Abstract
Objective
The selective amygdalohippocampectomy (AHC) has evolved to encompass a variety of techniques to resect the mesial temporal lobe. To date, there have been few large-scale evaluations of the trans-middle temporal gyrus selective AHC. The authors examine a large series of patients who have undergone trans-middle temporal gyrus AHC and assess its clinical and neuropsychological impact.
Methods
A series of 76 adult patients underwent selective AHC via the trans-middle temporal gyrus approach over a 10 year period, 19 of whom underwent pre and post-operative neuropsychological evaluation.
Results
Favorable seizure response rates were achieved (92% Engel class I or II), with very low surgical morbidity and no mortality. Postoperative neuropsychological assessment revealed a decline in verbal memory for the left AHC group. No post-operative memory decline was identified for the right AHC group but rather some improvements were noted within this group.
Conclusions
The trans-middle temporal gyrus selective AHC is a safe and effective choice for management of adult medically refractory epilepsy.
Keywords: selective amygdalohippocampectomy, epilepsy, neuropsychological outcomes, temporal stem
Introduction
The selective amygdalohippocampectomy (AHC) was first proposed in 1958 by Niemeyer 21. The technique was originally developed to spare unaffected brain tissue from surgery, thus minimizing the cognitive consequences of a full temporal lobe resection. With the evolution of functional imaging and progression of anatomic research there has been a new appreciation for the white matter tracts within the temporal lobe. This has elevated the appreciation for preservation of functional cortex and its deeper connections18,33,17,24. Since the AHC's initial inception, there have been several variants on the surgical approach for the selective resection of this region. The earliest approach, described by Niemeyer, was the transmiddle temporal gyrus, transventricular approach to the mediobasal temporal lobe. In an attempt to preserve neocortex, Yasargil later proposed a transsylvian approach in 1982 32. This approach however, may increase the risk for a vascular injury as it requires splitting the Sylvian fissure and exposing the distal middle cerebral artery branches. Still yet another approach was the subtemporal approach proposed by Hori in 1993 11. This approach was thought to have a functional advantage by penetrating the subtemporal cortex via the parahippocampal gyrus, but can involve undue cortical retraction and manipulation of large cortical veins. It is important to note that all approaches to a greater or lesser degree breach the cortical surface and white matter to arrive at the resectional targets of the procedure (i.e. the hippocampus, amygdala and parahippocampal gyrus). The transventricular approach transects the lateral surface of the middle temporal gyrus to gain access to the mesial structures. The transsylvian and subtemporal approaches both transect the cortex at the limen insula from either a medial or inferior trajectory, respectively (see Figure 1).
Figure 1.
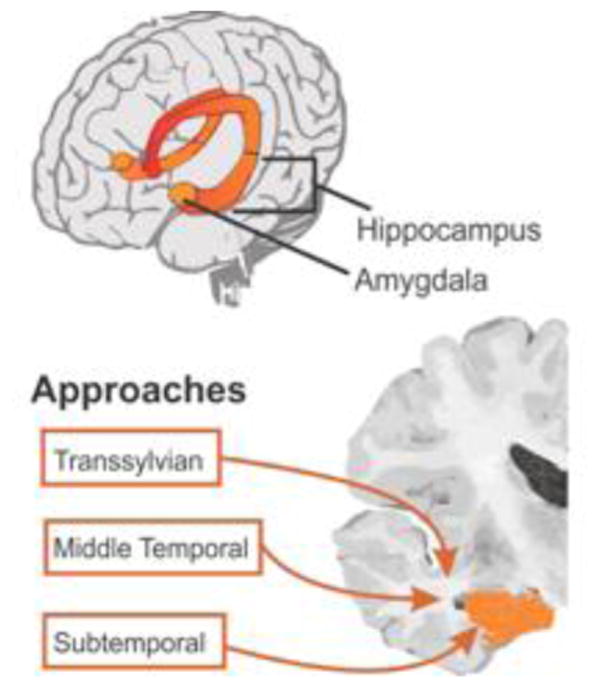
Comparison of the transsylvian, subtemporal and trans-middle temporal gyrus approaches to the resectional targets of the selective amygdalohippocampectomy within the mediobasal temporal lobe.
Regardless of the approach utilized, the selective AHC has been shown to provide durable clinical outcomes and decreased seizure burden for patients living with medically refractory epilepsy 29,12,20, 23,30,31,35,36. Though the trans-middle temporal gyrus approach was initially described in 1958, there have been only a limited number of modern clinical series that have assessed its clinical impact as measured by post-operative Engel classification, neuropsychological consequences and surgical complications 1,22,26. Herein, we have evaluated the trans-middle temporal approach to evaluate seizure response rates, neuropsychological outcomes and complications for consideration as a safe and effective choice with regard to seizure control and functional outcome.
Methods
Patient Population
The authors present a retrospective review of 76 consecutive adult patients ranging in age from 20 to 65 years old who underwent selective AHC via the trans-middle temporal gyrus approach for medically refractory mesial temporal lobe epilepsy over a 10 year period between 1997 and 2007. All patients had at least 1 year of follow-up available for review. A subset of 19 of these patients underwent both pre and post-operative neuropsychological evaluation. All patients underwent standard preoperative evaluation including MR imaging, inpatient video EEG monitoring with requirement for at least one electroclinical seizure captured during the monitoring period, interictal FDG-PET and neuropsychological evaluation. Patients were selected for a selective AHC without further invasive monitoring if, following multi-disciplinary review of their case, seizure semiology, scalp EEG and imaging data were concordant, suggesting a unilateral mesial temporal lobe onset due to hippocampal sclerosis. Radiographic criteria used to identify evidence of hippocampal sclerosis on MRI include hippocampal atrophy, hyperintensity on T2 weighted imaging or fluid attenuated inversion recovery (FLAIR) sequences and/or mesial temporal lobe hypometabolism on PET imaging. A single surgeon performed all operations (JD).
Surgical Technique
The trans-middle temporal gyrus approach was utilized to reach the mesial temporal lobe structures. This approach was selected by the operating surgeon (JD) based solely on training experience. The surgical technique for this procedure has been previously described by Niemeyer and in great detail by Wheatley 28. Briefly, utilizing neuronavigation (StealthStation, Medtronics, Minneapolis MN) the surgical incision and craniotomies are planned to allow posterior and inferiorly directed access to the mediobasal structures of the temporal lobe through a 15-20mm corticectomy within the anterior middle temporal gyrus. Once the ventricle is accessed, the microscope is brought in for high power magnification and illumination allowing identification of the lateral ventricular sulcus and the choroidal fissure, both of which serve as important reference landmarks during resection. Ultrasonic aspiration (CUSA, Integra Radionics, Burlington MA) continues anteriorly extending to the medial pia to allow subpial resection of the uncus and amygdala. Once resection is carried down over the tentorial edge, the 3rd cranial nerve and ipsilateral posterior communicating and posterior cerebral arteries are visualized through the medial pia. Focus is then turned posteriorly to allow resection of the hippocampus and parahippocampal gyrus back to the level of the colliculi.
Neuropsychological Assessment
A total of 19 patients underwent pre and post-surgical neuropsychological assessment. All 76 patients underwent preoperative neuropsychological assessment but those available for post-operative assessment were limited, frequently due to travel time constraints and the fact that many had returned to work post-operatively. Post-surgical assessments were completed approximately eight months post-surgery (mean 8.84 months, range 6-21 months). The patients' intellectual ability (IQ) was estimated by their scores on the Wechsler Test of Adult Reading (WTAR; 2001). Verbal memory was assessed by the California Verbal Learning Test-Second Edition (CVLT-II; 2000) and the Wechsler Memory Scale-Third Edition Logical Memory subtest (WMS-III LM; 1997). The CVLT-II is a list learning and memory test which requires the patient to learn a 16-item word list across 5 learning trials and then recall and recognize the learned list after a 20-minute delay. For this study total raw score for trials 1-5, short delay free recall, short delay cued recall, long delay free recall, long delay cued recall, recognition false positives, and recognition correct scores were calculated. The WMS-III LM subtest is a test requiring the patient to recall two stories immediately after they are read and to recall and recognize information from the stories after a 30-minute delay. Total immediate and delayed recall scores were calculated (LM I and LM II). Visual memory was assessed by the WMS-III Visual Reproduction subtest which is a test requiring the patient to recall five pictures of designs immediately after their presentation and after a 30-minute delay. Total immediate and delayed recall scores were calculated (VR I and VR II).
Data Analysis & Statistics
Patient data were organized in a database (Excel, Microsoft Corporation, Seattle, WA). The data were then analyzed using the Statistical Analysis Software (SAS Institute Inc. Cary, NC). Neuropsychological data were analyzed with repeated measures ANOVAs on the two groups (left and right AHC) pre and post-operatively. Statistics were considered significant if p-values were less than 0.05.
Results
Demographics
Table 1 demonstrates the demographic profile of patients included in this study. Our patient population was composed of 62 percent female patients and 38 percent male patients with an average age of 41 years at time of surgery, with an age range of 20-65 years. The average duration of pre-operative seizure activity was 28 years with a range of less than one year up to 62 years. Left and right sided procedures were roughly evenly distributed at 49 and 51 percent respectively.
Table 1. Patient Demographics.
| Characteristic | N= | Percent (%) |
|---|---|---|
| Gender | ||
| Male | 29 | 38.2 |
| Female | 47 | 61.8 |
| Age | ||
| 20-35 | 22 | 28.9 |
| 36-50 | 33 | 43.4 |
| 51-65 | 21 | 27.7 |
| Average Duration of Seizures (yrs) | ||
| Operative Side | ||
| Right | 39 | 51 |
| Left | 37 | 49 |
Surgical Outcomes
Table 2 delineates the seizure response rates utilizing the Engel Classification system 13 and histopathological diagnoses of the patients included in the study. All patients had at least 1 year of follow up available for review. The last observation was carried forward to 2 years for patients with between 1 and 2 years of follow up. Average duration of follow up was 39 months. Engel Classification was determined by clinical outpatient assessment by the patient's treating epileptologist. Sixty-six percent of our patients experienced post-surgical Engel Class I seizure response rates (51% with Ia, 5% with Ib, 5% with Ic and 4% with Id). Twenty-six percent of patients experienced post-surgical Engel class II seizure response rates (20% with IIa and 6% with IIb) for a total of 92 percent of patients experiencing Engel class I or II seizure response rates to surgical resection. Notably, no patients changed Engel Classification score between 2 years and last available follow up.
Table 2. Surgical Outcomes.
| Feature | N= | Percent (%) |
|---|---|---|
| Pathological Diagnosis | ||
| HS | 70 | 92.1 |
| Non Specific Gliosis | 5 | 6.6 |
| Normal | 1 | 1.3 |
| Average Duration of Follow Up (months) | 38.8 (range 12-96) | |
| Engel Class | ||
| Ia | 39 | 51.3 |
| Ib | 4 | 5.3 |
| Ic | 4 | 5.3 |
| Id | 3 | 3.9 |
| IIa | 15 | 19.7 |
| IIb | 5 | 6.6 |
| III | 4 | 5.3 |
| IV | 2 | 2.6 |
| Average Post-Resection Hospital Stay (days) | 2.6 (range 1-6) |
Ia: seizure free, Ib: simple partial seizures only, Ic: free from disabling seizures, Id: generalized convulsions with discontinuation of seizure medications only; IIa: initially free from but now rare disabling seizures, IIb: persistent rare disabling seizures; III: worthwhile improvement; IV: no worthwhile improvement12
Histopathological diagnoses were predominantly represented by hippocampal sclerosis in 92.1% of specimens. Non-specific gliosis was identified in 5 patients representing 6.6% of our patient sample. Normal hippocampal cytoarchitecture and immunohistochemical staining was identified in 1 patient or 1.3% of specimens submitted for histopathological review.
Neuropsychological Assessment
As stated previously, 19 patients underwent pre and post-operative neuropsychological assessment (6 underwent left AHC and 13 underwent right AHC). Table 3 shows that the left and right AHC groups were generally equivalent in terms of demographic information. More specifically, there were no significant differences between the left AHC and right AHC groups in terms of sex, age at surgery, age of seizure onset, education, or estimated pre-operative IQ.
Table 3. Patient Demographic Information from patients who underwent pre and post-operative Neuropsychological Testing.
| Characteristic | Left AHC | Right AHC | p value |
|---|---|---|---|
| Number of patients | 6 | 13 | |
| Percent of male patients | 17.0 | 15.4 | 1.000 |
| Age at surgery | 48.5 (13.4) | 39.6 (9.9) | 0.360 |
| Age of seizure onset | 13.5 (11.7) | 12.8 (15.3) | 0.570 |
| Education | 13.7 (2.7) | 12.2 (1.2) | 0.264 |
| Estimated preop IQ | 99.3 (13.5) | 89.9 (11.4) | 0.140 |
p<0.05
Repeated measures ANOVAs were completed on the two groups (left and right AHC) before surgery and at approximately six months post-surgery. As noted in Table 4 there was a significant finding for one verbal memory measure. More specifically, the time by surgery interaction was significant for Logical Memory first recall raw score. In other words, Table 4 demonstrates that the left AHC group experienced a decline in their scores on the Logical Memory first recall raw score after surgery, while the right AHC group improved on this measure after surgery. Additionally, the time by surgery interaction approached significance for the CVLT-II Trials 1-5 variable with the left AHC group scoring lower post-surgery, while the right AHC group scoring higher on this measure post-surgery.
Table 4. Pre and post-operative neuropsychological assessment results.
| Neuropsychological Test | Left AHC | Right AHC | p value |
|---|---|---|---|
| CVLT-II Trials 1-5 | Pre 43.6 (11.0) Post 38.6 (11.5) |
Pre 51.8 (9.6) Post 54.6 (9.4) |
0.055 |
| CVLT-II Short Delay Free Recall | Pre 8.4 (1.1) Post 5.3 (1.2) |
Pre 11.2 (1.0) Post 12.0 (1.1) |
0.066 |
| CVLT-II Short Delay Cued Recall | Pre 8.9 (1.0) Post 8.0 (.9) |
Pre 12.5 (.9) Post 13.0 (.9) |
0.630 |
| CVLT-II Long Delay Free Recall | Pre 8.0 (1.1) Post 6.1 (1.1) |
Pre 11.4 (1.0) Post 12.3 (1.0) |
0.101 |
| CVLT-II Long Delay Cued Recall | Pre 8.3 (1.0) Post 6.9 (1.0) |
Pre 12.4 (.9) Post 13.5 (.9) |
0.074 |
| CVLT-II Recognition-False Positives | Pre 4.1 (1.2) Post 8.0 (1.3) |
Pre .7 (1.0) Post .6 (1.1) |
0.132 |
| CVLT-II Recognition-Correct | Pre 14.2 (.6) Post 13.4 (.8) |
Pre 14.1 (.5) Post 14.6 (.6) |
0.194 |
| Logical Memory I raw | Pre 28.4 (3.8) Post 25.8 (3.1) |
Pre 33.4 (3.2) Post 38.2 (2.6) |
0.005* |
| Logical Memory II raw | Pre 13.9 (2.7) Post 13.1 (2.5) |
Pre 20.8 (2.3) Post 23.1 (2.1) |
0.192 |
| Visual Reproduction I raw | Pre 81.2 (4.1) Post 79.4 (4.0) |
Pre 72.1 (3.5) Post 74.5 (3.4) |
0.200 |
| Visual Reproduction II raw | Pre 54.7 (6.1) Post 55.8 (6.4) |
Pre 52.0 (5.2) Post 58.4 (5.4) |
0.424 |
CVLT-II: California Verbal Learning Test-Second Edition;
p<0.05
Further inspection of the data revealed that 5 out of 6 of the patients in the left AHC group experienced at least a one standard deviation decline on either the Logical Memory first recall score or the CVLT-II Trials 1-5 variable. Three of these 5 patients declined on Logical Memory, whereas 2 declined on the CVLT-II. In contrast, 1 of the 6 patients in the left AHC group showed a one standard deviation improvement on the Logical Memory first recall score post surgery (pre-surgical scaled score of 6 and post-surgical scaled score of 9).
In the right AHC group 8 out of 13 patients displayed at least a one standard deviation improvement on the Logical Memory first recall score and/or the CVLT-II Trials 1-5 variable after the resection (4 improved on Logical Memory and 5 improved on CVLT-II). One of the 13 right AHC patients displayed at least a one standard deviation improvement on both the Logical Memory first recall and on the CVLT-II (pre-surgical scaled score of 5 on the Logical Memory first recall and post-surgical scaled score of 9; pre-surgical CVLT-II t-score of 40 and post-surgical t-score of 66). One of the 13 right AHC patients displayed more than a standard deviation decline on the CVLT-II, but that same patient displayed a one standard deviation improvement on the Logical Memory first recall (pre-surgical CVLT-II t-score of 67 and post-surgical t-score of 39; pre-surgical scaled score of 7 for Logical Memory first recall and post-surgical scaled score of 10).
The time by surgery interaction was not significant for CVLT-II short delay free recall, CVLT-II short delay cued recall, CVLT-II long delay free recall, CVLT-II long delay cued recall, CVLT-II recognition correct, CVLT-II Recognition correct, or Logical Memory delayed trial raw score. Time by surgery interactions for VR I or VR II were not significant as also shown in Table 4.
Surgical Complications
Table 5 identifies the most frequently encountered post surgical complications experienced by the patients in the study. No post-operative complications were present in 97% of patients. The only encountered post-surgical complication was transient aphasia occurring in 2 out of 76 patients, representing 3% of our patient population. There were no subjective visual field deficits or deficits noted on bedside exam, but formal pre- and post-operative visual field testing was not performed.
Table 5. Surgical Complications.
| Complication | # of patients | Percent (%) |
|---|---|---|
| None | 74 | 97.3 |
| Transient Aphasia | 2 | 2.7 |
Discussion
Since its inception as a cortex-sparing procedure in cases of medial temporal lobe epilepsy, a variety of selective AHC approaches have been developed in an effort to spare the functional regions of cortex and the critical white matter tracts. With few variations, these different approaches have been subdivided into the trans-middle temporal gyrus, the transsylvian and the subtemporal approaches 11,21,32. The most relevant eloquent cortical sites in this region pertain to receptive speech classically known as Wernicke's area. Multiple studies have demonstrated that there is a moderate amount of topographic variability in its location 8,19,25. Predominantly however, receptive speech has been localized to superior and middle temporal gyri posterior to the central sulcus. With regard to the functional significance of white matter tracts, the temporal stem is an important anatomic structure that warrants caution. The microsurgical anatomy of the temporal stem as it relates to white matter tracts within and around the mesial temporal lobe has been described by Ebeling and von Cramon, Peltier et al and Martino et al 4,17,24. Their findings demonstrate that the temporal stem is a compilation of short and long association fiber tracts that connect the frontal and temporal lobes. It is comprised primarily of fibers giving rise to the anterior commissure, amygdalofugal fibers, the Inferior Occipitofrontal Fasciculus (IFOF), the corpus callosum, the uncinate fasciculus and Meyer's Loop. Taken together, all the described approaches have attempted to minimize the injury to these regions (and the associated language and vision related consequences), while best achieving seizure control with removal of the mesial temporal structures. In this study we have evaluated a large number of patients undergoing a trans-middle temporal gyrus approach to evaluate this balance of seizure freedom with functional outcome. Here we demonstrate that this surgical approach has an exceptionally low surgical morbidity and mortality, while still maintaining a high level of successful clinical outcomes.
Surgical Outcomes
Seizure response rates to surgery vary widely in the literature. Published studies include a wide range of patient populations with an equally broad range of follow-up duration. Given these variations, only generalizations can be made regarding the accepted norms of seizure response rates following resective surgery for medically intractable epilepsy. In general, the Engel class I seizure frequency can be expected in 60-80% of patients undergoing selective AHC for the management of epilepsy 1,2,12,20,29,34 with up to 90% of patients experiencing favorable seizure response classified as Engel class I or II 26. Particularly favorable outcome can be obtained in patient subpopulations demonstrating histopathologic evidence of hippocampal sclerosis at the time of surgical resection5. Our results are comparable, with 66 percent of our patients achieving an Engel Class I outcome and 92 percent of patients experiencing Engel class I or II outcome. Accepting these general norms, the data presented herein are within the expected range of reduction of seizure burden following surgery. The benefit with regard to reduction of seizure burden, coupled with the exceptionally low morbidity rate and 0% mortality rate further demonstrates the efficacy and safety of the trans-middle temporal gyrus approach to the selective AHC. Table 6 summarizes the previously published selective AHC literature with regard to approach utilized, seizure outcome as based on post-operative Engel classification, surgical complications and presence or absence of neuropsychological analysis. The present report adds to that body of literature confirming previously published results21 and provides additional support for the clinical efficacy and safety of the trans-middle temporal gyrus selective AHC.
Table 6. Summary of previously published selective amygdalohippocampectomy data.
| Approach | Reference | # of patients (n) | Seizure outcome as measured by postoperative Engel class for duration of follow up | Surgical complications | Neuropsychological Testing |
|---|---|---|---|---|---|
|
| |||||
| Trans-Middle Temporal Gyrus | Paglioli, et al. | n=81 | 92.6% Engel class I or II | 0/81 | (+) |
| Acar, et al. | n=39 | 82% Engel class I | 0/39 | (-) | |
| Tanriverdi, et al. | n=50 | 90% Engel class I or II | 1/50 | (-) | |
|
| |||||
| Transsylvian | Yasargil, et al. | n=73 | 93.1% Engel class I or II | 0/73 | (+) |
| Vajkoczy, et al. | n=32 | 97% Engel class I or II | 1/32 | (-) | |
|
| |||||
| Subtemporal | Little, et al. | n=23 | 87% Engel class I or II | 1/23 | (+)(+) |
| Hori, et al. | n=26 | 84% Engel class I or II | 3/26 | ||
Neuropsychological Outcomes
Consistent with previous work, our series demonstrates a hemispheric asymmetry in neuropsychological outcome dependent on which side the selective AHC was performed. Pre and post-surgical neuropsychological assessment shows patients who underwent left selective AHC experienced significant decline in verbal memory after surgery. This is consistent with many studies investigating memory outcome following left AHC 6,7,10,15 but inconsistent with others 16. Given the important role the dominant hippocampus plays in verbal episodic memory these results more likely reflect the impact of the resection of the mesial structures rather than penetration of middle temporal gyrus cortex. Given that speech can infrequently be located in the middle temporal gyrus 8, the transient aphasias noted in 2 patients in this series likely reflects a potential impact of a trans middle temporal approach. Patients who underwent right selective AHC experienced no remarkable declines in verbal or visual memory when comparing pre and post-operative test scores, which is consistent with some previous research 7, but not others. 10,16 The right AHC group demonstrated a statistically significant improvement on one test assessing verbal memory and approached a significant improvement on another test assessing verbal memory, which is consistent with previous research. 3,27
Study Limitations
Though this is a large series, there are some important limitations and caveats that merit attention. First, regarding the neuropsychological assessment component of this study, only a small subset of 19 patients underwent both preoperative and postoperative evaluation. Close inspection of test scores of all individuals assessed, however, did not reveal remarkable outliers that might have accounted for significant results. Second, this study does not address the issue of clinically meaningful change in cognition. That is, results show statistically significant verbal memory decline post surgery in the left AHC group, but statistical significance does not always translate into clinically meaningful change. To address this issue, future studies might consider completing reliable change analysis on their data, using regression-based norms for change, or assessing whether patients experienced a decline in their ability to perform functional activities. This study did not assess patient perception of cognitive decline post surgery, however, subjective reporting of cognition has limitations and can be affected by emotional factors such as depression 9,14,16. Lastly, this study does not answer the question of whether a selective procedure lessens post-operative cognitive decline when compared to a larger resection (i.e., anterior temporal lobectomy).
Conclusions
The authors present our experience with trans-middle temporal gyrus selective AHC for medically refractory epilepsy. Favorable seizure response rates are achieved with generally low morbidity. Post-operative neuropsychological assessment, however, did reveal a significant decline in verbal memory for the left selective AHC group, but no post-operative memory declines for the right selective AHC group. These statistically significant changes, however, will need to be further investigated to determine whether they are indeed clinically and functionally relevant.
Highlights.
Favorable seizure outcomes are achieved via the trans-middle temporal gyrus AHC.
Low surgical morbidity and no mortality was encountered in our review of 76 adult AHCs.
Neuropsych assessment revealed a decline in verbal memory for the left AHC group.
No post-operative memory changes were identified for the right AHC group.
The trans-middle temporal gyrus AHC is safe & effective for treatment of adult mTLE.
Abbreviations
- AHC
amygdalohippocampectomy
- IFOF
Inferior Occipitofrontal Fasciculus
- EEG
electroencephalogram
- FDG-PET
Fluorodeoxyglucose Positron Emission Tomography
- IQ
Intelligence Quotient
- WTAR
Wechsler Test of Adult Reading
- CVLT-II
California Verbal Learning Test-Second Edition
- WMS-III LM
Wechsler Memory Scale-Third Edition Logical Memory subtest
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers thatk apply to the journal pertain.
References
- 1.Acar G, Acar F, Miller J, Spencer DC, Burchiel KJ. Seizure outcome following transcortical selective amygdalohippocampectomy in mesial temporal lobe epilepsy. Stereotactic and functional neurosurgery. 2008;86:314–319. doi: 10.1159/000160154. [DOI] [PubMed] [Google Scholar]
- 2.Bate H, Eldridge P, Varma T, Wieshmann UC. The seizure outcome after amygdalohippocampectomy and temporal lobectomy. European journal of neurology : the official journal of the European Federation of Neurological Societies. 2007;14:90–94. doi: 10.1111/j.1468-1331.2006.01565.x. [DOI] [PubMed] [Google Scholar]
- 3.Baxendale S, Thompson P. Defining meaningful postoperative change in epilepsy surgery patients: measuring the unmeasurable? Epilepsy & behavior : E&B. 2005;6:207–211. doi: 10.1016/j.yebeh.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 4.Ebeling U, von Cramon D. Topography of the uncinate fascicle and adjacent temporal fiber tracts. Acta neurochirurgica. 1992;115:143–148. doi: 10.1007/BF01406373. [DOI] [PubMed] [Google Scholar]
- 5.Elsharkawy AE, Alabbasi AH, Pannek H, Oppel F, Schulz R, Hoppe M, et al. Long-term outcome after temporal lobe epilepsy surgery in 434 consecutive adult patients. Journal of neurosurgery. 2009;110:1135–1146. doi: 10.3171/2008.6.JNS17613. [DOI] [PubMed] [Google Scholar]
- 6.Gleissner U, Helmstaedter C, Schramm J, Elger CE. Memory outcome after selective amygdalohippocampectomy in patients with temporal lobe epilepsy: one-year follow-up. Epilepsia. 2004;45:960–962. doi: 10.1111/j.0013-9580.2004.42203.x. [DOI] [PubMed] [Google Scholar]
- 7.Gleissner U, Helmstaedter C, Schramm J, Elger CE. Memory outcome after selective amygdalohippocampectomy: a study in 140 patients with temporal lobe epilepsy. Epilepsia. 2002;43:87–95. doi: 10.1046/j.1528-1157.2002.24101.x. [DOI] [PubMed] [Google Scholar]
- 8.Haglund MM, Berger MS, Shamseldin M, Lettich E, Ojemann GA. Cortical localization of temporal lobe language sites in patients with gliomas. Neurosurgery. 1994;34:567–576. doi: 10.1227/00006123-199404000-00001. discussion 576. [DOI] [PubMed] [Google Scholar]
- 9.Hall KE, Isaac CL, Harris P. Memory complaints in epilepsy: an accurate reflection of memory impairment or an indicator of poor adjustment? A review of the literature. Clinical psychology review. 2009;29:354–367. doi: 10.1016/j.cpr.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Helmstaedter C, Richter S, Roske S, Oltmanns F, Schramm J, Lehmann TN. Differential effects of temporal pole resection with amygdalohippocampectomy versus selective amygdalohippocampectomy on material-specific memory in patients with mesial temporal lobe epilepsy. Epilepsia. 2008;49:88–97. doi: 10.1111/j.1528-1167.2007.01386.x. [DOI] [PubMed] [Google Scholar]
- 11.Hori T, Tabuchi S, Kurosaki M, Kondo S, Takenobu A, Watanabe T. Subtemporal amygdalohippocampectomy for treating medically intractable temporal lobe epilepsy. Neurosurgery. 1993;33:50–56. discussion 56-57. [PubMed] [Google Scholar]
- 12.Hori T, Yamane F, Ochiai T, Kondo S, Shimizu S, Ishii K, et al. Selective subtemporal amygdalohippocampectomy for refractory temporal lobe epilepsy: operative and neuropsychological outcomes. Journal of neurosurgery. 2007;106:134–141. doi: 10.3171/jns.2007.106.1.134. [DOI] [PubMed] [Google Scholar]
- 13.Jr EJ, editor. Outcome with respect to epileptic seizures. New York: Raven Press; 1993. [Google Scholar]
- 14.Liik M, Vahter L, Gross-Paju K, Haldre S. Subjective complaints compared to the results of neuropsychological assessment in patients with epilepsy: The influence of comorbid depression. Epilepsy research. 2009;84:194–200. doi: 10.1016/j.eplepsyres.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Lutz MT, Clusmann H, Elger CE, Schramm J, Helmstaedter C. Neuropsychological outcome after selective amygdalohippocampectomy with transsylvian versus transcortical approach: a randomized prospective clinical trial of surgery for temporal lobe epilepsy. Epilepsia. 2004;45:809–816. doi: 10.1111/j.0013-9580.2004.54003.x. [DOI] [PubMed] [Google Scholar]
- 16.Marino SE, Meador KJ, Loring DW, Okun MS, Fernandez HH, Fessler AJ, et al. Subjective perception of cognition is related to mood and not performance. Epilepsy & behavior : E&B. 2009;14:459–464. doi: 10.1016/j.yebeh.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martino J, Vergani F, Robles SG, Duffau H. New insights into the anatomic dissection of the temporal stem with special emphasis on the inferior fronto-occipital fasciculus: implications in surgical approach to left mesiotemporal and temporoinsular structures. Neurosurgery. 2010;66:4–12. doi: 10.1227/01.NEU.0000348564.28415.FA. [DOI] [PubMed] [Google Scholar]
- 18.Mayanagi Y, Watanabe E, Kaneko Y. Mesial temporal lobe epilepsy: clinical features and seizure mechanism. Epilepsia. 1996;37(Suppl 3):57–60. doi: 10.1111/j.1528-1157.1996.tb01823.x. [DOI] [PubMed] [Google Scholar]
- 19.Ojemann G, Ojemann J, Lettich E, Berger M. Cortical language localization in left, dominant hemisphere An electrical stimulation mapping investigation in 117 patients. J Neurosurg. 1989;71:316–326. doi: 10.3171/jns.1989.71.3.0316. [DOI] [PubMed] [Google Scholar]
- 20.Olivier A. Transcortical selective amygdalohippocampectomy in temporal lobe epilepsy. The Canadian journal of neurological sciences Le journal canadien des sciences neurologiques. 2000;27(Suppl 1):S68–76. doi: 10.1017/s031716710000069x. discussion S92-66. [DOI] [PubMed] [Google Scholar]
- 21.P N, editor. The transventricular amygdala-hippocampectomy in temporal lobe epilepsy. Springfield, IL: Charles C. Thomas; 1958. [Google Scholar]
- 22.Paglioli E, Palmini A, Portuguez M, Azambuja N, da Costa JC, da Silva Filho HF, et al. Seizure and memory outcome following temporal lobe surgery: selective compared with nonselective approaches for hippocampal sclerosis. Journal of neurosurgery. 2006;104:70–78. doi: 10.3171/jns.2006.104.1.70. [DOI] [PubMed] [Google Scholar]
- 23.Park TS, Bourgeois BF, Silbergeld DL, Dodson WE. Subtemporal transparahippocampal amygdalohippocampectomy for surgical treatment of mesial temporal lobe epilepsy Technical note. Journal of neurosurgery. 1996;85:1172–1176. doi: 10.3171/jns.1996.85.6.1172. [DOI] [PubMed] [Google Scholar]
- 24.Peltier J, Verclytte S, Delmaire C, Pruvo JP, Godefroy O, Le Gars D. Microsurgical anatomy of the temporal stem: clinical relevance and correlations with diffusion tensor imaging fiber tracking. Journal of neurosurgery. 2010;112:1033–1038. doi: 10.3171/2009.6.JNS08132. [DOI] [PubMed] [Google Scholar]
- 25.Sanai N, Mirzadeh Z, Berger MS. Functional outcome after language mapping for glioma resection. N Engl J Med. 2008;358:18–27. doi: 10.1056/NEJMoa067819. [DOI] [PubMed] [Google Scholar]
- 26.Tanriverdi T, Olivier A, Poulin N, Andermann F, Dubeau F. Long-term seizure outcome after mesial temporal lobe epilepsy surgery: corticalamygdalohippocampectomy versus selective amygdalohippocampectomy. Journal of neurosurgery. 2008;108:517–524. doi: 10.3171/JNS/2008/108/3/0517. [DOI] [PubMed] [Google Scholar]
- 27.Trenerry MR, Jack CR, Jr, Cascino GD, Sharbrough FW, So EL. Bilateral magnetic resonance imaging-determined hippocampal atrophy and verbal memory before and after temporal lobectomy. Epilepsia. 1996;37:526–533. doi: 10.1111/j.1528-1157.1996.tb00604.x. [DOI] [PubMed] [Google Scholar]
- 28.Wheatley BM. Selective amygdalohippocampectomy: the trans-middle temporal gyrus approach. Neurosurgical focus. 2008;25:E4. doi: 10.3171/FOC/2008/25/9/E4. [DOI] [PubMed] [Google Scholar]
- 29.Wiebe S, Blume WT, Girvin JP, Eliasziw M. A randomized, controlled trial of surgery for temporal-lobe epilepsy. The New England journal of medicine. 2001;345:311–318. doi: 10.1056/NEJM200108023450501. [DOI] [PubMed] [Google Scholar]
- 30.Wieser HG. Selective amygdalo-hippocampectomy for temporal lobe epilepsy. Epilepsia. 1988;29(Suppl 2):S100–113. doi: 10.1111/j.1528-1157.1988.tb05793.x. [DOI] [PubMed] [Google Scholar]
- 31.Wieser HG. Selective amygdalohippocampectomy: indications, investigative technique and results. Advances and technical standards in neurosurgery. 1986;13:39–133. doi: 10.1007/978-3-7091-7010-6_2. [DOI] [PubMed] [Google Scholar]
- 32.Wieser HG, Yasargil MG. Selective amygdalohippocampectomy as a surgical treatment of mesiobasal limbic epilepsy. Surgical neurology. 1982;17:445–457. doi: 10.1016/s0090-3019(82)80016-5. [DOI] [PubMed] [Google Scholar]
- 33.Wurm G, Wies W, Schnizer M, Trenkler J, Holl K. Advanced surgical approach for selective amygdalohippocampectomy through neuronavigation. Neurosurgery. 2000;46:1377–1382. doi: 10.1097/00006123-200006000-00016. discussion 1382-1373. [DOI] [PubMed] [Google Scholar]
- 34.Yasargil MG, Krayenbuhl N, Roth P, Hsu SP, Yasargil DC. The selective amygdalohippocampectomy for intractable temporal limbic seizures. Journal of neurosurgery. 2010;112:168–185. doi: 10.3171/2008.12.JNS081112. [DOI] [PubMed] [Google Scholar]
- 35.Yasargil MG, Teddy PJ, Roth P. Selective amygdalo-hippocampectomy Operative anatomy and surgical technique. Advances and technical standards in neurosurgery. 1985;12:93–123. doi: 10.1007/978-3-7091-7008-3_2. [DOI] [PubMed] [Google Scholar]
- 36.Yasargil MG, Ture U, Yasargil DC. Impact of temporal lobe surgery. Journal of neurosurgery. 2004;101:725–738. doi: 10.3171/jns.2004.101.5.0725. [DOI] [PubMed] [Google Scholar]


