2, with all double bonds displaying E configuration, was isolated by semi-preparative reverse phase HPLC (Scheme 9).38
Scheme 9.
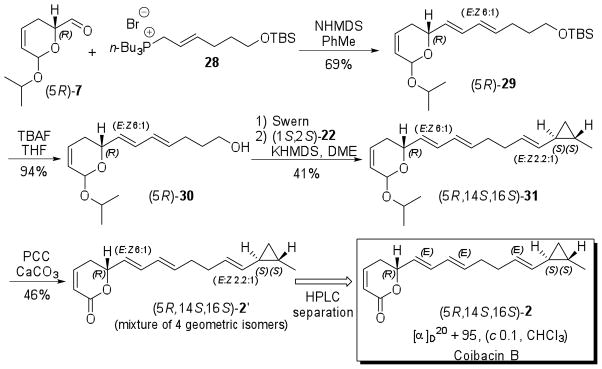
Synthesis of (5R,14S,16S)-2 (coibacin B).
Synthetic (5R,14S,16S)-2 and natural coibacin B displayed identical spectra within the limits of NMR data (see Supporting Information). As for coibacin A (1), the specific optical rotation for the synthetic sample of coibacin B (2) ([α]D20 + 95, c 0.1, CHCl3) was higher than that reported for the natural compounds ([α]D + 59, c 0.1, CHCl3). Fortunately, the CD curve of synthetic (5R,14S,16S)-2 closely resembles to that of natural coibacin A (1), providing strong evidence that this represents the correct isomer of coibacin B (2).
3. CONCLUSION
In summary, total syntheses of four coibacin A (1) stereoisomers displaying a trans-configured cyclopropane ring revealed the natural substance to possess 5R,16S,18S absolute configuration. The total synthesis of coibacin A (1) was carried out in 12 steps (longest linear route) and 3.4% overall yield. Additionally, we synthesized the correct isomer of coibacin B (2) based on the assignment of configuration for coibacin A (1). Studies aimed to assess the cytotoxic and anti-inflammatory properties of these compounds are underway.
4. EXPERIMENTAL SECTION
General Information
THF and DME were freshly distilled from sodium/benzophenone. DCM, DMF, HMPA and Et3N were freshly distilled over CaH2. PhMe and MeCN were dried over 4 Å molecular sieves for 48 h prior to use. TLC analyses were performed in silica gel plates and the spots were revealed using UV and/or the following solutions: phosphomolybdic acid in EtOH, vanillin in EtOH/H2SO4 and p-anisaldehyde in EtOH/AcOH/H2SO4. Flash column chromatography was carried out using silica gel 60 (35–60 μm). 1H-1H COSY (90°) and 1H-13C HSQC NMR experiments were used for confirmation of NMR peak assignments. The HPLC analyses were performed at room temperature with a photodiode array detector. All melting points were measured in open capillaries and were uncorrected. HRMS analyses were performed using ESI-TOF mass spectrometer. IUPAC names of the compounds were generated using ChemBioDraw Ultra 13.0; however, the usual numbering of the carbon atoms as shown in Scheme 1 was adopted to refer to the compounds and NMR peak assignments.
(S)-1-((tert-Butyldiphenylsilyl)oxy)pent-4-en-2-ol, (S)-14
To a solution of (R)-10 (3.35 g, 45.2 mmol) and imidazole (4.00 g, 58.8 mmol) in anhyd DCM (100 mL), was added TBDPSCl (15.4 mL, 54.3 mmol) at 0 °C and under N2. The solution was allowed to warm to rt and stirred for 2 h. The solution was quenched by the addition of water (100 mL). The layers were separated and the aqueous phase was extracted with Et2O (2 × 50 mL). The combined organic layers were washed with brine (100 mL), dried over Na2SO4, filtered and concentrated. The crude product was purified by flash column chromatography (hexanes/Et2O 9:1) to provide (S)-tert-butyl(oxiran-2-ylmethoxy)diphenylsilane (13.5 g, 43.3 mmol, 87%) as a colorless oil. [α]D20 − 2.6 (c 9.4, CHCl3). Lit.39 [α]D23 − 2.46 (c 9.07, CHCl3). Spectral data (1H NMR and 13C NMR) are in accordance with those reported in the literature.39
To a solution of (S)-tert-butyl(oxiran-2-ylmethoxy)diphenylsilane (13 g, 42 mmol) in anhyd THF (19 mL) and under N2 was added CuI (0.634 g, 3.32 mmol, 8 mol%) at rt. This suspension was stirred for 15 min. After that, the temperature was reduced to − 30 °C and vinylmagnesium bromide (100 mL, 100 mmol, 1M in THF) was added by syringe pump (over 1 h). The mixture was then stirred an additional hour at this temperature. The reaction was carefully quenched with saturated NH4Cl solution (100 mL) at − 30 °C, the cooling bath was removed and the reaction was stirred for more 20 min. The layers were separated and the aqueous phase was extracted with Et2O (2 × 100 mL). The combined organic layers were washed with brine (100 mL), dried over Na2SO4, filtered and concentrated under reduced pressure. The crude product was purified by flash column chromatography (hexanes/Et2O 7:3) to afford alcohol (S)-14 (13.4 g, 39.4 mmol, 94%) as a colorless oil. [α]D20 − 2.4 (c 9.6, CHCl3). Lit.40 [α]D20 − 2.6 (c 9.17, CHCl3). Spectral data (1H NMR and 13C NMR) are in accordance with those reported in the literature.40
(S)-6-(((tert-Butyldiphenylsilyl)oxy)methyl)-5,6-dihydro-2H-pyran-2-one, (S)-15
To a solution of alcohol (S)-14 (12.97 g, 38.08 mmol) and Et3N (10.6 mL, 76.2 mmol) in anhyd DCM (131 mL) was added freshly prepared acryloyl chloride41 (4.62 mL, 57.1 mmol) dropwise at 0 °C and under N2. At this time color change from light yellow to dark yellow was observed. The reaction was stirred for 1 h at the same temperature and then it was quenched by the addition of brine (50 mL) and saturated Rochelle’s salt solution (50 mL). The emulsion was stirred until complete layer separation (~ 3 h). The layers were separated and the aqueous phase was extracted with Et2O (2 × 100 mL). The combined organic layers were dried over Na2SO4, filtered and concentrated under reduced pressure. The crude product was purified by flash column chromatography (hexanes/Et2O 7:3) to give (S)-1-((tert-butyldiphenylsilyl)oxy)pent-4-en-2-yl acrylate (12.92 g, 32.74 mmol, 86%) as a yellow oil. [α]D20 − 9 (c 1.05, CHCl3). Lit.42 (antipode) [α]D26 + 8.3 (c 1, CHCl3). Spectral data (1H NMR and 13C NMR) are in accordance with those reported in the literature for its enantiomer.42
To a solution of (S)-1-((tert-butyldiphenylsilyl)oxy)pent-4-en-2-yl acrylate (4.00 g, 10.1 mmol) in anhyd DCM (1 L) at reflux was added Grubbs catalyst 1st generation (0.823 g, 1.03 mmol, 10 mol%) in three portions (275 mg, each hour) dissolved in anhyd DCM (10 mL). The reaction mixture was refluxed for 20 h. The temperature was cooled to rt and the solvent was evaporated under reduced pressure. The crude product was purified by flash column chromatography (hexanes/AcOEt 6:4) to provide lactone (S)-15 (3.08 g, 8.40 mmol, 83%) as a brown oil. [α]D20 − 48 (c 1.03, CHCl3). Lit.42 (antipode) [α]D26 + 47.8 (c 1, CHCl3). Spectral data (1H NMR and 13C NMR) are in accordance with those reported in the literature for its enantiomer.42
tert-Butyl(((2S)-6-isopropoxy-3,6-dihydro-2H-pyran-2-yl)methoxy)diphenylsilane, (5S)-16
To a solution of lactone (S)-15 (1.2 g, 3.27 mmol) in anhyd DCM (40 mL) and under N2 was added a solution of DIBALH (4 mL, 1.2 M in toluene) at − 78 °C. The reaction was stirred for 1 h at the same temperature and then it was quenched by the addition of saturated NaHCO3 solution (40 mL) and Rochelle’s salt solution (60 mL). The emulsion formed was stirred until complete phase separation (~ 3 h). The layers were separated and the aqueous phase was extracted with DCM (3 × 100 mL). The combined organic layers were dried over MgSO4, filtered and concentrated under reduced pressure. The product was used in the next step without purification.
The crude product was taken up in toluene (16 mL) and then isopropyl alcohol (8.00 mL, 105 mmol) and PPTS (0.025 g, 0.098 mmol, 3 mol%) were added at rt. The reaction mixture was stirred for 12 h at the same temperature and it was quenched by the addition of saturated NaHCO3 solution (20 mL). The mixture was stirred for 30 min. The layers were separated and the aqueous phase was extracted with DCM (3 × 40 mL). The combined organic layers were dried over Na2SO4, filtered and concentrated under reduced pressure. The product was purified by flash column chromatography (hexanes/AcOEt 6:4) to give (5S)-16 (1.16 g, 2.82 mmol, 86%, 2 steps) as a yellow oil and as a single isomer. [α]D20 − 28 (c 0.66, DCM). Lit.20 (antipode) [α]D24 + 28.2 (c 0.68, DCM). Spectral data (1H NMR and 13C NMR) are in accordance with those reported in the literature for its enantiomer.20,23
(R)-1-((tert-Butyldiphenylsilyl)oxy)pent-4-en-2-ol, (R)-14
(R)-tert-butyl(oxiran-2-ylmethoxy)diphenylsilane was prepared from (S)-10 (3.66 g, 49.5 mmol) by similar procedure as described above for its enantiomer. The crude product was purified by flash column chromatography (gradient elution, 2 to 4% of AcOEt in hexanes) to provide the expected product (13.6 g, 43.5 mmol, 88%) as a colorless oil. [α]D20 + 2.5 (c 2, CHCl3). Lit.43 [α]D20 + 2.3 (c 2, CHCl3). Spectral data (1H NMR and 13C NMR) are in accordance with those reported in the literature.43
Alcohol (R)-14 was obtained from (R)-tert-butyl(oxiran-2-ylmethoxy)diphenylsilane (13.4 g, 42.9 mmol) as described above for (S)-14. The crude product was purified by flash column chromatography (hexanes/AcOEt 75:25) to give (R)-14 (12.8 g, 37.6 mmol, 87%) as a colorless oil. [α]D20 + 3 (c 1, CHCl3). Lit.43 [α]D20 + 2.9 (c 1, CHCl3). Spectral data (1H NMR and 13C NMR) are in accordance with those reported in the literature.43
(R)-6-(((tert-Butyldiphenylsilyl)oxy)methyl)-5,6-dihydro-2H-pyran-2-one, (R)-15
(R)-1-((tert-butyldiphenylsilyl)oxy)pent-4-en-2-yl acrylate was prepared from (R)-14 (12.7 g, 37.3 mmol) using a similar procedure to that reported above for its enantiomer. The crude product was purified by flash column chromatography (hexanes/Et2O 8:2) to furnish the desired product (13.9 g, 35.2 mmol, 94%) as a yellow oil. [α]D20 + 11 (c 1, CHCl3). Lit.42 [α]D26 + 8.3 (c 1, CHCl3). Spectral data (1H NMR and 13C NMR) are in accordance with those reported in the literature.42
Lactone (R)-15 was obtained from (R)-1-((tert-butyldiphenylsilyl)oxy)pent-4-en-2-yl acrylate (5.0 g, 13 mmol) by similar procedure described for (S)-15. The crude product was purified by flash column chromatography (hexanes/AcOEt 7:3) to provide (R)-15 (3.81 g, 10.4 mmol, 82%) as a brown oil. [α]D20 + 41 (c 1, CHCl3). Lit.42 [α]D26 + 47.8 (c 1, CHCl3). Lit.43 [α]D23 + 34.2 (c 1.5, CHCl3). Spectral data (1H NMR and 13C NMR) are in accordance with those reported in the literature.42,43
tert-Butyl(((2R)-6-isopropoxy-3,6-dihydro-2H-pyran-2-yl)methoxy)diphenylsilane, (5R)-16
Compound (5R)-16 was prepared from (R)-15 (3.0 g, 8.4 mmol) as described for (5S)-16. The product was purified by flash column chromatography (hexanes/AcOEt 6:4) giving (5R)-16 (3.05 g, 7.42 mmol, 88%, 2 steps) as a yellow oil and as a single isomer. [α]D20 + 30 (c 0.65, DCM). Lit.20 [α]D24 + 28.2 (c 0.68, DCM). Spectral data (1H NMR and 13C NMR) are in accordance with those reported in the literature.20,23
Ethyl (E)-6-hydroxyhex-2-enoate, 17
To a solution of 1,4-butanediol (11) (3.10 g, 34.3 mmol) in DCM (1.2 L) was added respectively (ethoxycarbonylmethylene)triphenylphosphorane (14.3 g, 41.2 mmol) and MnO2 (10.0 g, 115 mmol) and the mixture was stirred at rt. After 24 h and 48 h, respectively, other two additional portions of MnO2 (2 × 10.0 g) were added. The mixture was stirred for a further 5 d and filtered through Celite® using DCM for washing. The solvent was evaporated under reduced pressure and the residue was treated with Et2O to allow precipitation of Ph3PO. After filtration and solvent evaporation, the crude product was purified by flash chromatography (hexanes/AcOEt 1:1), affording the E isomer 17 (4.595 g, 25.92 mmol, 75%) as the major product, together with its Z isomer (Z)-6-hydroxyhex-2-enoate (622 mg, 3.93 mmol, 11%) and the diester 18 (316 mg, 1.40 mmol, 4%) as byproducts. All compounds were obtained as colorless oils and their spectral data (IR, 1H NMR and 13C NMR) are in accordance with those reported in the literature.21
Diethyl (2E,6E)-octa-2,6-dienedioate, 18
A mixture of 17 (2.35 g, 14.9 mmol) and PCC (6.5 g, 30.1 mmol, ground with 12.9 g of silica) in DCM (0.6 L) was stirred at rt for 5 h. Imidazole was added (2.02 g, 29.7 mmol) and the reaction mixture was stirred for an additional 1 h. After addition of (ethoxycarbonylmethylene)triphenylphosphorane (12.4 g, 35.6 mmol), stirring was continued for 24 h. The mixture was filtered through Celite® using DCM for washing. The solvent was removed under reduced pressure and the crude material was treated with Et2O to promote precipitation of Ph3PO. After filtration, the solvent was evaporated and the residue was purified by flash chromatography (hexanes/AcOEt 8:2) to give E:E diester 18 (2.598 g, 11.48 mmol, 77%) and its Z:E isomer diethyl (2Z,6E)-octa-2,6-dienedioate (228 mg, 1.00 mmol, 7%) as colorless oils. Spectral data (IR, 1H NMR and 13C NMR) of 18 and its geometric isomer are in accordance with those reported in the literature.21
(2E,6E)-8-((tert-Butyldimethylsilyl)oxy)octa-2,6-dien-1-ol, 8
To a solution of 18 (2.598 g, 11.48 mmol) in anhyd DCM (50 mL) was slowly added via cannula a solution of DIBALH (9 mL, 50.5 mmol) in DCM (15 mL) at − 78 °C and under N2. The reaction mixture was stirred under reduced temperature for 2 h. After that, were added Et2O (150 mL) and saturated solution of Rochelle’s salt (50 mL), maintaining vigorous stirring for 1 h. After phase separation, the aqueous one was extracted with Et2O (2 × 50 mL). The combined organic phases were washed with brine (50 mL), dried over MgSO4, filtered, and concentrated under reduced pressure. The crude product was purified by flash chromatography (AcOEt) to afford the corresponding diol (2E,6E)-octa-2,6-diene-1,8-diol (1.539 g, 10.82 mmol, 94%) as a light yellow oil. Spectral data (IR, 1H NMR and 13C NMR) are in accordance with those reported in the literature.44
To a solution of (2E,6E)-octa-2,6-diene-1,8-diol (1.27 mg, 8.93 mmol) in anhyd DMF (18 mL) was added TBSCl (897 mg, 5.95 mmol) and imidazole (608 mg, 8.93 mmol). The resulting mixture was stirred at rt for 24 h and the reaction was quenched by addition of H2O (50 mL). After extraction with AcOEt (3 × 100 mL), the combined organic phases were dried over MgSO4, filtered, and concentrated under reduced pressure. The residue was purified by flash chromatography (gradient elution, 10 to 100% of AcOEt in hexanes) affording the mono-protected compound 8 (894 mg, 3.49 mmol, 59%), the bis-silyl ether (6E,10E)-2,2,3,3,14,14,15,15-octamethyl-4,13-dioxa-3,14-disilahexadeca-6,10-diene (251 mg, 0.677 mmol, 11%) as a byproduct, and recovery of starting material (532 mg, 3.74 mmol, 42%). Spectral data (IR, 1H NMR and 13C NMR) of the isolated compounds are in agreement with the literature data.44
(((2E,6E)-8-Bromoocta-2,6-dien-1-yl)oxy)(tert-butyl)dimethylsilane, 19
To a solution of 8 (611 mg, 2.38 mmol) in anhyd MeCN (14 mL) was added Ph3P (1.25 mg, 4.77 mmol), 2,6-lutidine (0.55 mL, 4.75 mmol) and CBr4 (1.58 g, 4.76 mmol), respectively, at 0 °C. The resulting solution was stirred at rt for 10 min. The reaction was quenched with H2O (50 mL) and extracted with Et2O (2 × 100 and 1 × 50 mL). The combined organic phases were washed with brine (50 mL), dried over MgSO4, filtered, and concentrated under reduced pressure. The crude product was purified by flash chromatography (hexanes/AcOEt 95:5) to give 19 (744 mg, 2.3 mmol, 98%) as a colorless oil. Rf: 0.64 (hexanes/AcOEt 95:5). IR (ATR) νmax/cm−1: 2928, 2856, 1472, 1254, 966, 835, 775. 1H NMR (500 MHz, CDCl3) δ: 0.06 (s, 6H), 0.90 (s, 9H), 2.14 (s,br, 4H), 3.93 (d, J = 5.0 Hz, 2H), 4.11 (d, J = 2.5 Hz, 2H), 5.49–5.73 (m, 4H). 13C NMR (125 MHz, CDCl3) δ: - 5.1 (2 C), 18.4, 26.0 (3 C), 31.4, 31.6, 33.3, 63.8, 126.8, 129.7, 130.3, 135.6. HRMS (ESI-TOF) m/z: [M + Na]+ Calcd for C14H27BrOSiNa 341.09067; Found 341.09049.
2-((((1S,2S)-2-Methylcyclopropyl)methyl)sulfonyl)benzo[d]thiazole, (1S,2S)-22
To a mixture of DME (2 mL, 19 mmol) and anhyd DCM (18 mL) was added a solution of diethylzinc (1 M in hexanes, 19 mL) at − 10 °C and under N2. Diiodomethane (3 mL, 37 mmol) was added dropwise and the solution was stirred at − 10 °C for 20 min. This fresh Zn(CH2I)2·DME solution was added by cannula over a solution of trans-crotyl alcohol (12) (540 mg, 7.50 mmol) and butylboronic acid N,N,N′,N′-tetramethyl-L-tartaric acid diamide ester, (4R,5R)-13 (2.2 g, 8.1 mmol), in DCM (37 mL) at − 10 °C and the mixture was stirred at 0 °C for 4 h. Saturated aqueous NH4Cl (50 mL) was added and the mixture was extracted with Et2O (4 × 50 mL). The combined organic phases were stirred with NaOH 2 M (100 mL) overnight. After phase separation, the organic one was washed with aqueous solution of HCl 1 M (50 mL), saturated solution of NaHCO3 (50 mL) and brine (50 mL). The organic phase was dried over MgSO4, filtered, and concentrated under reduced pressure (450 mbar). The crude product ((1S,2S)-2-methylcyclopropyl)methanol, (1S,2S)-9, was obtained as a light yellow oil and used in the next step without purification to avoid losses due to its volatility. The enantiomeric ratio was determined to be 94:6 after analysis of the corresponding Mosher’s ester by 1H NMR (500 MHz).
To a solution of (1S,2S)-9 obtained above in THF (15 mL) was successively added 2-mercaptobenzothiazole (1.0 g, 6.0 mmol), Ph3P (1.57 g, 5.97 mmol) and DIAD (1.3 mL, 6.6 mmol). The mixture was stirred at rt for 24 h and the solvent excess was removed under reduced pressure. Et2O was added to the residue and the precipitate was removed by filtration. After solvent evaporation, the crude material was partially purified by flash chromatography (hexanes/AcOEt 95:5) to give 2-((((1S,2S)-2-methylcyclopropyl)methyl)thio)benzo[d]thiazole, (1S,2S)-21 (1.033 g), as a light yellow oil. This intermediate was not characterized because it was still impure.
To a solution of (1S,2S)-21 (1.033 g) in EtOH (40 mL) was added a solution of ammonium molybdate tetrahydrate (540 mg, 0.437 mmol) in H2O2 30% v/v (2 mL) at 0 °C and under N2. The resulting mixture was stirred at rt for 18 h and the solvent was removed under reduced pressure. The residue was diluted with H2O (50 mL) and extracted with DCM (5 × 50 mL). The combined organic phases were washed with brine (50 mL), dried with MgSO4, evaporated, and the residue was purified by flash chromatography (hexanes/AcOEt 8:2) to afford (1S,2S)-22 (890 mg, 3.33 mmol, 39% for 3 steps, er 94:6) as a white solid. mp: 77–80 °C. Lit.25 mp: 94–96 °C. [α]D20 + 8 (c 1.13, CHCl3). Lit.25 [α]D20 + 7 (c 1.14, CHCl3). The enantiomeric ratio of (1S,2S)-22 was determined by chiral HPLC analysis26 and its spectral data (IR, 1H NMR and 13C NMR) are in agreement with the literature data.25
2-((((1R,2R)-2-Methylcyclopropyl)methyl)sulfonyl)benzo[d]thiazole, (1R,2R)-22
Sulfone (1R,2R)-22 was prepared from 12 in 31% overall yield and er 94:6 employing a similar procedure as described above for (1S,2S)-22, but using butylboronic acid N,N,N′,N′-tetramethyl-D-tartaric acid diamide ester, (4S,5S)-13. mp: 79–82 °C. [α]D20 − 8 (c 1.11, CHCl3). Lit.25 [α]D20 (antipode) + 7 (c 1.14, CHCl3). The enantiomeric ratio of (1R,2R)-22 was determined by chiral HPLC analysis26 and its spectroscopic data (IR, 1H NMR and 13C NMR) are in accordance with those reported for its enantiomer by Charette.25
2-((((trans)-2-Methylcyclopropyl)methyl)sulfonyl)benzo[d]thiazole (22)
Racemic sulfone 22 was produced from 12 in 83% overall yield using similar procedure as described for (1S,2S)-22, but employing Simmons–Smith cyclopropanation in place of asymmetric Charette’s reaction. mp: 84–86 °C. The spectra data of racemic 22 (IR, 1H NMR and 13C NMR) are in accordance with those reported for (1S,2S)-22 by Charette.25
tert-Butyl(((2E,6E,8E)-9-((2S)-6-isopropoxy-3,6-dihydro-2H-pyran-2-yl)nona-2,6,8-trien-1-yl)oxy)dimethylsilane, (5S)-23
To a solution of 19 (664 mg, 2.08 mmol) in anhyd MeCN (9.5 mL) was added n-Bu3P (0.77 mL, 3.1 mmol) and the mixture was stirred at rt for 12 h. After that, the excess of solvent was removed under reduced pressure and the crude product was maintained under high vacuum (0.2 mmHg) for 8 h. The tri-n-butylphosphonium salt 20 was used in the Wittig olefination without purification.
To a solution of dihydropyran (5S)-16 (1.41 g, 3.43 mmol) in anhyd THF (35 mL) at 0 °C and under N2 was added a solution of TBAF (3.6 mL, 1 M in THF). The solution was allowed to warm to rt and stirred for 3 h. The reaction was quenched by the addition of H2O (35 mL). The layers were separated and the aqueous layer was extracted with AcOEt (5 × 40 mL). The combined organic layers were dried over Na2SO4, filtered and concentrated under reduced pressure. The crude product was purified by column chromatography (hexanes/AcOEt 6:4) to give the corresponding alcohol ((2S)-6-isopropoxy-3,6-dihydro-2H-pyran-2-yl)methanol (0.492 g, 2.85 mmol, 83%) as a white solid. mp: 43–45 °C. [α]D20 − 47 (c 1, DCM). Lit.20 (antipode) [α]D24 + 40.4 (c 0.47, DCM). Spectral data (1H NMR and 13C NMR) are in accordance with those reported in the literature for its enantiomer.20
To a solution of oxalyl chloride (0.192 mL, 2.23 mmol) in anhyd DCM (10 mL) at − 78 °C and under N2, was added dropwise DMSO (0.227 mL, 3.12 mmol). After stirring for 15 min, a solution of the alcohol obtained above (0.240 g, 1.39 mmol) in anhyd DCM (4 mL) was added dropwise by cannula. The resultant white emulsion was stirred for more 15 min and then Et3N (1.0 mL, 6.9 mmol) was added at the reaction. After 5 min the mixture was allowed to warm to rt. The reaction was quenched by the addition of saturated NH4Cl solution (15 mL). The layers were separated and the aqueous phase was extracted with Et2O (3 × 25 mL). The organic layers were combined, washed with brine (20 mL), dried over Na2SO4, filtered and carefully concentrated (bath temperature: 30 °C, pressure 400–420 mbar) to prevent loss of the volatile aldehyde. The crude product (2S)-6-isopropoxy-3,6-dihydro-2H-pyran-2-carbaldehyde, (5S)-7, was obtained as a colorless oil which was used in the next step without purification because its instability. Rf: 0.57 (hexanes/AcOEt 7:3).
Wittig Olefination
To a solution of freshly prepared aldehyde (5S)-7 (236 mg, 1.39 mmol) and tri-n-butylphosphonium salt 20 (2.08 mmol) in anhyd PhMe (25 mL) was added dropwise a solution of NHMDS (2.8 mL, 2.8 mmol, 1 M in THF) at − 78 °C and under N2. The mixture was warmed slowly and stirred over 6 h. The reaction was quenched with H2O (50 mL) and extracted with AcOEt (3 × 100 mL). The combined organic phases were washed with brine (50 mL), dried over MgSO4 and the solvent was removed under reduced pressure. The crude product was purified by flash chromatography (hexanes/AcOEt 95:5) to furnish (5S)-23 (383 mg, 0.975 mmol, 70%) as a light yellow oil and as a 6:1 mixture of the two isomers at C-6/C-7 double bond. Rf: 0.50 (hexanes/AcOEt 95:5). IR (ATR) νmax/cm−1: 2958, 2926, 2895, 2851, 1254, 1125, 1099, 1028, 988, 836, 776. 1H NMR (500 MHz, CDCl3) δ: (major isomer) 0.06 (s, 6H, Si(CH3)2), 0.90 (s, 9H, C(CH3)3), 1.16 (d, J = 5.0 Hz, 3H, C(CH3)2), 1.22 (d, J = 5.0 Hz, 3H, C(CH3)2), 1.97–2.26 (m, 6H, H-4, H-10 and H-11), 4.00 (sept, J = 5.0 Hz, 1H, CH(CH3)2), 4.11 (d, J = 4.7 Hz, 2H, H-14), 4.42–4.46 (m, 1H, H-5), 5.10 (s,br, 1H, H-1), 5.52–5.72 (m, 5H, H-13, H-6, H-12, H-9 and H-2), 5.97–6.08 (m, 2H, H-3 and H-8), 6.22 (dd, J = 15.3 and 10.5 Hz, 1H, H-7). 13C NMR (125 MHz, CDCl3) δ: (major isomer) − 5.1 (2 CH3, Si(CH3)2), 18.4 (C, Si-C), 22.0 (CH3, C(CH3)2), 23.8 (CH3, C(CH3)2), 26.0 (3 CH3, C(CH3)3), 30.7 (CH2, C-4), 31.8 (CH2, C-11), 32.2 (CH2, C-10), 63.9 (CH2, C-14), 66.4 (CH, C-5), 69.4 (CH, C(CH3)2), 93.1 (CH, C-1), 126.1 (CH, C-2), 128.4 (CH, C-3), 129.7 (CH, C-13), 130.0 (CH, C-8), 130.2 (CH, C-12*), 130.8 (CH, C-6*), 131.1 (CH, C-7), 134.5 (CH, C-9); *indicates that assignments may be interchanged. HRMS (ESI-TOF) m/z: [M − OCH(CH3)2]+ Calcd for C20H33O2Si 333.22443; Found 333.22458.
(2E,6E,8E)-9-((2S)-6-Isopropoxy-3,6-dihydro-2H-pyran-2-yl)nona-2,6,8-trienal, (5S)-24
To a solution of (5S)-23 (0.375 g, 0.955 mmol) in anhyd THF (48 mL) was added a solution of TBAF (1 mL, 1 mmol, 1 M in THF) at 0 °C and under N2. The yellow solution was allowed to warm to rt and stirred for 2 h. The reaction was quenched by the addition of H2O (30 mL) and AcOEt (60 mL). The layers were separated and the aqueous phase was extracted with AcOEt (4 × 50 mL). The combined organic phases were dried over MgSO4, filtered and concentrated under reduced pressure. The product was used in the next step without purification. To a solution of the alcohol obtained above in anhyd DCM (95 mL) was added MnO2 (5 g, 57.3 mmol, ≥ 85% activated, Sigma-Aldrich) in 3 portions (every 24 hour). The reaction was allowed to stir for additional 24 h. The crude reaction mixture was directly filtered through a plug of silica gel, eluted with a mixture of hexanes/AcOEt 1:1, and concentrated under reduced pressure. The product was purified by flash chromatography (hexanes/AcOEt 6:4) to give (5S)-24 (227 mg, 0.821 mmol, 86 %, 2 steps) as a light yellow oil and as a 6:1 mixture of the two isomers at C-6/C-7 double bond. Rf: 0.54 (hexanes/AcOEt 1:1). IR (ATR) νmax/cm−1: 2959, 2922, 2850, 1691, 1124, 1099, 1026, 993, 668, 651. 1H NMR (500 MHz, CDCl3) δ: (major isomer) 1.19 (d, J = 6.1 Hz, 3H, C(CH3)2), 1.25 (d, J = 6.1 Hz, 3H, C(CH3)2), 2.01–2.14 (m, 2H, H-4), 2.32–2.36 (m, 2H, H-10), 2.45–2.49 (m, 2H, H-11), 4.02 (sept, J = 6.1 Hz, 1H, CH(CH3)2), 4.46–4.49 (m, 1H, H-5), 5.13 (s,br, 1H, H-1), 5.66–5.79 (m, 3H, H-6, H-9 and H-2), 6.00–6.03 (m, 1H, H-3), 6.09–6.17 (m, 2H, H-8 and H-13), 6.25 (dd, J = 15.4 and 10.5 Hz, 1H, H-7), 6.85 (dd, J = 15.6 and 6.7 Hz, 1H, H-12), 9.52 (d, J = 7.9 Hz, 1H, H-14). 13C NMR (125 MHz, CDCl3) δ: (major isomer) 22.0 (CH3, C(CH3)2), 23.8 (CH3, C(CH3)2), 30.6 (CH2, C-4), 30.7 (CH2, C-10), 32.2 (CH2, C-11), 66.2 (CH2, C-5), 69.5 (CH, C(CH3)2), 93.1 (CH, C-1), 126.1 (CH, C-2), 128.3 (CH, C-3), 130.5 (CH, C-7), 131.1 (CH, C-8), 131.9 (CH, C-9*), 132.5 (CH, C-6*), 133.3 (CH, C-13), 157.4 (CH, C-12), 193.9 (CHO, C-14); *indicates that assignments may be interchanged. HRMS (ESI-TOF) m/z: [M + Na]+ Calcd for C17H24O3Na 299.1623; Found 299.1637.
(2S)-6-Isopropoxy-2-((1E,3E,7E,9E)-10-((1S,2S)-2-methylcyclopropyl)deca-1,3,7,9-tetraen-1-yl)-3,6-dihydro-2H-pyran, (5S,16S,18S)-25
Julia Olefination with THF. To a solution of aldehyde (5S)-24 (16 mg, 0.058 mmol) and sulfone (1S,2S)-22 (23 mg, 0.086 mmol) in anhyd THF (2.5 mL) was added dropwise a solution of NHMDS (120 μL, 0.12 mmol, 1 M in THF) at − 78 °C and under N2. The light yellow resulting mixture was stirred for 10 min and the reaction was quenched with H2O (6 mL). The mixture was extracted with AcOEt (30 + 10 mL) and the organic layer was washed with brine (10 mL), dried over MgSO4, and concentrated under reduced pressure. The crude product was purified by flash chromatography (hexanes/AcOEt 95:5) to afford (5S,16S,18S)-25 as 1.7:1 E:Z mixture at C-14 double bond (12.4 mg, 0.0377 mmol, 65%), contaminated with minor amounts of the 6Z double bond isomers as a light yellow oil. Rf: 0.67 (hexanes/AcOEt 9:1). IR (ATR) νmax/cm−1: 2969, 2925, 1654, 1447, 1380, 1316, 1181, 1099, 1027, 985, 718. 1H NMR (500 MHz, CDCl3) δ: (major isomer) 0.47–0.50 (m, 1H), 0.53–0.57 (m, 1H), 0.72–0.79 (m, 1H), 1.04–1.10 (m, 1H), 1.06 (d, J = 6.0 Hz, 3H), 1.17 (d, J = 6.2 Hz, 3H), 1.24 (d, J = 6.2 Hz, 3H), 2.03–2.25 (m, 6H), 4.01 (sept, J = 6.2 Hz, 1H), 4.42–4.47 (m, 1H), 5.10 (s,br, 1H), 5.15 (dd, J = 14.8 and 8.9 Hz, 1H), 5.48–5.56 (m, 1H), 5.62 (d, J = 15.3 and 6.1 Hz, 1H), 5.68–5.76 (m, 2H), 5.94–6.09 (m, 4H), 6.22 (dd, J = 15.3 and 10.2 Hz, 1H), (second largest isomer) 0.53–0.57 (m, 2H), 0.72–0.79 (m, 1H), 1.04–1.10 (m, 1H), 1.09 (d, J = 5.8 Hz, 3H), 1.17 (d, J = 6.1 Hz, 3H), 1.24 (d, J = 6.2 Hz, 3H), 2.03–2.25 (m, 6H), 4.01 (sept, J = 6.2 Hz, 1H), 4.42–4.47 (m, 1H), 4.72 (t, J = 10.4 Hz, 1H), 5.10 (s,br, 1H), 5.48–5.56 (m, 1H), 5.68–5.76 (m, 2H), 5.87 (t, J = 10.9 Hz, 1H), 5.94–6.09 (m, 4H), 6.47 (dd, J = 14.8 and 11.2 Hz, 1H). 13C NMR (63 MHz, CDCl3) δ: (major isomer) 15.6 (2 C), 18.5, 22.0, 22.9, 23.9, 30.7, 32.3, 32.6, 66.4, 69.4, 93.1, 126.1, 127.5, 128.5, 130.0, 130.1, 130.7, 130.9, 131.2, 134.6, 136.1; (second largest isomer) 15.8, 16.0, 18.5, 19.3, 21.9, 23.9, 30.8, 32.2, 32.7, 62.7, 69.3, 92.8, 126.3, 126.5, 128.6, 130.0, 130.9, 131.2, 132.8, 134.2, 134.6, 136.2. HRMS (ESI-TOF) m/z: [M + Na]+ Calcd for C22H32O2Na 351.2300; Found 351.2288.
(S)-6-((1E,3E,7E,9E)-10-((1S,2S)-2-Methylcyclopropyl)deca-1,3,7,9-tetraen-1-yl)-5,6-dihydro-2H-pyran-2-one, (5S,16S,18S)-1
A mixture of PCC (323 mg, 1.50 mmol) and CaCO3 (600 mg, 6 mmol) in anhyd DCM (6 mL) was stirred at rt for 30 min. Then a solution of (5S,16S,18S)-25 (100 mg, 0.304 mmol) in DCM (13 mL) was added by cannula and the reaction mixture was stirred at rt for 1 h. After filtration over silica gel and solvent evaporation under reduced pressure, the crude product was purified by flash chromatography (gradient elution, 20 to 30% of AcOEt in hexanes) to give (5S,16S,18S)-1′ (19 mg, 0.067 mmol, 22%) as a mixture of E:Z isomers at C-14/C-15 double bond and contaminated with minor amounts of the 6Z isomers. This mixture was separated using preparative HPLC with SunFire™ Prep C18 OBD™ column (particle size 5 μm, dimensions 19 mm ×100 mm, 60% MeCN/40% H2O, 17 mL/min) to yield the major isomer with E-configured double bonds (5S,16S,18S)-1 (6.7 mg, tR 18.4 min) as a colorless oil. Rf: 0.41 (hexanes/AcOEt 7:3). [α]D20 − 10 (c 0.1, CHCl3). IR (ATR) νmax/cm−1: 2995, 2918, 1720, 1658, 1381, 1244, 986, 816. 1H NMR (500 MHz, CDCl3) δ: 0.47–0.51 (m, 1H, H-17), 0.54–0.58 (m, 1H, H-17), 0.73–0.80 (m, 1H, H-18), 1.05 (d, J = 6.0 Hz, 3H, H-19), 1.05–1.10 (m, 1H, H-16), 2.14–2.18 (m, 4H, H-10 and H-11), 2.43–2.45 (m, 2H, H-4), 4.92–4.96 (m, 1H, H-5), 5.15 (dd, J = 14.7 and 9.0 Hz, 1H, H-15), 5.50 (dt, J = 14.1 and 6.6 Hz, 1H, H-12), 5.64 (dd, J = 15.3 and 6.6 Hz, 1H, H-6), 5.77 (dt, J = 15.2 and 6.6 Hz, 1H, H-9), 5.94–6.06 (m, 4H, H-2, H-8, H-13 and H-14), 6.30 (dd, J = 15.3 and 10.4 Hz, 1H, H-7), 6.85–6.89 (m, 1H, H-3). 13C NMR (125 MHz, CDCl3) δ: 15.7 (CH2, C-17; CH, C-18), 18.5 (CH3, C-19), 22.8 (CH, C-16), 29.8 (CH2, C-4), 32.1 (CH2, C-11), 32.6 (CH2, C-10), 77.9 (CH, C-5), 121.6 (CH, C-2), 126.7 (CH, C-6), 127.4 (CH, C-14), 129.1 (CH, C-8), 129.9 (CH, C-12), 130.8 (CH, C-13), 133.7 (CH, C-7), 136.3 (CH, C-15), 136.8 (CH, C-9), 144.6 (CH, C-3), 164.0 (CO, C-1). HRMS (ESI-TOF) m/z: [M + Na]+ Calcd for C19H24ONa 307.1674; Found 307.1663.
(2S)-6-Isopropoxy-2-((1E,3E,7E,9E)-10-((1R,2R)-2-methylcyclopropyl)deca-1,3,7,9-tetraen-1-yl)-3,6-dihydro-2H-pyran, (5S,16R,18R)-25
Julia Olefination with DME. To a solution of aldehyde (5S)-24 (103 mg, 0.373 mmol) and sulfone (1R,2R)-22 (150 mg, 0.561 mmol) in anhyd DME (17 mL) was added dropwise a solution of NHMDS (0.78 mL, 0.78 mmol, 1 M in THF) at − 78 °C and under N2. The light yellow mixture was stirred for 10 min and the reaction was quenched with brine (50 mL). The mixture was extracted with AcOEt (3 × 50 mL) and the organic layer was dried over MgSO4 and concentrated under reduced pressure. The crude product was purified by flash chromatography (hexanes/AcOEt 95:5) to afford (5S,16R,18R)-25 as a 2.7:1 E:Z mixture at C-14 double bond (116 mg, 0.353 mmol, 95%), contaminated with minor amounts of the 6Z double bonds isomers as a light yellow oil. Rf: 0.67 (hexanes/AcOEt 9:1). The IR and NMR data of (5S,16R,18R)-25 are identical to those described for (5S,16S,18S)-25, despite their diastereoisomeric relationship. HRMS (ESI-TOF) m/z: [M + K]+ Calcd for C22H32O2K 367.2039; Found 367.2030.
(S)-6-((1E,3E,7E,9E)-10-((1R,2R)-2-Methylcyclopropyl)deca-1,3,7,9-tetraen-1-yl)-5,6-dihydro-2H-pyran-2-one, (5S,16R,18R)-1
A mixture of PCC (361 mg, 1.67 mmol) and CaCO3 (670 mg, 6.70 mmol) in anhyd DCM (7 mL) was stirred at rt for 2 h. Then a solution of (5S,16R,18R)-25 (111 mg, 0.338 mmol) in DCM (12 mL) was added by cannula and the reaction mixture was stirred at rt for 2 h. After filtration over silica gel and solvent evaporation under reduced pressure, the crude product was purified by flash chromatography (gradient elution, 20 to 30% of AcOEt in hexanes) to afford (5S,16R,18R)-1′ (28 mg, 0.098 mmol, 29%) as a mixture of E:Z isomers at C-14/C-15 double bond and contaminated with minor amounts of the 6Z isomers. This mixture was separated using preparative HPLC with SunFire™ Prep C18 OBD™ column (particle size 5 μm, dimensions 19 mm ×100 mm, 60% MeCN/40% H2O, 17 mL/min) to give the major isomer with E-configured double bonds (5S,16R,18R)-1 (12.9 mg, tR 18.4 min) as a colorless oil. Rf: 0.41 (hexanes/AcOEt 7:3). [α]D20 − 100 (c 0.1, CHCl3). The IR and NMR data of (5S,16R,18R)-1 are identical to those described for (5S,16S,18S)-1 despite their diastereoisomeric relationship. HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C19H25O2 285.1855; Found 285.1866.
tert-Butyl(((2E,6E,8E)-9-((2R)-6-isopropoxy-3,6-dihydro-2H-pyran-2-yl)nona-2,6,8-trien-1-yl)oxy)dimethylsilane, (5R)-23
To a solution of 19 (568 mg, 1.78 mmol) in anhyd MeCN (8 mL) was added n-Bu3P (0.70 mL, 2.8 mmol) and the mixture was stirred at rt for 12 h. After that, the excess of solvent was removed under reduced pressure and the crude product was maintained under high vacuum (0.2 mmHg) for 8 h. The tri-n-butylphosphonium salt 20 was used without purification.
Deprotection of (5R)-16 (3.05 g, 7.31 mmol) employing a similar procedure described above for its enantiomer provided the corresponding alcohol. The crude product was purified by column chromatography (hexanes/AcOEt 6:4) to give the desired product ((2R)-6-isopropoxy-3,6-dihydro-2H-pyran-2-yl)methanol (1.14 g, 6.62 mmol, 90%) as a white solid. mp: 42–44 °C. [α]D20 + 46 (c 1, DCM). Lit.20 [α]D24 + 40.4 (c 0.47, DCM). Spectral data (1H NMR and 13C NMR) are in accordance with those reported in the literature.20
Aldehyde (2R)-6-isopropoxy-3,6-dihydro-2H-pyran-2-carbaldehyde, (5R)-7, was prepared from the alcohol prepared above (0.205 g, 1.19 mmol) as described for (5S)-7. The crude product was obtained as a colorless oil that was used in the next step without purification because its instability. Rf: 0.57 (hexanes/AcOEt 7:3).
Wittig Olefination
Compound (5R)-23 was obtained from freshly prepared aldehyde (5R)-7 (202 mg, 1.19 mmol) and tri-n-butylphosphonium salt 20 (1.78 mmol) following the procedure described above for (5S)-23. The crude product was purified by flash chromatography (gradient elution, 0 to 10% of AcOEt in hexanes) to furnish (5R)-23 (368 mg, 0.937 mmol, 79%) as a light yellow oil and as a 5:1 mixture of the two isomers at C-6/C-7 double bond. Rf: 0.50 (hexanes/AcOEt 95:5). The IR and NMR data of (5R)-23 are identical to those described for its enantiomer (5S)-23. HRMS (ESI-TOF) m/z: [M + Na]+ Calcd for C23H40O3SiNa 415.2644; Found 415.2633.
(2E,6E,8E)-9-((2R)-6-Isopropoxy-3,6-dihydro-2H-pyran-2-yl)nona-2,6,8-trienal, (5R)-24
Aldehyde (5R)-24 was obtained from (5R)-23 (0.699 g, 1.78 mmol) using a similar procedure as described for (5S)-24 except for the reaction time of the MnO2 oxidation which was reduced from 96 h to 72 h by using of another batch of MnO2 (6.19 g, 71.2 mmol, ≥ 90% activated, Fluka Analytical). The product was purified by flash chromatography (hexanes/AcOEt 6:4) to give (5R)-24 (371 mg, 1.34 mmol, 75 %, 2 steps) as a light yellow oil and as a 5:1 mixture of the two isomers at C-6/C-7 double bond. Rf: 0.54 (hexanes/AcOEt 1:1). The IR and NMR data of (5R)-24 are identical to those described for its enantiomer (5S)-24. HRMS (ESI-TOF) m/z: [M + Na]+ Calcd for C17H24O3Na 299.1623; Found 299.1618.
(2R)-6-Isopropoxy-2-((1E,3E,7E,9E)-10-((1S,2S)-2-methylcyclopropyl)deca-1,3,7,9-tetraen-1-yl)-3,6-dihydro-2H-pyran, (5R,16S,18S)-25
Intermediate (5R,16S,18S)-25 was prepared from aldehyde (5R)-24 (105 mg, 0.380 mmol) and sulfone (1S,2S)-22 (150 mg, 0.561 mmol) as described for (5S,16R,18R)-25. The crude product was purified by flash chromatography (hexanes/AcOEt 95:5) to afford (5R,16S,18S)-25 as a 2.7:1 E:Z mixture at C-14 double bond (117 mg, 0.356 mmol, 94%), contaminated with minor amounts of the 6Z double bonds isomers as a light yellow oil. Rf: 0.67 (hexanes/AcOEt 9:1). The IR and NMR data of (5R,16S,18S)-25 are identical to those described for (5S,16S,18S)-25 despite their diastereoisomeric relationship. HRMS (ESI-TOF) m/z: [M + Na]+ Calcd for C22H32O2Na 351.2300; Found 351.2306.
(R)-6-((1E,3E,7E,9E)-10-((1S,2S)-2-Methylcyclopropyl)deca-1,3,7,9-tetraen-1-yl)-5,6-dihydro-2H-pyran-2-one, (5R,16S,18S)-1
Compound (5R,16S,18S)-1 was synthesized from (5R,16S,18S)-25 (111 mg, 0.338 mmol) using a similar procedure to that described for (5S,16R,18R)-1. The crude product was purified by flash chromatography (gradient elution, 20 to 30% of AcOEt in hexanes) to give (5R,16S,18S)-1′ (31 mg, 0.11 mmol, 33%) as a mixture of E:Z isomers at C-14/C-15 double bond and contaminated with minor amounts of the 6Z isomers. After separation in preparative HPLC with SunFire™ Prep C18 OBD™ column (particle size 5 μm, dimensions 19 mm ×100 mm, 60% MeCN/40% H2O, 17 mL/min) the major isomer with E-configured double bonds (5R,16S,18S)-1 (11.9 mg, tR 18.4 min) was obtained as a colorless oil. Rf: 0.41 (hexanes/AcOEt 7:3). [α]D20 + 100 (c 0.1, CHCl3). IR (ATR) νmax/cm−1: 2995, 2918, 1720, 1658, 1381, 1244, 986, 816. 1H NMR (500 MHz, CDCl3) δ: 0.47–0.51 (m, 1H, H-17), 0.54–0.58 (m, 1H, H-17), 0.73–0.80 (m, 1H, H-18), 1.05 (d, J = 6.0 Hz, 3H, H-19), 1.05–1.10 (m, 1H, H-16), 2.14–2.18 (m, 4H, H-10 and H-11), 2.43–2.45 (m, 2H, H-4), 4.92–4.96 (m, 1H, H-5), 5.15 (dd, J = 14.7 and 9.0 Hz, 1H, H-15), 5.50 (dt, J = 14.1 and 6.6 Hz, 1H, H-12), 5.64 (dd, J = 15.3 and 6.6 Hz, 1H, H-6), 5.77 (dt, J = 15.2 and 6.6 Hz, 1H, H-9), 5.94–6.06 (m, 4H, H-2, H-8, H-13 and H-14), 6.30 (dd, J = 15.3 and 10.4 Hz, 1H, H-7), 6.85–6.89 (m, 1H, H-3). 13C NMR (125 MHz, CDCl3) δ: 15.7 (CH2, C-17; CH, C-18), 18.5 (CH3, C-19), 22.8 (CH, C-16), 29.8 (CH2, C-4), 32.1 (CH2, C-11), 32.6 (CH2, C-10), 77.9 (CH, C-5), 121.6 (CH, C-2), 126.7 (CH, C-6), 127.4 (CH, C-14), 129.1 (CH, C-8), 129.9 (CH, C-12), 130.8 (CH, C-13), 133.7 (CH, C-7), 136.3 (CH, C-15), 136.8 (CH, C-9), 144.6 (CH, C-3), 164.0 (CO, C-1). HRMS (ESI-TOF) m/z: [M + Na]+ Calcd for C19H24O2Na 307.1674; Found 307.1678.
(2R)-6-Isopropoxy-2-((1E,3E,7E,9E)-10-((1R,2R)-2-methylcyclopropyl)deca-1,3,7,9-tetraen-1-yl)-3,6-dihydro-2H-pyran, (5R,16R,18R)-25
Intermediate (5R,16R,18R)-25 was synthesized from aldehyde (5R)-24 (105 mg, 0.380 mmol) and sulfone (1R,2R)-22 (145 mg, 0.543 mmol) as described for (5S,16R,18R)-25. The crude product was purified by flash chromatography (hexanes/AcOEt 95:5) to furnish (5R,16R,18R)-25 as a 2.7:1 E:Z mixture at C-14 double bond (118 mg, 0.359 mmol, 95%), contaminated with minor amounts of the 6Z double bonds isomers as a light yellow oil. Rf: 0.67 (hexanes/AcOEt 9:1). The IR and NMR data of (5R,16R,18R)-25 are identical to those described for (5S,16S,18S)-25. HRMS (ESI-TOF) m/z: [M + Na]+ Calcd for C22H32O2Na 351.2300; Found 351.2294.
(R)-6-((1E,3E,7E,9E)-10-((1R,2R)-2-Methylcyclopropyl)deca-1,3,7,9-tetraen-1-yl)-5,6-dihydro-2H-pyran-2-one, (5R,16R,18R)-1
Compound (5R,16R,18R)-1 was synthesized from (5R,16R,18R)-25 (110 mg, 0.335 mmol) using a similar procedure to that described above for (5S,16R,18R)-1. The crude product was purified by flash chromatography (gradient elution, 20 to 30% of AcOEt in hexanes) to furnish (5R,16R,18R)-1′ (23 mg, 0.081 mmol, 24%) as a mixture of E:Z isomers at C-14/C-15 double bond and contaminated with minor amounts of the 6Z isomers. After separation in preparative HPLC with SunFire™ Prep C18 OBD™ column (particle size 5 μm, dimensions 19 mm ×100 mm, 60% MeCN/40% H2O, 17 mL/min) the major isomer with E-configured double bonds (5R,16R,18R)-1 (6.2 mg, tR 18.4 min) was obtained as a colorless oil. Rf: 0.41 (hexanes/AcOEt 7:3). [α]D20 + 10 (c 0.1, CHCl3). The IR and NMR data of (5R,16R,18R)-1 are identical to those described for (5S,16S,18S)-1. HRMS (ESI-TOF) m/z: [M + Na]+ Calcd for C19H24O2Na 307.1674; Found 307.1663.
Ethyl (E)-6-hydroxyhex-2-enoate, 26
To a solution of 17 (1.54 g, 9.74 mmol) in anhyd DCM (40 mL) was added Et3N (3.3 mL, 23.4 mmol), DMAP (120 mg, 1.15 mmol) and TBSCl (1.76 g, 11.7 mmol), respectively, under N2. The resulting mixture was stirred at rt for 4.5 h and the solvent was removed under reduced pressure. The residue was purified by flash chromatography (hexanes/AcOEt 95:5) affording ethyl (E)-6-((tert-butyldimethylsilyl)oxy)hex-2-enoate (2.54 g, 9.32 mmol, 96%) as a colorless oil. Spectral data (1H NMR and 13C NMR) are in accordance with those reported in the literature.45
To a solution of ethyl (E)-6-((tert-butyldimethylsilyl)oxy)hex-2-enoate (2.35 g, 8.63 mmol) in anhyd DCM (40 mL) was slowly added via cannula a solution of DIBALH (3.9 mL, 21.6 mmol) in DCM (10 mL), at − 78 °C and under N2. The reaction mixture was stirred under reduced temperature for 2 h. After that, were added Et2O (100 mL) and saturated solution of Rochelle’s salt (50 mL), maintaining vigorous stirring for 30 min at 0 °C and for 1 h at rt. After phase separation, the aqueous one was extracted with Et2O (2 × 100 mL). The combined organic phases were dried over MgSO4, filtered, and concentrated under reduced pressure. The crude product was purified by flash chromatography (hexanes/AcOEt 7:3) to afford 26 (1.95 g, 8.46 mmol, 98%) as a colorless oil. Spectral data (1H NMR and 13C NMR) are in accordance with those reported in the literature.46
tert-Butyl(((4E,6E)-7-((2R)-6-isopropoxy-3,6-dihydro-2H-pyran-2-yl)hepta-4,6-dien-1-yl)oxy)dimethylsilane, (5R)-29
To a solution of 26 (1.00 g, 4.34 mmol) in anhyd MeCN (14 mL) was added 2,6-lutidine (1.0 mL, 8.9 mmol), Ph3P (2.28 mg, 8.69 mmol) and CBr4 (2.88 g, 8.68 mmol), respectively, at 0 °C. The resulting solution was stirred for 10 min and the reaction was quenched with H2O (50 mL) and extracted with Et2O (3 × 100 mL). The combined organic phases were washed with brine (50 mL), dried over MgSO4, filtered, and concentrated under reduced pressure. The crude product was purified by flash chromatography (gradient elution, 0 to 5% of AcOEt in hexanes) to give (E)-((6-bromohex-4-en-1-yl)oxy)(tert-butyl)dimethylsilane (27) (1.16 g, 3.97 mmol, 91%) as a colorless oil. Rf: 0.64 (hexanes/AcOEt 95:5). IR (ATR) νmax/cm−1: 2929, 2857, 1472, 1255, 1104, 835, 775. 1H NMR (250 MHz, CDCl3) δ: 0.04 (s, 6H), 0.89 (s, 9H), 1.54–1.65 (m, 2H), 2.04–2.17 (m, 2H), 3.60 (t, J = 6.3 Hz, 2H), 3.94 (d, J = 6.6 Hz, 2H), 5.63–5.85 (m, 2H). 13C NMR (63 MHz, CDCl3) δ: - 5.3 (2 C), 18.3, 25.9 (3 C), 28.4, 31.9, 33.5, 62.3, 126.6, 136.1. We were unable to obtain HRMS data of 27 using ESI-TOF and APCI mass spectrometry techniques.
To a solution of 27 (912 mg, 3.12 mmol) in anhyd MeCN (17.2 mL) was added n-Bu3P (1.15 mL, 4.66 mmol) and the mixture was stirred at rt for 12 h. After that, the excess of solvent was removed under reduced pressure and the crude product was maintained under high vacuum (0.2 mmHg) for 8 h. The tri-n-butylphosphonium salt 28 was employed in the Wittig reaction without purification.
Wittig Olefination
To a solution of freshly prepared aldehyde (5R)-7 (354 mg, 2.08 mmol) and tri-n-butylphosphonium salt 28 (3.12 mmol) in anhyd PhMe (38 mL) was added dropwise a solution of NHMDS (4.2 mL, 4.2 mmol, 1 M in THF) at − 78 °C and under N2. The mixture was stirred for 13 h at − 78 °C and for 1 h at rt. The reaction was quenched with H2O (50 mL) and extracted with AcOEt (100 mL and 2 × 50 mL). The combined organic phases were washed with brine (50 mL), dried over MgSO4 and the solvent was removed under reduced pressure. The crude product was purified by flash chromatography (hexanes/AcOEt 95:5) to furnish (5R)-29 (526 mg, 1.43 mmol, 69%) as a colorless oil and as a 6:1 mixture of the two isomers at C-6/C-7 double bond. Rf: 0.50 (hexanes/AcOEt 95:5). IR (ATR) νmax/cm−1: 2955, 2929, 2893, 2857, 1255, 1181, 1100, 1028, 988, 835, 775. 1H NMR (600 MHz, CDCl3) δ: (major isomer) 0.04 (s, 6H, Si(CH3)2), 0.89 (s, 9H, C(CH3)3), 1.17 (d, J = 6.2 Hz, 3H, C(CH3)2), 1.23 (d, J = 6.2 Hz, 3H, C(CH3)2), 1.57–1.63 (m, 2H, H-11), 1.99–2.04 (m, 1H, H-4), 2.07–2.10 (m, 1H, H-4), 2.12–2.16 (m, 2H, H-10), 3.61 (t, J = 6.4 Hz, 1H, H-12), 4.01 (sept, J = 6.2 Hz, 1H, CH(CH3)2), 4.43–4.47 (m, 1H, H-5), 5.11 (s,br, 1H, H-1), 5.62 (dd, J = 15.4 and 6.3 Hz, 1H, H-6), 5.70–5.75 (m, 2H, H-9 and H-2), 5.99–6.01 (m, 1H, H-3), 6.05 (dd, J = 15.4 and 10.6 Hz, 1H, H-8), 6.23 (dd, J = 15.4 and 10.6 Hz, 1H, H-7). 13C NMR (150 MHz, CDCl3) δ: (major isomer) − 5.3 (2 CH3, Si(CH3)2), 18.3 (C, Si-C), 22.0 (CH3, C(CH3)2), 23.9 (CH3, C(CH3)2), 26.0 (3 CH3, C(CH3)3), 28.9 (CH2, C-10), 30.7 (CH2, C-4), 32.3 (CH2, C-11), 62.5 (CH2, C-12), 66.4 (CH, C-5), 69.5 (CH, C(CH3)2), 93.1 (CH, C-1), 126.1 (CH, C-2), 128.5 (CH, C-3), 129.9 (CH, C-8), 130.7 (CH, C-6), 131.3 (CH, C-7), 135.0 (CH, C-9). HRMS (ESI-TOF) m/z: [M − C(CH3)2 + Na]+ Calcd for C18H32O3SiNa 347.20129; Found 347.20134.
(4E,6E)-7-((2R)-6-Isopropoxy-3,6-dihydro-2H-pyran-2-yl)hepta-4,6-dien-1-ol, (5R)-30
To a solution of (5R)-29 (520 mg, 1.42 mmol) in anhyd THF (71 mL) was added a solution of TBAF (1.5 mL, 1.5 mmol, 1 M in THF) at 0 °C and under N2. The yellow solution was allowed to warm to rt and stirred for 5.5 h. The reaction was quenched by the addition of H2O (50 mL) and AcOEt (3 × 100 mL). The combined organic layers were washed with brine (50 mL), dried over MgSO4, filtered and concentrated under reduced pressure. The crude product was purified by flash chromatography (hexanes/AcOEt 7:3) to give (5R)-30 (334.5 mg, 1.326 mmol, 94%) as a colorless oil and as a 6:1 mixture of the two isomers at C-6/C-7 double bond. Rf: 0.30 (hexanes/AcOEt 7:3). IR (ATR) νmax/cm−1: 3409, 2971, 2930, 2887, 1657, 1181, 1099, 1027, 989. 1H NMR (500 MHz, CDCl3) δ: (major isomer) 1.17 (d, J = 6.2 Hz, 3H, C(CH3)2), 1.23 (d, J = 6.2 Hz, 3H, C(CH3)2), 1.64–1.70 (m, 2H, H-11), 1.98–2.04 (m, 1H, H-4), 2.06–2.13 (m, 1H, H-4), 2.16–2.20 (m, 2H, H-10), 3.66 (t, J = 6.4 Hz, 1H, H-12), 4.00 (sept, J = 6.2 Hz, 1H, CH(CH3)2), 4.43–4.47 (m, 1H, H-5), 5.10 (s,br, 1H, H-1), 5.63 (dd, J = 15.4 and 6.3 Hz, 1H, H-6), 5.69–5.75 (m, 2H, H-9 and H-2), 5.99–6.01 (m, 1H, H-3), 6.07 (dd, J = 14.8 and 10.5 Hz, 1H, H-8), 6.23 (dd, J = 15.4 and 10.5 Hz, 1H, H-7). 13C NMR (125 MHz, CDCl3) δ: (major isomer) 22.0 (CH3, C(CH3)2), 23.8 (CH3, C(CH3)2), 28.9 (CH2, C-10), 30.7 (CH2, C-4), 32.1 (CH2, C-11), 62.4 (CH2, C-12), 66.4 (CH, C-5), 69.5 (CH, C(CH3)2), 93.1 (CH, C-1), 126.1 (CH, C-2), 128.4 (CH, C-3), 130.2 (CH, C-8), 131.0 (CH, C-6)*, 131.1 (CH, C-7)*, 134.4 (CH, C-9). *indicates that assignments may be interchanged. HRMS (ESI-TOF) m/z: [M − CH2(CH3)2 + H]+ Calcd for C12H17O3 209.11722; Found 209.11709.
(2R)-6-Isopropoxy-2-((1E,3E,7E)-8-((1S,2S)-2-methylcyclopropyl)octa-1,3,7-trien-1-yl)-3,6-dihydro-2H-pyran, (5R,14S,16S)-31
To a solution of oxalyl chloride (133 μL, 1.54 mmol) in DCM (16 mL) was added DMSO (160 μL, 2.19 mmol) at − 78 °C and under N2. After 15 min, was added dropwise (5R)-30 (240 mg, 0.951 mmol) in DCM (7 mL) and the mixture was stirred for a further 15 min. Et3N (0.7 mL, 4.8 mmol) was added and after 5 min the mixture was allowed to warm to rt. The reaction was quenched by the addition of saturated NH4Cl solution (20 mL). The layers were separated and the aqueous phase was extracted with Et2O (3 × 50 mL). The organic layers were combined, washed with brine (20 mL), dried over MgSO4, filtered and concentrated under reduced pressure. The crude product was obtained as a yellow oil, which was used in the next step without purification. Rf: 0.67 (hexanes/AcOEt 7:3).
To a solution of crude aldehyde obtained in the previous step and sulfone (1S,2S)-22 (390 mg, 1.46 mmol) in anhyd DME (40 mL) was added dropwise a solution of potassium hexamethyldisilazide (1.9 mL, 1.9 mmol, 1 M in THF) at − 78 °C and under N2. The dark brown resulting mixture was stirred for 30 min and the reaction was quenched with brine (100 mL). The mixture was extracted with AcOEt (3 × 100 mL) and the organic layer was washed with brine (50 mL), dried over MgSO4, and concentrated under reduced pressure. The crude product was purified by flash chromatography (hexanes/AcOEt 9:1) to afford (5R,14S,16S)-31 as 2.2:1 E:Z mixture at C-12 double bond (119.3 mg, 0.394 mmol, 41%), contaminated with minor amounts of the 6Z double bond isomers as a light yellow oil. Rf: 0.58 (hexanes/AcOEt 9:1). IR (ATR) νmax/cm−1: 2969, 2924, 1659, 1400, 1380, 1316, 1181, 1099, 1027, 988. 1H NMR (600 MHz, CDCl3) δ: (major isomer) 0.39–0.41 (m, 1H), 0.46–0.49 (m, 1H), 0.66–0.72 (m, 1H), 1.00–1.10 (m, 1H), 1.05 (d, J = 6.0 Hz, 3H), 1.17 (d, J = 6.2 Hz, 3H), 1.23 (d, J = 6.2 Hz, 3H), 1.99–2.28 (m, 6H), 4.01 (sept, J = 6.2 Hz, 1H), 4.43–4.47 (m, 1H), 5.01 (dd, J = 15.3 and 8.6 Hz, 1H), 5.11 (s,br, 1H), 5.44 (dt J = 15.3 and 6.7 Hz, 1H), 5.62 (d, J = 15.3 and 6.2 Hz, 1H), 5.68–5.76 (m, 2H), 5.99–6.11 (m, 2H), 6.23 (dd, J = 15.3 and 10.2 Hz, 1H); (second largest isomer) 0.46–0.49 (m, 2H), 0.76–0.72 (m, 1H), 1.00–1.10 (m, 1H), 1.07 (d, J = 5.9 Hz, 3H), 1.17 (d, J = 6.1 Hz, 3H), 1.23 (d, J = 6.2 Hz, 3H), 1.99–2.28 (m, 6H), 4.01 (sept, J = 6.2 Hz, 1H), 4.43–4.47 (m, 1H), 4.79 (t, J = 10.3 Hz, 1H), 5.11 (s,br, 1H), 5.26 (dt, J = 10.3 and 7.4 Hz, 1H), 5.60–5.65 (m, 1H), 5.68–5.76 (m, 2H), 5.99–6.11 (m, 2H), 6.21–6.27 (m, 1H). 13C NMR (150 MHz, CDCl3) δ: (major isomer) 15.1, 15.5, 18.5, 22.0, 22.4, 23.9, 30.7, 32.2, 32.8, 66.4, 69.5, 93.1, 126.1, 126.6, 128.5, 129.8, 130.8, 131.3, 134.1, 134.9; (second largest isomer) 15.1, 15.5, 18.6, 18.7, 22.0, 23.9, 27.3, 30.7, 32.8, 66.5, 69.5, 93.1, 126.1, 126.5, 128.5, 129.9, 130.8, 131.3, 134.2, 135.0. HRMS (ESI-TOF) m/z: [M + Na]+ Calcd for C20H30O2Na 325.21380; Found 325.21354.
(R)-6-((1E,3E,7E)-8-((1S,2S)-2-Methylcyclopropyl)octa-1,3,7-trien-1-yl)-5,6-dihydro-2H-pyran-2-one, (5R,14S,16S)-2
A mixture of PCC (395 mg, 1.82 mmol) and CaCO3 (730 mg, 7.28 mmol) in anhyd DCM (10 mL) was stirred at rt for 2 h. Then a solution of (5R,14S,16S)-31 (110 mg, 0.364 mmol) in DCM (10 mL) was added by cannula and the reaction mixture was stirred at rt for 2 h. After filtration over silica gel and solvent evaporation under reduced pressure, the crude product was purified by flash chromatography (hexanes/AcOEt 7:3) to afford (5R,14S,16S)-2′ (43 mg, 0.166 mmol, 46%) as a mixture of E:Z isomers at C-12/C-13 double bond and contaminated with minor amounts of the 6Z isomers. This mixture was separated using preparative HPLC with SunFire™ Prep C18 OBD™ column (particle size 5 μm, dimensions 19 mm × 100 mm, 55% MeCN/45% H2O, 17 mL/min) to yield the major isomer with E-configured double bonds (5R,14S,16S)-2 (14.5 mg, tR 18.6 min) as a colorless oil. Rf: 0.64 (hexanes/AcOEt 7:3). [α]D20 + 95 (c 0.1, CHCl3). IR (ATR) νmax/cm−1: 2996, 2925, 1720, 1382, 1244, 991, 961, 816. 1H NMR (500 MHz, CDCl3) δ: 0.38–0.42 (m, 1H, H-15), 0.46–0.49 (m, 1H, H-15), 0.66–0.71 (m, 1H, H-16), 0.99–1.05 (m, 1H, H-14), 1.04 (d, J = 6.2 Hz, 3H, H-17), 2.04–2.08 (m, 2H, H-11), 2.10–2.16 (m, 2H, H-10), 2.43–2.45 (m, 2H, H-4), 4.92–4.96 (m, 1H, H-5), 5.00 (dd, J = 15.2 and 8.6 Hz, 1H, H-13), 5.43 (dt, J = 15.3 and 6.9 Hz, 1H, H-12), 5.64 (dd, J = 15.3 and 6.6 Hz, 1H, H-6), 5.77 (dt, J = 15.2 and 6.6 Hz, 1H, H-9), 6.01–6.05 (m, 2H, H-2, H-8), 6.31 (dd, J = 15.4 and 10.5 Hz, 1H, H-7), 6.85–6.89 (m, 1H, H-3). 13C NMR (125 MHz, CDCl3) δ: 14.7 (CH, C-16), 14.8 (CH2, C-15), 18.5 (CH3, C-17), 22.4 (CH, C-14), 29.8 (CH2, C-4), 32.0 (CH2, C-11), 32.8 (CH2, C-10), 77.9 (CH, C-5), 121.7 (CH, C-2), 126.3 (CH, C-12), 126.5 (CH, C-6), 128.9 (CH, C-8), 133.7 (CH, C-7), 134.3 (CH, C-13), 137.1 (CH, C-9), 144.6 (CH, C-3), 164.0 (CO, C-1). HRMS (ESI-TOF) m/z: [M + Na]+ Calcd for C17H22O2Na 281.15120; Found 281.15065.
Supplementary Material
Acknowledgments
RAP, VMTC and CMA acknowledge FAPESP (research Grants 2009/51602-5, 11/00457-5 to VMT and 2010/08673-6 to CMA) and Institute of Chemistry/UNICAMP for financial and academic support. WHG and MJB acknowledge the Fogarty International Center (FIC) and International Cooperative Biodiversity Group (ICBG) for a grant based in Panama (U01 TW006634) and a FIC International Research Scientist Development Award (IRSDA) (K01 TW008002 to MJB).
The authors would like to thank Dr. Clécio F. Klitzke (LECO Corporation) and Marcos N. Eberlin (Thomson Laboratory) for HRMS analyses.
Footnotes
The authors declare no competing financial interest.
Experimental procedures, full spectroscopic data for new compounds, HPLC chromatograms and CD curves. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Gerwick WH, Moore BS. Chem Biol. 2012;19:85. doi: 10.1016/j.chembiol.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lewy DS, Gauss CM, Soenen DR, Boger DL. Curr Med Chem. 2002;9:2005. doi: 10.2174/0929867023368809. [DOI] [PubMed] [Google Scholar]
- 3.Stampwala SS, Bunge RH, Hurley TR, Willmer NE, Brankiewicz AJ, Steinman CE, Smitka TA, French JC. J Antibiot. 1983;36:1601. doi: 10.7164/antibiotics.36.1601. [DOI] [PubMed] [Google Scholar]
- 4.Tunac JB, Graham BD, Dobson WE. J Antibiot. 1983;36:1595. doi: 10.7164/antibiotics.36.1595. [DOI] [PubMed] [Google Scholar]
- 5.Kalesse M, Christmann M. Synthesis. 2002:981. [Google Scholar]
- 6.Kobayashi M, Higuchi K, Murakami N, Tajima H, Aoki S. Tetrahedron Lett. 1997;38:2859. [Google Scholar]
- 7.Murakami N, Wang W, Aoki M, Tsutsui Y, Higuchi K, Aoki S, Kobayashi M. Tetrahedron Lett. 1997;38:5533. [Google Scholar]
- 8.Maier ME. Nat Prod Rep. 2009;26:1105. doi: 10.1039/b809658a. [DOI] [PubMed] [Google Scholar]
- 9.Nicolaou KC, Snyder SA. Angew Chem Int Ed. 2005;44:1012. doi: 10.1002/anie.200460864. [DOI] [PubMed] [Google Scholar]
- 10.Balunas MJ, Grosso MF, Villa FA, Engene N, McPhail KL, Tidgewell K, Pineda LM, Gerwick L, Spadafora G, Kyle DE, Gerwick WH. Org Lett. 2012;14:3878. doi: 10.1021/ol301607q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerwick WH, Proteau PJ, Nagle DG, Hamel E, Blokhin A, Slate DL. J Org Chem. 1994;59:1243. [Google Scholar]
- 12.Edwards DJ, Marquez BL, Nogle LM, McPhail K, Goeger DE, Roberts MA, Gerwick WH. Chem Biol. 2004;11:817. doi: 10.1016/j.chembiol.2004.03.030. [DOI] [PubMed] [Google Scholar]
- 13.Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Anal Biochem. 1982;126:131. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 14.Barcelos RC, Pastre JC, Caixeta V, Vendramini-Costa DB, De Carvalho JE, Pilli RA. Bioorg Med Chem. 2012;20:3635. doi: 10.1016/j.bmc.2012.03.059. [DOI] [PubMed] [Google Scholar]
- 15.Bruder M, Vendramini-Costa DB, De Carvalho JE, Pilli RA. Bioorg Med Chem. 2013;21:5107. doi: 10.1016/j.bmc.2013.06.044. [DOI] [PubMed] [Google Scholar]
- 16.De Fátima A, Kohn LK, Antonio MA, De Carvalho JE, Pilli RA. Bioorg Med Chem. 2005;13:2927. doi: 10.1016/j.bmc.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 17.De Fátima A, Kohn LK, De Carvalho JE, Pilli RA. Bioorg Med Chem. 2006;14:622. doi: 10.1016/j.bmc.2005.08.036. [DOI] [PubMed] [Google Scholar]
- 18.Vendramini-Costa DB, De Castro IDB, Ruiz AL, Marquissolo C, Pilli RA, De Carvalho JE. Bioorg Med Chem. 2010;18:6742. doi: 10.1016/j.bmc.2010.07.053. [DOI] [PubMed] [Google Scholar]
- 19.Boger DL, Ichikawa S, Zhong W. J Am Chem Soc. 2001;123:4161. doi: 10.1021/ja010195q. [DOI] [PubMed] [Google Scholar]
- 20.Crimmins MT, King BW. J Am Chem Soc. 1998;120:9084. [Google Scholar]
- 21.Phillips DJ, Pillinger KS, Li W, Taylor AE, Graham AE. Chem Commun. 2006:2280. doi: 10.1039/b602329k. [DOI] [PubMed] [Google Scholar]
- 22.Charette AB, Juteau H. J Am Chem Soc. 1994;116:2651. [Google Scholar]
- 23.Although axial orientation of the isopropyl substituent can be assumed based on anomeric effect, we refrained from depict the stereochemistry at C-1 due to the lack of unambiguous evidence as the NOESY spectrum does show a weak correlation between H-1 and H-5.
- 24.Corrêa IR, Jr, Pilli RA. Angew Chem Int Ed. 2003;42:3017. doi: 10.1002/anie.200351347. [DOI] [PubMed] [Google Scholar]
- 25.Charette AB, Lebel H. J Am Chem Soc. 1996;118:10327. [Google Scholar]
- 26.Chiralpak® IA column (particle size 5 μm, dimensions 4.6 mm × 250 mm, 98% hexanes/2% i-PrOH, 1 mL/min). For chromatograms see supporting information.
- 27.Tamura R, Saegusa K, Kakihana M, Oda D. J Org Chem. 1988:53. [Google Scholar]
- 28.Aissa C. Eur J Org Chem. 2009:1831. [Google Scholar]
- 29.Liu P, Jacobsen EN. J Am Chem Soc. 2001;123:10772. doi: 10.1021/ja016893s. [DOI] [PubMed] [Google Scholar]
- 30.Smith AB, Brandt BM. Org Lett. 2001;3:1685. doi: 10.1021/ol0158922. [DOI] [PubMed] [Google Scholar]
- 31.The yield and diastereoisomeric ratio for preparation of (5S,16S,18S)-25 were not optimized because they were obtained before the studies shown in Table 1.
- 32.Li D, Zhao Y, Ye L, Chen C, Zhang J. Synthesis. 2010:3325. [Google Scholar]
- 33.SunFireTM Prep C18 OBDTM column (particle size 5 μm, dimensions 19 mm × 100 mm, 60% MeCN/40% H2O, 17 mL/min).
- 34.Chiralpak® IA (particle size 5 μm, dimensions 4.6 mm × 250 mm, 90% MeCN/10% MeOH, 0.7 mL/min). For chromatograms see supporting information.
- 35.Beecham AF. Tetrahedron. 1972;28:5543. [Google Scholar]
- 36.Nagle DG, Geralds RS, Yoo HD, Gerwick WH, Kim TS, Nambu M, White JD. Tetrahedron Lett. 1995;36:1189. [Google Scholar]
- 37.Gu L, Wang B, Kulkarni A, Geders TW, Grindberg RV, Gerwick L, Hakansson K, Wipf P, Smith JL, Gerwick WH, Sherman DH. Nature. 2009;459:731. doi: 10.1038/nature07870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.SunFireTM Prep C18 OBDTM column (particle size 5 μm, dimensions 19 mm × 100 mm, 55% MeCN/45% H2O, 17 mL/min).
- 39.Guivisdalsky PN, Bittman R. J Org Chem. 1989;54:4637. [Google Scholar]
- 40.Huckins JR, De Vicente J, Rychnovsky SD. Org Lett. 2007;9:4757. doi: 10.1021/ol7020934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stempel GH, Cross RP, Mariella RP. J Am Chem Soc. 1950;72:2299. [Google Scholar]
- 42.Kamal A, Balakrishna M, Reddy PV, Faazil S. Tetrahedron: Asymmetry. 2010;21:2517. [Google Scholar]
- 43.Hansen TV. Tetrahedron: Asymmetry. 2002;13:547. [Google Scholar]
- 44.Fox RJ, Lalic G, Bergman RG. J Am Chem Soc. 2007;129:14144. doi: 10.1021/ja075967i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kelly BD, Lambert TH. Org Lett. 2011;13:740. doi: 10.1021/ol102980t. [DOI] [PubMed] [Google Scholar]
- 46.Bahde RJ, Rychnovsky SD. Org Lett. 2008;10:4017. doi: 10.1021/ol801523r. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.