Abstract
Background
Myo-inositol given to preterm infants with respiratory distress has reduced death, increased survival without bronchopulmonary dysplasia (BPD) and reduced severe retinopathy of prematurity (ROP) in 2 randomized trials. Pharmacokinetic (PK) studies in extremely preterm infants are needed prior to efficacy trials.
Methods
Infants of 23–29 weeks gestation were randomized to a single intravenous (IV) dose of inositol at 60 or 120 mg/kg or placebo. Over 96 h, serum levels (sparse sampling population PK) and urine inositol excretion were determined. Population PK models were fit using a nonlinear mixed effects approach. Safety outcomes were recorded.
Results
A 1-compartment model that included factors for endogenous inositol production, allometric size based on weight, gestational age (GA) strata and creatinine clearance fit the data best. The central volume of distribution was 0.5115 l/kg, the clearance 0.0679 l/kg/h, endogenous production 2.67 mg/kg/h and the half life 5.22 h when modeled without the covariates. During the first 12 h renal inositol excretion quadrupled in the 120 mg/kg group, returning to near baseline after 48 h. There was no diuretic side-effect. No significant differences in adverse events occurred between the 3 groups (p > 0.05).
Conclusions
A single compartment model accounting for endogenous production satisfactorily described the PK of IV inositol.
INTRODUCTION
Inositol is ubiquitous in living organisms where it is largely present as a free sugar alcohol and also as a headgroup of membrane lipids. In addition, phosphoinositides and glycosyl-phoshatidylinositols have specific roles in signal transduction and in lipid-protein interactions (1–4). In its free form, inositol is a sugar alcohol present in human milk, widely available in the diet and classified as “Generally Regarded as Safe” for enteral administration by the Food and Drug Administration (FDA). In utero, early fetal serum inositol levels are 2–10 times higher than adult levels, and decrease gradually towards term (5–7). Inositol has been supplemented in infant formulas since the late 1990s at approximately 44 mg /100 kcal (350 mg/l), yet its clearance has not been studied in extremely preterm newborns. If an infant is not receiving enteral milk feedings, serum levels fall to levels substantially below those that would have been present in utero (5,6).
Hallman et al administered myo-inositol intravenously (IV) and/or enterally to preterm infants with respiratory distress syndrome (RDS) in two randomized trials and found increased survival, increased survival without bronchopulmonary dysplasia (BPD), reduced severe retinopathy of prematurity (ROP), reduced severe intraventricular hemorrhage (IVH) and no observed toxicity (8–11). An additional partially randomized trial of inositol supplemented formula in preterm infants (12) was also considered in the Cochrane review of inositol that concluded these findings warrant further study, particularly in today's younger gestation infants who remain at highest risk for these morbidities (13).
The endogenous production and metabolism of inositol, combined with dietary intake add complexity to a pharmacokinetic (PK) analysis. However, to guide dosing of IV inositol as a potential treatment in extremely preterms for further study, we need to better understand its disposition in this population.
RESULTS
Population Demographics
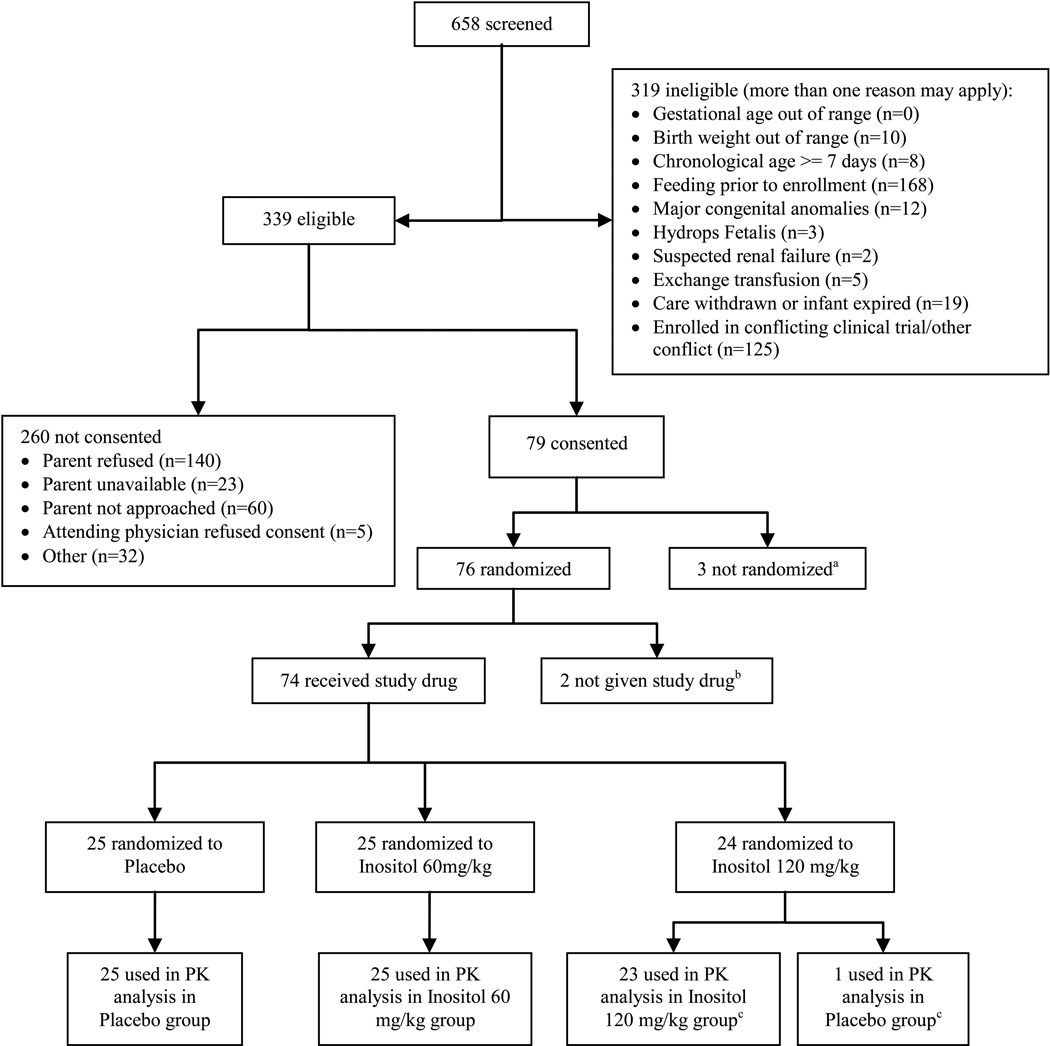
Figure 1 shows the number of infants screened, eligible and enrolled between June 2006 and December 2007 at 10 participating centers. Consent was obtained for 79, 76 were randomized, and 74 received study drug. Two infants did not complete the minimum of 4 specified blood samples (3 post drug infusion) and their randomizations were replaced with two additional enrollees from the same center and gestational age (GA) stratum, per protocol. Available data from the 2 replaced infants were included in the PK and safety analyses. One infant received placebo instead of the assigned 120 mg/kg dose, and for the PK analysis, this infant’s serum and urine data were included in the placebo group. However, this subject’s data on adverse events and clinical outcomes were included as randomized (intention to treat). The baseline characteristics of the enrolled infants were similar across all three groups, and the median ages at study drug infusion by group were between 2.4 and 3.2 days (Table 1).
Figure 1. Consort Diagram: Flow of enrolled, randomized and analyzed subjects.
a Two infants started feeding prior to randomization; 1 infant’s condition worsened prior to randomization resulting in loss of vascular access required for drug administration.
b Two infants identified as ineligible post-randomization (1 had < 600 grams birth weight, 1 developed severe IVH post-consent)
c One infant received placebo in error, samples included as placebo in the pharmacokinetic (PK) analyses, however clinical outcomes included as intention to treat.
Table 1.
Baseline Characteristics of Enrolled Infants by Treatment Group
| Characteristic | Placebo (N=25) |
Inositol 60 mg/Kg (N=25) |
Inositol 120 mg/Kg (N=24) |
P-value | |
|---|---|---|---|---|---|
| Gestational Age (weeks) | Median (range) | 26.9 (24.0–29.3) | 26.7 (23.7–29.7) | 27.1 (23.7–29.7) | 0.98 |
| Gestational Age Stratum | 27–29 weeks, N (%) | 12 (48%) | 12 (48%) | 13 (54%) | 0.88 |
| Birth Weight (g) | Median (range) | 995 (610–1470) | 1020 (640–1340) | 968 (690–1495) | 0.95 |
| Gender | Female, N (%) | 12 (48%) | 14 (56%) | 11 (46%) | 0.75 |
| Race, Non-Hispanic White | N (%) | 11 (44%) | 12 (48%) | 12 (50%) | 0.91 |
| Antenatal steroids | N (% yes) | 20 (80%) | 21 (84%) | 20 (83%) | 0.92 |
| Chorioamnionitis | N (% yes) | 8 (32%) | 5 (20%) | 5 (21%) | 0.54 |
| Apgar score, 1 minutea | Median (range) | 4.5 (1–9) | 6.5 (0–9) | 6 (1–9) | 0.51 |
| Apgar score, 5 minutea | Median (range) | 7 (4–9) | 7 (0–9) | 8 (1–9) | 0.47 |
| Age (days) at infusion | Median (range) | 3.2 (1.4–5.2) | 3.1 (1.4–5.1) | 2.4 (1.4–4.9) | 0.22 |
missing Apgar scores: infant born in ambulance (n=1); infant born in Mexico (n=1)
Safety
During the infusions, heart rate, blood pressure, and respiration did not differ between placebo and inositol infants at either dose (data not shown). The incidence of at least one adverse event was lowest for the higher dose of inositol: 80% in the placebo, 84% in the 60 mg/kg, and 54% in the 120 mg/kg group (p=0.05), (Table 2). The frequencies of sepsis, IVH, need for supplemental oxygen, need for mechanical ventilation and use of the specified medications did not differ significantly among the groups, nor did early specified clinical diagnoses during the first 4 days after infusion (Table 2). Serious adverse events occurred in 29% of all subjects, and were lowest in the 120 mg/kg group (17%, p=0.11), (data not shown, tables available from the authors). During hospitalization there were no significant differences between groups in the rates of expected preterm diagnoses as a whole (Table 3), nor between groups in the upper or lower GA strata. Clinical event rates were similar to historic data observed in this population in the NICHD Neonatal Research Network (14).
Table 2.
Clinical outcomes through Day 7 after infusion by treatment group
| Characteristic | Placebo (N=25) |
Inositol 60 mg/Kg (N=25) |
Inositol 120 mg/Kg (N=24) |
P-value | |
|---|---|---|---|---|---|
| Severe Adverse Events | |||||
| Died within 7 days of infusion | Yes (%) | 0 | 1 (4%) | 0 | >0.99 |
| Adverse events | |||||
| During Infusion | N | 25 | 25 | 24 | |
| At least 1 event | Yes | 4 (16%) | 3 (12%) | 1 (4%) | 0.52 |
| Total # events per infant | Median (range) | 0 (0–2) | 0 (0–2) | 0 (0–1) | 0.38 |
| After Infusion | N | 25 | 25 | 24 | |
| At least 1 event | Yes | 20 (80%) | 21 (84%) | 13 (54%) | 0.05 |
| Total # events | Median (range) | 2 (0–12) | 3 (0–16) | 1 (0–14) | 0.11 |
| Within 2 hours | |||||
| Apnea | Yes (%) | 1 (4%) | 1 (4%) | 2 (8%) | 0.69 |
| Perfusion change | Yes (%) | 3 (12%) | 1 (4%) | 1 (4%) | 0.52 |
| Clinical conditions within 4 days of infusion: | |||||
| Sepsis | n (%) | 2 (8%) | 1 (4%) | 3 (13%) | 0.52 |
| PDAa | n (%) | 8 (32%) | 5 (20%) | 7 (29%) | 0.65 |
| IVHb, clinical | n (%) | 1 (4%) | 2 (8%) | 1 (4%) | >0.99 |
| IVHb, imaged | n (%) | 3 (12%) | 4 (16%) | 4 (17%) | 0.92 |
| On supplemental oxygen | n (%) | 20 (80%) | 21 (84%) | 20 (83%) | >0.99 |
| On ventilator | n (%) | 16 (64%) | 19 (76%) | 15 (63%) | 0.56 |
| Concurrent Medications in 4 days after infusion (Study days 1–4): | |||||
| Surfactant | n (%) | 4 (16%) | 1 (4%) | 3 (13%) | 0.42 |
| Dopamine or Dobutamine | n (%) | 2 (8%) | 5 (20%) | 5 (21%) | 0.42 |
| Antibiotics | n (%) | 21 (84%) | 22 (88%) | 18 (75%) | 0.48 |
| Indomethacin, for IVHb prophylaxis | n (%) | 6 (24%) | 6 (24%) | 6 (25%) | >0.99 |
| Indomethacin, PDAa treatment | n (%) | 7 (28%) | 3 (12%) | 6 (25%) | 0.41 |
| Systemic Steroids | n (%) | 2 (8%) | 1 (4%) | 1 (4%) | >0.99 |
| Diuretics | n (%) | 3 (12%) | 7 (28%) | 5 (21%) | 0.37 |
PDA patent ductus arteriosus
IVH intraventricular hemorrhage
Table 3.
Clinical Outcomes and Adverse Events by Treatment group
| Characteristic a | Placebo (N=25) |
Inositol 60 mg/Kg (N=25) |
Inositol 120 mg/Kg (N=24) |
P-value | ||
|---|---|---|---|---|---|---|
| Died prior to discharge | n Yes (%) |
25 3 (12%) |
25 4 (16%) |
24 3 (12%) |
>0.99 | |
| BPD b (On O2 at 36 weeks PMA c) | n Yes (%) |
23 3 (13%) |
21 6 (29%) |
21 9 (43%) |
0.09 | |
| Days on Oxygen | n | 25 | 25 | 24 | 0.22 | |
| Median (range) | 32 (1–108) | 32 (1–120) | 53 (0–120) | |||
| PDA d | n Yes (%) |
25 13 (52%) |
25 7 (28%) |
24 11 (46%) |
0.20 | |
| If PDA d, Indomethacin Rx | n Yes (%) |
13 7 (54%) |
7 4 (57%) |
11 9 (82%) |
0.36 | |
| If PDA d, surgical ligation | n Yes (%) |
13 2 (15%) |
7 3 (43%) |
11 4 (36%) |
0.35 | |
| IVHe grade 3,4 | n Yes (%) |
25 3 (12%) |
24 5 (21%) |
23 1 (4%) |
0.22 | |
| Seizures treated > 72 hours | n Yes (%) |
25 0 |
25 1 (4%) |
24 2 (8%) |
0.31 | |
| Sepsis, late onset | n Yes (%) |
25 7 (28%) |
25 7 (28%) |
24 13 (54%) |
0.09 | |
| NEC f IIA or worse | n Yes (%) |
25 5 (20%) |
25 2 (8%) |
24 2 (8%) |
0.49 | |
| If NEC f, had surgery | n Yes (%) |
5 2 (40%) |
2 1 (50%) |
2 1 (50%) |
>0.99 | |
| Spontaneous GI perforation, no NEC f | n Yes (%) |
25 0 |
25 2 (8%) |
24 0 |
0.32 | |
| Hearing test, failed both ears | n Yes (%) |
21 2 (10%) |
17 0 |
19 1 (5%) |
0.77 | |
| ROP g | Final Status Known Received Surgery for ROP (%) |
8 4 (50%) |
8 2 (25%) |
9 1 (11%) |
0.21 | |
number of subjects in the denominator shown because of variation
BPD bronchopulmonary dysplasia,
PMA postmenstrual age (=gestational age + chronologic age),
PDA patent ductus arteriosus,
IVH intraventricular hemorrhage,
NEC necrotizing enterocolitis,
ROP retinopathy of prematurity.
Pharmacokinetic Analyses
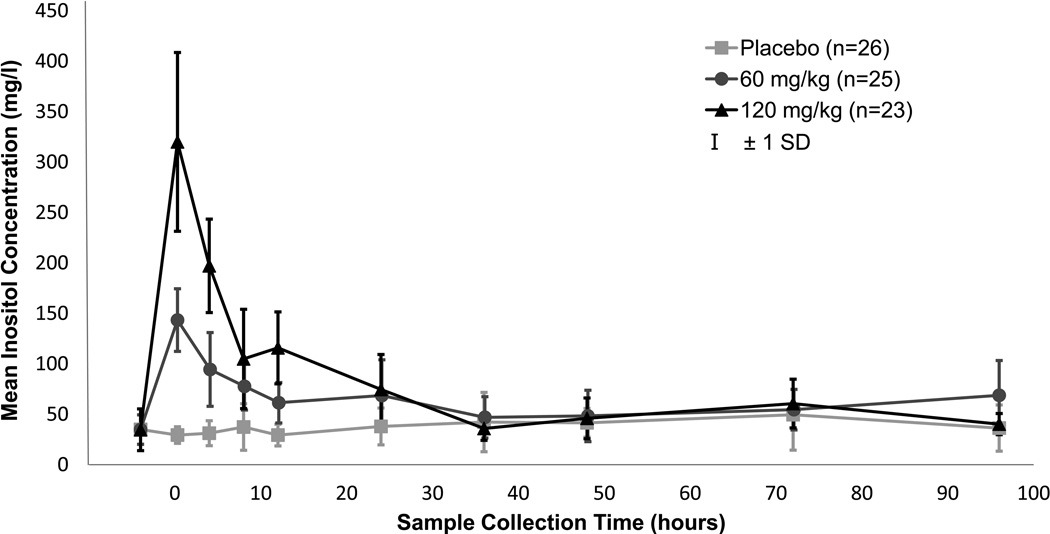
The raw mean serum levels rose in proportion to the dose given, gradually returned to baseline (Figure 2) and appeared consistent with the compartmental model under consideration. The pharmacokinetic analysis, which included endogenous production, revealed no improvement between 1 and 2 compartment models (p=0.38), so a 1 compartment model was used. In the measurement of residual error, a constant variance best fit the data. The relationships between the random effects were graphically studied by plotting uvi vs. uCli, uVi vs. uRi and uCli vs. uRi for all infants. A strong linear relationship was observed between the random effect estimates for clearance (Cl) and endogenous production (R) with no apparent relationship between the other two combinations of random effects. The random effects were then modeled only with correlation between Cl and R.
Figure 2. Serum Inositol Levels by Dose Group.
Samples were collected within scheduled windows, plus additional scavenged samples as available. For this graph, collection times were clustered as follows to obtain mean values: 0 h (baseline) = obtained prior to infusion; 0.3 h (end of infusion) = 0–2 h post-infusion; 4 h = 2–6 h post-infusion; 8 h = 6–10 h post-infusion; 12 h = 10–14 h post-infusion; 24 h= 14–30 h post-infusion; 36 h = 30–42 h post-infusion; 48 h = 43–60 h post-infusion; 72 h = 60–82 h post-infusion; 96 h = >82 h post-infusion. Square = placebo, circle = 60mg/kg, triangle= 120mg/kg, vertical bar = ± 1 SD.
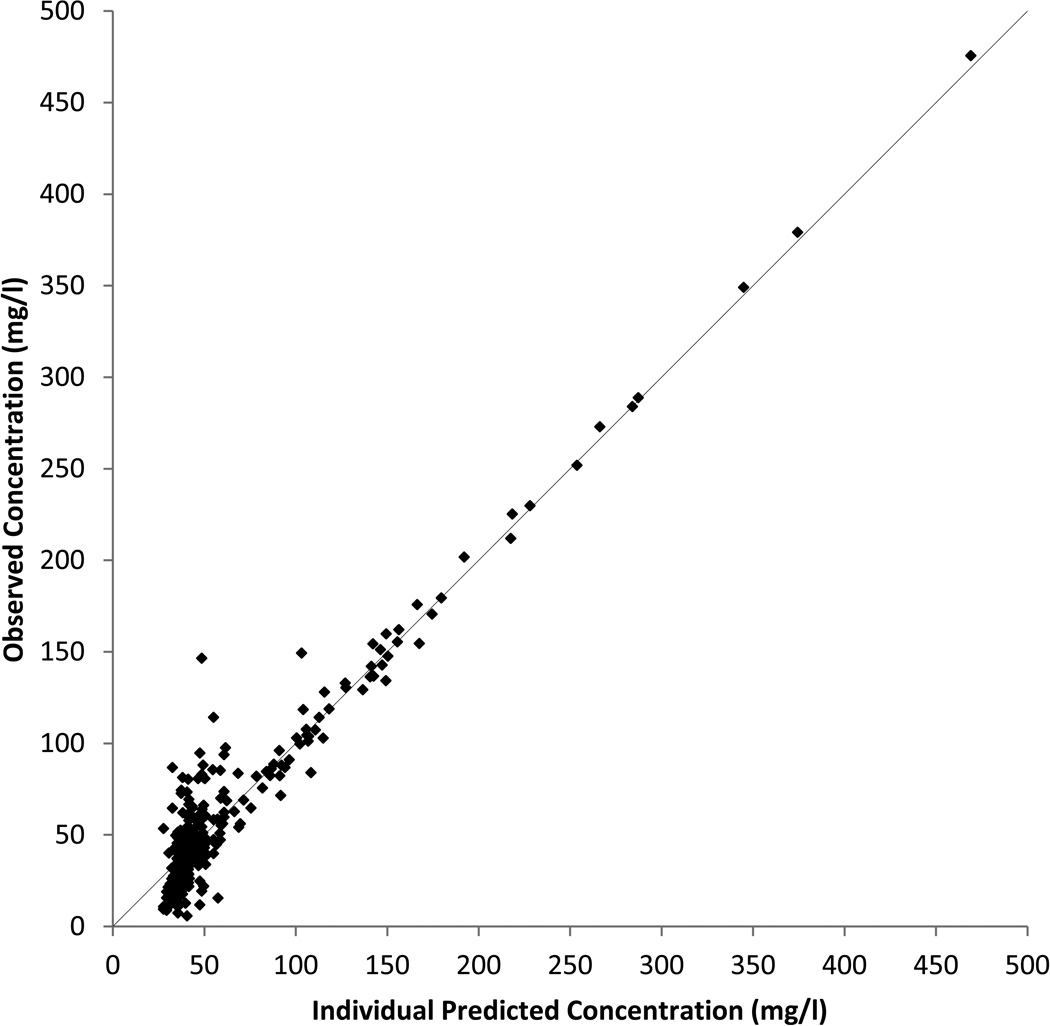
Table 4 presents the population-PK (Pop-PK) estimates for the 1-compartment model excluding any covariates with the associated random effect variance and correlation estimates in Table 5. Derived values for the elimination rate, the half-life and the apparent concentration associated with endogenous production are also shown in Table 4. The model appears to provide a good fit to the data as shown in Figure 3 with the observed and individual predicted values being well aligned. The mass of data points at the low values are primarily comprised of pre-dosing and placebo measurements combined with a smaller number of late time point measurements, all of which are expected to have low inositol concentrations. Residual plots comparing predicted and actual values, not included here, did not indicate any major model deficiencies.
Table 4.
Population Pharmacokinetic Parameter Estimates for a Typical Infant (Fixed Effects)
| Parameter | Units | Estimate | Standard Error |
|---|---|---|---|
| Model Parameters | |||
| V (volume) | L/kg | 0.5115 | 0.0345 |
| Cl (clearance) | L/kg/h | 0.0679 | 0.0064 |
| R (endogenous infusion rate) | mg/kg/h | 2.666 | 0.2762 |
| Standard deviation of the residual error | mg/L | 18.71 | 1.048 |
| Derived Values | |||
| k (elimination rate; Cl/V) | 1/h | 0.133 | 0.0154 |
| t1/2 (half-life; 0.693/k) | h | 5.22 | 0.605 |
| E (endogenous concentration; R/Cl) | mg/L | 39.25 | 1.655 |
Table 5.
Population Pharmacokinetic Random Effects Variances and Correlations
| Volume (uV) | Clearance (uCl) | Endogenous Infusion Rate (uR) |
|
|---|---|---|---|
| Volume (uV) | 0.08506 | - | - |
| Clearance (uCl) | 0.0a | 0.22636 | - |
| Endogenous Infusion Rate (uR) | 0.0 a | 0.87606 | 0.14492 |
Random effect variances on the diagonal and correlations between the random effects are displayed on the off diagonal.
Correlation set to 0.0 (zero) based upon review of plots of uVi vs. uCli, and uVi vs. uRi.
Figure 3. Observed vs. Individual Predicted Inositol Concentrations from the Pop-PK Analysis.
Predicted values were calculated for each observed data point using the individual characteristics in the model described in the PK section of the text.
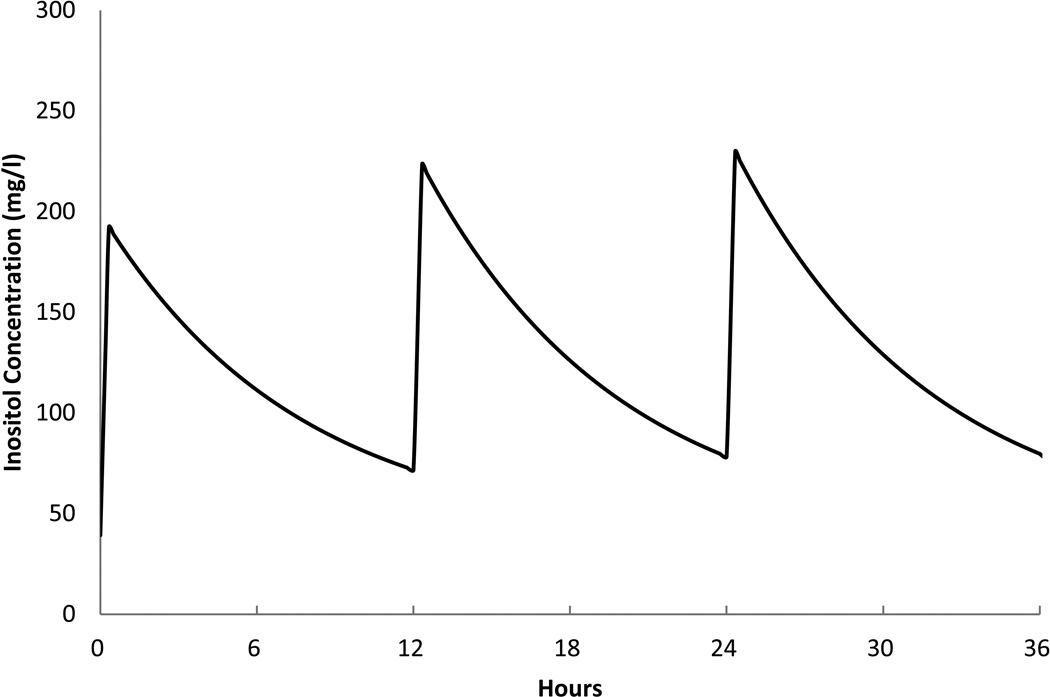
This model was used to assist the investigators to project the effect on serum levels of inositol from repeated dosing with a q12 h or q 24 h schedule using several different daily doses. As an example, Figure 4 shows the model's prediction for 80 mg/kg/day divided into two doses q12 h, over 36 h. While this study did not directly evaluate multiple dose administrations, the model's estimate represents probable serum levels over a short time period for planning future studies of repeated administration.
Figure 4. Predicted Serum Inositol Model.
The model described in the PK section (before covariates) was used to predict the pattern of serum levels for a typical infant given repeated doses of 80 mg/kg/day divided into 40 mg/kg every 12 h for 36 h.
The effect of the following covariates on each of the model parameters was tested: infant birth weight, allometric size at birth, GA strata at birth (23–26 weeks vs. 27–29 weeks), postmenstrual age, postnatal age, creatinine clearance and sex (15). Allometric size, a function of birth weight, entered the model for a parameter as a multiplicative scaling factor given by Fsize = (wi/w̃)βsize where wi is the birth weight, in grams, for the i-th infant, w̃ is the median birth weight of the infants in the study (997.5 g) and βsize is the estimated coefficient. The remaining covariates had an exponential multiplicative effect given by Fz = eβz×z where z is one of the covariates. Table 6 presents the mean and standard deviations for the continuous covariates while the two categorical covariates were both equally divided with 50% in each of their two categories.
Table 6.
Covariate Means and Standard Deviations
| Covariate | Mean | Standard Deviation |
|---|---|---|
| Birth weight (grams) | 986.8 | 245.0 |
| Size at birth (wi/w̃) | 0.989 | 0.246 |
| Postmenstrual age (weeks) | 27.3 | 1.72 |
| Postnatal age (weeks) | 0.44 | 1.15 |
| Creatinine clearance (ml/min) | 0.69 | 0.34 |
Each covariate was tested individually against the base model. Those covariates found to have a statistically significant effect on any of the three Pop-PK model parameters, R, Cl and V(V = volume of distribution), were then combined into a single model. Birth weight, postmenstrual age, postnatal age and sex had no significant effects on the model parameters. Allometric size, GA strata and creatinine clearance were found to have a statistically significant effect on one or more of the three model parameters (Table 7). The final model including all of the statistically significant covariates indicates that infants with a GA of 27–29 weeks had a lower volume of distribution than infants with a lower GA of 23–26 weeks, while clearance increased with increasing allometric size. The pathway of influence for creatinine clearance was less clear. When its effect was considered separately on each of the model parameters, V, Cl and R, it was statistically significant for Cl and R (p=.044 and p=.014, respectively) with a direction of effect that was physiologically plausible, increasing for Cl and decreasing for R. However, when creatinine clearance was allowed to simultaneously affect both Cl and R, then its direction of effect on Cl was the opposite of that expected with increased creatinine clearance leading to a reduction in Cl. In addition, the model with creatinine clearance affecting R alone was not significantly different from the model with creatinine clearance affecting both Cl and R (p=.292). Thus, the final covariate model included the effect of creatinine clearance solely on R. The estimated typical value for each of the three model parameters were affected as follows by the final covariate model:
where V*, Cl* and R* are the covariate adjusted model parameters and I(GA) is an indicator function that equals 0 (zero) for GA stratum 23–26 weeks and 1 (one) for stratum 27–29 weeks.
Table 7.
PK Model Parameter Estimates for a Typical Infant (Fixed Effects)
| Model Label | Parameter Name a |
Parameter Estimate (std. error) |
Covariate Estimate (standard error) |
−2 log likelihood (p-value e) |
||
|---|---|---|---|---|---|---|
| log(Size)b | GA Stratac | Creatinine Clearanced |
||||
| Base Model | Volume (V) | 0.5115 (0.0345) | 2708.2 (NA) | |||
| Clearance (Cl) | 0.0679 (0.0064) | |||||
| Endogenous Production (R) | 2.666 (0.2762) | |||||
| log(Size) | Volume (V) | 0.5177 (0.0359) | 2702.3 (0.02) | |||
| Clearance (Cl) | 0.0661 (0.0059) | 0.3544 (0.1460) | ||||
| Endogenous Production (R) | 2.542 (0.2566) | |||||
| GA Strata | Volume (V) | 0.5937 (0.0546) | −0.2776 (0.1286) | 2704.1 (0.04) | ||
| Clearance (Cl) | 0.0660 (0.0059) | |||||
| Endogenous Production (R) | 2.572 (0.2566) | |||||
| Creatinine Clearance | Volume (V) | 0.5096 (0.0341) | 2701.1 (0.03) | |||
| Clearance (Cl) | 0.0671 (0.065) | |||||
| Endogenous Production (R) | 3.273 (0.430) | −0.3252 (0.1260) | ||||
| All Covariates | Volume (V) | 0.5924 (0.0539) | −0.2777 (0.1257) | 2697.4 (0.03) | ||
| Clearance (Cl) | 0.0549 (0.0120) | 0.2158 (0.1963) | ||||
| Endogenous Production (R) | 3.040 (0.457) | −0.1898 (0.1583) | ||||
Units – V in L/kg, Cl in L/kg/h, R in mg/kg/h; where L: liters of serum, kg: kg’s of body weight, mg; mg’s of inositol, h: hours
log(Size): Natural logarithm of wi/w̃ where wi is the birth weight, in grams, for the i-th infant, w̃ is the median birth weight of the infants in the study (997.5 gm);
Gestational Age Strata: Reference cell 23–26 weeks; Comparison cell 27–29 weeks;
Creatinine Clearance: clearance in ml/min
p-value for testing the model including the covariates versus the base model without the covariates.
Urine excretion of inositol: Excretion in the placebo infants had a baseline rate of 26.6 ± 12.3 mg/kg/24 h (m ± sd) and showed an upward trend with age over the 96 h study to 36.8 ± 28.8 mg/kg/24 h on study day 4. Following inositol administration, excretion rose during the first 12 h collection and decreased in the second 12 h. Thereafter, excretion approached levels in the placebo group. Following the 120 mg/kg dose, average excretion was 67.3 ± 35.8 mg/kg in the first 12 h and 25.3 ± 14.4 mg/kg in the second 12 h. For the 60 mg/kg subjects 31.9 ± 13.4 mg/kg was excreted in the first 12 h and 18.0 ± 9.6 mg/kg in the second 12 h.
We examined the volume of urine output for evidence of a potential diuretic effect of the excreted inositol and found none. The mean volume of urine in the first 12 h after drug dosing when excretion was the highest was 40.7 ml/kg/12 h in placebo infants, 37.8 ml/kg/12 h in the 60 mg/kg group and 46.2 ml/kg/12 h in the 120 mg/kg group respectively, (p-value=0.1242 Kruskal-Wallis test).
DISCUSSION
These findings demonstrate among preterm infants of 23–29 weeks gestation that a single IV dose of 60 mg/kg or 120 mg/kg inositol given over 20 minutes results in an initial dose proportional increase in serum concentrations that persists between 24 to 36 h. Accommodating a parameter for endogenous production, the PK analysis showed a central volume of distribution of 0.5115 l/kg with a half life of 5.22 h when excluding any covariate effects. While increased urine inositol losses initially occurred, this did not result in a diuresis.
Previous controlled trials suggest supplemental inositol is safe and beneficial for preterm infants. The serum levels achieved in the key study demonstrating benefit was 126–153 mg/l during the first week of treatment (11). In this study inositol also appeared safe, however this study’s power to detect differences in uncommon adverse events is limited by the small sample size, and infants received only a single dose. While the higher rates of BPD and sepsis in the highest dose group were unexpected, have not been previously reported, and are not statistically significant, it will be important to monitor these event rates during future studies. To date, reported studies do not describe IV inositol supplementation for more than 8 days.
Hallman et al observed an apparent steady state serum concentration after the first of 5 days of 80 mg/kg/day dosing (11). The fall in serum levels in the 'wash out' period in the Hallman study suggested a half-life of 4–5 days (11), which differs substantially from our estimate (5.22 h) and is not explained, although fully established enteral intake of human milk in the Hallman studies may have contributed to supporting the serum levels despite discontinuing IV dosing. A PK study conducted with repeated dosing and a wider range of doses should help resolve these findings.
Brown and colleagues found that the plasma rate of appearance of inositol in term and late preterm infants was 121.7 mg/kg/day, well in excess of the usual daily enteral intake from preterm formulas or human milk (approximately 54 mg/kg/day) (16). Endogenous production from glucose occurs in many tissues, and enterally received inositol is actively transported across the intestinal mucosa where it is absorbed almost completely (17,18). The catabolic enzyme for inositol, myo-inositol oxidase, is localized almost exclusively to the renal cortex (19), but the activity of this enzyme is very low in the fetal and newly born renal cortex. It rises rapidly after birth as the renal blood flow redistributes from the renal medulla to the cortex. Thus, urinary losses of intact inositol are high in both the term and preterm newborn, but gradually fall to very low levels in the weeks following birth (4,20). Despite these developmental physiologic mechanisms that affect inositol concentrations, serum levels remain responsive to dietary intake decreasing in both tissue and serum when diets are low in inositol, and rising with supplemented diets (4).
We speculate that immature renal tubular transport at these stages of development is inadequate to reabsorb the amount of inositol filtered by the glomerulus, similar to glucose in extremely low birth weight newborns. The amount of filtered inositol reaching the renal tubules over a short period of time exceeded renal tubular reabsorption so the higher dose caused a greater renal loss. This pattern of renal excretion is expected to change rapidly postnatally, and could lead to reabsorption of a greater percentage of the dose and higher circulating concentrations rather than to faster clearance and shorter half-lives which occurs with most drugs administered to preterm newborns during maturation. With the expected increase in the catabolic myo-inositol oxidase enzyme in the renal cortex in the weeks after birth, remaining filtered inositol will likely be converted to d-glucuronic acid and no longer appear in the urine (19). These observations and remaining uncertainties are important to the design of future multi-dose efficacy trials. We plan to examine daily administration of inositol over longer periods of time. Different doses will be evaluated, and divided doses will be used in an attempt to reduce peak serum levels and therefore reduce urine losses. As we increase our understanding of how to influence serum levels, we can better test inositol’s safety, and potential to benefit extremely preterm infants.
METHODS
Design
A randomized, double-masked, placebo-controlled pharmacokinetic trial with sparse blood sampling was conducted by the Eunice Kennedy Shriver National Institutes of Child Health and Human Development Neonatal Research Network (NRN). Ten of the NRN Centers participated and enrolled subjects between June 2006 and December 2007. Randomization was performed centrally via computer within two pre-specified GA strata (230/7–266/7 weeks, and 270/7–296/7 weeks), and infants were monitored prospectively for toxicity and clinical outcomes during hospitalization.
Population
Eligible subjects were 230/7–296/7 weeks gestation, ≥ 600 g birth weight, had no major congenital anomalies, were between 12 h and 6 days of age at randomization, and had received no human milk or formula feedings since birth.
Intervention
Inositol was given as a single low (60 mg/kg) or high (120 mg/kg) dose of 5% myo-inositol (referred to as 'inositol' in the remaining text) intravenously over 20 minutes in a 1:1:1 randomization with placebo delivered in one of two volumes to maintain masking (5% glucose, USP, for IV administration). Drug or placebo was dispensed from the respective pharmacies in unit doses labeled as "inositol study drug", and all clinical and research personnel except for the pharmacist were masked to the study group. Inositol (hexahydroxycyclohexane) is manufactured from corn or rice bran and was provided as an isotonic, pyrogen and preservative-free, sterile 5% solution of myo-inositol in water containing 0.5 g sodium chloride per liter (8.55mM), pH 6.5–7.5.
Outcome Variables
Infants were continuously observed during the infusion by study personnel and vital signs recorded every 5 minutes for the first 30 minutes and thereafter at 15–30 minute intervals for 2 h. Six pre-specified clinical conditions and 7 concomitant medications were recorded for 4 days following infusion (culture positive sepsis, documented patent ductus arteriosus (PDA), clinically diagnosed PDA, supplemental oxygen, mechanical ventilation, (IVH); and surfactant, dopamine/dobutamine, antibiotics, indomethacin as prophylaxis for IVH, indomethacin as a treatment for PDA, systemic steroids, and diuretics).
Adverse events were recorded for 7 days and severity determined using a toxicity table modified for neonatal use from the National Cancer Institute. Baseline characteristics and demographic data as well as neonatal morbidities from birth through hospital discharge (or 120 days if sooner) were recorded from the medical record using the definitions of the Neonatal Research Network generic database. BPD was defined as receiving oxygen at 36 weeks post menstrual age (or at discharge if discharged before 36 weeks postmenstrual age (PMA));
IVH was classified according to Papile (21); late onset sepsis was defined as a positive culture from a normally sterile body fluid obtained after 72 h of age; and necrotizing enterocolitis (NEC) was defined as either Modified Bell’s IIA or worse not needing surgery, or requiring surgery (IIIB) (22).
Blood Sampling
Each subject contributed 4 blood samples (predose plus three post dose samples) according to a sparse sampling, Pop PK design (23,24). The pre-dose sample was within 4 h before infusion; and the 3 post dose samples were distributed among 9 sample windows: within 4 minutes of the end of infusion, 4±1, 8±1, 12 ±1, 24±2, 36±2, 48±2, 72±2 and 96±2 h after the start of the infusion. In addition, some scavenged serum samples from the clinical laboratories were obtained, with consent, to supplement the scheduled samples. The exact time of sampling in relation to the end of drug infusion was recorded for all samples. Blood (200 µL) was collected in serum separator micro-tubes, refrigerated until centrifuged, up to 60 h after sampling, and frozen at −70°C to −80°C until analysized. (Stability data at room temperature for up to a week are available from the authors upon request.)
Urine Collections
Six consecutive urine collections starting with the 8–12 h before the study drug were collected continuously for 96 h post infusion (the second two for 12 h each, then three for 24 h each). Urine volume was determined by the change in weight of each pre-weighed non-gel containing diaper/cotton balls within the given 12 or 24 h time period, assuming 1 g = 1ml. Urine was expressed from the diapers/cotton balls and pooled within each timed collection, and inositol and creatinine were determined on an aliquot of the pooled sample. Serum creatinine was measured at least twice during the 96 h by each institution’s clinical labs.
Enteral Inositol Intake
Following drug infusion, enteral intake was permitted when the infant was considered ready to begin feedings by the clinical team. Enteral intake (type and volume each day) was recorded for 4 days beginning with the day of study drug infusion. Samples of ingested milk were assayed for inositol to permit estimation of additional inositol intake during the sampling period.
Assay
Inositol isomers (myo-inositol, 1,5-anhydro-D-sorbitol, and d-chiro-inositol) were quantified from 25 or 50 µL samples using high performance liquid chromatography with electrochemical (pulsed amperometry) detection (method and validation documents available upon request from http://www.ttuhsc.edu/sop/research/internalgrants/CPET.aspx., Leff RD). Results of validation experiments showed the following assay performance parameters: lower limit of quantitation 1.0 mg/l; coefficient of variation from low to high concentrations was 10.2% to 13.4% between days, and 1.9% to 2.3% within a day. Validated test samples included serum; plasma collected using EDTA, sodium heparin, or lithium heparin; urine extracted from any of 5 common non-gel preterm infant diaper types with or without cotton balls; human milk and infant formulas. Serum, human milk and formulas contained only non-detectable or only barely detectable levels of the D-sorbitol and d-chiro-inositol isomers. Therefore, only myo-inositol levels are reported. The concentration of inositol in formulas was 356 (67) mg/l (m (±sd)), and in human milk was 287 (107) mg/l.
Statistical Analysis
The planned sample size was at least 36 infants in each GA stratum who received study drug and completed 4 blood samples. Baseline characteristics and clinical outcomes for all randomized infants were analyzed by study group using Chi Square tests, Fisher’s exact tests, ANOVA, or Kruskal-Wallis tests, where appropriate.
Pharmacokinetic Analysis
Pop-PK models were fit to the data using the nonlinear mixed effects approach in Monolix (Monolix Version 3.2. LIXOFT http://www.lixoft.com/, Paris, France, 2010). This approach accounts for the variability between infants in the model parameters, the correlation between measurements on the same infant at different occasions, as well the residual unexplained variability in serum concentrations (25).
As noted, inositol is produced endogenously and is present in human milk and infant formulas (26). Hence, the Pop-PK model had to account for an endogenous serum concentration of inositol not related to treatment. Apparent endogenous inositol production was included in the Pop-PK model similarly to Hayashi (27). The final model combines apparent endogenous production with a one-compartment IV-infusion model with linear elimination. The model for serum concentration is then
where, for the i-th infant, Ci(t) is the serum concentration at time t, Ri the apparent rate of inositol infusion due to the combination of endogenous production and feeding, Cli the clearance and Vi the apparent volume of distribution. T is the duration of the infusion period and t is the time after the start of the infusion, both in h; finally, εit is the residual error at time t. The steady-state endogenous concentration is then given by Ei = Ri/Cli for the i-th infant, which is used to measure the combined effect of endogenous inositol production and inositol intake from enteral feeding. It was not possible to separate these two sources of inositol since enteral intake was measured as a total amount fed over a day and not the amount fed at each occasion (feedings are given 8 times throughout the 24 h day). In addition, the enteral intake of inositol was very low during the 4 day study. There was minimal enteral intake of inositol in the first 12 h after the IV dose, and a maximum of 3.6 mg/kg in the first 24 h. Only 49% of subjects received any enteral intake in the 2nd 24 h period (mean intake of inositol 1.8 mg/kg/d, range 0–20) and the mean intakes on days 3 and 4 were 4.5 and 5.5 mg/kg, respectively. Because the calculated enteral intake of inositol from human milk or formula among those able to receive some feedings was so low during the first two study days, measured enteral intake was not included as a separate source in these models.
The between infant variability in the Pop-PK model parameters, Ri, Cli and Vi, is modeled using random effect variables (uR, uCl and uV) that approximate the individual trajectory over time of each infant’s concentration. The random effects are assumed to be normally distributed with means of 0 (zero) and variances and correlations that will be estimated. For example, the clearance for the i-th infant is modeled as Cli = Cl × euCli where Cl is the fixed-effect common to all infants and uCli is the random effect unique to the i-th infant. A similar formulation is used for Ri and Vi. Thus, the three model parameters are log-normal. Individual specific parameter estimates were obtained as the conditional modes, or the maximum a posteriori, of the Bayes estimates of the parameters. The fixed effects, R, Cl and V, are the median values of the parameters and are called the typical values for the population from which each infant’s parameters are derived. The residual error, εit, is assumed to be uncorrelated with the random effects and normally distributed with mean 0 (zero) and a variance that will be estimated. The quality of fit of a Pop-PK model was judged by visual examination of plots of observed vs. individual predicted concentrations and of residuals vs. individual predicted concentrations. Nested models were compared by referencing the improvement in the objective function (−2 log-likelihood) against the chi-square distribution with degrees of freedom equal to the difference in the number of estimated parameters between two models. Statistical significance was assessed at the level of p ≤ 0.05.
Ethical oversight
The institutional review boards of each center approved the protocol, and written informed consent was obtained for each participant. An independent Data Safety Monitoring Committee approved the protocol and monitoring plan before the study began and monitored the accumulating safety data at regular intervals. The FDA approved protocol (IND # 70510) was registered with ClinicalTrials.gov (NCT00349726).
Supplementary Material
ACKNOWLEDGMENTS
We are indebted to our medical and nursing colleagues and the infants and their parents who agreed to take part in this study. The following investigators, in addition to those listed as authors, contributed to this study and are listed in an online listing.
Funding:
The National Institutes of Health provided grant support through the Eunice Kennedy Shriver National Institute of Child Health and Human Development’s (NICHD) Neonatal Research Network and the Pediatric Pharmacology Research Units Network, with co-funding from the National Eye Institute and with support from the National Center for Research Resources, and the National Center for Advancing Translational Sciences. The content of the publication is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additional participants and grant numbers are cited in the Acknowledgments online.
The NICHD Pediatric Pharmacology Research Unit (PPRU) network provided support and advice in designing the PK study (JVA, JVDA, AAV, SK), analyzing the data (SK), and conducting the Inositol Assay (RL): Jacob V. Aranda, MD PhD FRCPC, Wayne State University (U10 HD37261); John van der Anker, MD, Children's National Medical Center (U10 HD45993); Steven E. Kern, PhD, College of Pharmacy, University of Utah Medical Center; Alexander A. Vinks, PharmD PhD FCP, Cincinnati Children’s Hospital Medical Center, Department of Pediatrics, University of Cincinnati (U10 HD37249); Richard D. Leff, PharmD FCCP, Texas Tech University Health Sciences Center, Pediatric Pharmacology Research & Development Center (U10 HD46000).
Footnotes
Disclosures:
None of the authors report any commercial, proprietary, or financial interest in any of the products described in this article. Abbott Nutrition Division, Abbott Laboratories, supplied the Inositol drug used in the study. NICHD is the Sponsor of the study and holds the Investigational New Drug number (IND).
Portions of this study were presented at the 2010 Pediatric Academic Societies Annual Meeting, Vancouver, Canada, May 1–4, 2010.
REFERENCES
- 1.Michell RH. Inositol derivatives: evolution and functions. Nat Rev Mol Cell Biol. 2008;9:151–161. doi: 10.1038/nrm2334. [DOI] [PubMed] [Google Scholar]
- 2.Sauer K, Cooke MP. Regulation of immune cell development through soluble inositol-1,3,4,5-tetrakisphosphate. Nat Rev Immunol. 2010;10:257–271. doi: 10.1038/nri2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okazaki IJ, Moss J. Characterization of glycosylphosphatidylinositol-anchored, secreted, and intracellular vertebrate mono-ADP-ribosyltransferases. Annu Rev Nutr. 1999;19:485–509. doi: 10.1146/annurev.nutr.19.1.485. [DOI] [PubMed] [Google Scholar]
- 4.Holub BJ. Metabolism and function of myo-inositol and inositol phospholipids. Annu Rev Nutr. 1986;6:563–597. doi: 10.1146/annurev.nu.06.070186.003023. [DOI] [PubMed] [Google Scholar]
- 5.Carver JD, Stromquist CI, Benford VJ, Minervini G, Benford SA, Barness LA. Postnatal inositol levels in preterm infants. J Perinatol. 1997;17:389–392. [PubMed] [Google Scholar]
- 6.Bromberger P, Hallman M. Myoinositol in small preterm infants: relationship between intake and serum concentration. J Pediatr Gastroenterol Nutr. 1986;5:455–458. doi: 10.1097/00005176-198605000-00021. [DOI] [PubMed] [Google Scholar]
- 7.Brusati V, Jóźwik M, Jóźwik M, et al. Fetal and maternal non-glucose carbohydrates and polyols concentrations in normal human pregnancies at term. Pediatr Res. 2005;58:700–704. doi: 10.1203/01.PDR.0000180549.86614.73. [DOI] [PubMed] [Google Scholar]
- 8.Hallman M, Järvenpää A-L, Pohjavuori M. Respiratory distress syndrome and inositol supplementation in preterm infants. Arch Dis Child. 1986;61:1076–1083. doi: 10.1136/adc.61.11.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hallman M, Arjomaa P, Hoppu K. Inositol supplementation in respiratory distress syndrome: relationship between serum concentration, renal excretion, and lung effluent phospholipids. J Pediatr. 1987;110:604–610. doi: 10.1016/s0022-3476(87)80561-9. [DOI] [PubMed] [Google Scholar]
- 10.Hallman M, Pohjavuori M, Bry K. Inositol supplementation in respiratory distress syndrome. Lung. 1990;168:877–882. doi: 10.1007/BF02718223. [DOI] [PubMed] [Google Scholar]
- 11.Hallman M, Bry K, Hoppu K, Lappi M, Pohjauori M. Inositol supplementation in premature infants with respiratory distress syndrome. N Engl J Med. 1992;326:1233–1239. doi: 10.1056/NEJM199205073261901. [DOI] [PubMed] [Google Scholar]
- 12.Friedman CA, McVey J, Borne MJ, et al. Relationship between serum inositol concentration and development of retinopathy of prematurity: a prospective study. J Pediatr Ophthalmol Strabismus. 2000;37:79–86. doi: 10.3928/0191-3913-20000301-06. [DOI] [PubMed] [Google Scholar]
- 13.Howlett A, Ohlsson A, Plakkal N. Inositol for respiratory distress syndrome in preterm infants. Cochrane Database of Syst Rev (online) 2012;(3) doi: 10.1002/14651858.CD000366.pub2. CD000366. [DOI] [PubMed] [Google Scholar]
- 14.Stoll BJ, Hansen NI, Bell EF, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126:443–456. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meibohm B, Läer S, Panetta JC, Barrett JS. Population pharmacokinetic studies in pediatrics: issues in design and analysis. AAPS J. 2005;7:E475–E487. doi: 10.1208/aapsj070248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown LD, Cheung A, Harwood JEF, Battaglia FC. Inositol and mannose utilization rates in term and late-preterm infants exceed nutritional intakes. J Nutr. 2009;139:1648–1652. doi: 10.3945/jn.109.109108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caspary WF, Crane RK. Active transport of myo-inositol and its relation to the sugar transport system in hamster small intestine. Biochim Biophys Acta. 1970;203:308–316. doi: 10.1016/0005-2736(70)90145-8. [DOI] [PubMed] [Google Scholar]
- 18.Nahapetian A, Young VR. Metabolism of 14C-phytate in rats: effect of low and high dietary calcium intakes. J Nutr. 1980;110:1458–1472. doi: 10.1093/jn/110.7.1458. [DOI] [PubMed] [Google Scholar]
- 19.Troyer DA, Schwertz DW, Kreisberg JI, Venkatachalam MA. Inositol phospholipid metabolism in the kidney. Annu Rev Physiol. 1986;48:51–71. doi: 10.1146/annurev.ph.48.030186.000411. [DOI] [PubMed] [Google Scholar]
- 20.Lewin LM, Melmed S, Passwell JH, et al. Myoinositol in human neonates: serum concentrations and renal handling. Pediatr Res. 1978;12:3–6. doi: 10.1203/00006450-197801000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92:529–534. doi: 10.1016/s0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- 22.Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am. 1986;33:179–201. doi: 10.1016/S0031-3955(16)34975-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duffull S, Waterhouse T, Eccleston J. Some consideration on the design of population pharmacokinetic studies. J Pharmacokinet Pharmacodyn. 2005;32:441–457. doi: 10.1007/s10928-005-0034-2. [DOI] [PubMed] [Google Scholar]
- 24.Mahmood I, Duan J. Population pharmacokinetics with a very small sample size. Drug Metabol Drug Interact. 2009;24:259–274. doi: 10.1515/dmdi.2009.24.2-4.259. [DOI] [PubMed] [Google Scholar]
- 25.Bertrand J, Comets E, Mentré F. Comparison of model-based tests and selection strategies to detect genetic polymorphisms influencing pharmacokinetic parameters. J Biopharm Stat. 2008;18:1084–1102. doi: 10.1080/10543400802369012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holub BJ. The nutritional importance of inositol and the phosphoinositides. N Engl J Med. 1992;326:1285–1287. doi: 10.1056/NEJM199205073261909. [DOI] [PubMed] [Google Scholar]
- 27.Hayashi N, Kinoshita H, Yukawa E, Higuchi S. Pharmacokinetic analysis of subcutaneous erythropoietin administration with nonlinear mixed effect model including endogenous production. Br J Clin Pharmacol. 1998;46:11–19. doi: 10.1046/j.1365-2125.1998.00043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.