Abstract
Social subordination in female macaques represents a well-described model of chronic psychosocial stress. Additionally, a length polymorphism (5HTTLPR) in the regulatory region of the serotonin (5HT) transporter (5HTT) gene (SLC6A4) is present in rhesus macaques, which has been linked to adverse outcomes similar to what has been described in humans with an analogous 5HTTLPR polymorphism. The present study determined the effects of social status and the 5HTTLPR genotype on 5HT1A receptor binding potential (5HT1A BPND) in brain regions implicated in emotional regulation and stress reactivity in ovariectomised female monkeys, and then assessed how these effects were altered by 17β-oestradiol (E2) treatment. Areas analyzed included the prefrontal cortex [anterior cingulate (ACC); medial prefrontal cortex (mPFC); dorsolateral prefrontal cortex; orbitofrontal prefrontal cortex], amygdala, hippocampus, hypothalamus and raphe nucleui. Positron emission tomography (PET) using p-[18F]MPPF was performed to determine the levels of 5HT1A BPND under a non-E2 and a 3-wk E2 treatment condition. The short variant (s-variant) 5HTTLPR genotype produced a significant reduction in 5HT1A BPND in the mPFC regardless of social status, and subordinate s-variant females showed a reduction in 5HT1A BPND within the ACC. Both these effects of 5HTTLPR were unaffected by E2. Additionally, E2 reduced 5HT1A BPND in the dorsal raphe of all females irrespective of psychosocial stress or 5HTTLPR genotype. Hippocampal 5HT1A BPND was attenuated in subordinate females regardless of 5HTTLPR genotype during the non-E2 condition, an effect that was normalised with E2. Similarly, 5HT1A BPND in the hypothalamus was significantly lower in subordinate females regardless of 5HTTLPR genotype, an effect reversed with E2. Together, the data indicate that the effect of E2 on modulation of central 5HT1A BPND may only occur in brain regions that show no 5HTTLPR genotype-linked control of 5HT1A binding.
Keywords: oestradiol, social subordination, psychosocial stress, 5HT1A receptor, 5HTTLPR, monkeys
Introduction
Exposure to chronic psychogenic stress is implicated in the etiology of adverse health outcomes in humans (1). One neuromodulatory system that is particularly affected in psychopathologies is the serotonergic system (2), which plays an important role in the control of mood and emotionality through receptors in corticolimbic circuits (3). For example, attenuation of central levels of the serotonin (5HT) 1A receptor in brain regions important for emotional regulation is associated with psychopathology in humans (4, 5) and behavioural depression in monkeys (6). Importantly, the serotonin transporter (5HTT) regulates 5HT transmission through removal of 5HT from the synaptic cleft and length variation in the polymorphic region in the gene (SLC6A4) encoding the 5HTT (5HTTLPR) in humans confers individual variability in vulnerability to the adverse effects of chronic stress (7). Specifically, the short variant (s-variant) 5HTTLPR reduces 5HTT transcription (7) and is associated with increased risk for psychopathology in people (8).
Women during their reproductive years are at higher risk for psychopathology than men (9), suggesting that gonadal hormones such as oestradiol (E2) might be important in the etiology of stress-induced adverse health outcomes in females. E2 is not only critical for the regulation of reproductive physiology in females, but also acts as a crucial modulator of behaviour (10). The ability of E2 to regulate physiology and behaviour in females is in part due to its capacity to modulate the activity of the serotonergic system (11). E2 alters the expression of the 5HTT (12) and 5HT receptors in females monkeys (11). Specifically, levels of the 5HT1A receptor in brain regions that regulate emotionality, including hippocampus-amygdala, brain stem, and cingulate cortex are modulated by E2, as replacement of E2 to ovariectomised macaque females reduces 5HT1A receptor protein and mRNA expression in the dorsal raphe (13, 14). A similar attenuation in 5HT1A receptors is noted in E2-treated ovariectomised rats (15). While the independent effects of either chronic psychosocial stress or E2 attenuate central 5HT1A receptors, it remains unclear how chronic psychosocial stress and E2 interact with 5HTTLPR genotype to influence central 5HT1A receptors.
Socially housed macaque monkeys provide a unique opportunity to study the effects of chronic psychosocial stress, 5HTTLPR genotype, and E2 on female physiology and behaviour. Female macaque monkeys, when housed socially, are organised by a linear dominance hierarchy in which subordinate females are harassed by more dominant females via contact and non-contact aggression (16). This continuous harassment results in dysregulation of the limbic-hypothalamic-pituitary-adrenal (LHPA) axis (17). Furthermore, a 5HTTLPR polymorphism, analogous to that seen in humans, is present in rhesus monkeys (18) that increases vulnerability to early life stress (19), and alters the central serotonergic system and behaviour (20, 21). The 5HTTLPR in adult female monkeys has been associated with differences in metabolism and emotional behaviour (22), and interacts with social status to alter physiological and behavioural responses to E2 in adult ovariectomised females (23, 24). Thus, the goal of the present study was to undertake a positron emission tomography (PET) analysis in socially stratified ovariectomised adult female monkeys to determine the effect of the social subordination and the 5HTTLPR on E2’s ability to modulate 5HT1A receptor binding potential (5HT1A BPND) in brain regions implicated in emotional regulation and stress reactivity.
Methods
Subjects
Ovariectomised adult female rhesus macaques (n=33), ranging in age from 10–16 years (Mean ± SEM: 12.5±0.38 years), housed in indoor-outdoor enclosures at the Yerkes National Primate Research Center (YNPRC) Field Station were subjects in the current study. Social groups consisted of four or five female members, including a single male. Females were ovariectomised as previously described (25) immediately proceeding group formation when females were ~8.5 years of age (22). All animals were fed ad libitum twice daily with Purina monkey chow (diet 5038; PMI, St Louis, MO, USA), supplemented with seasonal fruits and vegetables, and had continuous access to water. The Emory University Institutional Animal Care and Use Committee approved all procedures in accordance with the Animal Welfare Act and the US Department of Health and Human Services ‘Guide for Care and Use of Laboratory Animals.’
All subjects had previously been genotyped for 5HTTLPR alleles as either having both long promoter length alleles (l/l) or a short promoter length allele (l/s or s/s genotypes; s-variant) (22). As previously reported, social groups were formed by sequentially introducing unfamiliar and unrelated females together of similar 5HTTLPR genotypes, such that each group comprised of either all l/l females or of all s-variant females (22). Social dominance rankings were assessed via three 30-min behavioural observations during the control, no-E2 phase of the study using an established ethogram (22). Agonistic behaviours including contact (bite, slap) and non-contact (open-mouth threat, chase) aggression, as well submissive (withdrawal, grimaces) behaviours were noted to determine social ranking (22). While E2 increases the expression of aggressive and thus submissive behaviour in macaque groups, social ranks remain stable (24). Social rank in the current monkey groups were stable for 120 months after group formation, and remained stable throughout the completion of the current study.
In order to examine the extremes in the dominance hierarchy, the current study sample consisted of the first (n = 7) and second (n= 8) ranked females within each group as well as the fourth (n = 9) and fifth (n = 9) ranked females. Thus, the middle ranking females were excluded. Social status categorizations were determined by grouping females ranked 1 and 2 as dominant and females ranked 4 and 5 as subordinate according to previously described conventions (17). When considering the 5HTTLPR genotype, the resulting sample size for each genotype and social status category was 8 dominant l/l, 9 subordinate l/l, 7 dominant s-variant, and 9 subordinate s-variant females completed the current study.
Experimental Design
Animals were studied under a non-E2 replacement condition (control) and an experimental 3-week chronic E2 replacement condition (E2). The order of conditions was counterbalanced across groups, with a 3-week wash out period between each condition. All females had been without hormone replacement for at least four weeks before the beginning of the study. Females received E2 replacement by surgical implantation of a Silastic capsule filled with E2 subcutaneously between the scapulae. Capsules were based on body weight and achieved mid-follicular levels of E2 (mean 45.4 ± 4.15 pg/ml). Levels of E2 during replacement were not different between dominant and subordinate females (49.2±6.36 vs. 42.4±5.79 respectively; p>0.05) or between l/l and s-variant females (45.9±5.97 vs. 45.7±6.19 respectively; p>0.05). During the E2 condition, females were scanned on average 24.8 days after E2 replacement, and the duration of E2 replacement was not different between dominant and subordinate females (24.4±1.43 vs. 25.2±1.30 respectively; p>0.05). Scans during the control condition occurred on average 44.2 days after a 3-week washout period and this amount of time was not different between dominant and subordinate females (44.4±3.79 vs. 43.9±3.44 respectively; p>0.05).
PET imaging
The radioligand 4-(2′-methoxyphenyl)-1-[2′-(N-2″-pyridinyl)-p[18F]fluorobenzamido]ethylpiperazine (p-[18F]MPPF), a fluoro analog of WAY-100635, was used to assess the binding potential of central 5HT1A receptors as previously described in rhesus monkeys by our group (26). The YNPRC Radiochemistry Laboratory synthesised the p-[18F]MPPF with a radiochemical purity of over 99%. Binding potential (BPND) was defined as the ratio at equilibrium of bound p-[18F]MPPF to that of nondisplacable p-[18F]MPPF in the tissue of each region. BPND is a measure used to correlate receptor density to experimental conditions (26). An analog of WAY-100635 was used because WAY-100635 has been shown to be insensitive to the presence of endogenous 5HT (27).
Each female received two PET scans (control vs. E2 in a counterbalanced manner) separated by one month using p-[18F]MPPF. All PET scans were acquired at the YNPRC Field Station Imaging Center on a Siemens P4 microPET scanner (Siemens, Concorde Microsystems, Knoxville, TN, USA). Procedures were standardised across subjects to minimise stress due to temporary social separation and transport, and to control for its potential confounding effects on our measures. Only one animal per social group was scanned on any given day. Briefly, one the day of a scan, animals were removed from their social housing and transported immediately to the PET scanning facility within five minutes of being removed from the social group. Following the scan, animals were housed over night in a radioactive quarantine and returned to their home group the following day once radioactivity levels were undetectable (on average ~22 hours following initial removal from the group). Removal of animals from the group did not affect the social structure of groups.
For the PET scans, all subjects had an IV catheter placed for radioligand infusion and hydration fluids and were scanned supine with the head positioned to standardised coordinates and secured using a soft elastic wrap. All PET scans were done under isoflurane anaesthesia (1.2%, inhalation) and all subjects received a 5 min pump infusion of ~4 mCi of p-[18F]MPPF. Anaesthesia, heart rate, blood oxygenation, and respiration throughout the duration of the scanning period were monitored by YNPRC veterinarian staff. As previously described (26), data from each PET scan were combined into 21 frames and an image reconstructed. PET images for each subject were summed across frames and manually rigid-body registered to her own structural MRI image (see acquisition methods below) using in-house scripts written in IDL (26). Regions of interest (ROIs) were drawn manually on each individual subject’s MRI image and then transferred to the PET images (26). Time-activity curves and Logan analysis (26, 28) using the cerebellum as the reference region (26) were performed to generate 5HT1A BPND.
MRI imaging
T1-weighted structural MRI scans were collected at the YNPRC Imaging Center for each subject for PET scan co-registration. MRI scans were acquired under anaesthesia (1–1.5% isoflurane, inhalation to effect) using a 3 T Siemens scanner, an 8-channel phase array knee coil and a T1-weighted MPRAGE sequence (TI/TR/ TE = 950/3000/3.49 ms, FOV = 96 mm, eight averages) with a 0.5 × 0.5 × 0.5-mm3 voxel size. MRI images were reconstructed into 3D volumes and rigid-body registered to a rhesus monkey template (29) and then aligned to the Wisconsin 112RM-SL rhesus T1-atlas to allow for drawing of the ROIs in the Saleem and Logothetis rhesus macaque brain atlas space (30).
ROI drawing
Procedures and neuroanatomical definitions previously published in rhesus monkeys by our group were used to draw ROIs (26, 29). ROI tracing within structural MRI images in coronal and sagittal views was guided by rhesus macaque brain atlases (30). Regions of the prefrontal cortex (PFC) were drawn, including the medial PFC (mPFC), the dorsolateral PFC (dlPFC), the anterior cingulate cortex (ACC), and the orbitofrontal cortex (ofPFC). The amygdala, hippocampus, hypothalamus, and dorsal raphe nuclei in the brainstem were also traced. All of these brain regions have been implicated in the regulation of emotional and stress-related behaviour, contribute to LHPA axis regulation, and express 5HT1A (3). The cerebellum was drawn and used as a reference region following previously published protocols (26). Statistical analysis. Data were summarised as mean ± SEM. The main effects of social status (dominant vs. subordinate), 5HTTLPR (l/l vs. s-variant), and treatment (control vs. E2) and the interaction between these factors on 5HT1A BPND in all ROIs were analyzed by analysis of variance for repeated measures and post-hoc t-tests conducted when necessary. A test result with a p ≤ 0.05 was considered significant.
Results
Social Status Categorization
Figure 1A depicts overall rates of submission emitted and aggression received by monkeys at each social rank. As expected, rates of submission emitted by females as well as the levels of aggression received by each were directly related to their social rank such that lower-ranking females emitted more submissive behaviour (F 3, 29 = 4.41, p = 0.011) and received more aggression females (F 3, 29 = 3.52, p = 0.027) than high-ranking females (Figure 1A). Collapsing into social status categorizations according to previously described methods (17, 31) by grouping females ranked 1 and 2 as dominant and females ranked 4 and 5 as subordinate resulted in significant main effects of social status on the amount of submission emitted. Dominant females showed less submission emitted (F 1, 31 = 12.3, p = 0.001) and aggression received (F 1, 31 = 10.2, p = 0.003) compared to subordinate females (Figure 1B).
Figure 1.
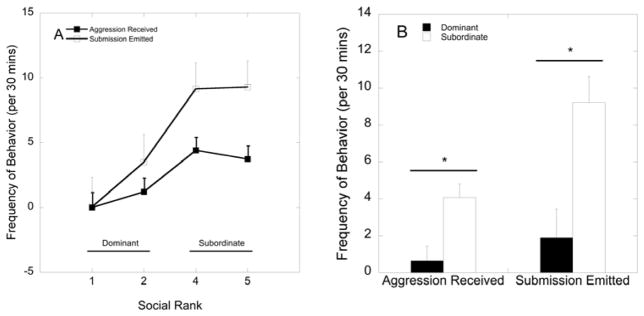
(A) Mean ± SEM frequency of aggression received and submission emitted by for each social dominant position. (B) Social status differences in the amount of aggression received and submission emitted. Subordinate females show increased rates of aggression received and submission emitted compared to dominant females (p<0.05) as denoted by asterisks.
5HT1A BPND
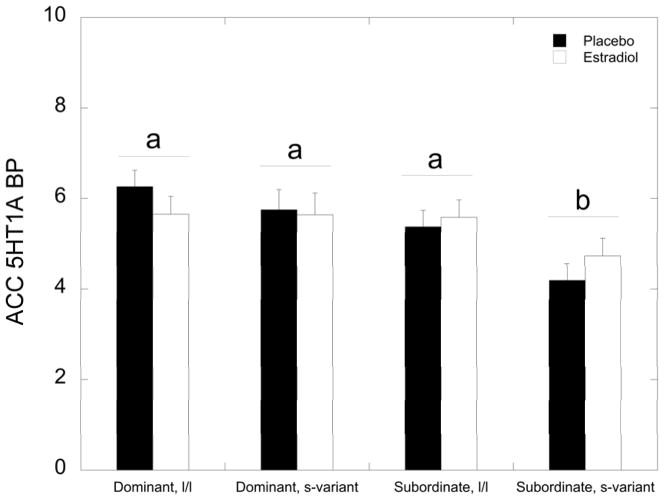
Within the ACC, there were significant main effects of status (F 1, 29 = 7.84, p = 0.009) and of 5HTTLPR (F 1, 29 = 6.49, p = 0.016). Dominant and l/l 5HTTLPR females had higher levels of 5HT1A BPND in the ACC compared to subordinate and s-variant females, respectively. While the status by 5HTTLPR interaction was not significant (F 1, 29 = 0.549, p = 0.465), subordinate s-variant females had attenuated 5HT1A BPND levels in the ACC compared to all other groups of females (Figure 2; p<0.001). There was no effect of E2 or its interaction with status and 5HTTLPR on 5HT1A BPND in the ACC (p>0.05).
Figure 2.
Mean ± SEM 5HT1A BPND in the ACC (anterior cingulate) during the placebo (closed bar) and the oestradiol (E2; open bar) for each group of females. Subordinate, s-variant females show decreased 5HT1A BPND in the ACC compared to all other groups of females as denoted by the letters (p<0.001).
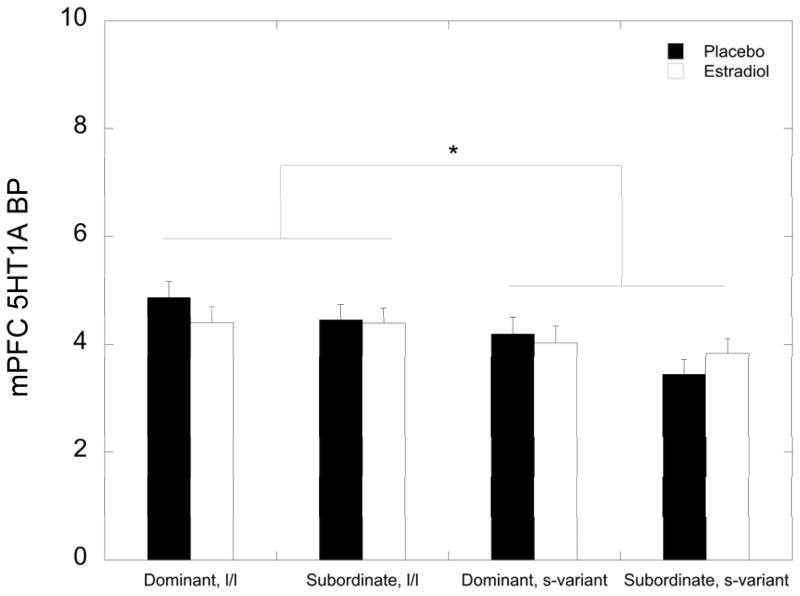
In the mPFC, while there was no main effect of status on 5HT1A BPND (F 1, 29 = 1.77, p = 0.194), there was a main effect of 5HTTLPR on 5HT1A BPND (F 1, 29 = 6.60, p = 0.016), with s-variant females having significantly lower binding levels than l/l females (3.87±0.184 vs. 4.53±0.177 respectively; Figure 3). There were no main effects of status or 5HTTLPR on 5HT1A BPND in the ofPFC or the dlPFC (p>0.05). There were no significant interactions between status and 5HTTLPR genotype in any of these prefrontal regions (p>0.05). Furthermore, there were no effects of E2, nor any interactions of E2 treatment with status or 5HTTLPR in the prefrontal regions (p>0.05).
Figure 3.
Mean ± SEM 5HT1A BPND in the (mPFC (medial prefrontal cortex) during the placebo (closed bar) and the oestradiol (E2; open bar) for each group of females. The asterisk shows that s-variant 5HTTLPR females show decreased levels of 5HT1A BPND in the mPFC compared to l/l variant females, regardless of treatment condition.
In the hippocampus there were no main effects of status, 5HTTLPR, or their interaction (p>0.05) on 5HT1A BPND. However, status significantly interacted with E2 treatment (F 1, 29 = 9.25, p = 0.005), as dominant females had higher levels of 5HT1A BPND than subordinate females during the placebo condition (p=0.05, η2=0.12; Figure 4A). Replacement of E2 in subordinate females normalised this status difference in 5HT1A BPND seen during the placebo, no-hormone condition by increasing 5HT1A BPND in subordinate females to levels seen in dominant females (Figure 4A). E2 treatment did not interact with 5HTTLPR (F 1, 29 = 3.58, p = 0.069), nor was there a three-way interaction among E2 treatment, status and 5HTTLPR (p>0.05). Analyses assessing the effects of status, 5HTTLPR, and E2 treatment in the other temporal region, the amygdala, revealed no significant main or interaction effects of these factors on 5HT1A BPND (p>0.05).
Figure 4.
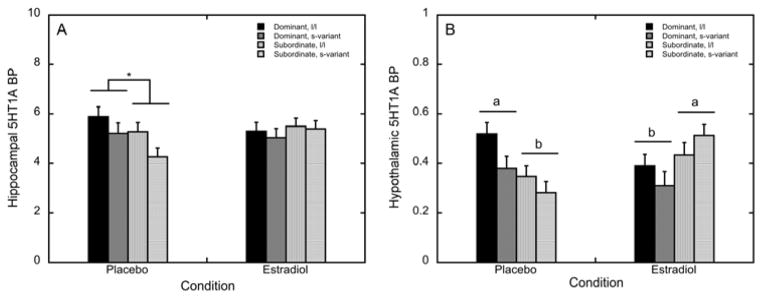
Mean ± SEM 5HT1A BPND during the placebo and the oestradiol (E2) for dominant, l/l (closed bars), dominant, s-variant (dark grey bars), subordinate l/l (light grey bars), and subordinate s-variant (open bars) females. (A) Hippocampal 5HT1A BPND is attenuated in subordinate females during the placebo condition compared to dominant females (denoted by asterisk), an effect that is not present during E2 replacement. (B) Letters denote that hypothalamic 5HT1A BPND is attenuated in subordinate females during the placebo condition compared to dominant females, an effect that is reversed upon E2 replacement.
5HT1A BPND in the hypothalamus was not affected by status, 5HTTLPR, or their interaction (p>0.05). While there was no main effect of E2 treatment either (F 1, 29 = 0.783, p = 0.384), we detected a significant E2 by status interaction effect on hypothalamic 5HT1A BPND (F 1, 29 = 14.2, p = 0.001: Figure 4B). Dominant females had significantly higher levels of 5HT1A BPND during the placebo condition than did subordinate females (p=0.007, η2=0.21; Figure 3B). This status difference was reversed in the E2 condition, as subordinate females on E2 showed greater 5HT1A BPND levels compared to dominant females (p=0.029, η2=0.14; Figure 4B). E2 replacement decreased hypothalamic 5HT1A BPND in dominant females (p=0.038; Figure 3B) while increasing hypothalamic 5HT1A BPND in subordinate animals (p=0.007; Figure 4B). E2 treatment did not interact with 5HTTLPR (F 1, 29 = 2.21, p = 0.148), nor was there a three-way interaction between E2 treatment, status and 5HTTLPR (p>0.05).
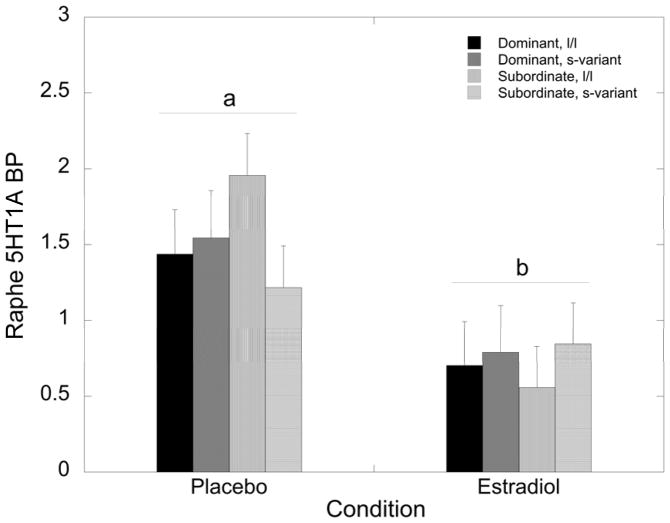
Dorsal raphe nuclei 5HT1A BPND was not significantly affected by status, 5HTTLPR, or their interaction (p>0.05). However, E2 replacement significantly attenuated 5HT1A BPND levels in the raphe (F 1, 29 = 9.06, p = 0.005; Figure 5) independently from status, 5HTTLPR or their interaction (p>0.05).
Figure 5.
Mean ± SEM 5HT1A BPND in the raphe nucleus during the placebo and the oestradiol (E2) for dominant, l/l (closed bars), dominant, s-variant (dark grey bars), subordinate l/l (light grey bars), and subordinate s-variant (open bars) females. Letters denote significant attenuation of 5HT1A BPND upon E2 replacement in all females.
Discussion
The current results indicate that replacement of mid-follicular levels of E2 in ovariectomised female rhesus macaques leads to alterations in 5HT1A BPND that are region-specific and dependent on 5HTTLPR or on an interaction between social status and E2 treatment. 5HTTLPR genotype influenced 5HT1A BPND in the mPFC and the ACC independent of E2 treatment. Specifically, 5HT1A binding in the mPFC was attenuated in females with the s-variant 5HTTLPR genotype irrespective of social status, and 5HT1A BPND in the ACC of subordinate females with the s-variant 5HTTLPR genotype was attenuated compared to all other females. In contrast, hypothalamic 5HT1A BPND in the absence of E2 was lower in subordinate females compared to dominant animals, and replacement of E2 led to a decrease of 5HT1A BPND in dominant animals and an increase of 5HT1A BPND in subordinate females. Hippocampal 5HT1A BPND was attenuated in subordinate females during the placebo condition compared to dominant females, an effect that is normalised upon E2 replacement. Furthermore, 5HT1A receptor binding did not differ in the dorsal raphe due to 5HTTLPR genotype or social subordination, and E2 treatment reduced 5HT1A binding in the raphe in all females, irrespective of status or 5HTTLPR genotype.
The 5HT1A receptor is a Gi-coupled receptor that inhibits adenylyl cyclase in most brain tissues (32). The 5HT1A receptor is an auto-receptor in the dorsal raphe nucleus and also acts postsynaptically in other brain regions to inhibit neuronal activity (33). Previous studies have demonstrated that E2 reduces 5HT1A auto-receptor levels in the dorsal raphe in female rats (15) and in female monkeys (13, 14). Although reductions in receptor binding potential measured with PET imaging may be indicative of a diverse range of functional changes, including changes in receptor density, binding or affinity, as well as receptor internalization or down-regulation, data from this study corroborate previous results showing that E2 reduces 5HT1A auto-receptor function in the dorsal raphe of rhesus females (13, 14). Moreover, we provide data indicating that this effect of E2 on 5HT1A binding in the dorsal raphe occurs independent of 5HTTLPR or social status.
Our findings also show that subordinate females, regardless of 5HTTLPR genotype, have reduced 5HT1A binding in the hippocampus and the hypothalamus in the absence of E2. However, E2 replacement increased 5HT1A BPND in these brain regions and seemed to normalise the effects of chronic psychosocial stress on 5HT1A binding in socially subordinate monkeys. In contrast to this, 5HT1A BPND in the mPFC appeared to be a function of 5HTTLPR genotype, with lower 5HT1A BPND in s-variant 5HTTLPR females irrespective of social status, and therefore independent of the effect of chronic psychosocial stress, and E2 treatment. E2 also had no effect on the reduced level of 5HT1A BPND in the ACC of subordinate females with the s-variant 5HTTLPR genotype. Therefore, although only subordinate s-variant 5HTTLPR females that are subject to chronic psychosocial stress show decreased 5HT1A binding in the cingulate, E2 does not modulate the effect of chronic stress on subordinate females with this 5HTTLPR genotype. Therefore, in the mPFC and the ACC, where 5HT1A binding is related to 5HTTLPR genotype rather than chronic psychosocial stress, E2 treatment does not exert any modulatory effect on 5HT1A BPND.
In the hypothalamus, 5HT1A BPND in the absence of E2 was significantly lower in subordinate females compared to dominant animals regardless of 5HTTLPR genotype, suggesting that this difference is likely due to the effects of chronic psychosocial stress. Replacement of E2 reversed this status effect, leading to a decrease in binding in dominant animals and an increase in subordinate females. The greater hypothalamic 5HT1A binding in subordinate compared to dominant animals under E2 corroborates our previous finding of increased hypothalamic 5HT1A binding in subordinate compared to dominant peripubertal female rhesus (26). Although the results in this study could not provide the spatial resolution to distinguish individual nuclei within the hypothalamus, previous data indicate that E2 attenuates the levels of 5HT1A mRNA in preoptic, supraoptic, periventricular and paraventricalar, ventromedial, and dorsalmedial nuclei of the hypothalamus in female macaques (14). In addition, it has been suggested that activation of central 5HT1A receptors increases LHPA activation in people with depression (34) and also ACTH secretion from the pituitary in rats, presumably by enhancing the release of hypothalamic CRH (35). We have shown that subordinate females have altered LHPA axis function in a no-E2 state, including blunted diurnal peak cortisol and cortisol responses to ACTH (17). Moreover, E2 replacement attenuates glucocorticoid negative feedback on the LHPA axis more so in subordinate compared to dominant females (36). Thus, the E2-mediated increase in hypothalamic 5HT1A BPND in subordinate females may be involved in the diminished glucocorticoid negative feedback seen in subordinate females upon treatment with E2 (36).
Hippocampal 5HT1A BPND was lower in subordinate females during the no-E2 condition than in dominant females, an effect that was normalised upon E2 replacement. These data indicate that E2 modulates the effects of chronic stress associated with social subordination (17, 37) on 5HT1A receptor binding potential in this region. It has been shown that prolonged social subordination lowers 5HT1A BPND in the hippocampus of humans exposed to long-term psychological stress (38) or with clinical depression (39). Indeed, chronic stress is linked to hippocampal damage and to deficits in hippocampal-mediated learning and memory (reviewed (40)). Moreover, E2 has been shown to have a protective effect on hippocampal anatomy as well as cognition in humans (41). Therefore, in this present study, the E2-related increase in 5HT1A BPND in the hippocampi of subordinate females could counter the detrimental effects of chronic stress in these females.
Although E2 increased 5HT1A BPND in the hypothalamus and hippocampus of subordinate female monkeys, it had no effect on differences in 5HT1A BPND associated with 5HTTLPR genotype, rather than with chronic stress, like in the mPFC. The mPFC exerts inhibitory control over the amygdala through glutamatergic projections to GABAergic neurons within the amygdala, and thus is crucial in the control of emotional behaviour (42, 43). In the mPFC the 5HT1A receptor is co-localised with the 5HT2A receptor on glutamatergic projection neurons where these two receptor subtypes act in unison to modulate the activity of this efferent pathway (44). Binding of prefrontal 5HT1A receptors in healthy adults is inversely correlated with anxiety levels (45) and binding is significantly reduced in patients with psychopathologies (4). Moreover, there is a significantly higher occurrence of anxiety disorders, such as PTSD (46), in people with the s-variant 5HTTLPR allele, as well as a significant association between the s-variant 5HTTLPR genotype and heightened amygdala responses to fearful stimuli (47). Therefore, decreased 5HT1A receptor binding in the mPFC of s-variant 5HTTLPR female monkeys may predispose them to increased emotional reactivity.
An overall difference in 5HT1A BPND due to both chronic social stress as well as s-variant 5HTTLPR was observed in the ACC, as subordinate females with the s-variant 5HTTLPR genotype showed lower 5HT1A BPND than all other groups of animals. Reduced 5HT1A BPND in the ACC has been linked to top-down regulation deficits of the stress response in people (38), and has been observed in women with postpartum depression (5). Thus, reduced ACC 5HT1A BPND only in s-variant 5HTTLPR subordinate females suggests a potential mechanism by which this 5HTTLPR genotype renders an increased vulnerability to the effects of chronic stress. This reduced 5HT1A binding in subordinate s-variant females was unchanged by E2 treatment, suggesting that E2 cannot alter the effect of chronic stress on ACC 5HT1A binding in these females. Furthermore, 5HT1A BPND in the mPFC was not affected by E2. While it has been shown that increases 5HT2A receptor density in frontal cortices and ACC in female rats (48) and increases 5HT2A receptor levels in the PFC and ACC in post-menopausal women (49), reports of E2 ability to increase 5HT1A receptors in these brain regions are sparse (15). The ability of E2 to modulate 5HT1A receptors in the PFC and ACC is mediated through the expression of estrogen receptors (ER) in these brain regions. ER alpha (ERα ) and beta (ERβ) are localised throughout the brain of rhesus macaques (50) and generally act to oppose each others’ actions (51). ERα is predominately expressed in the PFC in macaques (50), whereas ERβ is sparsely distributed in the PFC and expressed more in the hypothalamus and other sub-cortical regions (50). Thus, E2’s ability to alter region-specific 5HT1A levels in the current study could be due to region-specific differences in ER distribution. While our data suggests that ERα in the PFC and ACC does not influence 5HT1A binding, further studies are necessary to determine whether ERs are co-localised with cells that express 5HT1A receptors.
Another possibility for the lack of an effect of E2 on 5HT1A BPND in the mPFC and ACC is that the modulatory effects of E2 on central 5HT1A BPND cannot occur in regions where 5HTTLPR genotype is significantly associated with 5HT1A binding. A PET study by David et al. (52), using the same ligand as the present study, showed lower 5HT1A BPND in people with the s-variant 5HTTLPR in several regions of the frontal cortex and the ACC, as well as many other brain areas, indicating that the s-variant 5HTTLPR genotype directly affects the density and/or binding of the 5HT1A receptor in humans. In addition, a PET analysis in rhesus monkeys found lower 5HT1A binding in monkeys with the s-variant 5HTTLPR genotype in the raphe nuclei, occipital and parietal cortices (53). That study used a different 5HT1A ligand and tested intact-male and female monkeys together. Interestingly, multivariate analysis in that study showed a sex difference wherein females had higher amygdalar 5HT1A binding compared to males, and a strong statistical trend for sex differences in the same direction in the frontal cortex, hippocampus and the cingulate (53). There is considerable evidence that the 5HTTLPR genotype exerts key control of 5HT1A BPND in cortico-limbic areas of the primate brain. Our finding, that E2 is ineffective in altering 5HT1A BPND in the mPFC and ACC may indicate that 5HTTLPR genotype trumps E2 as the determining factor for controlling 5HT1A BPND in the cortex of primates. Thus, individuals with the s-variant of the 5HTTLPR who are subject to chronic psychosocial stress may be unresponsive to any effects of E2 treatment in these PFC regions, despite the effects of E2 on 5HT1A BPND in the hippocampus and hypothalamus.
In conclusion, the current data indicate that E2 restores 5HT1A BPND the hippocampus and the hypothalamus of subordinate monkeys to levels that equal or surpass those observed in dominant monkeys. Results also indicate that the s-variant 5HTTLPR is associated with reduced 5HT1A BPND in the mPFC and that this is not influenced by chronic stress or E2 treatment. Lastly, data indicate that 5HTTLPR genotype is important for determining 5HT1A BPND in the ACC. However, results suggest that social subordination may reduce 5HT1A BPND in the ACC of females with the s-variant 5HTTLPR, an effect that is not ameliorated by E2. This later finding in the ACC may be important in clarifying the complex interaction of 5HTTLPR genotype, stress reactivity, and E2 on emotional behaviour. These data, however, should be considered preliminary, as larger numbers of subjects are necessary to establish a link between the 5HTTLPR gene variant and the stress-induced alterations in sensitivity to E2 and 5HT1A expression. Overall, our data highlight the importance of accounting for gonadal hormones when assessing the effects of the 5HTTLPR and the adverse impact that chronic psychosocial stress may have on female physiology and behaviour.
Acknowledgments
We are thankful for the expert technical assistance of Jennifer Whitley, Shannon Bounar, Natalie Brutto and Jodi Godfrey. The project was supported by NIH grants MH 081816 (DT) and, in part, the Office of Research Infrastructure Programs (OD P51OD11132). The Yerkes NPRC is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, International.
References
- 1.McEwen BS. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. European journal of pharmacology. 2008;583(2–3):174–85. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ressler KJ, Nemeroff CB. Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depress Anxiety. 2000;12(Suppl):12–19. doi: 10.1002/1520-6394(2000)12:1+<2::AID-DA2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 3.Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nature reviews. 2009;10(6):397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drevets WC, Frank E, Price JC, Kupfer DJ, Holt D, Greer PJ, Huang Y, Gautier C, Mathis C. PET imaging of serotonin 1A receptor binding in depression. Biological psychiatry. 1999;46 (10):1375–87. doi: 10.1016/s0006-3223(99)00189-4. [DOI] [PubMed] [Google Scholar]
- 5.Moses-Kolko EL, Price JC, Thase ME, Meltzer CC, Kupfer DJ, Mathis CA, Bogers WD, Berman SR, Houck PR, Schneider TN, Drevets WC. Measurement of 5-HT1A receptor binding in depressed adults before and after antidepressant drug treatment using positron emission tomography and [11C]WAY-100635. Synapse (New York, NY. 2007;61(7):523–30. doi: 10.1002/syn.20398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shively CA, Friedman DP, Gage HD, Bounds MC, Brown-Proctor C, Blair JB, Henderson JA, Smith MA, Buchheimer N. Behavioral depression and positron emission tomography-determined serotonin 1A receptor binding potential in cynomolgus monkeys. Archives of general psychiatry. 2006;63(4):396–403. doi: 10.1001/archpsyc.63.4.396. [DOI] [PubMed] [Google Scholar]
- 7.Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science (New York, NY. 1996;274(5292):1527–31. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- 8.Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science (New York, NY. 2003;301(5631):386–9. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 9.Weissman MM, Olfson M. Depression in women: implications for health care research. Science (New York, NY. 1995;269(5225):799–801. doi: 10.1126/science.7638596. [DOI] [PubMed] [Google Scholar]
- 10.Pfaff DW, Vasudevan N, Kia HK, Zhu YS, Chan J, Garey J, Morgan M, Ogawa S. Estrogens, brain and behavior: studies in fundamental neurobiology and observations related to women’s health. Journal of Steroid Biochemistry & Molecular Biology. 2000;74(5):365–73. doi: 10.1016/s0960-0760(00)00114-x. [DOI] [PubMed] [Google Scholar]
- 11.Bethea CL, Lu NZ, Gundlah C, Streicher JM. Diverse actions of ovarian steroids in the serotonin neural system. Frontiers in neuroendocrinology. 2002;23(1):41–100. doi: 10.1006/frne.2001.0225. [DOI] [PubMed] [Google Scholar]
- 12.Lu NZ, Eshleman AJ, Janowsky A, Bethea CL. Ovarian steroid regulation of serotonin reuptake transporter (SERT) binding, distribution, and function in female macaques. Molecular psychiatry. 2003;8(3):353–60. doi: 10.1038/sj.mp.4001243. [DOI] [PubMed] [Google Scholar]
- 13.Pecins-Thompson M, Bethea CL. Ovarian steroid regulation of serotonin-1A autoreceptor messenger RNA expression in the dorsal raphe of rhesus macaques. Neuroscience. 1999;89(1):267–77. doi: 10.1016/s0306-4522(98)00326-1. [DOI] [PubMed] [Google Scholar]
- 14.Lu NZ, Bethea CL. Ovarian steroid regulation of 5-HT1A receptor binding and G protein activation in female monkeys. Neuropsychopharmacology. 2002;27(1):12–24. doi: 10.1016/S0893-133X(01)00423-7. [DOI] [PubMed] [Google Scholar]
- 15.Birzniece V, Johansson IM, Wang MD, Seckl JR, Backstrom T, Olsson T. Serotonin 5-HT(1A) receptor mRNA expression in dorsal hippocampus and raphe nuclei after gonadal hormone manipulation in female rats. Neuroendocrinology. 2001;74(2):135–42. doi: 10.1159/000054679. [DOI] [PubMed] [Google Scholar]
- 16.Bernstein IS, Gordon TP, Rose RM. Aggression and social controls in rhesus monkey (Macaca mulatta) groups revealed in group formation studies. Folia Primatol (Basel) 1974;21(2):81–107. doi: 10.1159/000155607. [DOI] [PubMed] [Google Scholar]
- 17.Michopoulos V, Reding KM, Wilson ME, Toufexis D. Social subordination impairs hypothalamic-pituitary-adrenal function in female rhesus monkeys. Hormones and behavior. 2012;62 (4):389–99. doi: 10.1016/j.yhbeh.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lesch KP, Meyer J, Glatz K, Flugge G, Hinney A, Hebebrand J, Klauck SM, Poustka A, Poustka F, Bengel D, Mossner R, Riederer P, Heils A. The 5-HT transporter gene-linked polymorphic region (5-HTTLPR) in evolutionary perspective: alternative biallelic variation in rhesus monkeys. Rapid communication. J Neural Transm. 1997;104(11–12):1259–66. doi: 10.1007/BF01294726. [DOI] [PubMed] [Google Scholar]
- 19.McCormack K, Newman TK, Higley JD, Maestripieri D, Sanchez MM. Serotonin transporter gene variation, infant abuse, and responsiveness to stress in rhesus macaque mothers and infants. Hormones and behavior. 2009;55(4):538–47. doi: 10.1016/j.yhbeh.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bennett AJ, Lesch KP, Heils A, Long JC, Lorenz JG, Shoaf SE, Champoux M, Suomi SJ, Linnoila MV, Higley JD. Early experience and serotonin transporter gene variation interact to influence primate CNS function. Molecular psychiatry. 2002;7(1):118–22. doi: 10.1038/sj.mp.4000949. [DOI] [PubMed] [Google Scholar]
- 21.Champoux M, Bennett A, Shannon C, Higley JD, Lesch KP, Suomi SJ. Serotonin transporter gene polymorphism, differential early rearing, and behavior in rhesus monkey neonates. Molecular psychiatry. 2002;7(10):1058–63. doi: 10.1038/sj.mp.4001157. [DOI] [PubMed] [Google Scholar]
- 22.Jarrell H, Hoffman JB, Kaplan JR, Berga S, Kinkead B, Wilson ME. Polymorphisms in the serotonin reuptake transporter gene modify the consequences of social status on metabolic health in female rhesus monkeys. Physiology & behavior. 2008;93(4–5):807–19. doi: 10.1016/j.physbeh.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Michopoulos V, Berga SL, Kaplan JR, Wilson ME. Social subordination and polymorphisms in the gene encoding the serotonin transporter enhance estradiol inhibition of luteinizing hormone secretion in female rhesus monkeys. Biology of reproduction. 2009;81(6):1154–63. doi: 10.1095/biolreprod.109.079038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Michopoulos V, Checchi M, Sharpe D, Wilson ME. Estradiol effects on behavior and serum oxytocin are modified by social status and polymorphisms in the serotonin transporter gene in female rhesus monkeys. Hormones and behavior. 2011;59(4):528–35. doi: 10.1016/j.yhbeh.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Michopoulos V, Berga SL, Wilson ME. Estradiol and progesterone modify the effects of the serotonin reuptake transporter polymorphism on serotonergic responsivity to citalopram. Exp Clin Psychopharmacol. 2011;19(6):401–8. doi: 10.1037/a0025008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Embree M, Michopoulos V, Votaw JR, Voll RJ, Mun J, Stehouwer JS, Goodman MM, Wilson ME, Sanchez MM. The relation of developmental changes in brain serotonin transporter (5HTT) and 5HT1A receptor binding to emotional behavior in female rhesus monkeys: effects of social status and 5HTT genotype. Neuroscience. 2013:22883–100. doi: 10.1016/j.neuroscience.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rabiner EA, Wilkins MR, Turkheimer F, Gunn RN, Udo de Haes J, de Vries M, Grasby PM. 5-Hydroxytryptamine1A receptor occupancy by novel full antagonist 2-[4-[4-(7-chloro-2,3-dihydro-1,4-benzdioxyn-5-yl)-1-piperazinyl]butyl]-1,2-benzi sothiazol-3-(2H)-one-1,1-dioxide: a[11C][O-methyl-3H]-N-(2-(4-(2-methoxyphenyl)-1-piperazinyl)ethyl)-N-(2-pyridinyl )cyclohexanecarboxamide trihydrochloride (WAY-100635) positron emission tomography study in humans. The Journal of pharmacology and experimental therapeutics. 2002;301(3):1144–50. doi: 10.1124/jpet.301.3.1144. [DOI] [PubMed] [Google Scholar]
- 28.Logan J, Fowler JS, Volkow ND, Wolf AP, Dewey SL, Schlyer DJ, MacGregor RR, Hitzemann R, Bendriem B, Gatley SJ, et al. Graphical analysis of reversible radioligand binding from time-activity measurements applied to [N-11C-methyl]-(−)-cocaine PET studies in human subjects. J Cereb Blood Flow Metab. 1990;10(5):740–7. doi: 10.1038/jcbfm.1990.127. [DOI] [PubMed] [Google Scholar]
- 29.Parr LA, Boudreau M, Hecht E, Winslow JT, Nemeroff CB, Sanchez MM. Early life stress affects cerebral glucose metabolism in adult rhesus monkeys (Macaca mulatta) Dev Cogn Neurosci. 2012;2(1):181–93. doi: 10.1016/j.dcn.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saleem KS, Logothetis N. A combined MRI and histology atlas of the rhesus monkey brain in stereotaxic coordinates. London; Burlington, MA: Academic Press; 2007. [Google Scholar]
- 31.Kaplan JR, Adams MR, Clarkson TB, Koritnik DR. Psychosocial influences on female ‘protection’ among cynomolgus macaques. Atherosclerosis. 1984;53(3):283–95. doi: 10.1016/0021-9150(84)90129-1. [DOI] [PubMed] [Google Scholar]
- 32.Raymond JR, Mukhin YV, Gettys TW, Garnovskaya MN. The recombinant 5-HT1A receptor: G protein coupling and signalling pathways. British journal of pharmacology. 1999;127(8):1751–64. doi: 10.1038/sj.bjp.0702723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sprouse JS, Aghajanian GK. (−)-Propranolol blocks the inhibition of serotonergic dorsal raphe cell firing by 5-HT1A selective agonists. European journal of pharmacology. 1986;128(3):295–8. doi: 10.1016/0014-2999(86)90782-x. [DOI] [PubMed] [Google Scholar]
- 34.Lesch KP. 5-HT1A receptor responsivity in anxiety disorders and depression. Prog Neuropsychopharmacol Biol Psychiatry. 1991;15(6):723–33. doi: 10.1016/0278-5846(91)90001-h. [DOI] [PubMed] [Google Scholar]
- 35.Gilbert F, Brazell C, Tricklebank MD, Stahl SM. Activation of the 5-HT1A receptor subtype increases rat plasma ACTH concentration. European journal of pharmacology. 1988;147(3):431–9. doi: 10.1016/0014-2999(88)90178-1. [DOI] [PubMed] [Google Scholar]
- 36.Wilson ME, Pazol K, Legendre A, Fisher J, Chikazawa K. Gonadal steroid modulation of the limbic - hypothalamic - pituitary - adrenal (LHPA) axis is influenced by social status in female rhesus monkeys. Endocrine. 2005;26(2) doi: 10.1385/ENDO:26:2:089. [DOI] [PubMed] [Google Scholar]
- 37.Michopoulos V, Higgins M, Toufexis D, Wilson ME. Social subordination produces distinct stress-related phenotypes in female rhesus monkeys. Psychoneuroendocrinology. 2012;37(7):1071–85. doi: 10.1016/j.psyneuen.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jovanovic H, Perski A, Berglund H, Savic I. Chronic stress is linked to 5-HT(1A) receptor changes and functional disintegration of the limbic networks. NeuroImage. 2011;55(3):1178–88. doi: 10.1016/j.neuroimage.2010.12.060. [DOI] [PubMed] [Google Scholar]
- 39.Savitz J, Lucki I, Drevets WC. 5-HT(1A) receptor function in major depressive disorder. Progress in neurobiology. 2009;88(1):17–31. doi: 10.1016/j.pneurobio.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lupien SJ, Maheu F, Tu M, Fiocco A, Schramek T. The effects of stress and stress hormones on human cognition: implications for the field of brain and cognition. Brain and cognition. 2007;65(3):209–37. doi: 10.1016/j.bandc.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 41.McEwen BS. Invited review: Estrogens effects on the brain: multiple sites and molecular mechanisms. J Appl Physiol. 2001;91(6):2785–801. doi: 10.1152/jappl.2001.91.6.2785. [DOI] [PubMed] [Google Scholar]
- 42.Quirk GJ, Likhtik E, Pelletier JG, Paré D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. The Journal of Neuroscience. 2003;23(25):8800–7. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, Macklin ML, Lasko NB, Cavanagh SR, Krangel TS. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Archives of general psychiatry. 2005;62(3):273. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- 44.Fisher PM, Price JC, Meltzer CC, Moses-Kolko EL, Becker C, Berga SL, Hariri AR. Medial prefrontal cortex serotonin 1A and 2A receptor binding interacts to predict threat-related amygdala reactivity. Biology of mood & anxiety disorders. 2011;1(1):1–11. doi: 10.1186/2045-5380-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tauscher J, Bagby RM, Javanmard M, Christensen BK, Kasper S, Kapur S. Inverse relationship between serotonin 5-HT1A receptor binding and anxiety: a [11C] WAY-100635 PET investigation in healthy volunteers. American Journal of Psychiatry. 2001;158(8):1326–8. doi: 10.1176/appi.ajp.158.8.1326. [DOI] [PubMed] [Google Scholar]
- 46.Lee HÄ, Lee MÄ, Kang RÄ, Kim H, Kim SÄ, Kee BÄ, Kim YH, Kim YÄ, Kim JB, Yeon BK. Influence of the serotonin transporter promoter gene polymorphism on susceptibility to posttraumatic stress disorder. Depression and Anxiety. 2005;21(3):135–9. doi: 10.1002/da.20064. [DOI] [PubMed] [Google Scholar]
- 47.Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, Egan MF, Weinberger DR. Serotonin transporter genetic variation and the response of the human amygdala. Science (New York, NY. 2002;297(5580):400–3. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- 48.Sumner BE, Fink G. Estrogen increases the density of 5-Hydroxytryptamine< sub> 2A</sub> receptors in cerebral cortex and nucleus accumbens in the female rat. The Journal of steroid biochemistry and molecular biology. 1995;54(1):15–20. doi: 10.1016/0960-0760(95)00075-b. [DOI] [PubMed] [Google Scholar]
- 49.Kugaya A, Epperson CN, Zoghbi S, van Dyck CH, Hou Y, Fujita M, Staley JK, Garg PK, Seibyl JP, Innis RB. Increase in prefrontal cortex serotonin 2A receptors following estrogen treatment in postmenopausal women. The American journal of psychiatry. 2003;160(8):1522–4. doi: 10.1176/appi.ajp.160.8.1522. [DOI] [PubMed] [Google Scholar]
- 50.Pau CY, Pau KY, Spies HG. Putative estrogen receptor beta and alpha mRNA expression in male and female rhesus macaques. Molecular and cellular endocrinology. 1998;146(1–2):59–68. doi: 10.1016/s0303-7207(98)00197-x. [DOI] [PubMed] [Google Scholar]
- 51.Rissman EF. Roles of oestrogen receptors alpha and beta in behavioural neuroendocrinology: beyond Yin/Yang. Journal of neuroendocrinology. 2008;20(6):873–9. doi: 10.1111/j.1365-2826.2008.01738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.David SP, Murthy NV, Rabiner EA, Munafó MR, Johnstone EC, Jacob R, Walton RT, Grasby PM. A functional genetic variation of the serotonin (5-HT) transporter affects 5-HT1A receptor binding in humans. The Journal of Neuroscience. 2005;25(10):2586–90. doi: 10.1523/JNEUROSCI.3769-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Christian BT, Wooten DW, Hillmer AT, Tudorascu DL, Converse AK, Moore CF, Ahlers EO, Barnhart TE, Kalin NH, Barr CS. Serotonin Transporter Genotype Affects Serotonin 5-HT1A Binding in Primates. The Journal of Neuroscience. 2013;33(6):2512–6. doi: 10.1523/JNEUROSCI.4182-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]