Abstract
The amygdala is widely recognized to play a central role in emotional processing. In nonhuman primates, the amygdala appears to be critical for generating appropriate behavioral responses in emotionally salient contexts. One common finding is that macaque monkeys that receive amygdala lesions as adults are behaviorally uninhibited in the presence of potentially dangerous objects. While control animals avoid these objects, amygdala-lesioned animals readily interact with them. Despite a large literature documenting the role of the amygdala in emotional processing in adult rhesus macaques, little research has assessed the role of the amygdala across the macaque neurodevelopmental trajectory. We assessed the behavioral responses of three-year-old (juvenile) rhesus macaques that received bilateral ibotenic acid lesions of the amygdala or hippocampus at two weeks of age. Animals were presented with salient objects known to produce robust fear-related responses in macaques (e.g., snakes and reptile-like objects), mammal-like objects that included animal-like features (e.g., eyes and mouths) but not reptile-like features (e.g., scales), and non-animal objects. The visual complexity of objects was scaled to vary the objects' salience. In contrast to control and hippocampus-lesioned animals, amygdale-lesioned animals were uninhibited in the presence of potentially dangerous objects. They readily retrieved food rewards placed near these objects and physically explored the objects. Furthermore, while control and hippocampus-lesioned animals differentiated between levels of object complexity, amygdala-lesioned animals did not. Taken together, these findings suggest that early damage to the amygdala, like damage during adulthood, permanently compromises emotional processing.
Keywords: amygdala, hippocampus, responsiveness, emotion, nonhuman primate, Macaca mulatta, Rhesus macaque
1. Introduction
The amygdala has long been recognized as important for the perception, generation and regulation of emotion. Research in both humans and nonhuman animals attests to the amygdala's broad role in emotion (for reviews: LeDoux, 2001; Phelps, 2006; Murray, 2007; Seymour & Dolan, 2008; Pessoa & Adolphs, 2010; Price & Drevets, 2010; Salzman & Fusi, 2010) in adult nonhuman primates, the amygdala appears critical for learning the emotional significance of new stimuli (e.g., Antoniadis, Winslow, Davis & Amaral, 2007; 2009), and for generating context-appropriate emotion-related behaviors during social interactions (e.g., Kling & Brothers, 1992; Emery et al., 2001; Machado & Bachevalier, 2006) and in the presence of provocative objects (e.g., Stefanaci, Clark & Zola, 2003, Mason et al., 2006; Machado et al., 2009). Despite a large body of research documenting the amygdala's role in emotion in mature animals, many questions remain about its role in emotional development. To investigate such questions, we have followed the emotional and social development of a cohort of rhesus macaque monkeys that includes animals that received neurotoxic lesions to the amygdala at two weeks of age, age-matched sham-operated control animals, and age-matched animals that received neurotoxic lesions to the hippocampus at two weeks of age. In the present experiment, we investigated the responsiveness of these animals to salient objects when the animals were juveniles (36 months of age; with sexual maturity occurring between 42 and 48 months of age and a life span of nearly three decades; Rowe, 1996).
A consistent finding in adult monkeys with amygdala lesions is that they are uninhibited in the presence of emotionally provocative or potentially dangerous objects (e.g., Aggleton & Passingham, 1981; Zola-Morgan et al., 1991; Meunier, et al., 1999; Stefanaci, Clark & Zola, 2003; Izquierdo, Suda & Murray, 2005; Mason et al., 2006; Machado et al., 2009; Chudasama, Izquierdo & Murray, 2009). A variety of objects are typically used in such testing to engender threat-based responding (the most common object used is a snake - usually a “life-like” toy); monkeys respond robustly to snakes even in the absence of prior experience with them (Nelson, Shelton & Kalin, 2003) leading many to believe that “fear” of snakes is biologically prepotent (Isbell, 2010; Öhman & Mineka, 2001). Researchers have long capitalized on this robust responding to explore the role of the amygdala and other neural structures in the generation of appropriate threat-based responses. Neurologically intact animals avoid emotionally provocative objects and refuse to take a desired food reward placed near such objects (Nelson, et al. 2003), but amygdala-lesioned animals readily interact with the same objects and retrieve proximate food rewards (Aggleton & Passingham, 1981; Mason et al., 2006; Machado et al., 2009). This lack of behavioral inhibition has been as attributed to a reduction or absence of “fear” (e.g., Meunier et al., 1999; Kalin et al., 2001) or defensive behavior in general (e.g., Izquierdo et al., 2005; Chudasama et al., 2009), a lack of “avoidance” (e.g., Machado, Kazama, & Bachevalier, 2009), or an increase in “tameness” (e.g., Zola-Morgan et al., 1991; Mason et al., 2006). Regardless of the mechanism underlying amygdala-lesioned animals' lack of behavioral inhibition, it seems clear that the amygdala is necessary for generating an appropriate behavioral response to emotionally relevant stimuli in mature animals.
Preliminary findings from our laboratory indicated that infant monkeys with bilateral amygdala damage display a similar lack of inhibition when exposed to novel or potentially dangerous objects (Prather et al., 2001). Subsequent studies documented similar effects of amygdala-lesions in infant (9 month old) and young (18 month old) rhesus macaques (Bliss-Moreau, et al., 2010); those animals are the subjects in the present study. Animals that received neurotoxic lesions to the amygdala at two weeks of age were significantly less inhibited in the presence of novel and emotionally provocative objects relative to age-matched control subjects and hippocampus-lesioned subjects. At both ages, control and hippocampus-lesioned subjects avoided objects, while amygdala-lesioned subjects physically touched them. These findings suggest that early damage to the amygdala disrupts normal emotional reactivity.
One of the strengths of this research program is that we can compare the behavior of animals that received amygdala-lesions as neonates not only to neurologically intact controls but also to animals that received hippocampus-lesions as neonates. In addition to serving as “operated controls” (thus allowing us to rule out general effects of the surgical procedures and ibotenic acid), our hippocampus-lesioned subjects allow for investigation of the role of the hippocampus in emotional responding. Little is known about the role of the hippocampus in emotional processing. The few existing studies that have tested emotional responding in hippocampus-lesioned macaques provide conflicting evidence. For example, Zola-Morgan and colleagues (1991) found that adult hippocampus-lesioned cynomolgus macaques (M. facicularis) behaved like control monkeys in the presence of provocative objects insofar as they were more aggressive and fearful of objects than were amygdala-lesioned subjects. In contrast, more recent evidence indicates that in the presence of emotionally provocative objects adult hippocampus-leisoned monkeys, as compared to controls, spent more time in proximity to objects, retrieved food items placed near the objects more quickly and were less defensive and avoidant (Chudasama, Wright, & Murray, 2008). In fact, both hippocampus-lesioned and amygdala-lesioned monkeys showed similarly reduced defensive behavior in the presence of provocative objects (Chudasama, et al., 2009) suggesting that the magnitude of impact of hippocampus damage on emotional reactivity is similar to that of amygdala-damage. When our subjects were tested at 9 months of age, hippocampus-lesioned animals behaved just like controls—they did not physically explore objects (Bliss-Moreau et al., 2010). The pattern of behavior changed at 18 months, however. In the presence of completely novel objects that were robustly provocative (moving objects), hippocampus-lesioned subjects behaved like control subjects insofar as they did not touch objects. In the presence of objects that were similar to those that had been seen before (e.g., a toy snake), hippocampus-lesioned animals touched objects like the amygdala-lesioned animals did. Clearly, further investigation of the role of the hippocampus in emotional processing is warranted.
In the present study, we investigated whether the changes that medial-temporal lobe damage produced on emotional responding persist over time. Animals previously tested at 9 and 18 months were tested for emotional responsivity again at 36 months of age. In order to further investigate our earlier finding that neonatal damage to the amygdala does not result in a failure to differentiate between stimulus salience or complexity (Bliss-Moreau et al., 2010), objects in the present study were presented at three levels of visual complexity. Finally, to investigate whether between-group behavioral variation was related specifically to the type of animal-like object displayed, we included both reptile-like and mammal-like objects.
2. Experimental Procedures
All experimental procedures were developed in consultation with the veterinary staff at the California National Primate Research Center. All protocols were approved by the University of California Davis Institutional Animal Care and Use Committee.
2.1 Animals and Living Conditions
Subject selection and rearing history has been fully described in other publications (Bauman, 2004a, 2004b; Bliss-Moreau et al., 2010). Briefly, subjects were 24 juvenile rhesus macaque monkeys (average age 34 months) that had previously received bilateral ibotenic acid lesions of either the amygdala (five females, three males) or hippocampus (five females, three males), or sham control operations (four females, four males). All surgeries were performed at 12-16 days after birth. The animals were returned to their mothers following surgery and housed in standard home cages (61 cm W × 66 cm D × 81 cm H). Throughout the course of infancy all subjects and their mothers participated in socialization groups. Each socialization group included six subjects (two from each lesion condition) and an adult male. Socialization groups met for three hours, five days a week. An adult female was added to the group when the subjects were weaned and separated from their mothers at six months of age. Beginning at one year of age, all animals were housed 24 hours per day in their socialization groups. At the time of the present experiment, all social groups were housed in large indoor chain-link enclosures (2.13m W × 3.35m D × 2.44m H).
One male amygdala-lesioned animal died at approximately 1 year of age due to unrelated causes (Bauman et al., 2004a) and was subsequently replaced with an alternative age-matched amygdala-lesioned male. The substitute subject was reared with his mother only for the first year of life. At 1 year of age, the animal was weaned and pair housed with an age-matched female until being introduced to his current cohort at approximately 1 year and 3 months of age.
2.2 Surgical Procedures
The surgical procedures summarized below are described in detail in previous publications (Bauman et al., 2004a, 2004b). Briefly, on the day of surgery, each infant was anesthetized with ketamine hydrochloride (15 mg/kg i.m.)i and medatomidine (30μg/kg), and then placed in an MRI-compatible stereotaxic apparatus (Crist Instruments Co., Inc., Damascus, MD). The infant's brain was imaged using a General Electric 1.5 T Gyroscan magnet, 1.0 mm thick sections were taken using a T1-weighted Inversion Recovery Pulse sequence (TR = 21, TE =7.9, NEX 3, FOV = 8cm, Matrix 256 × 256). From these images, we determined the location of the amygdala or hippocampus and calculated the coordinates for the ibotenic acid injections. Infants were ventilated and vital signs monitored throughout the surgery. A stable level of anesthesia was maintained using a combination of isoflurane (1.0% - varied as needed to maintain an adequate level of anesthesia) and intravenous infusion of fentanyl (7-10 μg/kg/hour). Following a midline incision, the skin was laterally displaced to expose the skull, two craniotomies were made over the amygdala or the hippocampus, depending on the pre determined lesion condition, and the dura was reflected to expose the surface of the brain. Ibotenic acid (IBO, Biosearch Technologies Inc., 10 mg/ml in 0.1 M phosphate buffered saline) was injected simultaneously bilaterally into the amygdala or hippocampus using 10 μl Hamilton syringes (26 gauge beveled needles) at a rate of 0.2 μl/min. Amygdala lesions required 7.0 to 12.0 uL of IBO per amygdala (modal number of injections= 8; range=6-8) and hippocampus lesions required 5.5 to 7.0 uL per hippocampus (number of injections=7). Ibotenic acid is a naturally occurring amino acid derived from the mushroom Amanita muscaria. It is a glutamate receptor agonist (Krogsgaard-Larsen, Nielsen, & Curtis, 1984) and a metabolic poison that causes highly localized damage to cell bodies while sparing the axons which pass through the targeted area and the terminals of nerves that do not originate in the targeted area (Schwarcz, Hökflet, Fuxe, et al., 1979).
Sham-operated controls underwent the same pre-surgical preparations, received a midline incision and the skull was exposed. The control animals were maintained under anesthesia for the average duration of the lesion surgeries and the fascia and skin were sutured in two separate layers. Following the surgical procedure, all infants were monitored by a veterinarian and returned to their mothers once they were fully alert.
2.3 Lesion Analysis
The animals are continuing behavioral testing and therefore have not been euthanized. T2-weighted MR images acquired ten days after surgery were used to examine the extent of the edema associated with the lesion. Images were collected using a General Electric 1.5 T Gyroscan magnet; 1.5 mm thick sections were taken using a T2-weighted Inversion Recovery Pulse sequence (TR = 4000, TE = 102, NEX 3, FOV = 8 cm, Matrix, 256 × 256). The hyper-intense T2-weighted signal for each of the sixteen experimental animals (eight amygdala lesion, eight hippocampus lesion) was evaluated to confirm the general target and extent of the lesions (i.e., amygdala lesion sparing the hippocampus or hippocampus lesion sparing the amygdala). T2-weighted images of coronal sections through the mid portion of the amygdala are illustrated in previous publications (Bauman et al., 2004a, 2004b), providing substantial reassurance that the ibotenic acid was injected and was focused in the amygdaloid complex or hippocampal formation. The extent of the targeted lesion was confirmed using histological evaluation in the one amygdala-lesioned animal that died due to an unrelated illness (see Figure 1).
Figure 1.
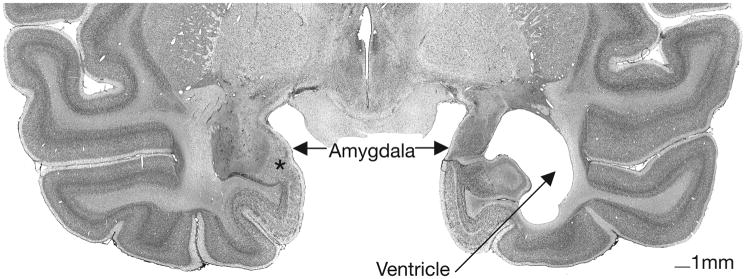
Nissl-stained coronal section through the caudal level in an amygdala-lesioned animals' anterior temporal lobe. Cell loss is nearly complete and the ventricle is expanded on the right side. Cell damage is visible on the left side. Asterisk on left side marks cell sparing in a small portion of the parvicellular division of the basal nucleus.
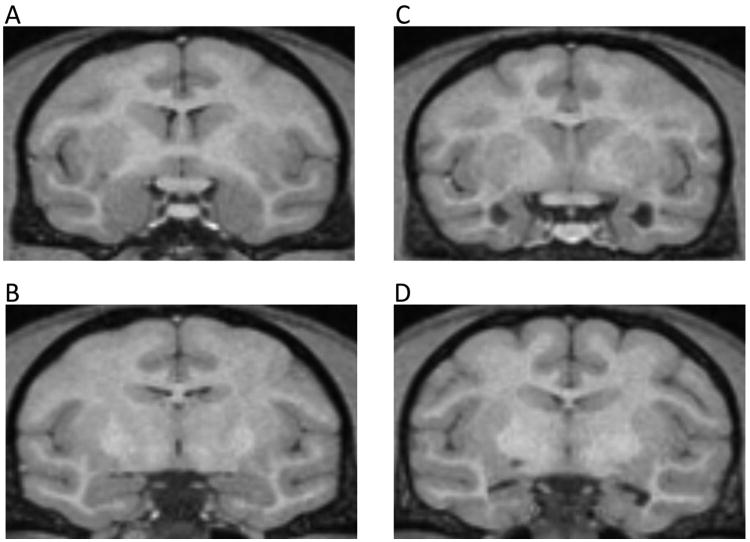
Additionally, lesion extent was further characterized in T1-weighted MRI images when animals were four years of age (shortly after the present experiment; see Table 1) (Machado, Snyder, Cherry, et al., 2008). Example MRI images can be found in Figure 2.
Table 1. Percent Atrophy Resulting From Ibotenic Acid Lesion.
| Amygdala-lesioned Animals | |||
|---|---|---|---|
| Amygdala Atrophy | |||
|
|
|||
| Animal Number | Left | Right | Average |
|
|
|
|
|
| 1 | 67.6 | 70.7 | 69.2 |
| 2 | 69.1 | 74.7 | 71.9 |
| 3 | 81.1 | 80.4 | 80.7 |
| 4 | 67.3 | 71.8 | 69.5 |
| 5 | 73.5 | 61.4 | 67.4 |
| 6 | 72.5 | 74.2 | 73.4 |
| 7 | 76.1 | 73.1 | 71.9 |
|
|
|||
| Hippocampus- lesioned Animals | |||
| Hippocampus Atrophy | |||
|
|
|||
| Animal Number | Left | Right | Average |
|
|
|
|
|
| 1 | 83.3 | 74.0 | 78.6 |
| 2 | 79.0 | 71.4 | 75.2 |
| 3 | 79.9 | 81.9 | 80.9 |
| 4 | 86.0 | 87.7 | 86.8 |
| 5 | 81.7 | 88.3 | 85.0 |
| 6 | 70.6 | 61.4 | 66.0 |
| 7 | 71.8 | 69.2 | 70.5 |
| 8 | 72.2 | 68.1 | 70.2 |
|
|
|||
Note: Data are the percent atrophy of the amygdaloid or hippocampal tissue for each case relative to the mean volume of those structures in the sham-operated controls (n=8). Left: percent tissue reduction in the left hemisphere; Right: percent tissue reduction in the right hemisphere; Average: Average percent reduction across both the left and right hemisphere. Adapted from Machado et al. (2008).
Figure 2.
T1 MRIs illustrating amygdala and hippocampus damage. Images in the left panel (A, B) are from the same control animal at (A) AP +18 and (B) AP +13. Images in the right panel illustrate (C) bilateral amygdala damage at AP +18 and (D) bilateral hippocampus damage at AP +13.
2.4 Behavioral Testing Procedure
2.4.1Testing Apparatus
Animals were tested in a modified lab care cage with which they were familiar (Experiment 2; Bliss-Moreau et al., 2010). The cage had a clear plastic front with two small openings through which animals could reach a solid platform on which the experimental objects were placed. An opaque guillotine door could be raised and lowered in front of the clear window allowing the researchers to shield the objects from view between trials. Two experimenters sat diagonally to the right of the testing cage and a camera was positioned directly in front of the cage.
2.4.2Test Cage Acclimation
Animals were acclimated to the test cage during the five days preceding the object responsiveness task. During each acclimation day, animals were placed individually in the test cage and presented with a single food reward (a mini marshmallow) on the platform. Each 30-second trial began when the opaque guillotine door was lifted and ended when the opaque door was closed. Criterion to move to the task phase was retrieval of the food item eight out of ten times on two consecutive days.
2.4.3 Object Responsiveness Task
Each animal completed nine testing days, with a three-day break in testing between days five and six. Each testing day included five trials. There were two types of trials—trials during which food reward was presented alone (called “food only trials”; trials 1 and 5) and trials during which a food reward was presented in the presence of an object (called “object trials”; trials 2-4). Therefore, there were 45 total trials—18 food only trials and 27 food plus object trials. Food rewards were always presented in the same position—centered on the platform, within arm's reach such that it was directly in front of the object on trials that included an object. Three categories of objects were presented—reptile-like objects, mammal-like objects and non-animal objects. In each category, there were three different specific objects (e.g., the reptile-like object category included a snake, a dinosaur and an alligator), for a total of nine different specific objects. Each object was presented at three levels of complexity.
Animals had previously seen a coiled snake (Bliss-Moreau et al., 2010), that was similar to the one used in this experiment but significantly smaller in size. All other objects were novel to the animals. Additionally, animals had no previous experience with other living mammals (with the exception of their human caretakers and experimenters) or reptiles because they were born, raised and housed indoors.
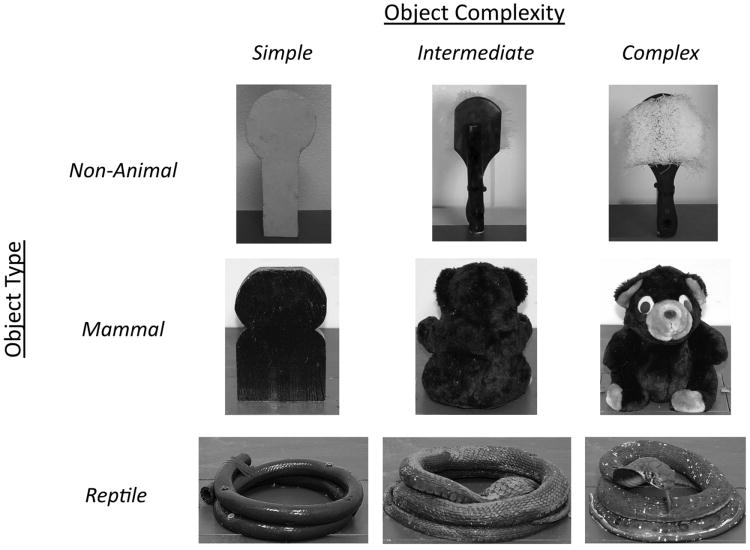
On each testing day, trials 2-4 consisted of the presentation of one object type (e.g., snake, bear, etc.) at three levels of object complexity (simple, medium, and complex) (See Figure 3 for examples of object sets).
Figure 3.
Salient objects. Examples of objects used during testing from each of the three types (non-animal, mammal, reptile) and each of the three levels of visual complexity (simple, intermediate, and complex).
The most complex form of the reptile-like and mammal-like objects were children's toys (alligator, snake, dinosaur, bear, red panda, lion) that were positioned on the platform so that the face of the toy faced the test cage. For the intermediate form of each mammalian object (i.e., bear, red panda, lion) and the intermediate dinosaur object, the objects were presented backwards (facing away from the test cage) so that the color and texture of the toy were visible but the facial features were not. Because the snake and alligator toys were relatively flat and the facial features could be seen even if the object was facing backwards, the intermediate forms were painted a solid color to cover the eye and facial features but not their texture. The head of the snake was positioned face down between the coils. For the complex version of non-animal objects (scrub brush, ice cube tray, book), objects were placed with the most prominent features facing towards the test cage. For the intermediate version of each non-animal object, objects were placed with the prominent features facing away from the test cage. For example, the scrub brush was presented with the bristles projecting towards the animal for the complex presentation form, but presented with the bristles facing away from the animal for the intermediate presentation form. The simplest form of each object was a piece of wood cut to approximate the shape and size of the most complex object and painted a solid color similar to the most prominent color of the corresponding complex object.
Each trial began when the opaque door was lifted and ended 30-seconds later when the opaque door was lowered (ITI = ∼30-seconds). The frequency and timing information (latency and/or duration of behavior) of the primary dependent variables (i.e., retrieval of food reward, and physical contact (exploration) of objects) as well as other species-typical behaviors (Table 2) were recorded using The Observer (Noldus Information Technology; Leesburg, VA).
Table 2. Behavioral Ethogram.
| Behavior | Description |
|---|---|
| Position in Cage | |
| Front | The animal's head or body is positioned in the front half of the testing cage for at least 3 seconds. |
| Rear | The animal's head or body is positioned in the rear half of the test cage for at least 3 seconds. |
| Nonspecific Activity | Animal does not remain in the front or rear of the cage. Default state in which each sample is started. |
| Exploratory Behavior | |
| Manual Exploration | Animal physically touches object. |
| Take Food | Animal takes food reward. |
| Emotion- related Expressions & Vocalizations | |
| Grimace | Exaggerated grin with teeth (scored as directed either to the object or the observer). |
| Lipsmack | Rapid lip movements with pursed or puckered lips (scored as directed either to the object or the observer). |
| Threat | Scored when one or more of these components are present: open mouth stare, head bobbing, ear flaps, bark vocalizations, or lunges (scored as direct either to the object or the observer). |
| Coo | Clear, soft sound, moderate in pitch and intensity; usually sounds like “whoooo.” |
| Scream | High-pitched vocalization with extreme high intensity; usually sounds like “eeeeeee”. |
| Grunt | Deep, muffled, low-intensity vocalization. |
| Bark | Low pitched, sharp, guttural sound. |
| Tooth Grind | Repetitive, audible rubbing of upper and lower teeth. |
| Stereotypies | |
| Backflip | Animal flips backwards at least 2 times in a row. |
| Bounce | Repetitive hopping or bouncing in the same place (must occur more than 3 times in a row to score). |
| Head-twist | Animal twists neck in a dramatic display; often seen when turning at corners and/or in conjunction with pacing. |
| Other Stereotypy | Repetitive motor pattern not described by any of the above definitions. |
| Pace | Repetitive undirected pacing with the same path repeated (must last longer than 3 seconds to score). |
| Salute | Animal covers hand over eye or holds hand over eye. |
| Self-bite | Biting oneself. |
| Self-clasp | Unusual holding of body part or limb with another body part. |
| Spin | Repetitive twirling or spinning for at least 2 rotations |
| Swinging | Repetitive swinging in the same place (must last at least 3 seconds to score). |
| Miscellaneous | |
| Cage Shake | Vigorous shaking of cage bars, or animals slams body against cage. |
| Self-sex | Anogenital exploration of self. |
| Self-groom | Grooming oneself. |
| Yawn | Open mouth, exposing teeth |
| Scratch | Animal scratches own body. |
2.4.4 Data Analysis Strategy
Means for frequency and latency/duration of behavior were computed for each trial type. Data were log10(x+1) transformed in cases where they were not normally distributed (i.e., latency to retrieve food when no object was present, frequency and duration to explore objects). For the purposes of interpretation, raw data (means and variance indices) are presented; log transformed data are available upon request. On trials during which animals did not take the food or explore the objects, missing latency data was replaced with the length of the trial (30 seconds). Data were subjected to ANOVA. Lesion condition was the between subjects factor for all analyses. Object complexity (simple, intermediate, complex) and object type (reptile, mammal, non-animal) were repeated measures. Following ANOVAs, we assessed pair-wise between group differences using a series of Mann-Whitney comparisons on the raw data; p-values associated with the Mann-Whitney tests are presented in parentheses.
3. Results
3.1 Test Cage Acclimation
With the exception of one amygdala-lesioned female, all animals met the criterion to retrieve a single food reward (a mini marshmallow) within 30 seconds on 8 out of 10 trials over two consecutive days. The animal that did not meet the criterion was excluded from subsequent analyses.
3.2 Object Responsiveness Task
We assessed differences in behavior related to lesion group, object type, and object complexity (and their interactions). Significant, meaningful, differences between lesion groups were only found in food retrieval and object exploration behaviors. There were no group differences in any of the other behaviors that were recorded (see Table 2). Therefore, we focus the presentation and interpretation of the results on food retrieval and object exploration only.
3.2.1 Retrieval of Food Reward
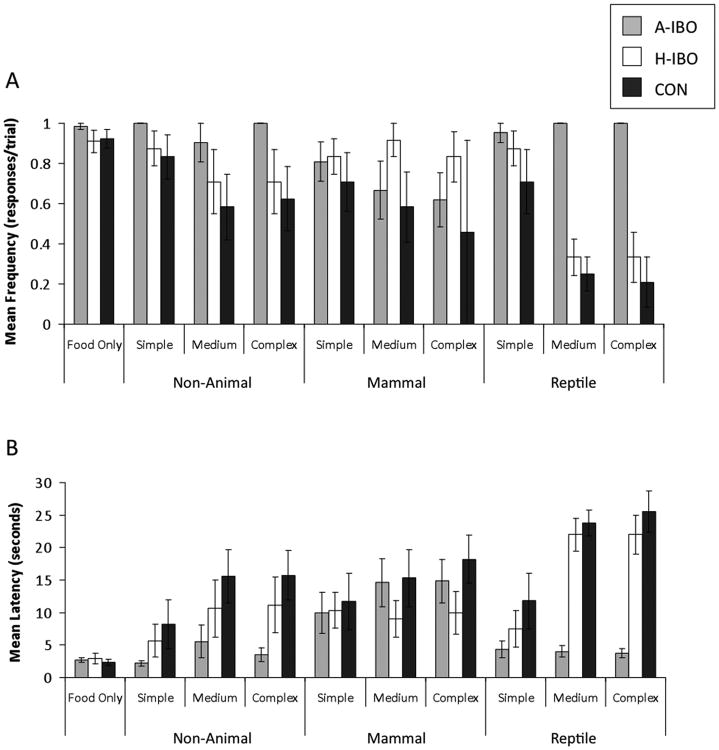
There were no lesion group differences in food retrieval on trials when no object was present—frequency: F(2, 22)=.73, p<.49, ηp2=.068; latency: F(2, 22)=.31, p<.74, ηp2=.030. (Figure 4)
Figure 4.
Food retrieval. a) Mean frequency of food retrieval. b) Mean latency of food retrieval. Means ± SEM. A-IBO: Amygdala-lesioned animals. H-IBO: Hippocampus-lesioned animals. CON: Control.
Overall, the amygdala-lesioned animals tended to retrieve food more frequently and faster than both control and hippocampus-lesioned animals in the presence of all objects—frequency: F(2, 20)=3.097, p<.067, η2=.236; latency: F(2, 20)=2.95, p<.080, ηp2=.228. Between group comparisons indicated that amygdala-lesioned animals retrieved food significantly more frequently and faster than control animals (frequency: p<.016; latency: p<.028). Hippocampus-lesioned animals' food retrieval behavior did not differ significantly from amygdala-lesioned animals, nor from control animals.
Food retrieval behavior differed across object types—frequency: F(2, 40)=5.594, p<.007, ηp2=.217; latency: F(2, 40)=7.56, p<.002, ηp2=.274. Food was retrieved most frequently and fastest in the presence of non-animal objects. The effect of object type differed by lesion condition—frequency: F(4, 40)=6.66, p<.0001, ηp2=.400; latency: F(4, 40)=6.69, p<.0001, ηp2=.401. Evaluation of the marginal means indicated that both hippocampus-lesioned and control animals' rate and speed of retrieval were the same for non-animal and mammal-like objects, but food was retrieved significantly less frequently and slower for reptile-like objects. In contrast, amygdala-lesioned animals retrieved food significantly less and slower on trials with mammal-like objects compared to non-animal and reptile-like objects. Interestingly, there were no lesion group differences when only mammal-like objects were considered—frequency: F(2, 20)=1.414, p<.267, ηp2=.141; latency: F(2, 20)=.665, p<.530, ηp2=.061.
Object complexity influenced food retrieval behavior. In general, animals retrieved food most frequently in the presence of the simple objects and least frequently in the presence of complex objects—frequency: F(2, 40)=25.946, p<.0001, ηp2=.565; latency: F(2, 40)=43.50, p<.0001, ηp2=.685. As with the effect of object type, the effect of object complexity was influenced by lesion condition—frequency: F(2, 40)=3.622, p<.013, ηp2=.266; latency: F(4, 40)=4.85, p<.003, ηp2=.327. Evaluation of the marginal means indicated that amygdala-lesioned animals did not modulate their behavior based on object complexity. In contrast, both hippocampus-lesioned and control animals retrieved food significantly more frequently and significantly faster on simple as compared to medium and complex trials.
Object type and object complexity interacted to influence food retrieval. Food retrieval was most frequent and fastest on trials with simple non-animal objects and least frequent and slowest on trials with medium and complex reptiles—frequency: F(4, 80)=4.055, p<.005, ηp2=.169; latency: F(4, 80)=4.27, p<.003, ηp2=.176. Interestingly, this pattern of effects held only for control and hippocampus-lesioned subjects but not for amygdala-lesioned subjects, indicating that these animals did not respond to differences in the objects' features—frequency: F(8, 80)=3.279, p<.003, ηp2=.247; latency: F(8, 80)=3.90, p<.001, ηp2=.281.
3.2.2 Object Exploration
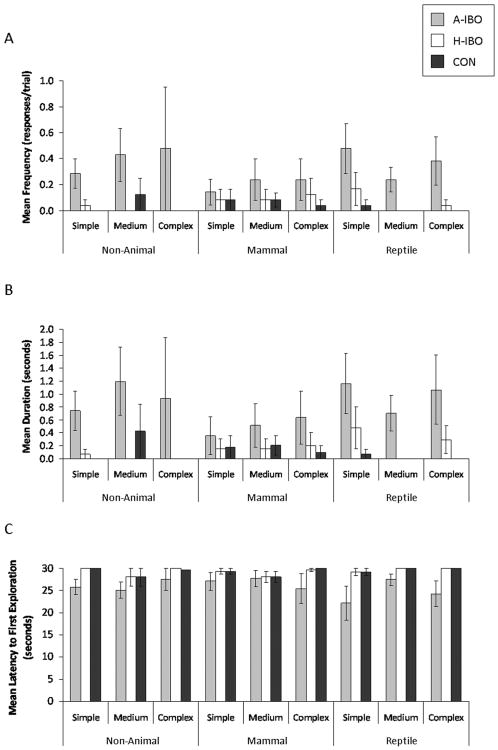
The amygdala-lesioned animals' lack of responsiveness to specific features was also evident in data on exploration, although the frequency with which animals physically explored was low across all trial types (See Figure 5). The amygdala-lesioned animals explored objects most frequently, for longest, and were quickest to first explore—frequency: F(2, 20)=3.98, p<.035, ηp2=.285 (amygdala-lesioned > control, p<.082; amygdala-lesioned > hippocampus-lesioned, p≤.05); duration: F(4, 20)=4.27, p<.029, ηp2=299 (amygdala-lesioned > control, p<.094; amygdala-lesioned > hippocampus-lesioned, p<.067); latency: F(4, 20)=3.07, p<.069, ηp2=.235 (amygdala-lesioned < hippocampus-lesioned, p<.045). No other effects were significant; neither object type, nor object complexity influenced animals' propensity to explore objects.
Figure 5.
Object exploration. a) Mean frequency of exploration. b) Mean duration of exploration. c) Mean latency to first exploration. Means ± SEM. A-IBO: Amygdala-lesioned animals. H-IBO: Hippocampus-lesioned animals. CON: Control.
4. Discussion
Consistent with previous assessments of other monkeys with neonatal amygdala lesions (Prather et al., 2001), with previous assessments of this cohort (Bliss-Moreau et al., 2010) and studies of responsiveness in adult amygdala-lesioned monkeys (e.g., Mason et al., 2006; Machado et al., 2009; etc.), juvenile monkeys that received lesions to the amygdala as neonates showed a lack of species-typical behavioral inhibition in the presence of emotionally provocative objects. Compared to neurologically intact control and hippocampus-lesioned animals, amygdala-lesioned animals had a greater propensity to physically touch objects and more readily retrieved foods presented in proximity to objects.
The amygdala-lesioned animals' pattern of physical exploration has been remarkably stable across time. These same animals were more likely to physically explore objects when tested at 9 and 18 months (Bliss-Moreau et al., 2010). In the present study we also found lesion group effects for physical exploration of objects, though it is notable that the rates and durations of manual exploration were extremely low. This is consistent with findings that neurologically intact rhesus macaques become less likely to physically explore their environments as they mature (Reinhardt, 1990). Despite low frequencies of exploration in the current study, amygdala-lesioned animals still explored more frequently and for longer periods of time than control and hippocampus-lesioned animals.
The amygdala-lesioned animals, as compared to control and hippocampus-lesioned animals, also retrieved a food reward placed near emotionally salient objects both more quickly and most frequently. This finding provided another indication that they were behaviorally uninhibited in the presence of salient objects. These findings are consistent with testing performed with adult animals with amygdala lesions (e.g., Aggleton & Passingham, 1981; Zola-Morgan et al., 1991; Meunier, et al., 1999; Stefanaci et al., 2003; Izquierdo et al. 2005; Mason et al., 2006; Machado et al., 2009; Chudasama et al. 2009).
Another similarity in the patterns of behavior between animals that received damage to the amygdala as neonates (the present subjects) and animals that received damage to the amygdala as adults (Mason et al., 2006) is that they did not modulate their behavior based on object complexity. While control animals' rate and speed of food retrieval decreased as object complexity increased, amygdala-lesioned animals' behavior did not change as object complexity increased. On measures of food retrieval, amygdala-lesioned animals were the fastest and retrieved food most frequently, while control animals were the slowest and retrieved food least frequently. This pattern differs from what was observed when these animals were 9 and 18 months of age (Bliss- Moreau et al., 2010). At those time points, all animals modulated their behavior based on stimulus complexity (Bliss-Moreau et al., 2010).
Perhaps the most unexpected finding in the current study was that the amygdale-lesioned animals were sensitive to the mammal-like objects. They retrieved food more slowly and less frequently when presented with mammalian objects as compared to reptile objects. In contrast, hippocampus-lesioned animals and control animals retrieved food more quickly in the presence of non-animal and mammal-like objects compared to reptile-like objects. As a result, there were no lesion group differences in food retrieval behavior on mammal-like object trials— amygdala-lesioned, control and hippocampus-lesioned animals retrieved food at equal rates and speeds. In other words, some property of the mammal-like objects resulted in the amygdala lesioned animals generating more “normal” behavioral regulation. One possible explanation is that the mammal-like objects engendered “normal” social responding because most resembled conspecifics since they had visible mammal eye-spots (as opposed to small slit eyes of the reptile-like objects), had four legs, and were furry.
Previous research with this cohort indicated that amygdala-lesioned animals' repertoire of social behaviors was largely intact (i.e., they have the capacity to generate a broad repertoire of species-typical behaviors in social contexts) and during the first year of life they actually generated more prosocial behavior towards conspecifics (Bauman, et al., 2004b). Interestingly, during the course of social interactions, the amygdala-lesioned animals produced more fear-related behaviors (e.g., grimacing, screaming) than control or hippocampus-lesioned animals (Bauman, et al., 2004b). Thus, despite the loss of their amygdala, they appeared to be expressing fear in social contexts. The neurobiological substrate for this unusual social fear remains elusive. This history raises the possibility, however, that the lower food retrieval in the presence of mammal-like objects may be due to a residual fear or threat-related processing generated in the presence of conspecifics; at the very least, this interfering processing may delay behaviors directed at retrieving the food. In this view, the amygdala-lesioned animals' respond “normally” only to objects that have similarity with conspecifics. Investigating what specific characteristics of the mammal-like objects elicited more normal behavioral inhibition and the specific regulatory mechanism underlying the inhibition are potentially fruitful avenues for future research. Regardless of the mechanism underlying the amygdala-lesioned animals' responding to mammal-like objects, the paradoxical finding allows us to rule out the possibility that amygdala lesioned animals food retrieval and exploration behavior on the other trial types was driven by global impairments in visual processing or attention which would have also impaired their responding to mammal-like objects.
Notably, hippocampus-lesioned animals in the present study behaved like controls both in their low rates of physical exploration and their food retrieval behavior, suggesting that early damage to the hippocampus does not disrupt normal juvenile emotional responding to salient objects. This pattern of findings is similar to what was observed when these animals were 9 months of age (Bliss-Moreau et al., 2010). Interestingly, behavior observed in this experiment differs from what was observed when the animals were 18 months of age. At 18 months of age, hippocampus-lesioned animals touched, albeit at low rates, objects that were similar to ones they had seen previously (e.g., a toy snake) (Bliss-Moreau et al., 2010) leading us to conclude that their emotional processing was compromised to some degree, although not to the extent that amygdala-lesioned animals' emotional processing was compromised. Investigating whether that compromise maintained, became exacerbated, or resolved across their neural developmental trajectories was one purpose of the present experiment. It is possible that neural reorganization that occurred between the 18 month and 36 month test periods subserved the development of normative emotional responding for the hippocampus-lesioned (but not amygdala-lesioned) animals since their impairments at 18 months of age were relatively mild in magnitude. This is feasible because we know that the hippocampus-lesioned animals' brains, in particular, underwent neural reorganization allowing for intact spatial memory in the absence of hippocampi (Lavenex et al. 2007). Importantly, the present findings with our neonatally hippocampus-lesioned animals stand in contrast to the findings of Chudasama and colleagues (Chudasama et al., 2009 Chudasama et al., 2010) who found that adult animals that sustained hippocampus damage as adults, as compared to controls, retrieved food faster and were less defensive in the presence of provocative objects. It is possible, even plausible, that the our hippocampus-lesioned animals behaved normally as juveniles because their brains were able to undergo neural reorganization in order to accommodate for their deficits because the damage to their hippocampi occurred so early in their neurodevelopmental trajectory. Understanding the specifics patterns of neural reorganization in these animals is a future goal.
In summary, the present study demonstrated that damage to the amygdala, but not the hippocampus, at two weeks of age perturbs emotional behavior observed at three years of age. These findings, in concert with previous assessments of this cohort's emotional responding at 9 and 18 months of age (Bliss-Moreau, et al., 2010), suggest that early damage to the amygdala impedes emotional responding across the developmental trajectory.
Acknowledgments
This research was supported by a grant from the National Institute of Mental Health (R37MH57502) and by the base grant of the California National Primate Research Center (RR00169). This work was also supported through the Early Experience and Brain Development Network of the MacArthur Foundation. We thank the veterinary and husbandry staff of the California National Primate Research Center for excellent care of the animal subjects. We thank Dr. Pierre Lavenex, Jeffrey Bennett and Pamela Tennant for assistance with surgical procedures, Melissa Marcucci for assistance with behavioral data collection, and Dr. Christopher Machado for comments on an earlier draft.
Comprehensive list of abbreviations
- A-IBO
Subjects with amygdala-lesions created with ibotenic acid
- ANOVA
Analysis of variance
- CON
Neurologically intact control subjects
- D
Depth
- FOV
Field of view
- H-IBO
Subjects with hippocampus-lesions created with ibotenic acid
- H
Height
- IBO
ibotenic acid
- ITI
Inter-trial Interval
- MR
Magnetic resonance
- MRI
Magnetic resonance imagining
- TE
Echo time
- TR
Repetition time
- W
Width
- F
F ratio statistic
- p
observed significance level
- ηp2
partial eta-squared
Footnotes
On average, males weighed .57 kg (± .024 SEM) and females weighted .54 kg (± .019 SEM) at the time of surgery. There were no significant differences in weight based on either sex or lesion condition.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggleton JP, Passingham RE. Syndrome produced by lesions of the amygdala in monkeys (Macaca mulatta) Journal of Comparative and Physiological Psychology. 1981;95:961–977. doi: 10.1037/h0077848. [DOI] [PubMed] [Google Scholar]
- Antoniadis EA, Winslow JT, Davis M, Amaral DG. The nonhuman primate amygdala is necessary for the acquisition but not the retention of fear-potentiated startle. Biological Psychiatry. 2009;65:241–248. doi: 10.1016/j.biopsych.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniadis EA, Winslow JT, Davis M, Amaral DG. Role of the primate amygdala in fear-potentiated startle: effects of chronic lesions in the rhesus monkey. Journal of Neuroscience. 2007;27:7386–7396. doi: 10.1523/JNEUROSCI.5643-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman MD, Lavenex P, Mason WA, Capitanio JP, Amaral DG. The development of mother-infant interactions after neonatal amygdala lesions in rhesus monkeys. Journal of Neuroscience. 2004a;24:711–721. doi: 10.1523/JNEUROSCI.3263-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman MD, Lavenex P, Mason WA, Capitanio JP, Amaral DG. The development of social behavior following neonatal amygdala lesions in rhesus monkeys. Journal of Cognitive Neuroscience. 2004b;16:1388–1411. doi: 10.1162/0898929042304741. [DOI] [PubMed] [Google Scholar]
- Bliss-Moreau E, Toscano JE, Bauman MD, Mason WA, Amaral DG. Neonatal amygdala or hippocampus lesions influence responsiveness to objects. Developmental Psychobiology. 2010;52:487–503. doi: 10.1002/dev.20451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y, Izquierdo A, Murray EA. Distinct contributions of the amygdala and hippocampus to fear expression. European Journal of Neuroscience. 2009;30:2327–2337. doi: 10.1111/j.1460-9568.2009.07012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y, Wright KS, Murray EA. Hippocampal lesions in rhesus monkeys disrupt emotional responses but not reinforcer devaluation effects. Biological Psychiatry. 2007;63:1084–1091. doi: 10.1016/j.biopsych.2007.11.012. [DOI] [PubMed] [Google Scholar]
- Emery NJ, Capitanio JP, Mason WA, Machado CJ, Mendoza SP, Amaral DG. The effects of bilateral lesions of the amygdala on dyadic social interactions in rhesus monkeys (Macaca mulatta) Behavioral Neuroscience. 2001;115:515–544. [PubMed] [Google Scholar]
- Isbell LA. The fruit, the tree, and the serpent: Why we see so well. Cambridge, MA: Harvard University Press; 2009. [Google Scholar]
- Izquierdo A, Suda RK, Murray EA. Comparison of the effects of bilateral orbital prefrontal cortex lesions and amygdala lesions on emotional responses in rhesus monkeys. The Journal of Neuroscience. 2005;25:8534–8542. doi: 10.1523/JNEUROSCI.1232-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ, Kelly AE. The primate amygdala mediates acute fear but not the behavioral and physiological components of anxious temperament. Journal of Neuroscience. 2001;21:2067–2074. doi: 10.1523/JNEUROSCI.21-06-02067.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kling AS, Brothers LA. The amygdala and social behavior. In: Aggleton JP, editor. The amygdala: Neurobiological aspects of emotion, memory and mental dysfunction. Hoboken, NJ: Wiley-Liss; 1992. pp. 353–377. [Google Scholar]
- Krogsgaard-Larsen P, Nielsen EO, Curtis DR. Ibotenic acid analogues. Synthesis and biological and in vitro activity of conformationally restricted agonists at central excitatory amino acid receptors. Journal of Medical Chemistry. 1984;27:585–591. doi: 10.1021/jm00371a005. [DOI] [PubMed] [Google Scholar]
- Lavenex P, Banta Lavenex P, Amaral DG. Spatial relational learning persists following neonatal lesions in macaque monkeys. Nature Neuroscience. 2007;10:234–239. doi: 10.1038/nn1820. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Machado CJ, Bachevalier J. The impact of selective amygdala, orbital frontal cortex, or hippocampal formation lesions on established social relationships in Rhesus monkeys (Macaca mulatta) Behavioral Neuroscience. 2006;120:761–786. doi: 10.1037/0735-7044.120.4.761. [DOI] [PubMed] [Google Scholar]
- Machado CJ, Kazama AM, Bachevalier J. Impact of amygdala, orbitofrontal, or hippocampal lesions on threat avoidance and emotional reactivity in nonhuman primates. Emotion. 2009;9:147–163. doi: 10.1037/a0014539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado CJ, Snyder AZ, Cherry SR, Lavenex P, Amaral DG. Effects of neonatal amygdala or hippocampus lesions on resting brain metabolism in the macaque monkey: a microPET imagining study. Neuroimage. 2008;15:832–846. doi: 10.1016/j.neuroimage.2007.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason WA, Capitanio JP, Machado CJ, Mendoza SP, Amaral DG. Amygdalectomy and responsiveness to novelty in Rhesus monkeys (Macaca mulatta): Generality and individual consistency of effects. Emotion. 2006;6:73–81. doi: 10.1037/1528-3542.6.1.73. [DOI] [PubMed] [Google Scholar]
- Meunier M, Bachevalier J, Murray EA, Málková L, Mishkin M. Effects of aspiration versus neurotoxic lesions of the amygdala on emotional responses in monkeys. European Journal of Neuroscience. 1999;11:4403–4418. doi: 10.1046/j.1460-9568.1999.00854.x. [DOI] [PubMed] [Google Scholar]
- Murray EA. The amygdala, reward and emotion. Trends in Cognitive Sciences. 2007;11:389–497. doi: 10.1016/j.tics.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Shelton SE, Kalin NH. Individual differences in the responses of naïve rhesus monkeys to snakes. Emotion. 2003;3:3–11. doi: 10.1037/1528-3542.3.1.3. [DOI] [PubMed] [Google Scholar]
- Öhman A, Mineka S. Fears, phobias, and preparedness: Toward an evolved module of fear and fear learning. Psychological Review. 2001;108:483–522. doi: 10.1037/0033-295x.108.3.483. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Adolphs R. Emotion processing and the amygdala: from a ‘low road’ to ‘many roads’ of evaluating biological significance. Nature Reviews Neuroscience. 2010;11:773–782. doi: 10.1038/nrn2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA. Emotion and cognition: Insights from studies of the human amygdala. Annual Review of Psychology. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- Prather MD, Lavenex P, Mauldin-Jourdain ML, Mason WA, Capitanio JP, Mendoza SP, Amaral DG. Increased social fear and decreased fear of objects in monkeys with neonatal amygdala lesions. Neuroscience. 2001;106:653–658. doi: 10.1016/s0306-4522(01)00445-6. [DOI] [PubMed] [Google Scholar]
- Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35:192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt V. Time budget of caged rhesus monkeys exposed to a companion, a PVC perch and a piece of wood for an extended time. American Journal of Primatology. 1990;20:51–56. doi: 10.1002/ajp.1350200108. [DOI] [PubMed] [Google Scholar]
- Salzman CD, Fusi S. Emotion, cognition and mental state representation in amygdala and prefrontal cortex. Annual Review of Neuroscience. 2010;33:173–202. doi: 10.1146/annurev.neuro.051508.135256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarcz R, Hökflet T, Fuxe K, Jonsson G, Goldstein M, Terenius L. Ibotenic acid induced neuronal degeneration: a morphological and neurochemical study. Experimental Brain Research. 1979;37:199–216. doi: 10.1007/BF00237708. [DOI] [PubMed] [Google Scholar]
- Seymour B, Dolan R. Emotion, decision making and the amygdala. Neuron. 2010;58:662–671. doi: 10.1016/j.neuron.2008.05.020. [DOI] [PubMed] [Google Scholar]
- Stefanacci L, Clark RE, Zola SM. Selective neurotoxic amygdala lesions in monkeys disrupt reactivity to food and object stimuli and have limited effects on memory. Behavioral Neuroscience. 2003;117:1029–1043. doi: 10.1037/0735-7044.117.5.1029. [DOI] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR, Alverez-Royo P, Clower RP. Independence of memory functions and emotional behavior: Separate contributions of the hippocampal formation and the amygdala. Hippocampus. 1991;1:207–220. doi: 10.1002/hipo.450010208. [DOI] [PubMed] [Google Scholar]