Abstract
Changes in sulfur-based redox metabolite profiles in multiple tissues of long-lived Snell dwarf mice were compared with age- and sex-matched controls. Plasma methionine and its oxidation products, hypotaurine and taurine, were increased in Snell dwarfs while cystine and glutathione levels were decreased, leading to an oxidative shift in the redox potential. Sexual dimorphism in renal cystathionine β-synthase (CBS) activity was observed in control mice but not in Snell dwarfs. Instead, female Snell mice exhibited ~2-fold higher CBS activity, comparable to levels seen in male Snell dwarf and in control mice. Taurine levels were significantly higher in kidney and brain of Snell dwarf versus control mice. Methionine adenosyltransferase (MAT) was higher in liver of Snell dwarfs, and the higher concentration of its product, S-adenosylmethionine, was correlated with elevated global DNA methylation status. Application of a mathematical model for methionine metabolism revealed that the metabolite perturbations in Snell dwarfs could be explained by decreased methionine transport, increased MAT and increased methyltransferase activity. Our study provides a comprehensive map of systemic differences in the sulfur network between Snell dwarfs and controls, providing the necessary foundation for assessment of nutrition-linked metabolic status in long-lived versus control animals.
Keywords: Snell dwarf mice, sulfur metabolism, redox, sexual dimorphism, global DNA methylation, mathematical model
1. Introduction
Since redox homeostasis is fundamentally important for cellular activities, it is not surprising that perturbations in redox balance are a common thread connecting the etiology of many complex diseases as well as natural cellular processes such as aging and cell death (Banjac et al., 2008; Bharath et al., 2002; Finkel, 2003; Menon and Goswami, 2007). However, what is generally missing in our understanding of oxidative stress-induced changes under natural and pathophysiological conditions is their specificity. Thus, while resistance to oxidative stress is a common denominator of life extension by caloric restriction and anti-aging mutations (Bokov et al., 2009; Harper et al., 2006; Hauck et al., 2002), the redox metabolic nodes involved in this response remain to be identified.
The sulfur metabolic network underlies cellular methylation and redox capacities and thereby influences these two important homeostatic mechanisms (Martinov et al., 2010). It connects the fate of the essential amino acid, methionine, to cysteine, via the transsulfuration pathway (Fig. 1). Alternatively, the methionine backbone can circulate in the methionine cycle furnishing S-adenosylmethionine (AdoMet)1, a major cellular methyl group donor. Dietary restriction of methionine, like caloric restriction, increases longevity in rodents (Miller et al., 2005; Orentreich et al., 1993; Richie et al., 2004). The cystine/cysteine couple represents an important redox node particularly in the extracellular compartment (Banerjee, 2012; Jones et al., 2004). Cysteine is a key junction intermediate and can be diverted to glutathione (GSH), taurine and H2S biosynthesis. GSH, a tripeptide, is a major antioxidant and the GSH/GSSG (oxidized glutathione) redox node is a significant determinant of the ambient redox poise, particularly in the intracellular compartment (Banerjee, 2012). Taurine is an oxidized sulfonic acid derivative of cysteine present at very high intracellular concentrations. It is believed to function as an organic osmolyte, but it is not known whether this is its only or even its major function (Huxtable, 1992). Another product of sulfur metabolism is H2S, a gaseous signaling molecule (Kabil and Banerjee, 2010), which is associated with increased lifespan in Caenorhabditis elegans (Miller and Roth, 2007). The transsulfuration pathway is needed for expression of the effects of dietary restriction on lifespan in Drosophila (Kabil et al., 2011). The expression and activity of CBS (cystathionine β-synthase), the first enzyme in the transsulfuration pathway, are increased in flies maintained on reduced nutrient conditions. Over-expression of CBS enhances lifespan in flies on an unrestricted diet, suggesting a role for CBS as a positive regulator of fruit fly lifespan (Kabil et al., 2011).
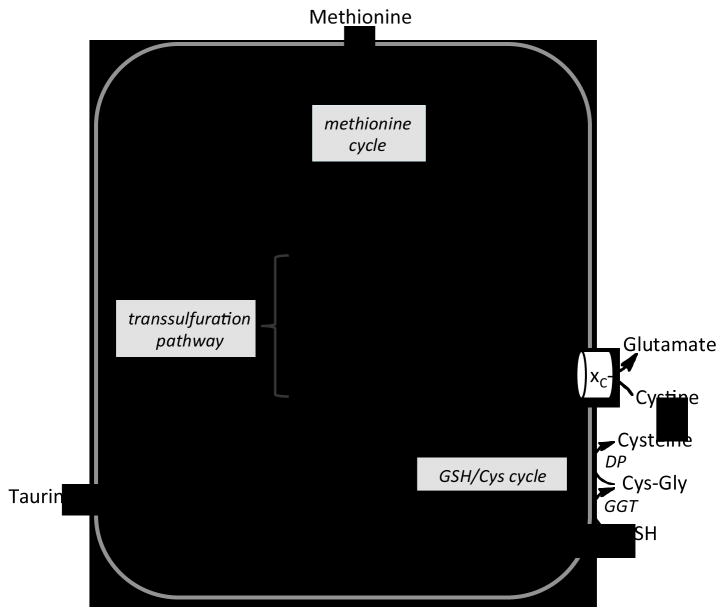
Fig. 1.
Scheme showing liver methionine metabolism. Methionine, an essential amino acid, enters the cell and is metabolized via the methionine cycle to AdoMet in a reaction catalyzed by methionine adenosyltransferase (MAT). Functional methyltransferases (MT) and glycine-N-methyltransferase (GNMT) use AdoMet as a methyl group donor and generate AdoHcy, which is hydrolyzed by the action of adenosylhomocysteine hydrolase (AHC) to adenosine and homocysteine. The latter is used in the methionine cycle via the action of methionine synthase (MS) or betaine homocysteinemethyltransferase (BHMT). Alternatively, homocysteine can be converted via the action of the transsulfuration pathway enzymes, cystathionine β-synthase (CBS) and γ-cystathionase (CSE), to cystathionine and cysteine, respectively. CBS and CSE are also involved in H2S biogenesis. Cysteine can be oxidized to either taurine or sulfate or converted to GSH via the actions of γ-glutamylcysteine ligase (GCL) and GSH synthetase (GS). The turnover of GSH occurs via the transmembrane GSH/cysteine cycle and is initiated by its export. Two membrane-bound enzymes, γ-glutamyltranspeptidase (GGT) and a dipetidase (DP), cleave GSH to its component amino acids. Cysteine is oxidized to cystine in the extracellular compartment and can be imported by the glutamate/cysteine antiporter, xC−.
Slow-aging mouse lines are useful models for studying mechanisms underlying longevity. The Snell dwarf mice, which are characterized by defects in production of growth hormone, insulin-like growth factor-1 (IGF-1), thyroid hormones, and prolactin, have a >40% increase in lifespan and show signs of decelerated aging in many tissues (Flurkey et al., 2001). There is strong evidence that there are differences in sulfur and redox metabolism between long-lived and normal mice. In the Ames dwarf mice, some metabolite changes in the remethylation and transsulfuration pathways have been reported (Uthus and Brown-Borg, 2003; Uthus and Brown-Borg, 2006), along with an increase in catalase activity (Brown-Borg et al., 1999). A more limited study of the growth hormone receptor knockout mouse provided evidence for alterations in the methionine cycle rather than in transsulfuration metabolism (Brown-Borg et al., 2009). However, much of the analysis in this study was restricted to mRNA expression levels and to the activities of two γ-glutamyl cycle enzymes (γ-glutamyltranspeptidase and glutamyl-cysteine ligase).
In the current study, we have employed a targeted metabolomic approach to determine the levels of key metabolites in the methylation cycle (methionine, AdoMet and S-adenosylhomocysteine (AdoHcy)), the transsulfuration pathway (cysteine), the γ-glutamyl cycle (GSH, and plasma GSH, GSSG, cysteine and cystine), and the oxidation pathway (hypotaurine and taurine) in multiple tissues in Snell dwarf mice versus wild-type controls. Compared to previous studies on Ames dwarf mice (Uthus and Brown-Borg, 2003; Uthus and Brown-Borg, 2006), our metabolite analysis is more extensive and includes a broader range of tissues, namely plasma, muscle, and adipose in addition to liver, kidney and brain. The metabolite inventory was complemented by global DNA methylation status and Western blot analyses of enzymes at critical regulatory points in the pathway (MAT (methionine adenosyltransferase), GNMT (glycine N-methyl transferase), CBS (cystathionine β-synthase)) and the cystine transporter, xC− in liver. The metabolic data obtained from liver of control and dwarf mice were analyzed using a quantitative mathematical model of liver methionine metabolism (Korendyaseva et al., 2008), providing valuable insights into potential sites of regulation underlying the observed differences in the sulfur network.
2. Materials and methods
2.1. Animals
Snell dwarf and control mice were produced by a cross between (DW/J x C3H/HeJ)F1 –dw/+ parents. Homozygous dw/dw dwarf progeny were compared to littermate controls in each experiment, using both dw/+ and +/+ mice as indistinguishable controls. After weaning at age 21–23 days, mice were housed in single-sex groups of 3–4, in mixed-genotype cages to minimize thermal stress on the cold-sensitive dw/dw dwarf homozygotes, as previously described (Flurkey et al., 2001). Mice had free access to laboratory chow and water. The mice were maintained in pathogen-free animal facilities at the University of Michigan, and the pathogen-free status was verified quarterly by virological surveillance of sentinel mice. Mice were used for tissue collection between 9–12 months of age. All procedures for animal handling were performed in accordance with the protocols approved by the University Committee for the Care and Use of Animals at the University of Michigan.
2.2. Tissue sample collection
Mice were anaesthetized between 10 am and noon with intraperitonial injection of pentobarbital, (2.5 mg per animal) and blood, liver, kidney, and brain were collected immediately. Blood (~500–1000 μl) was collected by retro-orbital bleeding into sample tubes containing heparin (APP Pharmaceuticals) at a final concentration of 20–40 U/ml of blood. The tubes were shaken and stored on ice till the sample collection was completed. Then, the tubes were shaken and centrifuged for 10 min at 2,000xg at 4 °C. Plasma was collected into separate tubes and stored at −80 °C until analysis. Liver, kidney and brain were flash frozen immediately following harvesting, and stored in liquid nitrogen at −80 °C until further use. Equal numbers of males and females were used for both wild-type and Snell dwarf groups for blood, liver, kidney, and brain collection. Muscle and adipose tissue samples were collected only from males.
2.3. Tissue sample preparation
Plasma samples were thawed and vortexed, and aliquots were mixed with equal volumes of metaphosphoric acid solution (16.8 mg/ml HPO3, 2 mg/ml EDTA, 9 mg/ml NaCl) or 10% trichloroacetic acid (TCA) solution. The mixtures were vortexed, denatured proteins were sediment by centrifugation and the supernatants were used for metabolite analysis.
Each frozen organ (except for Snell mouse kidneys) was pulverized in liquid nitrogen using a porcelain mortar and pestle and the powder was collected in weighed sample tubes containing 300 μl of metaphosphoric acid solution or 5% TCA for metabolite analysis, 500 μl of 1.15% KCl for CBS activity assays, or 500 μl lysis buffer (containing 20 mM HEPES, pH 7.5, 25 mMKCl, 0.5% NONIDET P-40, 10 μl/ml protease inhibitor cocktail for mammalian cells and tissue extracts (Sigma), 25 μg/ml tosyllysinechloromethylketone, and 17 μg/ml phenylmethylsulfonyl fluoride) for Western blot analysis. Due to their small size, Snell mouse kidneys were homogenized directly in sample tubes containing one of the solutions described above. Tubes were weighed before and after addition of kidney, and the kidney homogenate was used for analysis or stored at −80 °C until further use. The difference between the initial weight and the weight with tissue powder (or with Snell dwarf kidney) was used to estimate the amount of tissue in the sample. During sample preparation, tissues were diluted 5- to 10-fold (w/v). Tubes containing tissue homogenates with metaphosphoric acid and TCA were centrifuged to sediment the denatured proteins and the supernatants were used for metabolite analysis. Tubes containing homogenate prepared in 1.15% KCl solution were centrifuged and the clear supernatants were used for CBS activity determination. Tubes containing homogenate prepared in lysis buffer were centrifuged and the supernatants were used for protein and Western blot analysis.
2.4. Metabolite analysis
Concentrations of glutamate, aspartate, cysteine, cystine, GSH, and GSSG were measured in protein-free metaphosphoric acid extracts using HPLC after sample derivatization with 2,3-dinitrofluorobenzene, as described previously (Garg et al., 2010). Concentrations of glutamine, taurine, hypotaurine, and methionine were measured in protein-free TCA extracts using HPLC after sample derivatization with o-phthaldialdehyde as described elsewhere (Garg et al., 2010; Vitvitsky et al., 2011). The redox potentials for the cystine/cysteine and GSSH/GSH couples were calculated using the Nernst equation: where E°′ values of −250 mV for the cystine/cysteine and −265 mV for GSSG/GSH couples at pH 7.4 and 37°C were used (Schafer and Buettner, 2001; Yan et al., 2009). R in the Nernst equation represents the gas constant, T, the absolute temperature, and F, Faraday’s constant. Red and Ox denote the reduced (cysteine or GSH) and oxidized (cystine or GSSG) species in a given redox couple.
Concentrations of AdoMet and AdoHcy in liver TCA extracts were measured following a modification of the procedure described earlier (Wise et al., 1997). Samples were analyzed by HPLC on a Waters Spherisorb 5 μ ODS2 column (4.6 × 250 mm) under isocratic conditions at a flow rate of 1 ml/min with optical monitoring at 260 nm. The mobile phase contained 40 mM NaH2PO4 (pH 4.0), 10 mM heptanesulfonic acid sodium salt, and 17% (v/v) methanol. Samples (100 μl) were injected into the column without additional treatment. AdoHcy and AdoMet were eluted with retention times of 11 and 19 min respectively. Calibration of the columns was performed using AdoHcy and AdoMet solutions of known concentrations.
2.5. CBS activity
CBS activity was measured in 1.15% KCl tissue extracts using the ninhydrin assay as described elsewhere (Kashiwamata and Greenberg, 1970).
2.6. Western-blot analysis
For Western blot analysis protein concentration in tissue or cell extracts was determined using the Bradford assay (BioRad) and bovine serum albumin as standard according to the vendor’s protocol. For each sample, an equal amount of protein was loaded on to a 10% SDS-polyacrylamide gel. After separation, the proteins were transferred to a PVDF membrane followed by blocking of unspecific binding sites and treatment with primary antibodies. For CBS analysis, polyclonal anti-human CBS chicken antibodies developed in Aves Labs (Tigard, OR) against recombinant human protein purified in our laboratory were used. For MATI/III analysis, polyclonal anti-human MAT1A rabbit antibodies were purchased from Protein Tech Group Inc. (Chicago, IL). For GNMT analysis, polyclonal anti-human GNMT rabbit antibodies, a gift from Dr. Natalia Krupenko (Medical University of South Carolina, Charleston, SC) were used. For xc− analysis, commercial polyclonal rabbit antibodies developed against mouse xCT, the catalytic subunit of xc− was purchased from Novus Biological (Littleton, CO). Horseradish peroxidase conjugated anti-chicken IgY goat antibodies (Aves Labs) and anti-rabbit IgG donkey antibodies (GE Healthcare) were used as secondary antibodies. Signals were visualized using the chemiluminescent peroxidase substrate kit SuperSignal West Dura (Thermo Scientific). After documentation of the Western blot results, the PVDF membranes were stained with Ponceau S and the intensity of the staining was used to assess equal loading/transfer. For quantification of protein expression data, we first measured the intensities of specific protein bands as integrals of the optical density within band area. Band intensities were normalized to protein staining intensities in the PVDF membrane area corresponding to the analyzed protein. Band intensities and protein staining intensities were measured using ImageJ software.
2.7. DNA methylation analysis
DNA was extracted from frozen liver samples using GenElite™ Mammalian Genomic DNA Miniprep Kit (Sigma) according to the vendor’s protocol. The final DNA concentration in the samples was between 53–177 μg/ml. The DNA was denatured and digested as described (Chango et al., 2009; Fraga et al., 2002) to obtain a mixture of nucleotides. To this end, DNA samples were heated for 3 min at 99 °C and then immediately cooled down on ice. Next, 75 μl of DNA sample was mixed with 10 μl of 0.5 M ammonium acetate buffer (pH 5.2), 5 μl of 20 mM ZnSO4, and 10 μl of nuclease P1 solution (2 U). Nuclease P1 from Penicillium citrinum (Sigma) was dissolved in ammonium acetate buffer (pH 5.2) to a concentration of 200 U/ml. The mixture was incubated overnight at 37 °C and then subjected to HPLC analysis as described previously (Christman, 1982; Ramsahoye, 2002). Samples (50 μl) were injected into a Phenomenex Luna 3 μ C18 column (4.6 × 150 mm) and eluted isocratically with 50 mM ammonium phosphate buffer, pH 5.8 at a flow rate of 1 ml/min. The eluant was monitored at 271 nm. All nucleotides eluted within 35 min and 5-Me-dCMP (5-methyl-2′-deoxycytidine 5′-monophosphate) eluted at 6.8 min. The level of DNA methylation was determined as the ratio of the peak area of 5-Me-dCMP to the sum of peak areas for 5-Me-dCMP + dCMP.
2.8. Mathematical model
The difference in liver sulfur metabolism between normal and Snell dwarf mice was analyzed using a quantitative mathematical model of liver methionine metabolism described previously(Korendyaseva et al., 2008). The model comprises a set of differential equations in which the concentrations of methionine and of other intermediates of methionine metabolism are included as described below.
Here, [Met], [AdoMet], [AdoHcy], [Hcy], [MTHF], [CH2-THF] represent the intracellular concentrations of methionine, S-adenosylmethionine, S-adenosylhomocysteine, homocysteine, 5-CH3-tetrahydrofolate, and 5,10-methylene-tetrahydrofolate, respectively. [Ado] represents the intracellular adenosine concentration, F denotes the pool of folates interconnected via reversible enzymatic reactions, and F0 denotes the total concentration of all intracellular folates except dihydrofolate. Vt is the rate of transmembrane methionine transport, Vprot is the net rate of methionine consumption due to protein synthesis, VMATI, VMATIII, VGNMT, VMeth, VAHC, VCBS, VMS, VBHMT are the rates of reactions catalyzed by MATI, MATIII, GNMT, functional methylases, adenosylhomocysteinase (AHC), CBS, MS, and BHMT, respectively. Consumption of AdoMet for polyamine synthesis was not taken into account in this model as its rate is ~1% of the flux into methionine metabolism in normal liver (Scalabrino et al., 1978). Initially, the rate of methionine transport across cell membrane was assumed to be fast and reversible (Korendyaseva et al., 2008). As a result, the intracellular and extracellular methionine concentrations in the model were equal to each other.
In this study, the model was modified slightly. The extracellular methionine concentration was considered as a parameter (rather than a variable) and the rate of methionine transport across the cell membrane was described by a linear function of the difference between extracellular and intracellular methionine concentrations. This approach provides a good description of the methionine transport kinetics in hepatocytes at physiological methionine concentrations (Korendyaseva et al., 2010).
Here [Met]ext denotes the extracellular (plasma) methionine concentration and At denotes methionine transport activity.
2.9. Statistical analysis
In plasma, liver, kidney and brain, each analyte was evaluated using a two-factor analysis of variance, in which sex, genotype, and the Interaction between sex and genotype were taken as predictor variables. In Tables 1, 2 and 5, p-values represent the significance of the difference between normal and dwarf mice for the genotype term. In Table 4, the p-value indicates the significance of the interaction term to assess whether the dwarf gene has an effect that differs between male and female mice. Table 4 also includes results of a post-hoc Sidak test, which shows p-values for specific contrasting pairs (e.g. normal versus dwarf females) after adjustment for multiple comparisons. The statistical differences for Western blot and DNA methylation and for muscle and adipose tissue were calculated using two-sample t-test.
Table 1.
Metabolite concentrations in plasma of normal and Snell dwarf mice.a
| Plasma | Normal | Dwarf | p |
|---|---|---|---|
| Taurine | 367 ± 27 | 727 ± 35 | < 0.0001 |
| Hypotaurine | 4.9 ± 0.5 | 8.1 ± 0.7 | < 0.002 |
| Methionine | 96 ± 5 | 153 ± 15 | < 0.001 |
| Cystine | 62 ± 3 | 33 ± 3 | < 0.0001 |
| Cysteine | 19 ± 1 | 16 ± 2 | > 0.2 |
| Eh(Cystine/Cysteine) | −88 ± 2 | −92 ± 2 | >0.1 |
| GSH | 10 ± 2 | 5 ± 1 | < 0.02# |
| GSSG | 7 ± 2 | 6 ± 1 | >0.6 |
| Eh(GSSG/GSH) | −114 ± 4 | −98 ± 3 | < 0.002 |
| Glutamate | 61 ± 7 | 39 ± 2 | < 0.006 |
| Glutamine | 606 ± 22 | 493 ± 33 | < 0.009 |
| Aspartate | 19 ± 1 | 20 ± 2 | > 0.4 |
Data represent the mean ± SEM (standard error of the mean) from 16 normal and 16 dwarf mice, with equal numbers of males and females in each group. P-values correspond to the effect of the Snell dwarf genotype in a two factor ANOVA adjusting for differences between males and females. Concentrations are expressed in units of μmoles/liter for plasma metabolites and in mV for redox potentials.
Indicates an effect that is seen in one sex only (see Table 4).
Table 2.
Metabolite concentrations in tissues of normal and Snell dwarf mice.a
| Sample | Normal | n | Dwarf | n | p |
|---|---|---|---|---|---|
| Liver | |||||
| Taurine | 21600 ± 703 | 16 | 20600 ± 1427 | 16 | > 0.5 |
| Hypotaurine | 140 ± 30 | 16 | 74 ± 4 | 16 | < 0.02# |
| Methionine | 91 ± 5 | 16 | 80 ± 6 | 16 | > 0.1 |
| Cysteine | 129 ± 12 | 16 | 116 ± 5 | 16 | > 0.3 |
| GSH | 8400 ± 270 | 16 | 6700 ± 160 | 16 | < 0.0001 |
| GSSG | 410 ± 21 | 16 | 310 ± 15 | 16 | < 0.0005 |
| Eh(GSSG/GSH) | −242 ± 1 | 16 | −239 ± 1 | 16 | > 0.08 |
| AdoMet | 72 ± 5 | 8 | 126 ± 15 | 8 | < 0.004 |
| AdoHcy | 46 ± 8 | 8 | 106 ± 5 | 8 | < 0.0001 |
| Glutamate | 1900 ± 127 | 16 | 2000 ± 101 | 16 | > 0.8 |
| Glutamine | 4530 ± 243 | 16 | 4090 ± 491 | 16 | > 0.4 |
| Aspartate | 730 ± 27 | 16 | 920 ± 46 | 16 | < 0.003 |
| Kidney | |||||
| Taurine | 13400 ± 350 | 8 | 19400 ± 1280 | 4 | < 0.001 |
| Hypotaurine | 167 ± 20 | 8 | 171 ± 34 | 4 | > 0.9 |
| Methionine | 111 ± 5 | 8 | 166 ± 32 | 4 | < 0.05 |
| b Cystine | 90 ± 12 | 8 | 118 ± 13 | 8 | > 0.1 |
| Cysteine | 520 ± 64 | 8 | 591 ± 64 | 8 | > 0.4 |
| GSH | 2800 ± 110 | 8 | 2300 ± 240 | 8 | > 0.06 |
| GSSG | 280 ± 13 | 8 | 190 ± 30 | 8 | < 0.02 |
| Eh(GSSG/GSH) | −218 ± 1 | 8 | −217 ± 1 | 8 | > 0.9 |
| Glutamate | 6200 ± 390 | 8 | 5600 ± 470 | 8 | > 0.3 |
| Glutamine | 2340 ± 160 | 8 | 2510 ± 250 | 4 | > 0.4# |
| Aspartate | 1700 ± 110 | 8 | 2000 ± 180 | 8 | > 0.1 |
| Brain | |||||
| Taurine | 11000 ± 460 | 8 | 15200 ± 470 | 8 | < 0.0001 |
| Hypotaurine | 108 ± 8 | 8 | 137 ± 5 | 8 | < 0.006 |
| Methionine | 81 ± 5 | 8 | 105 ± 6 | 8 | < 0.01 |
| Cysteine | 59 ± 3 | 8 | 54 ± 3 | 8 | > 0.3 |
| GSH | 2160 ± 20 | 8 | 2220 ± 30 | 8 | > 0.1 |
| GSSG | 34 ± 3 | 8 | 42 ± 2 | 8 | < 0.03 |
| Eh(GSSG/GSH) | −239 ± 1 | 8 | −237 ± 1 | 8 | >0.5 |
| Glutamate | 13200 ± 172 | 8 | 12500 ± 124 | 8 | < 0.005# |
| Glutamine | 8370 ± 345 | 8 | 7440 ± 309 | 8 | < 0.04 |
| Aspartate | 4200 ± 111 | 8 | 3000 ± 89 | 8 | < 0.0001 |
| Muscle | |||||
| Taurine | 42800 ± 600 | 9 | 51400 ± 1100 | 6 | < 0.0001 |
| Hypotaurine | 142 ± 25 | 9 | 30 ± 20 | 6 | < 0.01 |
| Methionine | 113 ± 4 | 9 | 152 ± 17 | 6 | < 0.01 |
| Cysteine | 36 ± 2 | 9 | 35 ± 4 | 8 | > 0.8 |
| GSH | 581 ± 46 | 9 | 730 ± 64 | 8 | < 0.04 |
| GSSG | 24 ± 3 | 3 | 30 ± 3 | 2 | > 0.1 |
| Eh(GSSG/GSH) | −212 ± 4 | 3 | −216 ± 5 | 2 | > 0.3 |
| Glutamate | 1100 ± 200 | 9 | 1390 ± 70 | 8 | > 0.1 |
| Glutamine | 1870 ± 70 | 9 | 1730 ± 110 | 6 | > 0.2 |
| Aspartate | 490 ± 49 | 9 | 516 ± 44 | 8 | > 0.3 |
Data represent the mean ± SEM (standard error of the mean) in μmoles per kg of wet tissue for metabolites and in mV for redox potential. The number of animals in normal and dwarf groups is denoted by n. Each group of animals contained an equal number of males and females except for the muscle samples, which were collected only from males. P-values correspond to the effect of the Snell dwarf genotype in a two factor ANOVA adjusting for differences between males and females except muscle samples where the p-values were calculated using a two sample t-test.
Cystine levels in liver, brain, and muscle were below our limit of detection.
Indicates an effect that is seen in one sex only (see Table 4).
Table 5.
Tissue CBS activity in normal versus Snell dwarf mice.a
| Organ | Normal | Dwarf | n | p |
|---|---|---|---|---|
| Liver | 110 ± 4 | 108 ± 4 | 8 | > 0.6 |
|
| ||||
| Brain | 7.3 ± 0.2 | 10.0 ± 0.4 | 8 | < 0.0001 |
|
| ||||
| b Kidney(total) | 36 ± 4 | 52±3 | 8 | < 0.0002 |
| Kidney (male) | 47±4 | 57±4 | 4 | >0.08 |
| Kidney (female) | 27±3 | 48±2 | 4 | <0.001 |
Data represent the mean ± SEM values in units of mmol h−1 kg tissue−1. n indicates the number of animals, p indicates statistical significance. Liver and brain groups contained equal number of males and females.
CBS activity in normal male and female kidney is significantly different (p<0.006); the difference in CBS activity in dwarf males and female kidney shows borderline significance (p=0.048); CBS activity in normal male and dwarf female kidney is not significantly different (p>0.8).
Table 4.
Sexual dimorphism in effects of Snell dwarf genea
| Tissue | Metabolite | Normal male | Dwarf male | Normal female | Dwarf female | p | Sidak test |
|---|---|---|---|---|---|---|---|
| Plasma | GSH | 6.6 ± 0.6 | 5.4 ± 1.3 | 14 ± 3 | 5.3 ± 0.8 | 0.053 | NFb> DF(p = 0.02) NM = DM |
| Liver | Hypotaurine | 213 ± 49 | 72 ± 5 | 68 ± 7 | 75 ± 7 | 0.007 | NM > DM(p = 0.003) NF = DF |
| Kidney | Glutamine | 2700 ± 100 | 2200 ± 200 | 2000 ± 100 | 2800 ± 400 | 0.004 | DF > NF(p < 0.05) DM = NM |
| Brain | Glutamate | 13500 ± 100 | 12500 ± 200 | 12800 ± 200 | 12600 ± 200 | 0.03 | DM < NM(p < 0.006) DF = NF |
The table lists metabolites (mean ± SEM in μmoles per kg of wet tissue) for which the Snell dwarf gene alters metabolite concentration in one sex but not in the other. Examples were selected based on a significant interaction term in a two-factor ANOVA in which metabolite was dependent on sex, genotype, and the p value for their interaction (except that for GSH in plasma, the interaction p = 0.053). The Sidak post-hoc test shows significance for the specific comparisons of interest (DM versus NM, and DF versus NF), the equals sign indicates the absence of a significant effect.
NF, NM, DF and DM denote normal female, normal male, dwarf female and dwarf male respectively.
3. Results
3.1. Tissue and plasma metabolite analysis
Significant differences were observed in the concentrations of plasma sulfur metabolites, taurine, hypotaurine, methionine, cystine and GSH (Table 1). While taurine (2-fold), hypotaurine (1.6-fold) and methionine (1.6-fold) were significantly higher in Snell dwarf mice, the concentrations of cystine (1.9-fold) and GSH (2-fold) were lower. The shifts in the redox-active sulfur metabolites resulted in an oxidative shift in the GSSG/GSH redox potential (from −114±4 mV in wild-type to −98±3 mV in Snell dwarfs, p<0.002), while the cystine/cysteine redox potential was not significantly different. Our HPLC analysis also provides information on the concentrations of three other amino acids: glutamate, glutamine and aspartate. Of these, the first two influence the cysteine pool either directly (glutamate, by virtue of a shared glutamate-cystine antiporter, xC− (McBean, 2002)) or indirectly (glutamine can be converted to glutamate), and both amino acids were significantly lower in plasma from Snell dwarf mice.
Of the sulfur metabolites in liver, hypotaurine (1.9-fold), GSH (1.3-fold) and GSSG (1.3-fold) were significantly lower in Snell dwarf mice (Table 2). The GSSG/GSH redox potential was unchanged due to the parallel changes in the reduced and oxidized pools. Liver is chiefly responsible for the systemic regulation of methionine levels, and excess methionine switches liver methionine metabolism from a recycling mode via the methionine cycle to a disposal mode (Martinov et al., 2000). This switch is associated with an increase in liver AdoMet levels, which in turn up-regulates AdoMet consumption via GNMT and activates transsulfuration via allosteric regulation of CBS (Martinov et al., 2010). Since plasma methionine levels are higher in Snell dwarf mice, we examined the AdoMet and AdoHcy levels in liver. Significant increases in the concentrations of both metabolites were observed, with AdoMet and AdoHcy being 1.8- and 2.3-fold higher, respectively in liver of Snell dwarf mice (Table 2).
In kidney, taurine (1.5-fold) and methionine (1.3-fold) concentrations were higher in Snell dwarfs while the GSSG (1.5-fold) concentration was lower. The GSSG/GSH redox potential was unaffected. In brain, a larger number of differences in metabolite levels was observed between Snell dwarf versus wild-type mice compared to the other tissues. The concentrations of methionine (1.3-fold), taurine (1.4-fold), hypotaurine (1.3-fold) and GSSG (1.2-fold) were increased, but the GSSG/GSH redox potential was unaffected. Additionally, the concentrations of glutamate, glutamine and aspartate were lower in brain of Snell dwarf mice. In muscle, taurine (1.2-fold), methionine (1.3-fold) and GSH (1.3-fold) levels were significantly increased in Snell dwarf mice while the hypotaurine (5-fold) concentration was decreased. The GSSG/GSH redox potential was unaffected (Table 2).
Interestingly, in adipose tissue all metabolite concentrations appeared to be higher in Snell dwarf samples when expressed in μmoles per kg of wet tissue (Table 3). However, this difference could be misleading if adipocytes from normal versus dwarf mice have different proportions of fat deposits per unit cell volume. In this situation, normalization of metabolite data in adipose tissue to wet tissue weight would produce an underestimation of metabolite concentrations if a larger proportion of the cell volume is occupied by insoluble fat. To address this concern, we repeated the metabolite analysis in adipose tissue but normalized the data to soluble protein amount, a measure of the cytoplasmic volume. Based on this normalization, only hypotaurine (6-fold), methionine (2-fold), and cysteine (3-fold) levels were significantly increased in adipose tissue of Snell dwarf mice (Table 3).
Table 3.
Metabolite concentrations in adipose tissue of normal and Snell dwarf mice.a
| Sample | Normal | n | Dwarf | n | p |
|---|---|---|---|---|---|
| μmoles/kg | |||||
| Taurine | 1430 ± 100 | 9 | 2140 ± 29 | 7 | < 0.02 |
| Hypotaurine | 53 ± 35 | 9 | 228 ± 54 | 7 | < 0.01 |
| Methionine | 11 ± 1 | 9 | 33 ± 5 | 7 | < 0.001 |
| Cysteine | 7 ± 2 | 9 | 38 ± 12 | 8 | < 0.01 |
| GSH | 98 ± 14 | 9 | 263 ± 57 | 8 | < 0.01 |
| GSSG | 16 ± 2 | 3 | 21 ± 4 | 3 | > 0.1 |
| Eh(GSSG/GSH) | −346 ± 9 | 3 | −377 ± 5 | 3 | < 0.02 |
| Glutamate | 226 ± 32 | 9 | 676 ± 190 | 8 | < 0.02 |
| Glutamine | 216 ± 23 | 9 | 320 ± 39 | 7 | < 0.02 |
| Aspartate | 64 ± 11 | 9 | 229 ± 75 | 8 | < 0.02 |
| μmoles/g prot. | |||||
| Taurine | 158 ± 13 | 6 | 158 ± 13 | 4 | > 0.9 |
| Hypotaurine | 4 ± 3 | 6 | 23 ± 7 | 4 | < 0.01 |
| Methionine | 1.1 ± 0.2 | 6 | 2.1 ± 0.3 | 4 | < 0.01 |
| Cysteine | 0.34 ± 0.03 | 6 | 1.0 ± 0.3 | 5 | < 0.02 |
| GSH | 9.7 ± 1.3 | 6 | 10.3 ± 2.2 | 5 | > 0.8 |
| Glutamate | 25 ± 5 | 6 | 21 ± 4 | 5 | > 0.4 |
| Glutamine | 23 ± 1 | 6 | 25 ± 2 | 4 | > 0.4 |
| Aspartate | 5.4 ± 0.8 | 6 | 4.0 ± 0.6 | 5 | > 0.2 |
Data represent the mean ± SEM (standard error of the mean) in μmoles per kg of wet tissue and in μmoles per g of soluble protein for metabolites and in mV for redox potential. “n” indicates the number of animals in normal and dwarf groups. All samples were collected from males. P-values correspond to the effect of the Snell dwarf genotype and were calculated using two sample t-Test.
3.2. Sexually dimorphic expression of select metabolites
We evaluated the significance of sexual dimorphism in tissue metabolite levels by means of a two-factor ANOVA test, taking into account sex, genotype, and the significance of the genotype-sex interaction term (Table 4). Plasma GSH levels were 2-fold higher in normal females compared to males. In contrast, GSH levels in Dwarf males and females were comparable and similar to the lower value seen in normal males. In liver, hypotaurine was 3-fold higher in normal males versus females, a difference that was not seen in the Snell dwarf mice where the hypotaurine concentration in both sexes was comparable to the lower value found in normal females. In kidney, glutamine levels were 1.4-fold higher in Dwarf compared to normal females, a difference that was not seen between normal and dwarf males. A small but significant difference in brain glutamate levels between dwarf and normal males was observed.
3.3. Tissue CBS levels
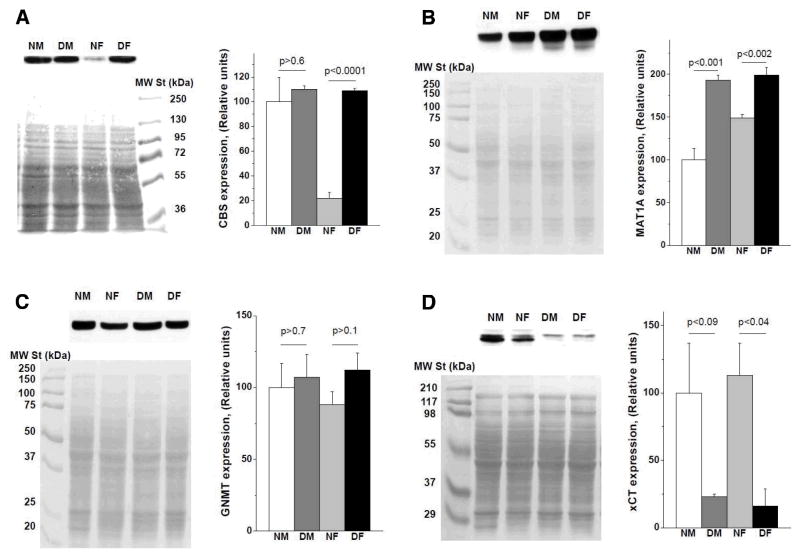
Excess methionine in mammalian tissues is disposed via the transsulfuration pathway. The committing enzyme in this pathway is CBS. To assess whether increased plasma methionine levels in Snell dwarf mice are associated with changes in tissue CBS levels, we measured CBS activity in liver, kidney and brain (Table 5). No difference was observed in liver CBS activity between normal and Snell dwarf mice. In contrast, brain CBS activity was ~1.4-fold higher in Snell dwarf compared to normal mice. In kidney, the picture was more complicated. It has been noted previously (Vitvitsky et al., 2004; Vitvitsky et al., 2007) that CBS activity is significantly higher in normal males than in normal females, a result we confirm in the Snell dwarf and control mice (Table 5). The Snell dwarf genotype elevates kidney CBS activity by 78% in females (p < 0.001) while the 21% difference in males did not reach statistical significance (p>0.08) (Table 5). The difference between control and dwarf CBS activity, pooled across gender, is significant at p <0.0002. Kidney CBS activity data were correlated with CBS protein expression levels (Fig. 2A). Similar CBS expression was observed in kidney of normal males, Snell dwarf males and Snell dwarf females, but was significantly lower in normal females (Fig. 2A).
Fig. 2.
Comparison of expression levels of enzymes in the sulfur network in wild-type (DW/J x C3H/HeJ)F2 and Snell dwarf mice. (A) Renal expression of CBS. Hepatic expression of (B) MATI/III, (C) GNMT and (D) the xCT subunit of the xC− antiporter. Each panel shows results of Western blot analysis (top left), the corresponding PVDF membrane stained with Ponceau S as an evidence of equal loading and transfer (bottom left) and a bar graph showing quantification of the protein expression data presented as mean±SEM (right). Data were obtained from four independent analyses for CBS, MATI/III, and GNMT, and from two independent analyses for xCT. Numbers above the horizontal lines show statistical significance of the difference between the corresponding vertical bars.
3.4. Hepatic expression of MAT, GNMT, and xCT
Since AdoMet and AdoHcy levels are perturbed in liver of Snell dwarf mice, we analyzed protein expression of the two key enzymes that regulate methionine metabolism, MAT (sum of MAT I and MAT III isoforms) and GNMT. MAT expression was significantly higher in liver of Snell dwarf compared to normal mice (Fig. 2B). Sexually dimorphic expression of MAT was observed in normal mice, with females exhibiting 1.5-fold higher MAT than males. However, this difference was not observed in Snell dwarf mice, where MAT expression was 1.3-fold higher in females and 2-fold higher in males compared to normal mice (Fig. 2B).
GNMT catalyzes the disposal of excess AdoMet by converting it to sarcosine and AdoHcy. No difference was observed in steady-state GNMT levels between normal and Snell dwarf mice (Fig. 2C). GNMT is typically present at 0.5–1% of soluble liver protein (Wagner et al., 1985) and exhibits substrate level regulation (Martinov et al., 2000).
Since hepatic GSH levels were lower in Snell dwarf mice and GSH synthesis is limited by cysteine availability, we also examined levels of the xCT subunit of the cystine transporter, xC− and found it to be significantly lower in Snell dwarf compared to normal mice, with levels reduced ~5-fold in Snell liver (Fig. 2D).
3.5. Global hepatic DNA methylation levels
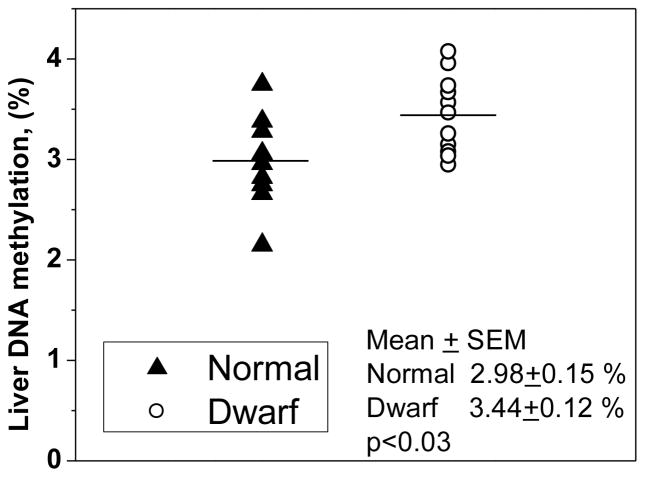
AdoMet is a ubiquitous methyl-group donor and is a substrate for many transmethylases. Since changes in hepatic AdoMet levels observed in Snell dwarf mice could potentially affect methylation homeostasis, we examined the global DNA methylation status. Snell dwarf mice exhibited significantly higher hepatic global DNA methylation levels (p<0.025) than normal mice (Fig. 3).
Fig. 3.
Comparison of global hepatic DNA methylation in wild-type and Snell dwarf mice. Symbols show individual data obtained in liver samples of nine normal (triangles) and eleven Snell dwarf (circles) mice and horizontal bars indicate the mean levels. Five normal males, four normal females, five dwarf males and six dwarf females were used in this study. No difference was observed between normal males and females or between dwarf males and females.
3.6. Mathematical model analysis of perturbations in hepatic methionine metabolism in Snell dwarf mice
To understand the mechanisms underlying the significant differences observed between normal and Snell dwarf mice in their sulfur metabolism, we applied a mathematical model developed to describe hepatic methionine metabolism in rodents (Korendyaseva et al., 2008). As developed originally, the model described the operation of the sulfur network at a physiologically relevant methionine concentration of 50 μM (Korendyaseva et al., 2008), which approximates the levels seen in rat liver and in plasma of C57BL/6J mice (Finkelstein et al., 1982; Finkelstein and Martin, 1986; Jacobs et al., 2001; Vitvitsky et al., 2004). In this study, methionine levels in plasma and liver of control mice were found to be higher, i.e. ~90–100 μM (Tables 1,2). At this methionine concentration, the original model switches to the “high” or methionine disposal mode with dramatically increased hepatic AdoMet production, which accumulates up to 1 mM concentration (Korendyaseva et al., 2008). During the transition from the “low” to the “high” mode, the methionine cycle switches from its reliance on the MATI isoform with a low Michaelis constant for methionine to the MATIII isoform with high Michaelis constant for methionine (Martinov et al., 2000). The MATI and MATIII isoforms have the same subunit molecular mass but differ in their oligomeric organization. Since the steady-state methionine level in the (DW/J x C3H/HeJ)F2 mouse strain is apparently higher than in the C57BL/6J mouse strain, the mathematical model had to be adjusted by decreasing the sensitivity of the MAT isoforms I and III to methionine. To this end, we increased the Michaelis constant for methionine for MATI from 25 μM to 50 μM and for MATIII from 1.2 mM to 2.4 mM (Table 6). With these modifications, we estimated the transmembrane methionine transport activity using an intracellular methionine concentration of 91 μM and a plasma methionine concentration of 96 μM. Finally, to fit the concentrations of AdoMet and AdoHcy in the model with the experimental values, we increased MATI and MATIII activities by 30% and the activity of functional methyltransferases by 15%. With these adjustments, a good fit was achieved between the theoretical concentrations of methionine, AdoMet, and AdoHcy and the experimentally obtained intracellular concentrations of these metabolites (Table 6).
Table 6.
Mathematical model parameter adjustment to describe methionine metabolism in liver of control and Snell dwarf mice.a
| Parameter/Variable | Original model | Control mice | Snell dwarf mice | ||||
|---|---|---|---|---|---|---|---|
| Theoretical value | Expt. value | Theoretical values | Expt. value | ||||
| First step | Second step | Third step | |||||
| (μM) | 25 | 50 | - | 50 | 50 | 50 | - |
| (mM) | 1.2 | 2.4 | - | 2.4 | 2.4 | 2.4 | - |
| AMATI (mmol/h kg tissue) | 2.2 | 2.9 | - | 4.6 | 4.6 | 6.1 | - |
| (mmol/h kg tissue) | 15.8 | 20.5 | - | 32.8 | 32.8 | 43.1 | - |
| At(1/h) | - | 160 | - | 160 | 20 | 27 | - |
| AMeth (mmol/h kg tissue) | 13 | 15 | - | 15 | 15 | 29 | - |
| [Met]ext, (μM) | 50 | 96 | 96±5 | 153 | 153 | 153 | 153±15 |
| [Met] (μmol/kg tissue) | 50 | 90 | 91±5 | 114 | 80 | 84 | 80±6 |
| [AdoMet] (μmol/kg tissue) | 60 | 74 | 72±5 | 1758 | 205 | 129 | 126±15 |
| [AdoHcy] (μmol/kg tissue) | 35 | 49 | 46±8 | 340 | 71 | 98 | 106±5 |
| VCBS (mmol/h kg tissue) | 0.62 | 0.83 | - | 6.13 | 1.3 | 1.7 | - |
-MATI Michaelis constant for methionine, -half-saturated methionine concentration for MATIII, AMATI-MATI activity, -MATIII activity, At -activity of methionine transport across hepatocyte cell membrane, AMeth- activity of functional methyltransferases, VCBS -rate of CBS reaction. Experimental data represent the mean±SEM.
To simulate hepatic methionine metabolism in Snell dwarf mice, we first increased the extracellular methionine concentration to the experimentally determined value of 153 μM and increased the MATI and MATIII activities to 160% of their values in normal (DW/J x C3H/HeJ)F2 mice, which is approximately equal to the average increase in MATI/III expression in Snell dwarf liver compared to normal mice (Fig. 2B). We assumed that changes in the activities of MATI/III are proportional to the changes in protein expression levels. These perturbations switch the model to the “high” or methionine disposal mode with high AdoMet and AdoHcy concentrations and a high CBS reaction rate (Table 6).
Next, we adjusted the intracellular methionine concentration to the experimentally observed value. To this end, we allowed the following activities to vary: methionine transport, functional methyltransferases, MS, BHMT and MTHFR. We found that the only mechanism by which the experimentally observed value of intracellular methionine concentration could be simulated was via an 8-fold decrease in the activity of transmembrane methionine transport in Snell dwarf versus normal mice (Table 6). Next, we simulated the experimentally obtained AdoMet and AdoHcy values by varying the same activities as in the previous round with the exception of the methionine transporter. The best result was obtained with a 2-fold increase in the activity of functional methyltransferases. In the final iteration, the best fit of the experimental data in Snell dwarf mice was modeled with the following changes: 1.9-fold increase in the activity of functional methyltransferases, 6-fold decrease in the activity of transmembrane methionine transport and a 2-fold increase in MATI/III activity (Table 6). The model also predicts an ~2-fold increase in the CBS reaction rate in Snell dwarf compared to normal mice (Table 6).
4. Discussion
Specific signaling networks, which can be influenced by metabolic changes, e.g. in cellular methylation and redox status, have the potential to regulate the aging process. In this study, we have mapped differences in the sulfur metabolite network in liver, kidney, brain, muscle, and adipose tissue of long-lived Snell dwarf mice and wild-type controls. Our results reveal that sulfur metabolism in the Snell dwarf strain is affected systemically (Tables 1 – 3). A 2-fold lower GSH concentration in plasma resulted in an oxidative shift in the extracellular redox potential from −114 mV in wild-type to −98 mV in Snell dwarf mice. In contrast, a statistically significant difference in the plasma cystine/cysteine redox potential was not seen between the Snell dwarf and wild-type mice (Table 1). The significant increase in plasma methionine in Snell dwarf mice was associated with an increase in methionine levels in all tissues except liver. The meaning of this correlation is presently unclear. Furthermore, a significant increase in plasma taurine in Snell dwarf mice was associated with an increase in this metabolite in kidney, brain, and muscle but not in liver. Interestingly, in all tissues with the exception of the adipose, methionine levels were of the same order as in plasma (~100 μmoles kg−1) while tissue taurine levels were up to 100-fold higher compared to plasma levels. Taurine, a sulfonic acid, is a six-electron oxidation product of cysteine and its generation is accompanied by the capture of reducing equivalents albeit via a pathway that is incompletely described. Hence, the general rise in taurine levels in Snell dwarf mice points to the possible increased use of sulfur/methionine metabolism as an energy source.
Since the liver plays a major role in organismal methionine regulation, we analyzed hepatic sulfur metabolite parameters in greater detail than in other tissues. In addition to lower hypotaurine, GSH, and GSSG levels, significantly higher AdoMet and AdoHcy levels were observed in liver of Snell dwarf mice (Table 2). The activity of CBS (Table 4) and expression of GNMT protein (Fig. 2C) were unaffected. In contrast, the expression of MAT was significantly higher (Fig. 2B) while expression of the cystine transporter xC− was dramatically lower (Fig. 2D) in liver of Snell dwarf mice. The increase in liver AdoMet concentration can be ascribed to increased expression of MAT with no change in GNMT in Snell dwarf mice. AdoMet regulates several steps in liver methionine metabolism and serves as a substrate for multiple methylation reactions in cells (Korendyaseva et al., 2008; Martinov et al., 2010). An increase in liver AdoMet is expected to elicit changes in the rate of methylation reactions. Indeed, we find a small, but statistically significant increase in global liver DNA methylation in Snell dwarf mice (Fig. 3). Identification of specific loci that exhibit epigenetic changes in Snell dwarf mice compared to their wild-type controls might help elucidate potential regulatory targets associated with the increased life span of these mice.
Aberrations in methionine metabolism have been previously observed in the long-lived Ames dwarf mice, which, like the Snell dwarfs, are deficient in growth hormone and IGF-1, as well as thyroid hormones and prolactin. Liver GSH levels are decreased and AdoHcy levels are increased in Ames dwarf mice as in Snell dwarf mice (Brown-Borg et al., 1999; Uthus and Brown-Borg, 2003). On the other hand, liver AdoMet levels are reportedly lower in Ames dwarf mice (Uthus and Brown-Borg, 2003) in contrast to Snell dwarf mice. Growth hormone reduces MAT levels (Pan and Tarver, 1967) and GNMT (Aida et al., 1997), and the activities of these enzymes were found to be significantly elevated in liver of Ames dwarf mice (Uthus and Brown-Borg, 2003). In Snell dwarf mice, MAT expression was increased while GNMT expression was unaffected. However, no difference was detected in liver global DNA methylation between normal and Ames dwarf mice (Uthus and Brown-Borg, 2003) in contrast with our results. It is likely that differences in experimental approach and sensitivity between these two studies accounted for the observed differences in the global DNA methylation results. Also, we did not observe a difference in liver CBS activity between wild-type and Snell dwarf mice (Table 5), in contrast to the 2-fold increase reported in Ames dwarf mice (Uthus and Brown-Borg, 2003). Hence, the sulfur metabolic profiles of two long-lived mouse models, Snell and Ames dwarf mice, appear to be distinct in some respects. The difference between our results and those published for the Ames dwarf mice may reflect a combination of effects of background genes and husbandry conditions that might warrant further investigation.
Using our previously described mathematical model of liver methionine metabolism (Korendyaseva et al., 2008) we find that the Snell dwarf mouse liver data set can be readily simulated with an ~2-fold increase in MAT activity, an ~2-fold increase in functional methyltransferase activity and an ~6-fold decrease in transmembrane methionine transport (Table 6). We distinguish between functional methyltransferases, i.e. enzymes that use AdoMet to methylate various targets, e.g. proteins, lipids, DNA and small molecules from GNMT, an enzyme that serves as a bypass under conditions of AdoMet excess, to resupply AdoHcy to the methionine cycle (Fig. 1). The increase in MAT activity is consistent with the experimentally observed increase in MAT protein expression in Snell dwarf mice (Fig. 2B). An increase in the functional methyltransferases activity is in turn consistent with the increase in liver global DNA methylation in Snell dwarf mice (Fig. 3). On the other hand, a decrease in methionine transport activity predicted by the model needs to be verified. Additionally, the model predicts an ~2-fold increase in the CBS reaction rate (Table 6) and hence, a corresponding increase in metabolic flux in the transsulfuration pathway in Snell dwarf liver. An increase in the CBS reaction rate can result from an increase in homocysteine (i.e. substrate) and/or AdoMet (i.e. allosteric activator) concentrations. In liver of Snell dwarf mice, an increase in cysteine synthesis from methionine via the transsulfuration pathway would compensate for the decreased transport of cystine predicted by the significant down-regulation of the transporter, xC−, resulting in cysteine levels being similar to those seen in wild-type controls. Liver is the main organ where taurine is synthesized from cysteine. The substantial increase in plasma taurine levels in Snell dwarf mice suggests activation of taurine synthesis and its export from hepatocytes in Snell dwarf mice.
The predicted decrease in transmembrane methionine transport in Snell hepatocytes can partially explain the contradiction between high plasma methionine levels in long-lived Snell dwarf mice and the longevity of methionine-restricted animals. Decreased transmembrane methionine transport can produce functional methionine restriction in liver. Interestingly, the change in GSH levels and in the cysteine/cystine pools in plasma and organs of Snell dwarf mice resemble the changes observed in methionine-restricted animals, e.g. lower GSH in liver and kidney and no change in brain GSH compared to controls (Richie et al., 2004; Richie et al., 1994). We speculate that elevated plasma methionine levels in Snell dwarf mice might be a consequence of limited uptake of methionine by tissues.
While several examples of sexual dimorphism in metabolite levels were observed (Table 3), the basis for these is not known. Sexual dimorphic expression and activity of renal CBS seen in other mouse strains (Vitvitsky et al., 2004; Vitvitsky et al., 2007) was not observed in Snell dwarf mice. Rather, the Snell dwarf females exhibit a kidney sulfur metabolite pattern that resembles the profile seen in control males (Table 5). Although significant differences in renal GSH and GSSG levels were seen between Snell dwarf and control mice, the magnitude of these differences was small and therefore did not translate into a major difference in the ambient GSSG/GSH redox potential (Table 2). In contrast, brain GSH levels are unaffected in Snell dwarf mice while GSSG was slightly higher leading to only a marginal change in the redox potential.
Brain taurine levels were 1.4-fold higher in Snell dwarfs compared to controls (Table 2). Interestingly, supplementation of aged mice with taurine reportedly resulted in significant improvement in memory acquisition and retention associated with age-related decrements in the inhibitory GABAergic system (El Idrissi, 2008). This study suggests a protective role for taurine in ameliorating age-related cognitive decline (El Idrissi et al., 2009). We speculate that elevated brain taurine levels might protect IGF-1 deficient mice from cognitive failure in old age. There is evidence for delayed impairment of cognitive failure in Ames dwarf mice, and also in growth hormone receptor knockout mice, which like Ames and Snell have very low serum IGF-1 levels (Kinney et al., 2001a; Kinney et al., 2001b). Concentrations of the excitatory neurotransmitter, glutamate and its “precursor”, glutamine, were significantly lower in brain of Snell dwarf mice compared to controls. Regional differences in rat brain glutamate levels are known to exist (Liu et al., 2009). Since whole tissue metabolite levels were measured in our study, it is unclear what pools of glutamate are affected in brain of Snell dwarf mice. As glutamate plays an important role in modulating synaptic plasticity, learning and memory, which decline with age, detailed mapping of glutamate pools in brain regions in Snell dwarf versus wild-type mice is warranted.
In summary, our data reveal systemic alterations in the sulfur network in Snell dwarf mice as evidenced by changes in metabolite levels in plasma and in several tissues. Together with the known beneficial effects of methionine restriction on lifespan extension in rodents (Miller et al., 2005; Richie et al., 2004; Richie et al., 1994), our data identify some nodes within the sulfur network that might underlie metabolic changes associated with increased longevity. Unfortunately, a comparable metabolite profile is not available for methionine-restricted animals, and plasma and tissue methionine levels in these animals were not reported. However, the changes in GSH levels and in the cysteine/cystine pools in plasma and organs of Snell dwarf mice resemble the changes observed in methionine-restricted rats (Richie et al., 2004; Richie et al., 1994).
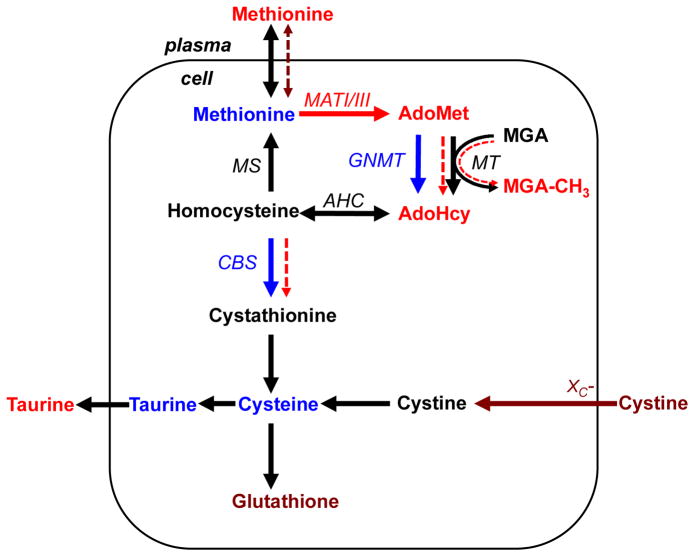
The salient differences in the hepatic sulfur network between Snell dwarf and control mice are summarized in Figure 4. An increase in liver MATI/III levels in dwarf mice results in elevated AdoMet that is associated in turn, with increased global DNA methylation and AdoHcy levels. The mathematical model simulates the metabolic changes in dwarf mice by predicting an ~6-fold decrease in transmembrane methionine transport activity and an ~2-fold increase in functional methyltransferase activity. Decreased transport under conditions of increased plasma methionine is predicted to give rise to normal methionine levels in liver while the predicted increase in methyltransferase activity is correlated with an increase in global DNA methylation. These results suggest that epigenetic changes are correlated with perturbations in sulfur metabolism in the long-lived Snell dwarf mice. The mathematical model also predicts an ~2-fold increase in the CBS reaction rate and hence, an increase in cysteine synthesis from methionine via the transsulfuration pathway. This would compensate for decreased import of cystine as a consequence of the significant down-regulation of the xC-transporter and result in cysteine levels being comparable to those in wild-type animals.
Fig. 4.
Summary of metabolic data obtained in liver of wild-type (DW/J x C3H/HeJ)F2 and Snell dwarf mice. Arrows show enzymatic reactions (metabolic fluxes). The red, maroon and blue colors denote metabolites or enzymes whose levels are increased, decreased, or unchanged, respectively in liver of Snell dwarf mice. Black is used to denote metabolites or enzymes that were not analyzed in this study. The mathematical model reveals (dashed arrows) that changes in sulfur metabolism in Snell dwarf mice can be accounted for by a decrease in membrane permeability to methionine, an increase in functional methyltransferase activity and an increase in the CBS reaction rate (without an increase in CBS activity). The abbreviations used are described in Figure 1 legend with the exception of: MGA-methyl group acceptor, MGA-CH3- methylated methyl group acceptor.
Another interesting finding of our study is that expression of sexual dimorphism in the sulfur network is suppressed in Snell dwarf with respect to several parameters, e.g. kidney CBS activity and expression, plasma GSH, liver hypotaurine, and brain glutamate levels (Fig. 2A, Table 4, 5). There is strong evidence that sexual dimorphism in patterns of hepatic gene expression often reflects responses to differences in pituitary growth hormone pulse patterns (Waxman and Holloway, 2009). Male mice, like male rats and humans, have a circadian pattern of growth hormone production that includes periodic peaks and troughs, a pattern distinct from the lower amplitude variations seen in females (Jansson et al., 1985). Some genes, over-expressed in females, are actively suppressed by the male growth hormone pulse pattern, while others require at least some growth hormone production for transcription, and are thus silenced in both male and female mice with pituitary mutations (Amador-Noguez et al., 2005). The absence of pituitary growth hormone in male and female dwarf mice is likely to disrupt these sex-specific endocrine circuits and may thus contribute to the loss of sexual dimorphism in some of the sulfur metabolic pathways as seen in our study.
Acknowledgments
This work was supported by grants from the National Institutes of Health (HL58984 to RB and AG031736 and AG013283 to RAM). We thank Lisa Burmeister and Sabrina van Roekel for technical assistance.
Footnotes
Abbreviations Used: 5-Me-dCMP, 5-methyl-2′-deoxycytidine-5′-monophosphate; AdoHcy, S-adenosylhomocysteine; AdoMet, S-adenosylmethionine; AHC, adenosylhomocysteine hydrolase (adenosylhomocysteinase); BHMT, betainehomocysteinemethyltransferase; CBS, cystathionineβ-synthase; CSE, γ-cystathionase; DP, dipeptidase; GCL, γ-glutamylcysteine ligase; GGT, γ-glutamyltranspeptidase; GNMT, glycine-N-methyltransferase; GS, glutathionesynthetase; GSH, reduced glutathione; GSSG, oxidized glutathione; MAT (MATI/III), methionine adenosyltransferase isoforms I and III; MS, methionine synthase; MT, functional methyltransferases; xC−, glutamate/cystineantiporter.
Contributor Information
Victor Vitvitsky, Email: victorv@umich.edu.
Michael Martinov, Email: martinov.michael@gmail.com.
Fazoil Ataullakhanov, Email: ataullakhanov.fazly@gmail.com.
Richard A. Miller, Email: millerr@umich.edu.
Ruma Banerjee, Email: rbanerje@umich.edu.
References
- Aida K, Tawata M, Negishi M, Onaya T. Mouse glycine N-methyltransferase is sexually dimorphic and regulated by growth hormone. Horm Metab Res. 1997;29:646–9. doi: 10.1055/s-2007-978982. [DOI] [PubMed] [Google Scholar]
- Amador-Noguez D, Zimmerman J, Venable S, Darlington G. Gender-specific alterations in gene expression and loss of liver sexual dimorphism in the long-lived Ames dwarf mice. Biochem Biophys Res Commun. 2005;332:1086–100. doi: 10.1016/j.bbrc.2005.05.063. [DOI] [PubMed] [Google Scholar]
- Banerjee R. Redox outside the box: linking extracellular redox remodeling with intracellular redox metabolism. J Biol Chem. 2012;287:4397–402. doi: 10.1074/jbc.R111.287995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banjac A, Perisic T, Sato H, Seiler A, Bannai S, Weiss N, Kolle P, Tschoep K, Issels RD, Daniel PT, Conrad M, Bornkamm GW. The cystine/cysteine cycle: a redox cycle regulating susceptibility versus resistance to cell death. Oncogene. 2008;27:1618–28. doi: 10.1038/sj.onc.1210796. [DOI] [PubMed] [Google Scholar]
- Bharath S, Hsu M, Kaur D, Rajagopalan S, Andersen JK. Glutathione, iron and Parkinson’s disease. Biochem Pharmacol. 2002;64:1037–48. doi: 10.1016/s0006-2952(02)01174-7. [DOI] [PubMed] [Google Scholar]
- Bokov AF, Lindsey ML, Khodr C, Sabia MR, Richardson A. Long-lived ames dwarf mice are resistant to chemical stressors. J Gerontol A Biol Sci Med Sci. 2009;64:819–27. doi: 10.1093/gerona/glp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown-Borg HM, Bode AM, Bartke A. Antioxidative mechanisms and plasma growth hormone levels: potential relationship in the aging process. Endocrine. 1999;11:41–8. doi: 10.1385/ENDO:11:1:41. [DOI] [PubMed] [Google Scholar]
- Brown-Borg HM, Rakoczy SG, Sharma S, Bartke A. Long-living growth hormone receptor knockout mice: potential mechanisms of altered stress resistance. Exp Gerontol. 2009;44:10–9. doi: 10.1016/j.exger.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chango A, Abdel Nour AM, Niquet C, Tessier FJ. Simultaneous determination of genomic DNA methylation and uracil misincorporation. Med Princ Pract. 2009;18:81–4. doi: 10.1159/000189803. [DOI] [PubMed] [Google Scholar]
- Christman JK. Separation of major and minor deoxyribonucleoside monophosphates by reverse-phase high-performance liquid chromatography: a simple method applicable to quantitation of methylated nucleotides in DNA. Anal Biochem. 1982;119:38–48. doi: 10.1016/0003-2697(82)90662-5. [DOI] [PubMed] [Google Scholar]
- El Idrissi A. Taurine improves learning and retention in aged mice. Neurosci Lett. 2008;436:19–22. doi: 10.1016/j.neulet.2008.02.070. [DOI] [PubMed] [Google Scholar]
- El Idrissi A, Boukarrou L, Splavnyk K, Zavyalova E, Meehan EF, L’Amoreaux W. Functional implication of taurine in aging. Adv Exp Med Biol. 2009;643:199–206. doi: 10.1007/978-0-387-75681-3_20. [DOI] [PubMed] [Google Scholar]
- Finkel T. Oxidant signals and oxidative stress. Curr Opin Cell Biol. 2003;15:247–54. doi: 10.1016/s0955-0674(03)00002-4. [DOI] [PubMed] [Google Scholar]
- Finkelstein JD, Kyle WE, Harris BJ, Martin JJ. Methionine metabolism in mammals: Concentration of metabolites in rat tissues. J Nutr. 1982;112:1011–1018. doi: 10.1093/jn/112.5.1011. [DOI] [PubMed] [Google Scholar]
- Finkelstein JD, Martin JJ. Methionine metabolism in mammals: Adaptation to methionine excess. J Biol Chem. 1986;261:1582–1587. [PubMed] [Google Scholar]
- Flurkey K, Papaconstantinou J, Miller RA, Harrison DE. Lifespan extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production. Proc Natl Acad Sci U S A. 2001;98:6736–41. doi: 10.1073/pnas.111158898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraga MF, Uriol E, Borja Diego L, Berdasco M, Esteller M, Canal MJ, Rodriguez R. High-performance capillary electrophoretic method for the quantification of 5-methyl 2′-deoxycytidine in genomic DNA: application to plant, animal and human cancer tissues. Electrophoresis. 2002;23:1677–81. doi: 10.1002/1522-2683(200206)23:11<1677::AID-ELPS1677>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Garg SK, Yan Z, Vitvitsky V, Banerjee R. Analysis of sulfur-containing metabolites involved in redox and methionine metabolism. In: Das DK, editor. Methods in Redox Signaling. Mary Ann Liebert; New Rochelle: 2010. pp. 7–11. [Google Scholar]
- Harper JM, Salmon AB, Chang Y, Bonkowski M, Bartke A, Miller RA. Stress resistance and aging: influence of genes and nutrition. Mech Ageing Dev. 2006;127:687–94. doi: 10.1016/j.mad.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauck SJ, Aaron JM, Wright C, Kopchick JJ, Bartke A. Antioxidant enzymes, free-radical damage, and response to paraquat in liver and kidney of long-living growth hormone receptor/binding protein gene-disrupted mice. Horm Metab Res. 2002;34:481–6. doi: 10.1055/s-2002-34787. [DOI] [PubMed] [Google Scholar]
- Huxtable RJ. Physiological actions of taurine. Physiol Rev. 1992;72:101–63. doi: 10.1152/physrev.1992.72.1.101. [DOI] [PubMed] [Google Scholar]
- Jacobs RL, Stead LM, Brosnan ME, Brosnan JT. Hyperglucagonemia in rats results in decreased plasma homocysteine and increased flux through the transsulfuration pathway in liver. J Biol Chem. 2001;276:43740–7. doi: 10.1074/jbc.M107553200. [DOI] [PubMed] [Google Scholar]
- Jansson JO, Eden S, Isaksson O. Sexual dimorphism in the control of growth hormone secretion. Endocr Rev. 1985;6:128–50. doi: 10.1210/edrv-6-2-128. [DOI] [PubMed] [Google Scholar]
- Jones DP, Go YM, Anderson CL, Ziegler TR, Kinkade JM, Jr, Kirlin WG. Cysteine/cystine couple is a newly recognized node in the circuitry for biologic redox signaling and control. FASEB J. 2004;18:1246–8. doi: 10.1096/fj.03-0971fje. [DOI] [PubMed] [Google Scholar]
- Kabil H, Kabil O, Banerjee R, Harshman LG, Pletcher SD. Increased transsulfuration mediates longevity and dietary restriction in Drosophila. Proc Natl Acad Sci U S A. 2011;108:16831–6. doi: 10.1073/pnas.1102008108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabil O, Banerjee R. The redox biochemistry of hydrogen sulfide. J Biol Chem. 2010;285:21903–21907. doi: 10.1074/jbc.R110.128363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwamata S, Greenberg DM. Studies on cystathionine synthase of rat liver. Properties of the highly purified enzyme. Biochim Biophys Acta. 1970;212:488–500. doi: 10.1016/0005-2744(70)90255-x. [DOI] [PubMed] [Google Scholar]
- Kinney BA, Coschigano KT, Kopchick JJ, Steger RW, Bartke A. Evidence that age-induced decline in memory retention is delayed in growth hormone resistant GH-R-KO (Laron) mice. Physiol Behav. 2001a;72:653–60. doi: 10.1016/s0031-9384(01)00423-1. [DOI] [PubMed] [Google Scholar]
- Kinney BA, Meliska CJ, Steger RW, Bartke A. Evidence that Ames dwarf mice age differently from their normal siblings in behavioral and learning and memory parameters. Horm Behav. 2001b;39:277–84. doi: 10.1006/hbeh.2001.1654. [DOI] [PubMed] [Google Scholar]
- Korendyaseva TK, Kuvatov DN, Volkov VA, Martinov MV, Vitvitsky VM, Banerjee R, Ataullakhanov FI. An allosteric mechanism for switching between parallel tracks in mammalian sulfur metabolism. PLoS Comput Biol. 2008;4:e1000076. doi: 10.1371/journal.pcbi.1000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korendyaseva TK, Martinov MV, Dudchenko AM, Vitvitsky VM. Distribution of methionine between cells and incubation medium in suspension of rat hepatocytes. Amino Acids. 2010;39:1281–9. doi: 10.1007/s00726-010-0563-x. [DOI] [PubMed] [Google Scholar]
- Liu P, Jing Y, Zhang H. Age-related changes in arginine and its metabolites in memory-associated brain structures. Neuroscience. 2009;164:611–28. doi: 10.1016/j.neuroscience.2009.08.029. [DOI] [PubMed] [Google Scholar]
- Martinov MV, Vitvitsky VM, Banerjee R, Ataullakhanov FI. The logic of the hepatic methionine metabolic cycle. Biochim Biophys Acta. 2010;1804:89–96. doi: 10.1016/j.bbapap.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinov MV, Vitvitsky VM, Mosharov EV, Banerjee R, Ataullakhanov FI. A substrate switch: A new mode of regulation in the methionine metabolic pathway. J Theor Biol. 2000;204:521–532. doi: 10.1006/jtbi.2000.2035. [DOI] [PubMed] [Google Scholar]
- McBean GJ. Cerebral cystine uptake: A tale of two transporters. Trends Pharmacol Sci. 2002;23:299–302. doi: 10.1016/s0165-6147(02)02060-6. [DOI] [PubMed] [Google Scholar]
- Menon SG, Goswami PC. A redox cycle within the cell cycle: ring in the old with the new. Oncogene. 2007;26:1101–9. doi: 10.1038/sj.onc.1209895. [DOI] [PubMed] [Google Scholar]
- Miller DL, Roth MB. Hydrogen sulfide increases thermotolerance and lifespan in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2007;104:20618–22. doi: 10.1073/pnas.0710191104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA, Buehner G, Chang Y, Harper JM, Sigler R, Smith-Wheelock M. Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-I and insulin levels, and increases hepatocyte MIF levels and stress resistance. Aging Cell. 2005;4:119–25. doi: 10.1111/j.1474-9726.2005.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orentreich N, Matias JR, DeFelice A, Zimmerman JA. Low methionine ingestion by rats extends life span. J Nutr. 1993;123:269–74. doi: 10.1093/jn/123.2.269. [DOI] [PubMed] [Google Scholar]
- Pan F, Tarver H. Effects of diet and other factors on methionine adenosyltransferase levels in rat liver. J Nutr. 1967;92:274–280. doi: 10.1093/jn/92.2.274. [DOI] [PubMed] [Google Scholar]
- Ramsahoye BH. Measurement of genome wide DNA methylation by reversed-phase high-performance liquid chromatography. Methods. 2002;27:156–61. doi: 10.1016/s1046-2023(02)00069-5. [DOI] [PubMed] [Google Scholar]
- Richie JP, Jr, Komninou D, Leutzinger Y, Kleinman W, Orentreich N, Malloy V, Zimmerman JA. Tissue glutathione and cysteine levels in methionine-restricted rats. Nutrition. 2004;20:800–5. doi: 10.1016/j.nut.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Richie JP, Jr, Leutzinger Y, Parthasarathy S, Malloy V, Orentreich N, Zimmerman JA. Methionine restriction increases blood glutathione and longevity in F344 rats. Faseb J. 1994;8:1302–7. doi: 10.1096/fasebj.8.15.8001743. [DOI] [PubMed] [Google Scholar]
- Scalabrino G, Poso H, Holtta E, Hannonen P, Kallio A, Janne J. Synthesis and accumulation of polyamines in rat liver during chemical carcinogenesis. Int J Cancer. 1978;21:239–45. doi: 10.1002/ijc.2910210217. [DOI] [PubMed] [Google Scholar]
- Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30:1191–212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- Uthus EO, Brown-Borg HM. Altered methionine metabolism in long living Ames dwarf mice. Exp Gerontol. 2003;38:491–8. doi: 10.1016/s0531-5565(03)00008-1. [DOI] [PubMed] [Google Scholar]
- Uthus EO, Brown-Borg HM. Methionine flux to transsulfuration is enhanced in the long living Ames dwarf mouse. Mech Ageing Dev. 2006;127:444–50. doi: 10.1016/j.mad.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitvitsky V, Dayal S, Stabler S, Zhou Y, Wang H, Lentz SR, Banerjee R. Perturbations in homocysteine-linked redox homeostasis in a murine model for hyperhomocysteinemia. Am J Physiol Regul Integr Comp Physiol. 2004;287:R39–46. doi: 10.1152/ajpregu.00036.2004. [DOI] [PubMed] [Google Scholar]
- Vitvitsky V, Garg SK, Banerjee R. Taurine biosynthesis by neurons and astrocytes. J Biol Chem. 2011;286:32002–10. doi: 10.1074/jbc.M111.253344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitvitsky V, Prudova A, Stabler S, Dayal S, Lentz SR, Banerjee R. Testosterone regulation of renal cystathionine beta-synthase: implications for sex-dependent differences in plasma homocysteine levels. Am J Physiol Renal Physiol. 2007;293:F594–600. doi: 10.1152/ajprenal.00171.2007. [DOI] [PubMed] [Google Scholar]
- Wagner C, Briggs WT, Cook RJ. Inhibition of glycine N-methyltransferase activity by folate derivatives: implications for regulation of methyl group metabolism. Biochem Biophys Res Commun. 1985;127:746–52. doi: 10.1016/s0006-291x(85)80006-1. [DOI] [PubMed] [Google Scholar]
- Waxman DJ, Holloway MG. Sex differences in the expression of hepatic drug metabolizing enzymes. Mol Pharmacol. 2009;76:215–28. doi: 10.1124/mol.109.056705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise CK, Cooney CA, Ali SF, Poirier LA. Measuring S-adenosylmethionine in whole blood, red blood cells and cultured cells using a fast preparation method and high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl. 1997;696:145–52. doi: 10.1016/s0378-4347(97)00213-2. [DOI] [PubMed] [Google Scholar]
- Yan Z, Garg SK, Kipnis J, Banerjee R. Extracellular redox modulation by regulatory T cells. Nat Chem Biol. 2009;5:721–723. doi: 10.1038/nchembio.212. [DOI] [PMC free article] [PubMed] [Google Scholar]