Abstract
BACKGROUND
Chronic alcohol consumption reduces brain serotonin and alters the synaptic mechanisms involved in memory formation. Hippocampal 5-HT1A receptors modulate these mechanisms, but the neuroadaptive response of 5HT1A receptors to chronic alcohol self-administration is not well understood.
METHODS
Hippocampal tissue from monkeys that voluntarily self-administered ethanol for 12 months (n=9) and accompanying controls (n=8) were prepared for in vitro receptor autoradiography and laser capture microdissection. The 5-HT1A receptor antagonist, [3H]MPPF, and the agonist, [3H]8-OH-DPAT, were used to measure total and G-protein coupled 5-HT1A receptors respectively. The expression of the genes encoding the 5-HT1A receptor and its trafficking protein Yif1B was measured in microdissected dentate gyrus (DG) granule cells and CA1 pyramidal neurons.
RESULTS
An increase in G-protein coupled, but not total, receptors was observed in the posterior pyramidal cell layer of CA1 in ethanol drinkers compared to controls. Chronic ethanol self-administration was also associated with an up-regulation of total and G-protein coupled 5-HT1A receptors in the posterior DG polymorphic layer. Changes in receptor binding were not associated with concomitant changes in 5-HT1A receptor mRNA expression. Chronic ethanol self-administration was associated with a significant increase in Yif1B gene expression in posterior CA1 pyramidal neurons.
CONCLUSIONS
Chronic, ethanol self-administration up-regulates hippocampal 5-HT1A receptor density in a region-specific manner that does not appear to be due to alterations at the level of transcription but instead may be due to increased receptor trafficking. Further exploration of the mechanisms mediating chronic ethanol-induced 5-HT1A receptor up-regulation and how hippocampal neurotransmission is altered is warranted.
Keywords: serotonin, nonhuman primate, heaving drinking, hippocampus, Yif1B
1. INTRODUCTION
The hippocampus plays an important role in the development of alcohol dependence. It modulates mesolimbic dopamine system-mediated reward via projections to several brain regions including the nucleus accumbens (Friedman et al., 2002; Haber and Knutson, 2009) and plays a significant role in the acquisition and retrieval of drug-related memories, which are thought to drive compulsive drug-seeking and continued consumption (Hyman and Malenka, 2001; Robbins et al., 2008). Many of the neurobiological processes altered by chronic alcohol also mediate memory formation (Kauer and Malenka, 2007; Nestler et al., 2002), including long-term potentiation (LTP; Roberto et al., 2002).
Serotonin plays a well-recognized role in regulating alcohol consumption (LeMarquand et al., 1994; Johnson, 2004; Sari et al., 2011). For example, chronic ethanol exposure is associated with a decrease in serotonin levels (Wu et al., 1986; Bare et al., 1998) and likewise, low serotonin is associated with increased ethanol preference and intake (Murphy et al., 1982; Gongwer et al., 1989; Linnoila et al., 1989). Furthermore, degeneration of ascending serotonergic neurons has been observed in the brains of alcoholics (Halliday et al., 1993) suggesting that low levels of serotonin may be a risk factor for, as well as a consequence of, chronic ethanol consumption.
Among the various receptors that mediate the effects of serotonin within the hippocampus, G-protein coupled 5-HT1A heteroreceptors have drawn particular interest. Located on the dendrites and axon hillock of dentate gyrus granule cells and CA1/2 pyramidal neurons (Azmitia et al., 1996), 5-HT1A receptors decrease cell firing (Pugliese et al., 1998) and modulate neuroadaptive responses, like LTP, in hippocampal neurons (Kojima et al., 2003).
Despite their pivotal role, there is a paucity of information regarding the relationship between chronic ethanol and hippocampal 5-HT1A receptors. Ulrichsen (1991) reported that exposing rats to ethanol for five days resulted in reduced hippocampal 5-HT1A receptor binding to the agonist, [3H]8-OH-DPAT compared to ethanol naïve controls. Nevo et al. (1995) reported similar results using both [3H]8-OH-DPAT and the antagonist, [3H]WAY 100635 in rats exposed to 14 days of ethanol compared to controls. This combination is particularly advantageous because it permits quantification of the entire 5-HT1A receptor population including G-protein coupled and uncoupled receptors. Nevo et al. (1995) did not, however, report a subregion and layer specific analysis of the hippocampus. Because the discrete functional subdivisions of the region each contribute uniquely to the hippocampal microcircuitry, a more anatomically comprehensive approach is warranted (Amaral and Lavenex, 2007).
In contrast to existing rodent studies, Martinez et al. (2009) reported no difference in hippocampal 5-HT1A receptor availability in alcohol dependent individuals versus controls using positron emission tomography. Technical resolution, ethanol exposure parameters, time since last ethanol exposure, and species differences may contribute to the discrepant findings. Nevertheless, the effect of ethanol on the expression of 5-HT1A receptors remains unclear.
Little attention has focused on the mechanism(s) underlying ethanol’s effect on 5HT1A receptor density, which may include changes in receptor gene expression or trafficking. The present study takes advantage of a well-characterized nonhuman primate model that accurately reflects human alcohol consumption (Grant et al., 2008; Vivian et al., 2001) to investigate the effects of chronic ethanol self-administration on hippocampal 5-HT1A receptors using a comprehensive subregion, layer and level specific approach.
2. MATERIALS AND METHODS
2.1 Subjects
The subjects and ethanol self-administration methods used in the current study have been described in detail previously (Burnett et al., 2012). Nine individually housed adult male cynomolgus macaques (Macaca fascicularis) voluntarily self-administered ethanol, water and banana flavored food pellets (Research Diets Incorporated, New Brunswick, NJ) 22 hr/day for 12 months using an operant panel permanently attached to their home cage. Each animal’s intake varied throughout the study with average daily intakes ranging from 1.17–4.25 g/kg (see Burnett et al., 2012 for details). Blood ethanol concentrations (BECs) were taken every fifth day at 7 hr following the onset of the drinking session. Intakes at the time of blood draw correlated positively with BEC (r2=0.87; p<0.01).
Controls consisted of two separate groups of ethanol naïve monkeys. Four “caloric controls” were housed in the same room as the ethanol group, received the same diet, and self-administered a volume of a maltose-dextrin solution that was matched in calories to the ethanol-caloric intake of an assigned ethanol monkey. Four “housing controls” were housed in a room identical to the ethanol self-administration room and remained on regular laboratory diet (Purina monkey chow).
Monkeys were necropsied at the end of their final 22-hour self-administration session as described previously (Burnett et al., 2012). The brain was quickly removed, blocked, flash frozen, and stored at −80°C until ready for sectioning. All procedures were approved by the Wake Forest University School of Medicine and Oregon Health & Sciences University Institutional Animal Care and Use Committees and adhered to NIH’s Guide for the Care and Use of Laboratory Animals.
2.2 In vitro receptor autoradiography
Blocks containing the hippocampus were sectioned coronally at 20 microns using a cryostat maintained at −20°C. Sections were thaw mounted to Frost-plus slides, placed on wet ice and then in a desiccator overnight at 4°C before being placed at −80°C.
5-HT1A receptor density was determined using the agonist [3H]8-hydroxy-2-(di-n-propyl)aminotetralin (8-OH-DPAT) or antagonist [3H]4-(2′-methoxy-)-phenyl-1-[2′-(N-2″-pyridal)-p-fluorobenzamido]ethyl-piperazine (MPPF; PerkinElmer, Inc., San Jose, CA). Specific binding was determined in two adjacent sections at two levels along the rostrocaudal extent of the hippocampus corresponding to approximately A8–10 (midrostrocaudal) and A4–6 (caudal) in the atlas of Szabo and Cowan (1984) per animal. Nonspecific binding (NSB) was determined using a single adjacent section per level per animal. The anterior and posterior hippocampus are distinguished from each other based on functional differences, but recent work suggests that the hippocampus can be divided into at least three structurally and functionally distinction regions designated as anterior, posterior and intermediate (Fanselow and Dong, 2010). While additional work is needed to clearly define these boundaries, the levels chosen for the present study correspond approximately to the intermediate and posterior hippocampus respectively.
Labeling with [3H]8-OH-DPAT was performed using the procedures of Lu and Bethea (2002). Sections were preincubated for 30 minutes at room temperature (RT) in 170 mM Tris-HCl buffer containing 4 mM CaCl2 (pH 7.6). Total binding was determined by incubation for 60 minutes in the same buffer containing 2 nM [3H]8-OH-DPAT (187.4 Ci/mmol), 0.01% L-ascorbic acid, 10 uM pargyline and 10 uM fluoxetine. NSB was determined in the presence of 2 uM serotonin. Sections were subsequently washed in ice-cold preincubation buffer (2 × 4 minutes) followed by a rinse in ice-cold distilled water.
Labeling with [3H]MPPF was performed using the procedures of Hensler (2002). Sections were preincubated for 30 minutes at RT in 170 mM Tris-HCl buffer (pH 7.6). Total binding was determined by incubation for 90 minutes in the same buffer containing 10 nM [3H]MPPF (74.2 Ci/mmol). NSB was determined in the presence of 10 uM WAY 100635. Sections were subsequently washed in ice-cold preincubation buffer (2 × 5 minutes) followed by two rinses in ice-cold distilled water for one minute and 30 seconds, respectively.
Sections were dried overnight in a hood under a stream of cool air and then apposed with [3H] standards (American Radiolabeled Chemicals, Inc., St. Louis, MO) to [3H]Hyperfilm for 4 weeks for [3H]8-OH-DPAT and 5 weeks for [3H]MPPF. Films were developed with Kodak GBX developer, stopbath and Kodak Rapid Fixer.
Autoradiograms were analyzed by quantitative densitometry using MCID (Imaging Research, St. Catherines, Ontario, Canada). Curves from [3H] standards were used to convert optical density values to tissue equivalent values (fmol/mg wet weight tissue). Two adjacent measurements of total binding were taken from each section, in each region, at each level (i.e., four measurements per region per level per animal). Specific binding was determined by subtracting NSB from total binding in adjacent sections. Adjacent nissl stained sections were used to confirm the anatomical placement of the measurements taken.
2.3 LCM, RNA isolation & cDNA synthesis
Sections adjacent to those used for in vitro receptor autoradiography were cut coronally at 10 microns, thaw mounted to plain glass slides, placed on dry ice and stored at −80°C until ready for processing.
Cells for microdissection were visualized using an RNA-compatible nissl stain. Sections were thawed to RT for 30 seconds and then submerged in 75% ethanol, nuclease free water (NFW), 0.005 M thionin, NFW, 75%, 95%, and 100% ethanol for 30 seconds each, dehydrated in xylene for five minutes and air dried under a hood for five minutes.
Individual CA1 pyramidal neurons and the entire dentate gyrus (DG) granule cell layer were microdissected using the Arcturus XT LCM Instrument (Life Technologies Corporation, Carlsbad, CA). Cells of interest were identified by neuroanatomical location, size and morphology. Average dissection parameters were: pyramidal neurons: power=68, duration=8, spot size=18–20; granule cell layer: power=75, duration=50, spot size=65. Approximately 1,200–1,600 pyramidal neurons and 4–5 granule cell layers were microdissected from 4–6 sections per level per animal.
After dissection, cells were lysed and RNA was extracted using the Arcturus PicoPure Frozen RNA Isolation Kit (Life Technologies Corporation). Isolated RNA was eluted into 30 uL of elution buffer. Samples from a single subject were pooled together and RNA was quantified using NanoDrop 1000 (Thermo Scientific, Wilmington, DE). RNA quality was assessed for each subject from adjacent sections using the Agilent 2100 Bioanalyzer with RNA 6000 Pico Kit (Agilent Technologies, Santa Clara, CA). Total RNA (100 ng) was transcribed for each subject using random primers and Superscript III (Life Technologies Corporation). Aliquots of RNA from each subject for each discrete cell population were pooled prior to reverse transcription for use as standards. An additional pooled aliquot was reverse transcribed in the absence of Superscript III to serve as a no reverse transcription control (NRTC) for detection of genomic DNA. Resulting complimentary DNA (cDNA) was diluted 1:50 for qPCR and standards were generated from the pooled cDNA and serially diluted in two fold dilutions from 1:10 to 1:320.
2.4 qPCR
Standard Human Taqman Gene Expression Assays (Applied Biosystems, Carlsbad, CA) were used for the measurement of 5-HT1A receptor and Yif1B levels as well as endogenous controls. The assays used and their percent homology with the Macaca genus, as determined by NCBI Genome BLAST, are as follows: 5-HT1A receptor (HTR1A) – Hs00265014_s1 (97%); Yif1B (YIF1B) – Hs00293051_m1 (96%); 18S –Hs99999901_s1 (99%); β-actin (ACTB) – Hs99999903_m1 (97%); β-2 microglobulin (B2M) – Hs99999907_m1 (92%); glyceraldehyde-3-phosphate dehydrogenase (GAPDH) – Hs99999905_m1 (97%); β-glucuronidase (GUSB) – Hs99999908_m1 (94%); hypoxanthine phosphoribosyltransferase 1 (HPRT1) – Hs99999909_m1 (99%); phosphoglycerate kinase 1 (PGK1) – Hs99999906_m1 (97%); peptidylprolyl isomerase A (cyclophilin A) (PPIA) – Hs99999904_m1 (97%); ribosomal protein, large (RPLPO) – Hs99999902_m1 (99%); TATA box binding protein (TBP) – Hs99999910_m1 (96%); transferrin receptor (TFRC) – Hs99999911_m1 (95%).
ABI Prism 7900HTS real-time detector (Applied Biosystems) was used to analyze 384-well plates containing 0.5 uL Taqman Gene Expression Assay (20X), 5.5 uL ABsolute QPCR ROX Mix (Thermo Scientific) and 4.5 uL diluted cDNA mixed together in each well. In addition to the NRTC, a no template control (NTC) consisting of water instead of cDNA was used. Samples were run in triplicate using thermocycle conditions and quantified according to methods described previously (O’Conner et al., 2007). Endogenous controls (ECs) were selected for each experiment from a set of eleven candidate reference transcripts (see above) using the geNorm algorithm (Vandesompele et al., 2002). HPRT1, PGK1, PPIA, and TBP were used as ECs for experiments using DG granule cells while 18S, HPRT1, PGK1, and PPIA were used for CA1 pyramidal neurons. Data for each gene of interest were expressed as mean quantity over geometric mean of the mean quantity of the selected ECs for each experiment. Normalized values are expressed as percent control.
2.5 Statistical analysis
SigmaStat 3.1 (Systat Software, Inc., Point Richmond, CA) was used for statistical analyses. All data were analyzed following log transformation for normalization. Two-way repeated measures analysis of variance was employed for each region measured in receptor autoradiography experiments with group (ethanol versus control) and level (intermediate versus posterior) as the factors and level as the repeated measure. Post-hoc pairwise multiple comparisons were conducted using a Bonferroni t-test. Data from each discrete cell population in the gene expression experiments were analyzed separately in a similar manner. Linear regression was used to identify the relationship between binding and gene expression data.
3. RESULTS
3.1 Comparison of caloric and housing controls
There were no significant differences between control groups for any region in [3H]MPPF binding or 5-HT1A receptor or Yif1B gene expression. Consequently, data from both groups were pooled and used as a single control group to assess the effect of ethanol self-administration on these measures. A single difference between the two control groups was observed in [3H]8-OH-DPAT binding in the DG granule cell layer (DGgcl) [F(1,15)=5.988, p=0.050; Figure 1]. A closer examination of the data revealed that the effect was driven by two housing controls that exhibited far greater (49%) [3H]8-OH-DPAT binding than the other six controls and all nine ethanol drinkers. Based on these findings, the two outliers were removed from all comparisons of [3H]8-OH-DPAT binding. The six remaining controls were pooled and used for comparison against the ethanol group.
Figure 1. Between group comparison of [3H]8-OH-DPAT binding in the dentate gyrus granule cell layer.
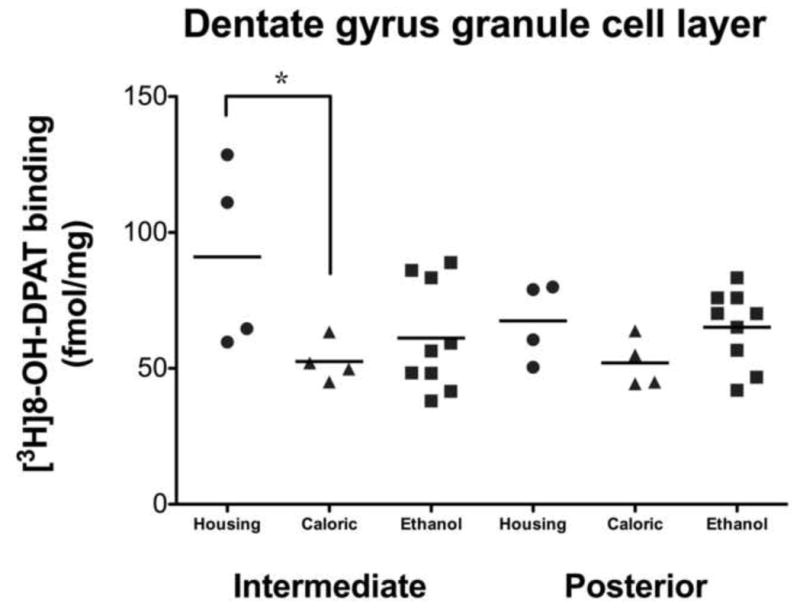
Two way repeated measures ANOVA uncovered a significant difference in [3H]8-OH-DPAT binding between housing and caloric control groups in the dentate gyrus granule cell layer at the intermediate level only (*p=0.050). This effect was driven by two housing controls that exhibited far greater (49%) binding than the other six controls and all nine ethanol drinkers.
3.2 Effect of chronic ethanol on [3H]MPPF binding
[3H]MPPF binding reflected the normal distribution of 5-HT1A receptors reported in the primate hippocampus (Figure 2B and 2C; Varnas et al., 2004) with the greatest density in CA1/CA2 and moderate to high levels in the dentate gyrus (DG). Based on this distribution, measurements were taken in all four layers of CA1: stratum oriens (so), pyramidal cell layer (pcl), stratum radiatum (sr) and stratum lacunosum molecular (slm), and all three layers of the DG: molecular layer (mol), granule cell layer (gcl) and polymorphic layer (pol).
Figure 2. Effect of chronic ethanol on hippocampal [3H]MPPF binding.
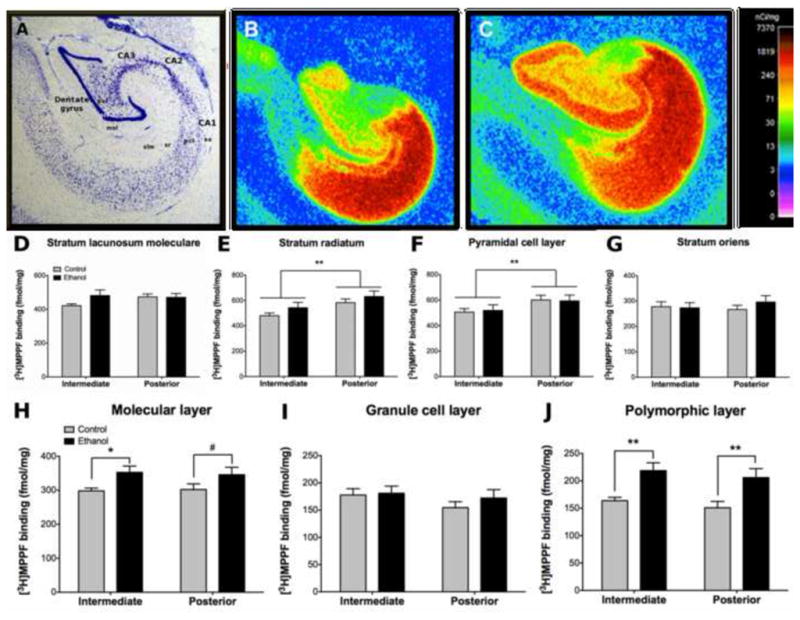
(A) Nissl stained section outlining the subregions and layers of the hippocampus. Representative control (B) and ethanol (C) autoradiograms showing high [3H]MPPF binding in CA1 and moderate to high binding in the dentate gyrus. Both ethanol and controls exhibited greater binding in posterior CA1 than intermediate levels in the stratum radiatum (E) and pyramidal cell layer (F). No differences were observed in the stratum lacunosum moleculare (D) or stratum oriens (G). Ethanol drinkers exhibited greater binding than controls in the molecular (H) and polymorphic (J) layers at both intermediate and posterior levels of the dentate gyrus. No differences were observed in the dentate gyrus granule cell layer (I). *p≤0.05; **p≤0.01; #p≤0.10. Abbreviations: CA1–3 – cornu ammonis 1–3; DG – dentate gyrus; gcl – granule cell layer; mol – molecular layer; pcl – pyramidal cell layer; pol – polymorphic layer; slm – stratum lacunosum moleculare; so – stratum oriens, sr – stratum radiatum.
A main effect of level was observed in CA1sr and CA1pcl [F(1,33)=29.947, p<0.001; F(1,33)=47.348, p<0.001 respectively]. Post-hoc comparisons revealed greater [3H]MPPF binding in the posterior than the intermediate level in both layers for both groups (p<0.01). No main effect of level was observed in CA1so or CA1slm. No main effect of group was observed for any of the layers in CA1 (Figure 2E–G).
A main effect of group was observed in DGmol [F(1,33)=4.628, p=0.048] and DGpol [F(1,33)=14.184, p=0.002]. Post-hoc comparisons revealed greater [3H]MPPF binding in the ethanol group than controls in the intermediate DGmol (18%; p=0.046) and both intermediate (34%; p=0.007) and posterior (37%; p=0.003) DGpol. Ethanol drinkers exhibited greater [3H]MPPF binding in posterior DGmol (14%) but this effect did not reach statistical significance (p=0.096; Figure 2H–J).
3.3 Effect of chronic ethanol on [3H]8-OH-DPAT binding
The distribution of hippocampal [3H]8-OH-DPAT binding was similar to that of [3H]MPPF (Figure 3B and 3C) so measurements were taken from the same layers and subregions. Measurements were not taken, however, from one section for three control animals due to tissue loss resulting in the average of two measurements instead of four for each region for these subjects.
Figure 3. Effect of chronic ethanol on hippocampal [3H]8-OH-DPAT binding.
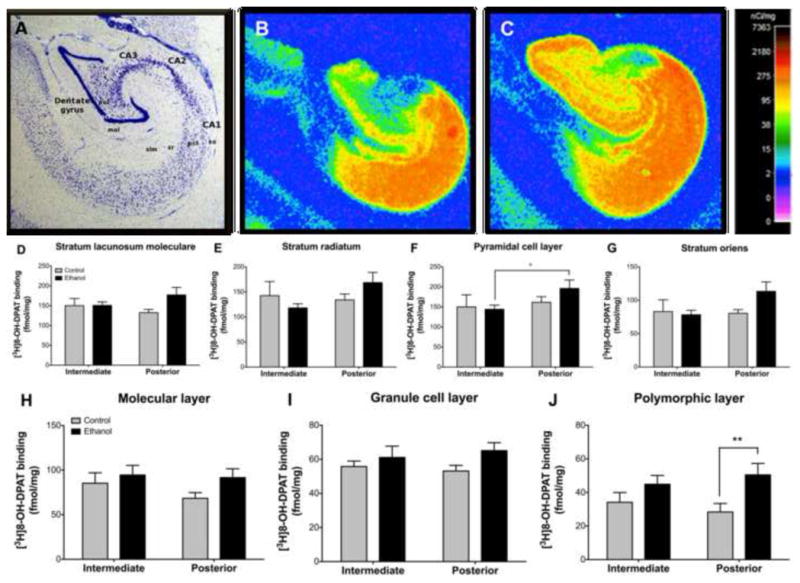
(A) Nissl stained section outlining the subregions and layers of the hippocampus. Representative control (B) and ethanol (C) autoradiograms showing high [3H]8-OH-DPAT binding in CA1 and moderate to high binding in the dentate gyrus. Binding was greater in ethanol drinkers in posterior CA1 than intermediate in the pyramidal cell layer (F) while controls exhibited similar binding between levels for both layers. No differences were observed in the stratum radiatum (E), stratum oriens (G) or stratum lacunosum moleculare (D). In the dentate gyrus, ethanol drinkers exhibited greater binding in the polymorphic layer (J) at the posterior level while no differences were observed in the molecular (H) or granule cell (I) layers. *p≤0.05; **p≤0.01. Abbreviations: CA1–3 – cornu ammonis 1–3; DG – dentate gyrus; gcl – granule cell layer; mol – molecular layer; pcl – pyramidal cell layer; pol – polymorphic layer; slm – stratum lacunosum moleculare; so – stratum oriens, sr – stratum radiatum.
A main effect of level was observed in CA1pcl [F(1,29)=5.513, p=0.035]. Like the [3H]MPPF findings, post-hoc comparisons indicated that the ethanol group exhibited greater binding in the posterior than the intermediate level (p=0.022), while controls exhibited similar densities between levels. No effect of group or level was observed in CA1so, CA1sr or CA1slm (Figure 3D–G).
A main effect of group was observed in DGpol [F(1,29)=8.680, p=0.011] with the ethanol group exhibiting greater binding at both intermediate (32%) and posterior (78%) levels compared to controls. This effect reached significance only at the posterior level (p=0.006). No effect of group or level was observed in DGmol or DGgcl (Figure 3H–J).
3.4 Effect of chronic ethanol on 5-HT1A receptor gene expression
A main effect of level was observed in HTR1A expression in CA1 pyramidal neurons [F(1,33)=5.462, p=0.034; Figure 4]. Post-hoc comparisons revealed significantly lower HTR1A expression in CA1 of ethanol drinkers in the intermediate than the posterior level (p=0.040) while controls exhibited similar HTR1A expression between levels. No effect of level or group was observed in the DG. In addition, no significant correlation was observed between [3H]MPPF or [3H]8-OH-DPAT binding and HTR1A expression in areas where an effect of ethanol was observed on receptor density (data not shown).
Figure 4. Effect of chronic ethanol on relative HTR1A and Yif1B mRNA expression.
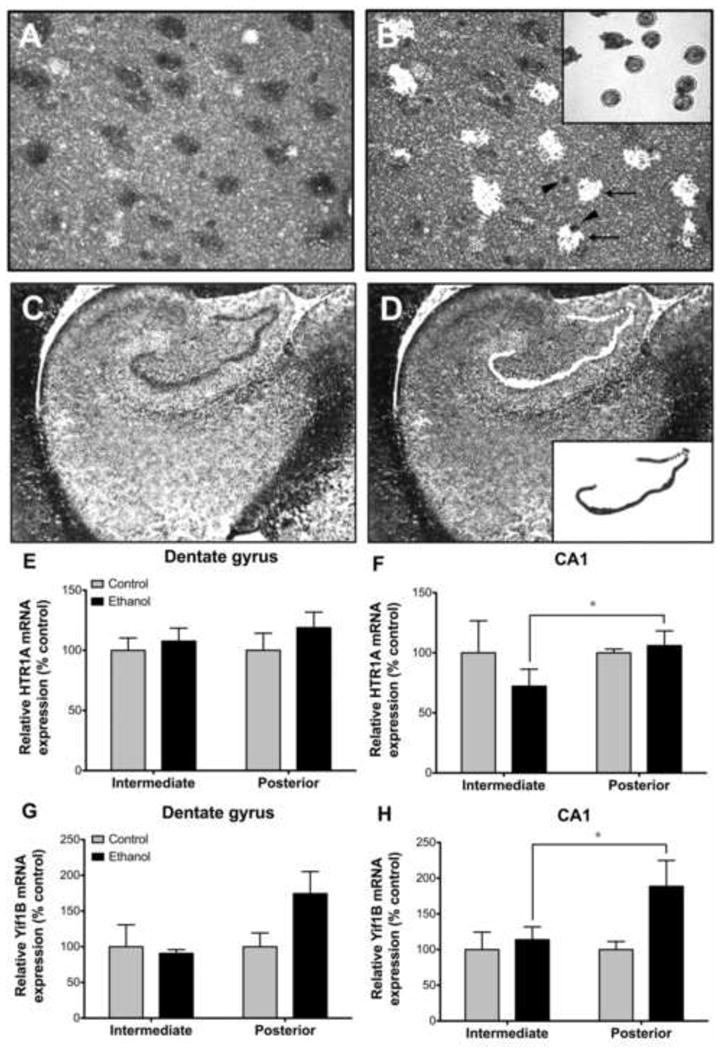
Individual CA1 pyramidal neurons (A) and the dentate gyrus granule cell layer (C) were identified based on location, size and morphology in nissl stained sections. The discrete cell populations were laser capture microdissected (B, D) after which the isolated cells were visualized on the LCM cap (insets in B and D). Note that non-neuronal cell populations remain undissected (arrow heads in B) from cells of interest (arrows in B) using this technique. Chronic ethanol had no effect on HTR1A (E) or Yif1B (G) expression in the dentate gyrus. HTR1A expression was significantly lower in ethanol drinkers in intermediate than posterior CA1 pyramidal neurons (F). In contrast, Yif1B expression was significantly greater in posterior than intermediate CA1 pyramidal neurons of ethanol drinkers (H). *p≤0.05.
3.5 Effect of chronic ethanol on YIF1B gene expression
A main effect of level was observed in CA1 pyramidal neurons [F(1,32)=6.964, p=0.019; Figure 4] with the ethanol group exhibiting greater YIF1B gene expression in the posterior than intermediate hippocampus (p=0.024) whereas controls exhibited a similar magnitude of expression at both levels. A similar effect of level was noted in the DG although it did not reach statistical significance [F(1,33)=3.398, p=0.085]. No effect of group was observed for either cell population. No significant correlation was observed between YIF1B gene expression and 5-HT1A receptor density measures (data not shown).
3.6 Relationship between hippocampal 5-HT1A receptors and ethanol intake
Despite the numerous findings of a main effect of daily voluntary ethanol consumption on 5-HT1A receptor density listed above, no significant correlations were found between daily ethanol intake (g/kg/day) or BECs taken at 7 hrs after the onset of every fifth session averaged over the 12 months of 22 hr/day access or lifetime ethanol intake (mL or g/kg) and any measure of receptor binding or gene expression. The absence of a correlation with global measures of ethanol intake suggests either that these measures are not sensitive to endpoint measurements of 5-HT1A receptor binding and gene expression or that a larger number of subjects are needed to observe significant correlations.
4. DISCUSSION
Chronic ethanol self-administration of 1.2–4.2 g/kg (approximately 7–17 drinks/day) up-regulated 5-HT1A receptor density in specific subregions, layers and levels of the hippocampus. These changes could not be accounted for by alterations in receptor gene expression, but alterations in receptor trafficking may have played a regionally specific role since ethanol induced an increase YIF1B gene expression in posterior CA1, where there was also an increase in 5-HT1A receptor binding.
4.1 5-HT1A receptor density
Total 5-HT1A receptor density, measured by [3H]MPPF, was greater in posterior than intermediate CA1sr and CA1pcl in both ethanol drinkers and controls, indicating the presence of layer-specific rostrocaudal differences in 5-HT1A receptor density. In addition, greater [3H]8-OH-DPAT binding was observed in the CA1pcl of ethanol drinkers compared to controls, suggesting an ethanol-induced 5-HT1A receptor upregulation in this layer due to an apparent transition from uncoupled reserve receptors to functionally coupled receptors.
We did not observe rostro-caudal differences in 5-HT1A binding in the DG. Nevertheless, chronic ethanol self-administration produced a similar layer-specific up-regulation of 5-HT1A receptors with greater total receptor number in both DGmol and DGpol. [3H]8-OH-DPAT binding was also greater in the posterior DGpol suggesting that the ethanol-induced increase in total 5-HT1A receptors in this region is due to an increase in coupled receptors. In contrast, the lack of a corresponding increase in [3H]8-OH-DPAT binding in DGmol and intermediate DGpol suggests that the ethanol-induced increase in 5-HT1A receptors in these regions is not due to an increase in coupled receptors. It is unclear from the present data whether the increase in uncoupled 5-HT1A receptors in these layers is due to an increase in receptor reserve or desensitized receptors that have not been fully degraded. Although the effect did not reach statistical significance, [3H]8-OH-DPAT binding was greater in the DGmol and intermediate DGpol in the ethanol group than in controls suggesting that increased receptor reserve is the more likely explanation.
The 5-HT1A receptor up-regulation observed in the present study challenges previous studies in rodents that report a decrease in hippocampal 5-HT1A receptors following chronic ethanol exposure (Nevo et al., 1995; Ulrichsen, 1991). Both studies, however, exposed animals to ethanol in a non-contingent manner, which is known to produce different effects from voluntary drug self-administration (Hemby et al., 1997; Jacobs et al., 2003). In addition, the exposure paradigms lasted 5–10 days while the present study lasted 12 months. Furthermore, in rodents, 5-HT1A receptors are most abundant in the DGmol and moderately abundant in CA1 whereas primates exhibit the reverse relationship (Duncan et al., 1998; Kohler et al., 1986). Thus, species and methodological differences therefore likely contribute to the contradictory results.
Chronic ethanol produces a decrease in extracellular serotonin levels in the brain (Borg et al., 1985; Branchey et al., 1981) that may be due to ethanol-induced degeneration of serotonergic fibers (Halliday et al., 1993). Indeed, we have reported decreased hippocampal serotonin transporter (SERT) density in these same animals (Burnett et al., 2012), and SERT density has been reported as an accurate indicator of serotonin fiber density (Nielsen et al., 2006). These findings suggest that the up-regulation of 5-HT1A receptors reported here may be a compensatory response to decreased serotonin. Similar to our current findings, the effect of chronic ethanol on SERT was greatest in the DG (Burnett et al., 2012) further supporting this conclusion.
An increase in 5-HT1A r receptor density would result in increased inhibition of excitatory neurotransmission. Given the 5-HT1A receptor’s role in inhibiting LTP (Kojima et al., 2003), the proposed increase in receptor activation may also contribute to the reduced LTP production associated with chronic ethanol. Additional work is necessary to determine the specific functional consequences of 5-HT1A receptor upregulation in these regions.
4.2 5-HT1A receptor gene expression
We found no between group differences in HTR1A expression in any comparison. Similarly, Nevo et al. (1995) found no difference in HTR1A expression following chronic ethanol despite altered receptor density. Kinoshita et al. (2003) did not observe a difference in CA1 HTR1A mRNA levels, but did report a significant decrease in HTR1A expression in both CA3 and DG in rats following five days of intra-gastric ethanol exposure. Again, experimental methods may contribute to these contradictory findings.
Dissociation between protein and gene expression is commonly reported (Gry et al., 2009; Guo et al., 2008). Moreover, regulation of 5-HT1A receptor gene expression is complex (Raymond et al., 1999), and under the control of numerous transcription factors (Albert et al., 2011). The effect of chronic ethanol on these transcription factors remains unknown, although stress and depression have both been associated with differential control of regulators of 5-HT1A receptor transcription (Iyo et al., 2009; Szewczyk et al., 2009, 2010), suggesting that similar alterations are plausible following chronic ethanol.
DG granule cells and CA1 pyramidal neurons are the primary sites of 5-HT1A receptor localization in the hippocampus, however, a small population of 5-HT1A receptors have been reported on hippocampal interneurons (Azmitia et al., 1992, 1996; Aznar et al., 2003) and astrocytes (Azmitia et al., 1992, 1996). The possibility, therefore, remains that these cell types contribute to the observed changes in 5-HT1A receptor density. Indeed, in the rodent, interneurons expressing 5-HT1A receptors are reportedly localized to the DGpol (Aznar et al., 2003), where we observed the most pronounced effects. Whether primate DGpol interneurons similarly express 5-HT1A receptors is unknown. Given the considerable differences in 5-HT1A receptor distribution between rodents and primates (Duncan et al., 1998) this possibility should be considered cautiously. Alternatively, DGpol 5-HT1A receptors may reside not only on granule cell apical dendrites, but also on their basal dendrites, which are present only in primates (Seress et al., 1987).
4.3 5-HT1A receptor trafficking
Yif1B was recently shown to enable trafficking of 5-HT1A receptors from the Golgi apparatus to neuronal dendrites (Carrel et al., 2008). Increased YIF1B expression in posterior but not intermediate CA1 pyramidal neurons of ethanol drinkers parallels the up-regulation of 5-HT1A receptor density in this group, suggesting that increased receptor trafficking may be responsible for some of the observed changes in receptor density. There was, however, no correlation between YIF1B expression and 5-HT1A receptor density. While very little is known about Yif1B, it is unlikely that it is specific to the 5-HT1A receptor. In addition, up to six isoforms of Yif1B have been sequenced to date and the functional differences of each of these isoforms, their distribution, and interaction with the 5-HT1A receptor are currently unknown.
4.4 Conclusions
The present study provides strong evidence that chronic ethanol self-administration upregulates hippocampal 5-HT1A receptors in primates in a layer, subregion and level specific manner. The data are limited, however, by the presence of two different control groups. While nonhuman primate models afford researchers greater control over many of the confounds experienced in human research, they, nevertheless, exhibit greater individual variability than commonly used inbred rodent models. The strength of the model, however, lies in the remarkable similarities between human and nonhuman primate neurobiology and behavior. Still, the presence of several outliers in the [3H]8-OH-DPAT experiment require that these data in particular be interpreted somewhat cautiously. Replication of the present study, using a single control group and a larger sample will help to increase the power of the comparisons made and allow firmer conclusions to be drawn. Nevertheless, these data make a unique contribution to the existing literature with the use of sophisticated methodologies and a comprehensive anatomical analysis. In addition, the self-administration procedure used here results in daily drinking to intoxication over long periods of time, which models well human alcoholic drinking.
Alterations in 5-HT1A receptor density are likely to have significant functional consequences. Altered 5-HT1A-mediated inhibitory modulation of the mossy fiber projection from DG granule cells to CA3 via up-regulation of 5-HT1A receptors in the DGpol is likely to have a pervasive effect on signaling throughout the rest of the hippocampal microcircuitry. Likewise, given the essential role of the CA1 region in hippocampal LTP and subsequent memory formation (Lisman et al., 2012), upregulation of CA1 5-HT1A receptors is expected to have significant functional consequences at both cellular and behavioral levels.
The mechanism(s) behind chronic ethanol-induced changes in hippocampal 5-HT1A receptor density are not well understood. Data from the present study suggest that receptor trafficking may play a more prominent role than changes in gene expression. Additional research examining the effects of chronic ethanol on transcriptional regulators of the 5-HT1A receptor and 5-HT1A trafficking proteins will be necessary to better understand the effects of ethanol on this system. Doing so may aid in identifying more effective drug targets for the treatment of alcohol dependence.
Acknowledgments
ROLE OF FUNDING SOURCE
Funding for this study was provided by NIAAA grants AA14106 (DPF), AA013510 (KAG), AA109431 (KAG) and AA018901 (EJB). NIAAA had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
This study was supported by NIAAA grants AA14106 (DPF), AA013510 (KAG), AA109431 (KAG) and AA018901 (EJB). The authors would like to thank Scot McIntosh for technical assistance.
Footnotes
CONTRIBUTORS
EJ Burnett, DP Friedman and SE Hemby worked together to design the experiments. KA Grant was responsible for the behavioral component of the experiment and provided the brain tissue that formed the basis of the experiments described. EJ Burnett carried out the experiments and data analysis with the assistance of AT Davenport and SE Hemby. EJ Burnett wrote the first draft of the manuscript. All authors participated in manuscript revisions and have approved the final manuscript.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albert PR, Le François B, Millar AM. Transcriptional dysregulation of 5-HT1A autoreceptors in mental illness. Mol Brain. 2011;4:21. doi: 10.1186/1756-6606-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DG, Lavenex P. Hippocampal neuroanatomy. In: Anderson P, Morris R, Amaral D, Bliss T, O’Keefe J, editors. The Hippocampus Book. Oxford University Press; New York: 2007. pp. 37–114. [Google Scholar]
- Azmitia EC, Gannon PJ, Kheck NM, Whitaker-Azmitia PM. Cellular localization of the 5-HT1A receptor in primate brain neurons and glial cells. Neuropsychopharmacology. 1996;14:35–46. doi: 10.1016/S0893-133X(96)80057-1. [DOI] [PubMed] [Google Scholar]
- Aznar S, Qian Z, Shah R, Rahbek B, Knudsen GM. The 5-HT1A serotonin receptor is located on calbindin- and parvalbumin-containing neurons in the rat brain. Brain Res. 2003;959:58–67. doi: 10.1016/s0006-8993(02)03727-7. [DOI] [PubMed] [Google Scholar]
- Bare DJ, McKinzie JH, McBride WJ. Development of rapid tolerance to ethanol-stimulated serotonin release in the ventral hippocampus. Alcohol Clin Exp Res. 1998;22:1272–1276. [PubMed] [Google Scholar]
- Borg S, Kvande H, Liljeberg P, Mossberg D, Valverius P. 5-Hydroxyindoleacetic acid in cerebrospinal fluid in alcoholic patients under different clinical conditions. Alcohol. 1985;2:415–418. doi: 10.1016/0741-8329(85)90106-5. [DOI] [PubMed] [Google Scholar]
- Burnett EJ, Davenport AT, Grant KA, Friedman DP. The effects of chronic ethanol self-administration on hippocampal serotonin transporter density in monkeys. Front Psychiatry. 2012;3:38. doi: 10.3389/fpsyt.2012.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrel D, Masson J, Al Awabdh S, Capra CB, Lenkei Z, Hamon M, Emerit MB, Darmon M. Targeting of the 5-HT1A serotonin receptor to neuronal dendrites Is mediated by Yif1B. J Neurosci. 2008;28:8063–8073. doi: 10.1523/JNEUROSCI.4487-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan GE, Knapp DJ, Breese GR, Crews FT, Little KY. Species differences in regional patterns of 3H-8-OH-DPAT and 3H-zolpidem binding in the rat and human brain. Pharmacol Biochem Behav. 1998;60:439–448. doi: 10.1016/s0091-3057(98)00018-5. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel D, Khanna JM, Kalant H, LeBlanc AE. Effect of acute and chronic ethanol administration on serotonin turnover in rat brain. Psychopharmacologia. 1974;37:91–100. doi: 10.1007/BF00437416. [DOI] [PubMed] [Google Scholar]
- Friedman DP, Aggleton JP, Saunders RC. Comparison of hippocampal, amygdala, and perirhinal projections to the nucleus accumbens: combined anterograde and retrograde tracing study in the macaque brain. J Comp Neurol. 2002;450:345–365. doi: 10.1002/cne.10336. [DOI] [PubMed] [Google Scholar]
- George SR, O’Dowd BF, Lee SP. G-protein-coupled receptor oligomerization and its potential for drug discovery. Nat Rev Drug Discov. 2002;1:808–820. doi: 10.1038/nrd913. [DOI] [PubMed] [Google Scholar]
- Gongwer MA, Murphy JM, McBride WJ, Lumeng L, Li T-K. Regional brain contents of serotonin, dopamine and their metabolites in the selectively bred high- and low-alcohol drinking lines of rats. Alcohol. 1989;6:317–320. doi: 10.1016/0741-8329(89)90089-x. [DOI] [PubMed] [Google Scholar]
- Grant KA, Leng X, Green HL, Szeliga KT, Rogers LS, Gonzales SW. Drinking typography established by scheduled induction predicts chronic heavy drinking in a monkey model of ethanol self-administration. Alcohol Clin Exp Res. 2008;32:1824–1838. doi: 10.1111/j.1530-0277.2008.00765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gry M, Rimini R, Strömberg S, Asplund A, Pontén F, Uhlén M, Nilsson P. Correlations between RNA and protein expression profiles in 23 human cell lines. BMC Genomics. 2009;10:365. doi: 10.1186/1471-2164-10-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Xiao P, Lei S, Deng F, Xiao GG, Liu Y, Chen X, Li L, Wu S, Chen Y, Jiang H, Tan L, Xie J, Zhu X, Liang S, Deng H. How is mRNA expression predictive for protein expression? A correlation study on human circulating monocytes. Acta Biochim Biophys Sin (Shanghai) 2008;40:426–436. doi: 10.1111/j.1745-7270.2008.00418.x. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2009;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday G, Ellis J, Heard R, Caine D, Harper C. Brainstem serotonergic neurons in chronic alcoholics with and without the memory impairment of Korsakoff’s psychosis. J Neuropathol Exp Neurol. 1993;52:567–579. doi: 10.1097/00005072-199311000-00003. [DOI] [PubMed] [Google Scholar]
- Hemby SE, Co C, Koves TR, Smith JE, Dworkin SI. Differences in extracellular dopamine concentrations in the nucleus accumbens during response-dependent and response-independent cocaine administration in the rat. Psychopharmacology (Berl) 1997;133:7–16. doi: 10.1007/s002130050365. [DOI] [PubMed] [Google Scholar]
- Hensler JG. Differential regulation of 5-HT1A receptor-G protein interactions in brain following chronic antidepressant administration. Neuropsychopharmacol. 2002;26:565–573. doi: 10.1016/S0893-133X(01)00395-5. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC. Addiction and the brain: the neurobiology of compulsion and its persistence. Nat Rev Neurosci. 2001;2:695–703. doi: 10.1038/35094560. [DOI] [PubMed] [Google Scholar]
- Iyo AH, Kieran N, Chandran A, Albert PR, Wicks I, Bissette G, Austin MC. Differential regulation of the serotonin 1 A transcriptional modulators five prime repressor element under dual repression-1 and nuclear-deformed epidermal autoregulatory factor by chronic stress. Neuroscience. 2009;163:1119–1127. doi: 10.1016/j.neuroscience.2009.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs EH, Smit AB, de Vries TJ, Schoffelmeer AN. Neuroadaptive effects of active versus passive drug administration in addiction research. Trends Pharmacol Sci. 2003;24:566–573. doi: 10.1016/j.tips.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Johnson BA. Role of the serotonergic system in the neurobiology of alcoholism: implications for treatment. CNS Drugs. 2004;18:1105–1118. doi: 10.2165/00023210-200418150-00005. [DOI] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Kinoshita H, Jessop DS, Roberts DJ, Hishida S, Harbuz MS. Chronic ethanol administration and withdrawal decreases 5-HT1A mRNA, but not 5-HT4 expression in the rat hippocampus. Pharmacol Toxicol. 2003;93:100–102. doi: 10.1034/j.1600-0773.2003.930208.x. [DOI] [PubMed] [Google Scholar]
- Köhler C, Radesäter AC, Lang W, Chan-Palay V. Distribution of serotonin-1A receptors in the monkey and the postmortem human hippocampal region. A quantitative autoradiographic study using the selective agonist [3H]8-OH-DPAT. Neurosci Lett. 1986;72:43–48. doi: 10.1016/0304-3940(86)90615-4. [DOI] [PubMed] [Google Scholar]
- Kojima T, Matsumoto M, Togashi H, Tachibana K, Kemmotsu O, Yoshioka M. Fluvoxamine suppresses the long-term potentiation in the hippocampal CA1 field of anesthetized rats: an effect mediated via 5-HT1A receptors. Brain Res. 2003;959:165–168. doi: 10.1016/s0006-8993(02)03756-3. [DOI] [PubMed] [Google Scholar]
- LeMarquand D, Pihl RO, Benkelfat C. Serotonin and alcohol intake, abuse, and dependence: findings of animal studies. Biol Psychiatry. 1994;36:395–421. doi: 10.1016/0006-3223(94)91215-7. [DOI] [PubMed] [Google Scholar]
- Linnoila M, De Jong J, Virkkunen M. Family history of alcoholism in violent offenders and impulsive fire setters. Arch Gen Psychiatry. 1989;46:613–616. doi: 10.1001/archpsyc.1989.01810070039006. [DOI] [PubMed] [Google Scholar]
- Lisman J, Yasuda R, Raghavachari S. Mechanisms of CaMKII action in long-term potentiation. Nat Rev Neurosci. 2012;13:169–182. doi: 10.1038/nrn3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu NZ, Bethea CL. Ovarian steroid regulation of 5-HT1A receptor binding and G protein activation in female monkeys. Neuropsychopharmacology. 2002;27:12–24. doi: 10.1016/S0893-133X(01)00423-7. [DOI] [PubMed] [Google Scholar]
- Martinez D, Slifstein M, Gil R, Hwang DR, Huang Y, Perez A, Frankle WG, Laruelle M, Krystal J, Abi-Dargham A. Positron emission tomography imaging of the serotonin Ttansporter and 5-HT1A receptor in alcohol dependence. Biol Psychiatry. 2009;65:175–180. doi: 10.1016/j.biopsych.2008.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy JM, McBride WJ, Lumeng L, Li T-K. Regional brain levels of monoamines in alcohol-preferring and -nonpreferring lines of rats. Pharmacol Biochem Behav. 1982;16:145–149. doi: 10.1016/0091-3057(82)90026-0. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Common molecular and cellular substrates of addiction and memory. Neurobiol Learn Mem. 2002;78:637–647. doi: 10.1006/nlme.2002.4084. [DOI] [PubMed] [Google Scholar]
- Nevo I, Langlois X, Laporte AM, Kleven M, Koek W, Lima L, Maudhuit C, Martres MP, Hamon M. Chronic alcoholization alters the expression of 5-HT1A and 5-HT1B receptor subtypes in rat brain. Eur J Pharmacol. 1995;281:229–239. doi: 10.1016/0014-2999(95)00238-g. [DOI] [PubMed] [Google Scholar]
- Nielsen K, Brask D, Knudsen GM, Aznar S. Immunodetection of the serotonin transporter protein is a more valid marker for serotonergic fibers than serotonin. Synapse. 2006;59:270–276. doi: 10.1002/syn.20240. [DOI] [PubMed] [Google Scholar]
- O’Connor JA, Muly EC, Arnold SE, Hemby SE. AMPA receptor subunit and splice variant expression in the DLPFC of schizophrenic subjects and rhesus monkeys chronically administered antipsychotic drugs. Schizophr Res. 2007;90:28–40. doi: 10.1016/j.schres.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paila YD, Kombrabail M, Krishnamoorthy G, Chattopadhyay A. Oligomerization of the serotonin(1A) receptor in live cells: a time-resolved fluorescence anisotropy approach. J Phys Chem B. 2011;115:11439–11447. doi: 10.1021/jp201458h. [DOI] [PubMed] [Google Scholar]
- Pugliese AM, Passani MB, Corradetti R. Effect of the selective 5-HT1A receptor antagonist WAY 100635 on the inhibition of e. p.s.ps produced by 5-HT in the CA1 region of rat hippocampal slices. Br J Pharmacol. 1998;124:93–100. doi: 10.1038/sj.bjp.0701807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Ersche KD, Everitt BJ. Drug addiction and the memory systems of the brain. Ann N Y Acad Sci. 2008;1141:1–21. doi: 10.1196/annals.1441.020. [DOI] [PubMed] [Google Scholar]
- Roberto M, Nelson TE, Ur CL, Gruol DL. Long-term potentiation in the rat hippocampus is reversibly depressed by chronic intermittent ethanol exposure. J Neurophysiol. 2002;87:2385–2397. doi: 10.1152/jn.2002.87.5.2385. [DOI] [PubMed] [Google Scholar]
- Sari Y, Johnson VR, Weedman JM. Role of the serotonergic system in alcohol dependence: from animal models to clinics. Prog Mol Biol Transl Sci. 2011;98:401–443. doi: 10.1016/B978-0-12-385506-0.00010-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seress L, Mrzljak L. Basal dendrites of granule cells are normal features of the fetal and adult dentate gyrus of both monkey and human hippocampal formations. Brain Res. 1987;405:169–174. doi: 10.1016/0006-8993(87)91003-1. [DOI] [PubMed] [Google Scholar]
- Szabo J, Cowan WM. A stereotaxic atlas of the brain of the cynomolgus monkey (Macaca fascicularis) J Comp Neurol. 1984;222:265–300. doi: 10.1002/cne.902220208. [DOI] [PubMed] [Google Scholar]
- Szewczyk B, Albert PR, Burns AM, Czesak M, Overholser JC, Jurjus GJ, Meltzer HY, Konick LC, Dieter L, Herbst N, May W, Rajkowska G, Stockmeier CA, Austin MC. Gender-specific decrease in NUDR and 5-HT1A receptor proteins in the prefrontal cortex of subjects with major depressive disorder. Int J Neuropsychopharmacol. 2009;12:155–168. doi: 10.1017/S1461145708009012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrichsen J. Alterations in serotonin receptor subtypes in ethanol-dependent rats. Alcohol Alcohol. 1991;26:567–573. doi: 10.1093/oxfordjournals.alcalc.a045160. [DOI] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:34. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnas K, Halldin C, Hall H. Autoradiographic distribution of serotonin transporters and receptor subtypes in human brain. Hum Brain Mapp. 2004;22:246–260. doi: 10.1002/hbm.20035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivian JA, Green HL, Young JE, Majerksy LS, Thomas BW, Shively CA, Tobin JR, Nader MA, Grant KA. Induction and maintenance of ethanol self-administration in cynomolgus monkeys (Macaca fascicularis): long-term characterization of sex and individual differences. Alcohol Clin Exp Res. 2001;25:1087–1097. [PubMed] [Google Scholar]
- Woehler A, Wlodarczyk J, Ponimaskin E. Specific oligomerization of the 5-HT1A receptor in the plasma membrane. Glycoconj J. 2009;26:749–756. doi: 10.1007/s10719-008-9187-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu PH, Naranjo CA, Fan T. Chronic ethanol inhibits rat hippocampal “stimulus-secretion” coupling mechanism for 5-hydroxytyptamine in vitro. Neurochem Res. 1986;11:801–812. doi: 10.1007/BF00965205. [DOI] [PubMed] [Google Scholar]


