Abstract
Objective
To examine trends in medical management of men with BPH/LUTS in relation to sentinel events specific to particular medication regimens.
Methods
Using the National Ambulatory Medical Care Survey (1993–2010), we identified outpatient visits by men with BPH/LUTS. We ascertained prescriptions for medical therapy and distinguished between treatment with α-blocker (AB) monotherapy, 5-α reductase inhibitor monotherapy, combination therapy, and anticholinergic therapy. We evaluated temporal trends in prescription patterns, and assessed for changes after sentinel events related to each regimen (e.g., FDA approval for tamsulosin and AB monotherapy). Finally, we used multivariable logistic regression to determine factors associated with each treatment strategy.
Results
From 1993–2010, there were over 101 million outpatient visits for men with a diagnosis of BPH/LUTS. Among these visits, use of BPH medication increased from 14% of visits in 1993–1995 to over 40% of visits in 2008–2010 (p<0.001). After tamsulosin was FDA approved, providers were twice as likely to prescribe ABs (OR 2.35, 95% CI 1.60 – 3.43). Providers were five times as likely to prescribe combination therapy after level 1 evidence supported its use (OR 5.13, 95% CI 3.35 – 7.86).
Conclusions
Over the past 15 years, there has been a steady increase in use of medications to manage men with BPH. Providers seem to have readily adopted novel medications and treatment regimens in response to FDA approval and supportive level 1 evidence.
Keywords: prostatic hyperplasia, drug therapy, cross-sectional studies, ambulatory care
INTRODUCTION
Over the past two decades, lower urinary tract symptoms due to benign prostatic hyperplasia (BPH/LUTS) has transitioned from an acute surgical condition to a chronic medical condition.1 Over this time, a number of events have changed the landscape of medical management of BPH/LUTS, including Food and Drug Administration (FDA) approval of novel medications (e.g., selective alpha-blockers (AB)) and publication of level 1 evidence showing superior efficacy of certain treatment regimens (e.g., the Medical Therapy of Prostatic Symptoms trial (MTOPS)).2
Despite this shift towards medical management, trends of prescribing patterns for BPH/LUTS in the United States have not been well characterized at a population level. In addition, although medical practice for other conditions has been responsive to clinical trial results3, it is unclear whether providers caring for men with BPH/LUTS respond similarly. Furthermore, prescribing patterns for BPH medication regiments in relation to FDA approval of novel medications have not been previously described.
To better understand how sentinel events like FDA approval and randomized clinical trial results are associated with practice patterns for medical management of BPH/LUTS in the United States, we used a population-based, nationally representative survey to characterize medication use at outpatient visits by men diagnosed with BPH/LUTS. From there, we aimed to evaluate if prescribing patterns vary in relation to these major events. An evaluation of the impact of FDA approval and dissemination of level 1 evidence has on practice patterns will be informative for efforts to support multicenter clinical trials and optimize care for men with BPH/LUTS.
MATERIALS AND METHODS
Data Source and Subjects
For this study, we used data from the National Ambulatory Medical Care Survey (NAMCS).4 This survey, conducted by the Centers for Disease Control and Prevention, is an annual three-stage probability sample of outpatient visits to non-federally employed office-based physicians. Weighted estimates from the NAMCS can be extrapolated to outpatient office visits in the United States in total.
On the record form from each sampled visit, up to three physician-coded diagnoses are listed using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) system. In addition, the visit record captures the patient’s stated reasons for the visit. Using this information, we identified 8,313 visits between 1993 and 2010 by men with either (a) a diagnosis of BPH (n = 3363), (b) a complaint of LUTS (n=3004), or (c) both (n=1946). (Appendix 1) We then excluded visits by patients younger than 40 years (n = 616). We also excluded visits associated with a diagnosis of urinary tract infection (UTI) (n = 527) and primary hypertension (n = 312). Finally, we excluded visits to providers who were not primary care providers or urologists (n = 245) for a final cohort of 6,613 visits. (Appendix 1)
Outcomes
The NAMCS captures whether any new or continued medications are prescribed at each sampled visit. Our primary outcomes of interest were prescription of one of four treatment regimens at a visit: (a) AB monotherapy, (b) 5-alpha reductase inhibitor (5ARI) monotherapy, (c) combination therapy (AB+5ARI), and (d) any anticholinergic therapy (AC). We also wanted to assess trends in use of specific drug types. To do so, we created binary indicator variables for specific ABs (doxazosin, terazosin, tamsulosin, alfuzosin, silodosin), 5ARIs (finasteride and dutasteride), ACs (flavoxate, trospium, oxybutynin, oxybutynin extended release, tolterodine, darifenacin, solifenacin, fesoterodine), and one combination pill (dutasteride + tamsulosin). (Appendix 2) We included generic and proprietary names in our search. We categorized ACs as first (oxybutynin, flavoxate), second (tolterodine, oxybutynin XR) or third generation (trospium, darifenacin, solifenacin, fesoterodine).
Sentinel events
We evaluated how specific events were associated with adoption of the four treatment regimens of interest, defined a priori to our analysis. In the absence of randomized trials demonstrating superior efficacy of one AB versus any other, we used the FDA approval date for tamsulosin as the sentinel event tied to AB monotherapy (January 1993 – April 1997 vs. May 1997 – December 2010).5 For 5ARI monotherapy, we assessed prescription prevalence before and after the approval of dutasteride (January 1993 – November 2001 vs. December 2001 – January 2010).6 For combination therapy, we examined patterns before and after the publication of MTOPS trial results in December 2003.2 Finally, the major event we linked to AC use was publication of a well-publicized randomized clinical trial confirming the safety and efficacy of ACs for men with LUTS in November 2006 (i.e., the TIMES study).7
Statistical Analysis
For all analyses, we applied the NAMCS sampling weights, clusters, and stratification to correct estimates and account for complex survey design. In our initial analytic step, we described annual trends in use of specific regimens of BPH medications (i.e., ABs alone, 5-ARIs alone, combination therapy, and ACs alone) and specific medication types among visits by men with BPH/LUTS. Of note, we aggregated individual years to improve statistical reliability for some analyses, which required (a) at least 30 raw visits in the denominator or (b) a standard error less than 30% of weighted estimates.4 The median number of raw visits per year was 348 (interquartile range 296 – 419). Based on this, data was aggregated by either two-year groups (alpha-blockers), three-year groups (overall use and 5-ARIs), or six-year groups (anticholinergics) based on the overall prevalence of each medication class. Chi-square testing was used to assess statistical significance of trends over time. We then used parametric statistics to evaluate for any associations between specific factors from the NAMCS data and BPH medication use. These included factors at the patient level (e.g., age, race, insurance type), practice level (e.g., geographic region, urban location), and provider specialty (i.e., urologist vs. primary care).
Next, we fit multivariable logistic regression models to estimate the association between factors of interest and our four primary outcomes: AB monotherapy, 5ARI monotherapy, combination therapy, and any AC therapy. In the model for each medication type, we included patient and geographic factors, provider specialty, and the respective time-dependent variable as described above.
Statistical analyses were performed with STATA version 11.2, using 2-sided significance testing with type I error rate set at 5%. This analysis, based entirely on publically available de-identified data, was exempt from our Institutional Review Board’s oversight.
RESULTS
From 1993 through 2010, there were an estimated 101 million visits by men over 40 years old with BPH/LUTS (unadjusted n = 6,613). Among these visits, 28% had a prescription for one or more BPH medications. Patients that were older than 75 (32% vs. 22% less than 60 years old, p<0.01), had Medicare/Medicaid insurance coverage (33% vs. 27% private insurance, p<0.01), and treated by urologists (32% vs. 22%, p<0.01) were more likely to receive medical therapy for BPH. Use of medical therapy did not vary significantly based on race, geographic region, or rural/urban status (all p>0.15).
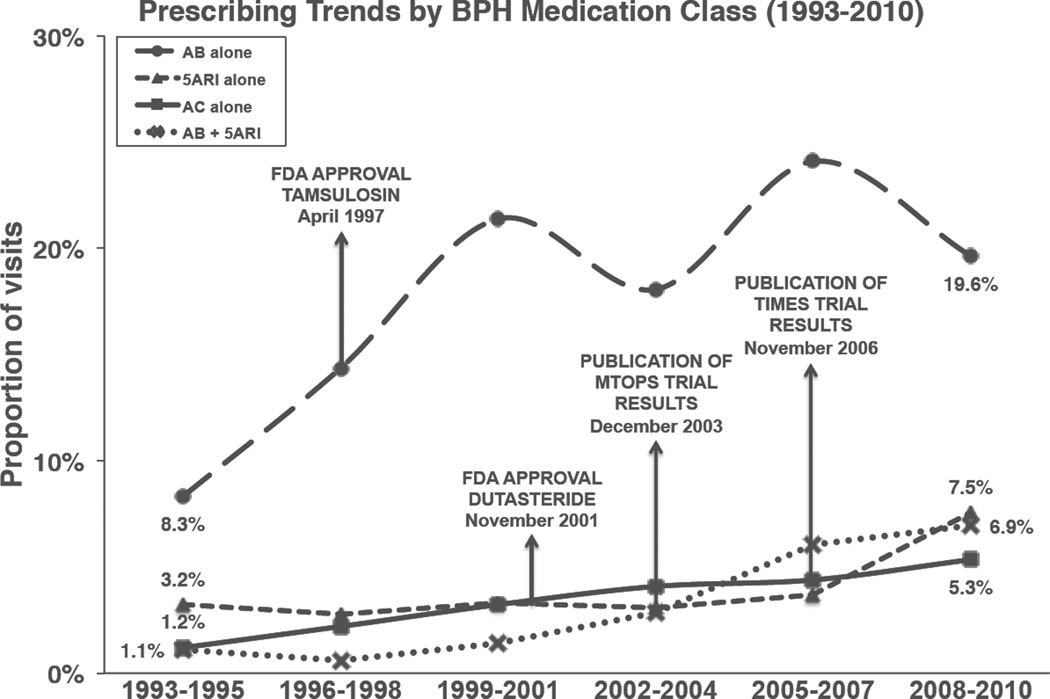
The most common treatment strategy was AB monotherapy, given at 18% of visits overall. The proportion of visits with AB monotherapy increased significantly from 8.3% in 1993–1995 to 19.6% in 2008–2010 (Figure 1, p<0.01). Treatment with other medication regimens was uncommon. However, 5-ARIs monotherapy (from 3.2% to 7.5%), combination therapy (1.1% to 6.9%) and ACs (1.2% to 5.3%) all increased significantly over time (all p<0.01).
Figure 1.
Trends in medical management of BPH/LUTS in the United States (1993–2010) This figure displays trends over time of four different treatment regimens for BPH: (a) AB monotherapy (circle marker, dashed line), (b) 5ARI monotherapy (triangle marker, dashed line), (c) AC therapy (square marker, solid line), and (d) combination therapy with AB and 5ARI (X marker, dotted line). The y-axis displays years, aggregated in groups of three years to ensure statistical reliability. The x-axis displays the proportion of outpatient visits by men with BPH/LUTS where each particular treatment regimen was prescribed.
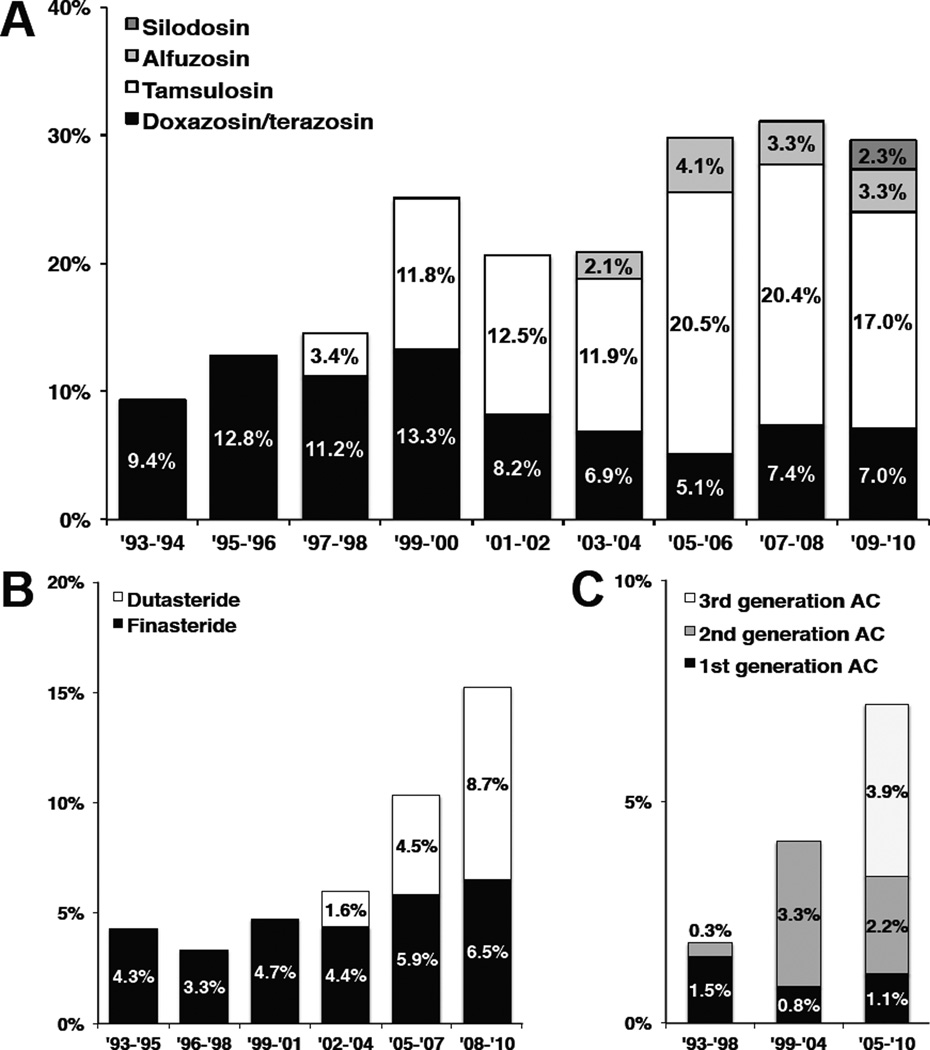
When we looked at individual medication types, we found that use of tamsulosin increased over time, peaking at 69% of AB prescriptions (20.5% overall visits) in 2005–2006, until it accounted for 58% of AB prescriptions in 2009–2010 (17.0% of overall visits) (Figure 2). In addition, the non-selective ABs (i.e., doxazosin and terazosin) still represented 24% of AB medications (7.0% of overall visits) prescribed in 2009–2010. Finally, medications that were more novel represented the majority of prescriptions for 5ARIs (dutasteride 57% in 2008–2010) and ACs (3rd generation 54% in 2005–2010) in contemporary time periods.
Figure 2.
Trends in medication types used for men with BPH/LUTS (1993 – 2010) This figure demonstrates trends in prescriptions for individual drug types in each medication class, including (a) ABs (Figure 2A), (b) 5ARIs (Figure 2B) and (c) ACs (Figure 2C). The y-axis displays the year of visit, aggregated for statistical reliability. The x-axis represents the proportion of visits where each medication type was prescribed. Alpha-blocker types included doxazosin/terazosin (black), tamsulosin (white), alfuzosin (light gray), and silodosin (dark gray). Types of 5ARIs included finasteride (black) and dutasteride (white). Anticholinergic types were grouped based on time period they were developed; these included 1st generation (oxybutynin, flavoxate; black), 2nd generation (tolterodine, oxybutynin XR; dark gray), and 3rd generation ACs (trospium, darifenacin, solifenacin, fesoterodine; light gray).
Estimates from our multivariable models showed variation in use of different treatment regimens based on patient and provider factors are shown in Table 1. Patients who were older than 75 were significantly more likely to be prescribed 5ARI monotherapy (OR 2.68, 95% CI 1.17 – 6.15) and combination therapy (OR 3.68, 95% CI 1.90 – 7.10). Non-white patients were more likely to receive combination therapy (OR 1.87, 1.02 – 3.44). Anticholinergics were more likely to be prescribed to patients with Medicare/Medicaid coverage (OR 1.69, 95% CI 1.23 – 2.32). Though more likely to prescribe BPH medications in general, urologists were not more likely to administer AB or 5ARI monotherapy overall (both p>0.05). We could not generate reliable estimates of odds of combination or AC therapy associated with provider specialty.
Table 1.
Patient and geographic characteristics associated with use of specific BPH medication regimens.
| Covariate | AB monotherapy | 5ARI monotherapy | Combination therapy | Any AC therapy | ||||
|---|---|---|---|---|---|---|---|---|
| % visits |
Multivariable OR (95% CI) |
% visits |
Multivariable OR (95% CI) |
% visits |
Multivariable OR (95% CI) |
% visits |
Multivariable OR (95% CI) |
|
| Age | ||||||||
| < 60 years | 16.4% | 1.00 (N/A) | 2.3% | 1.00 (N/A) | 1.2% | 1.00 (N/A) | 2.8% | 1.00 (N/A) |
| 60 – 75 years | 18.9% | 1.17 (0.89 – 1.54) | 4.4% | 2.10 (0.97 – 4.53) | 3.4% | 2.95 (1.60 – 5.46) | 4.5% | 1.02 (0.64 – 1.62) |
| 75+ years | 19.1% | 1.18 (0.86 – 1.61) | 4.9% | 2.68 (1.17 – 6.15) | 4.2% | 3.68 (1.90 – 7.10) | 5.3% | 1.10 (0.67 – 1.80) |
| Race | ||||||||
| White | 18.2% | 1.00 (N/A) | 4.0% | 1.00 (N/A) | 2.8% | 1.00 (N/A) | 4.3% | 1.00 (N/A) |
| Non-white | 19.5% | 1.13 (0.80 – 1.60) | 3.9% | 1.17 (0.58 – 2.38) | 4.9% | 1.87 (1.02 – 3.44) | 3.8% | 0.82 (0.51 – 1.32) |
| Insurance | ||||||||
| Private | 18.3% | 1.00 (N/A) | 4.2% | 1.00 (N/A) | 2.6% | 1.00 (N/A) | 3.0% | 1.00 (N/A) |
| Medicare or Medicaid | 19.7% | 0.96 (0.76 – 1.21) | 4.4% | 0.68 (0.45 – 1.04) | 4.0% | 0.81 (0.53 – 1.24) | 5.8% | 1.69 (1.23 – 2.32) |
| Other a | 16.2% | 1.02 (0.67 – 1.56) | 2.0% | 0.47 (0.20 – 1.10) | 1.0% | 0.55 (0.21 – 1.45) | 1.5% | 0.60 (0.27 – 1.35) |
| Region | ||||||||
| South | 17.9% | 1.00 (N/A) | 3.6% | 1.00 (N/A) | 3.2% | 1.00 (N/A) | 4.9% | 1.00 (N/A) |
| Northeast | 20.0% | 1.16 (0.89 – 1.52) | 5.2% | 1.60 (0.99 – 2.59) | 4.0% | 1.51 (0.98 – 2.32) | 3.8% | 0.81 (0.50 – 1.31) |
| Midwest | 17.9% | 1.07 (0.81 – 1.42) | 4.3% | 1.24 (0.79 – 1.96) | 2.2% | 0.88 (0.51 – 1.51) | 4.4% | 0.88 (0.57 – 1.35) |
| West | 17.6% | 1.04 (0.80 – 1.33) | 3.0% | 0.87 (0.51 – 1.48) | 2.8% | 1.04 (0.59 – 1.81) | 3.5% | 0.91 (0.57 – 1.46) |
| MSA residence | ||||||||
| Outside MSA | 17.7% | 1.00 (N/A) | 5.0% | 1.00 (N/A) | 3.2% | 1.00 (N/A) | 4.3% | 1.00 (N/A) |
| Within MSA | 18.4% | 1.07 (0.81 – 1.33) | 3.8% | 1.50 (0.94 – 2.39) | 3.0% | 1.35 (0.88 – 2.06) | 4.2% | 0.98 (0.64 – 1.48) |
| Provider specialty | ||||||||
| PCP | 16.0% | 1.00 (N/A) | 3.1% | 1.00 (N/A) | 1.3% | 1.00 (N/A) | 1.9% | 1.00 (N/A) |
| Urologist | 19.5% | 1.24 (0.96 – 1.61) | 4.4% | 1.18 (0.72 – 1.94) | 3.9% | 2.58 (1.18 – 5.66) | 5.5% | 3.68 (1.88 – 7.21) |
Includes worker’s compensation, self-pay, no charge visits, and unknown. Bolded values are statistically significant with p<0.05. Italicized values are not statistically reliable due to standard error > 30% of estimate.
Table 2 demonstrates use of individual BPH treatment regimens in relation to sentinel events of interest. Patients were twice as likely to be prescribed AB monotherapy after tamsulosin was approved (OR 2.35, 95% CI 1.60 – 3.43) and 50% more likely to be prescribed 5ARI monotherapy after dutasteride was approved (OR 1.52, 95% CI 1.03 – 2.27). We noted that men were over five times as likely to receive combination therapy for their BPH after MTOPS was published (OR 5.13, 95% CI 3.35 – 7.86). We also noted increased odds of AC prescriptions after the TIMES trial (OR 1.93, 95% CI 1.40 – 2.65).
Table 2.
Use of BPH treatment regimens in relation to FDA approval of new medications or publication of supportive level 1 evidence
| Treatment regimen |
Sentinel Event | Time Period | % visits by men with BPH/LUTS |
Multivariable OR* (95% CI) |
|---|---|---|---|---|
| AB monotherapy | FDA approval of tamsulosin (April 1997) | Pre-tamsulosin approval | 10.2% | 1.00 (N/A) |
| Post-tamsulosin approval | 20.7% | 2.35 (1.60 – 3.43) | ||
| 5ARI monotherapy | FDA approval of dutasteride (November 2001) | Pre-dutasteride approval | 3.1% | 1.00 (N/A) |
| Post-dutasteride approval | 5.0% | 1.52 (1.03 – 2.27) | ||
| Combination therapy (AB + 5ARI) | Publication of MTOPS study (December 2003) | Pre-MTOPS publication | 1.3% | 1.00 (N/A) |
| Post-MTOPS publication | 6.6% | 5.13 (3.35 – 7.86) | ||
| Any AC therapy | Publication of TIMES study (November 2006) | Pre-TIMES publication | 3.5% | 1.00 (N/A) |
| Post-TIMES publication | 7.5% | 1.93 (1.40 – 2.65) |
Model includes respective time variable, patient age, patient race, patient insurance, geographic region, MSA status, and provider specialty
DISCUSSION
Our findings provide the first long term look at trends in medical management of men with BPH/LUTS using population-based data from the United States. Historically, transurethral resection of the prostate was the only reliable means of management of symptomatic or obstructive BPH.1 Trends in surgical management of BPH eventually showed a rapid drop-off after ABs were introduced to the market1, stabilized for a short period (in the setting of novel laser technology)8,9, and then decreased again after 2005.8 Over this same period, we found that prescriptions for BPH medications have been increasingly more common over time, rising from 14% in 1993–1995 to nearly 40% of visits in 2008–2010. We also noted a significant increase in the odds of prescriptions of novel medications after FDA approval (i.e. AB and 5ARI monotherapy), and new treatment regimens after positive clinical trial findings (i.e., AB-5ARI combination and AC therapies).
In the field of urology, there are multiple examples of underutilization of treatments supported by level 1 evidence, including the use of perioperative mitomycin after bladder resection for patients with non-invasive bladder cancer10–12 and use of neoadjuvant chemotherapy prior to cystectomy for muscle-invasive bladder cancer.13,14 Perhaps our most important finding was that combination therapy with alpha-blockers and 5-alpha reductase inhibitors was over five times as likely to be prescribed at visits for BPH/LUTS following the publications from the MTOPS trial. Though finasteride had been available since 1992, MTOPS demonstrated that combination therapy with finasteride plus doxazosin was more effective than either agent alone, and could delay progression of disease among men with BPH.2 These findings were confirmed by the results from another placebo-controlled trial assessing the efficacy tamsulosin combined with dutasteride published in 2011.15 Furthermore, we identified increased use of AC therapy after publication of the TIMES trial (albeit of a smaller magnitude compared to the increased use of AB+5ARI therapy). A similar response of practice patterns to clinical trial results has previously been shown for prescribing patterns of cardiovascular medications after myocardial infarction3 and statins for coronary heart disease prevention.16 We are encouraged by our similar findings of increased adoption of combination and AC therapy for men with BPH/LUTS after level 1 evidence became available.
In addition to clinical trial data supporting different treatment regimens for men with BPH, the introduction of advanced, novel medications provides an opportunity for adoption of therapies with potentially improved side effect profiles and efficacy. The availability of tamsulosin was a major breakthrough for medical management of men with BPH/LUTS, due to comparable efficacy, improved side effect profile, and more optimal dosing compared to existing ABs.17 Along those lines, we found that AB monotherapy regimens were markedly more common after the tamsulosin was introduced to the market. However, the other AB types that were subsequently approved after tamsulosin represented a minority of overall AB prescriptions. There are scant reports of randomized clinical trials comparing different specific alpha-blocker types head-to-head, with no differences reported between different AB types.18,19 Our finding that newer medications were not rapidly adopted suggests there may only be marginal clinical improvements with these drugs, compared to tamsulosin. On the other hand, the fact that nearly 25% of AB prescriptions in 2008–2010 were for non-selective doxazosin and terazosin highlights the existence of barriers to adoption of more novel alpha-blockers. We also noted that dutasteride was rapidly adopted to become the most common 5ARI in 2008–2010, despite an absence of improved efficacy or side effect profile compared to finasteride.20 This finding highlights that there are other factors involved in the adoption of “me too” medications, including pharmaceutical marketing tactics directed at providers.21–23 Along these lines, the FDA also approved direct-to-consumer marketing in 1997, which eventually resulted in nearly $30 billion worth of marketing for novel medications in 2005.24 We would be remiss not to acknowledge the impact that these advertising campaigns have had on adoption of newer pharmaceutical agents, particularly during the timeframe of our analysis. To address these issues, some have stressed the importance of “pragmatic clinical trials” focused on post-regulatory comparative effectiveness assessments, in order to help guide treatment selection of medications with similar efficacy and side effect profiles.25
It is important to view our findings in the context of certain limitations of the dataset and analysis. First, we are unable to assess a number of confounding factors that are integral to the shared decision to prescribe therapy for men with BPH/LUTS. These include severity of disease and LUTS, prostate size, prior medical and surgical treatments of BPH, and patient and provider preferences, among others. Second, because the analysis is at the visit-level, patient level outcomes cannot be tracked longitudinally, especially in terms of adherence to particular therapies. Third, the observational nature of the data prevents us from making any firm causal links between outcomes of interest and their respective sentinel events. Finally, the nature of the dataset required aggregation of multiple years of data for some analyses, and prevented us from assessing secular trends in fine detail.
Despite these limitations, our population-based analysis with a complex survey design is generalizable to the outpatient experience in the United States, writ large. We feel that the greatest clinical implication of this study is our observation that level 1 evidence from multicenter, randomized clinical trials can be associated with positive trends in urologic practice patterns. Though there continue to be examples of delayed adoption of proven treatments for certain urologic conditions, our findings should encourage policymakers and funding agencies to continue to support multicenter clinical trials in order to optimize urologic care for men with BPH/LUTS. Furthermore, the fact that other medications (i.e., dutasteride) were rapidly adopted after their introduction, without significant literature supporting improved outcomes for men with BPH, sheds light on the importance of continued comparative effectiveness research to guide coverage decisions for the multitude of medical therapies available for men with BPH/LUTS.
Supplementary Material
Acknowledgments
This research was supported by the National Institutes of Health Clinical Training in Urology grant for CPF. (T32DK007782).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Holtgrewe HL. Current trends in management of men with lower urinary tract symptoms and benign prostatic hyperplasia. Urology. 1998;51:1–7. doi: 10.1016/s0090-4295(98)00048-x. [DOI] [PubMed] [Google Scholar]
- 2.McConnell JD, Roehrborn CG, Bautista OM, et al. The Long-Term Effect of Doxazosin, Finasteride, and Combination Therapy on the Clinical Progression of Benign Prostatic Hyperplasia. N Engl J Med. 2003;349:2387–2398. doi: 10.1056/NEJMoa030656. [DOI] [PubMed] [Google Scholar]
- 3.Lamas GA, Pfeffer MA, Hamm P, et al. Do the Results of Randomized Clinical Trials of Cardiovascular Drugs Influence Medical Practice? N Engl J Med. 1992;327:241–247. doi: 10.1056/NEJM199207233270405. [DOI] [PubMed] [Google Scholar]
- 4.National Ambulatory Medical Care Survey. National Center for Health Statistics. [Accessed June 2013]; Available at: http://www.cdc.gov/nchs/ahcd.htm.
- 5.Approval Letter: Flomax. Center for Drug Evaluation and Research. [Accessed May 31 2013]; Available at: http://www.accessdata.fda.gov/drugsatfda_docs/nda/pre96/020579_s000.pdf.
- 6.Approval Letter: Avodart. Center for Drug Evaluation and Research. [accessed May 31 2013]; Available at: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2001/21319_Duagen_approv.PDF.
- 7.Kaplan SA, Roehrborn CG, Rovner ES, et al. Tolterodine and Tamsulosin for Treatment of Men With Lower Urinary Tract Symptoms and Overactive Bladder: A Randomized Controlled Trial. JAMA. 2006;296:2319–2328. doi: 10.1001/jama.296.19.2319. [DOI] [PubMed] [Google Scholar]
- 8.Malaeb BS, Yu X, McBean AM, et al. National Trends in Surgical Therapy for Benign Prostatic Hyperplasia in the United States (2000-2008) Urology. 2012 doi: 10.1016/j.urology.2011.11.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schroeck FR, Hollingsworth JM, Kaufman SR, et al. Population Based Trends in the Surgical Treatment of Benign Prostatic Hyperplasia. The Journal of Urology. 2012;188:1837–1841. doi: 10.1016/j.juro.2012.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sylvester RJ, Oosterlinck W, van der Meijden APM. A single immediate postoperative instillation of chemotherapy decreases the risk of recurrence in patients with stage Ta-T1 bladder cancer: a meta-analysis of published results of randomized clinical trials. J Urol. 2004;171:2186–2190. doi: 10.1097/01.ju.0000125486.92260.b2. [DOI] [PubMed] [Google Scholar]
- 11.Cookson MS, Chang SS, Oefelein MG, et al. National Practice Patterns for Immediate Postoperative Instillation of Chemotherapy in Nonmuscle Invasive Bladder Cancer. J Urol. 2012;187:1571–1576. doi: 10.1016/j.juro.2011.12.056. [DOI] [PubMed] [Google Scholar]
- 12.Chamie K, Saigal CS, Lai J, et al. Compliance with guidelines for patients with bladder cancer - Chamie. Cancer. 2011;117:5392–5401. doi: 10.1002/cncr.26198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vale CL. Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data: Advanced Bladder Cancer (ABC) Meta-Analysis Collaboration. Eur Urol. 2005;48:202–206. doi: 10.1016/j.eururo.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 14.Raj GV, Karavadia S, Schlomer B, et al. Contemporary use of perioperative cisplatin-based chemotherapy in patients with muscle-invasive bladder cancer. Cancer. 2010;117:276–282. doi: 10.1002/cncr.25429. [DOI] [PubMed] [Google Scholar]
- 15.Montorsi F, Roehrborn C, Penit JG. The effects of dutasteride or tamsulosin alone and in combination on storage and voiding symptoms in men with lower urinary tract symptoms (LUTS) and benign prostatic hyperplasia (BPH): 4-year data from the Combination of Avodart and Tamsulosin (CombAT) study. BJU Int. 2011;107:1426–1431. doi: 10.1111/j.1464-410X.2011.10129.x. [DOI] [PubMed] [Google Scholar]
- 16.Mamdani MM, Tu JV. Did the major clinical trials of statins affect prescribing behaviour? Can Med J Assoc. 2001;164:1695–1696. [PMC free article] [PubMed] [Google Scholar]
- 17.Wilt TJ, MacDonald R, Nelson D. Tamsulosin for treating lower urinary tract symptoms compatible with benign prostatic obstruction: a systematic review of efficacy and adverse effects. J Urol. 2002;167:177–183. [PubMed] [Google Scholar]
- 18.Tsujii T. Comparison of prazosin, terazosin and tamsulosin in the treatment of symptomatic benign prostatic hyperplasia: A short-term open, randomized multicenter study. Int J Urol. 2000;7:199–205. doi: 10.1046/j.1442-2042.2000.00175.x. [DOI] [PubMed] [Google Scholar]
- 19.Chapple CR. A Comparison of Varying α-Blockers and Other Pharmacotherapy Options for Lower Urinary Tract Symptoms. Reviews in Urology. 2005;7:S22–S30. [PMC free article] [PubMed] [Google Scholar]
- 20.Nickel JC, Gilling P, Tammela TL, et al. Comparison of dutasteride and finasteride for treating benign prostatic hyperplasia: the Enlarged Prostate International Comparator Study (EPICS) BJU Int. 2011;108:388–394. doi: 10.1111/j.1464-410X.2011.10195.x. [DOI] [PubMed] [Google Scholar]
- 21.Kessler DA, Rose JL, Temple RJ, et al. Therapeutic-class wars--drug promotion in a competitive marketplace. N Engl J Med. 1994;331:1350–1353. doi: 10.1056/NEJM199411173312007. [DOI] [PubMed] [Google Scholar]
- 22.Wazana A. Physicians and the pharmaceutical industry. JAMA. 2000;283:373–380. doi: 10.1016/s0002-9394(00)00678-4. [DOI] [PubMed] [Google Scholar]
- 23.Guldal D, Semin S. The influences of drug companies' advertising programs on physicians. Int J of Health Svc. 2000;30:585–596. doi: 10.2190/GYW9-XUMQ-M3K2-T31C. [DOI] [PubMed] [Google Scholar]
- 24.Donohue JM, Cevasco M, Rosenthal MB. A Decade of Direct-to-Consumer Advertising of Prescription Drugs. N Engl J Med. 2007;357:673–681. doi: 10.1056/NEJMsa070502. [DOI] [PubMed] [Google Scholar]
- 25.Ware JH, Hamel MB. Pragmatic trials-guides to better patient care. N Engl J Med. 2011 doi: 10.1056/NEJMp1103502. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.