Abstract
Background
Lifestyle and socioeconomic status have been implicated in the prevalence of hypertension; thus, we evaluated factors associated with hypertension in a cohort of blacks and whites with similar socioeconomic status characteristics.
Methods and Results
We evaluated the prevalence and factors associated with self-reported hypertension (SR-HTN) and ascertained hypertension (A-HTN) among 69 211 participants in the Southern Community Cohort Study. Multivariable logistic regression models were used to estimate the odds ratios (ORs) and 95% confidence intervals (CIs) for factors associated with hypertension. The prevalence of SR-HTN was 57% overall. Body mass index was associated with SR-HTN in all race-sex groups, with the OR rising to 4.03 (95% CI, 3.74–4.33) for morbidly obese participants (body mass index, >40 kg/m2). Blacks were more likely to have SR-HTN than whites (OR, 1.84; 95% CI, 1.75–1.93), and the association with black race was more pronounced among women (OR, 2.08; 95% CI, 1.95–2.21) than men (OR, 1.47; 95% CI, 1.36–1.60). Similar findings were noted in the analysis of A-HTN. Among those with SR-HTN and A-HTN who reported use of an antihypertensive agent, 94% were on at least one of the major classes of antihypertensive agents, but only 44% were on ≥2 classes and only 29% were on a diuretic. The odds of both uncontrolled hypertension (SR-HTN and A-HTN) and unreported hypertension (no SR-HTN and A-HTN) were twice as high among blacks as whites (OR, 2.13; 95% CI, 1.68–2.69; and OR, 1.99; 95% CI, 1.59–2.48, respectively).
Conclusions
Despite socioeconomic status similarities, we observed suboptimal use of antihypertensives in this cohort and racial differences in the prevalence of uncontrolled and unreported hypertension, which merit further investigation.
Keywords: African Continental Ancestry Group, European Continental Ancestry Group, hypertension, prevalence
Hypertension is a well-established risk factor for all-cause and cardiovascular disease (CVD) mortality, accounting for an estimated 7.5 million deaths per year or 13.5% of total annual deaths worldwide.1,2 The public health significance of hypertension provided the impetus for the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7).3 Moreover, in the report of the recent landmark Global Burden of Disease Study, hypertension now tops the list of risk factors for death and disability worldwide,4 further highlighting the need to better characterize the risk factors for hypertension, specifically unreported and uncontrolled hypertension. Substantial evidence indicates that blacks have a higher prevalence of hypertension than whites5–7 and that severe hypertension and hypertension complicated by target organ damage are more common in blacks and lead to higher rates of CVD and adverse events.8 Black and white hypertensive patients have also been noted to have differential response to therapeutic strategies, comprising dietary interventions9 and antihypertensive drugs,10–12 including modulators of the renin-aldosterone-angiotensin system.13–17
Recent efforts to determine reasons for racial disparities in the prevalence of hypertension and related adverse sequelae have included assessments of the impact of socioeconomic status (SES), including social environment.18–25 In this context, a unique opportunity to enhance our understanding is presented by the Southern Community Cohort Study (SCCS), a prospective epidemiological cohort study examining racial differences in cancer and other chronic diseases such as CVD, diabetes mellitus, and hypertension in a population of blacks and whites with similar and well-documented SES indexes (eg, income, education, employment, and health insurance status).26–28 We have evaluated factors associated with the prevalence of hypertension among 69 211 blacks and whites with self-reported data and in a subset of 16 932 individuals in whom the presence of hypertension was ascertained using actual blood pressure (BP) measurements. This is the largest evaluation of factors associated with prevalent self-reported hypertension (SR-HTN) and ascertained hypertension (A-HTN) in a population of blacks and whites residing in the southeastern United States, a region known for its high CVD risk factor prevalence and attendant morbidity and mortality.
Methods
Study Population
The SCCS is an ongoing epidemiological study that enrolled adult participants (age, 40–79 years) resident within 12 states in southeastern United States from March 2002 until September 2009. This population-based study is a prospective, combined cross-sectional and longitudinal observational study. The cross-sectional analyses, which pertain to baseline findings, include the present study. Approximately 86% of participants were enrolled through urban and rural community health centers, which provide primary health care for medically underserved populations, and the remaining 14% were enrolled through mail-based general population sampling.28 The present report focuses on 69 211 black and white community health center participants, which ensured similarities in socioeconomic characteristics and access to health care at cohort entry regardless of race. The reason for the restriction to black and white participants was that the sample size for other racial groups was too small for stable statistical analysis. The educational and income levels of the participants are low compared with other established cohorts, with less than a high school education in one third of community health center–enrolled participants and an annual household income of <$15 000 in 61% of participants. Details of the rationale, design, and population of the SCCS have been described.28 SCCS participants provided written informed consent, and the Institutional Review boards of Vanderbilt University Medical Center and Meharry Medical College approved all SCCS protocols.
Data Collection
All enrollees completed a baseline, computer-assisted personal interview (available at www.southerncommunitystudy.org) that ascertained information about demographic, socioeconomic, and anthropometric characteristics; personal and family medical histories; diet; exercise; tobacco and alcohol use; and medication use. The reliability of the SCCS questionnaire has been verified through a series of validation studies.28 Participants who enrolled in 2004 or later and responded affirmatively to the question of whether a doctor ever told them they have high BP were asked additional follow-up questions on the use (and names) of prescription medications for BP control. During community health center enrollment, BP measurements were obtained for a subset of 16 932 black and white participants.
The indexes of SES were total household income in the previous year (<$15 000, $15 000–$24 999, $25 000–$49 999, $50 000–$99 999, and ≥$100 000), highest level of education completed (less than 12th grade, high school/vocational school, some college/junior college, completed college, and graduate school), and health insurance status (none, any private insurance, Medicaid only, Medicare only, military only, and other). We calculated body mass index (BMI) using participants’ self-reported weight and height at baseline. A BMI of <18.5 kg/m2 defines underweight, 25 to 29.9 kg/m2 defines overweight, 30 to 39.9 kg/m2 defines obesity, and >40 kg/m2 defines morbid obesity. Smoking was categorized as never, former (smoked 100+ cigarettes in their lifetime but do not currently smoke), and current <10, 10 to 19, and ≥20 cigarettes/d. Alcohol consumption was classified as heavy (>3 drinks/d), moderate (≤3 drinks/d), or none during the past year. Finally, information was obtained on self-reported family history of heart disease and personal history of myocardial infarction or coronary bypass surgery, stroke, depression, diabetes mellitus, and high cholesterol.
Statistical Analysis
We evaluated the distribution of baseline characteristics of participants by race and sex in the whole population (Table 1), and then among those with A-HTN but no SR-HTN (Table 2), and finally among those with A-HTN and SR-HTN (Table 3). Crude frequency distributions of categorical variables were compared using χ2 tests, whereas t tests or ANOVA was used for continuous variables. The presence of A-HTN was defined as systolic BP (SBP) >140 mm Hg or diastolic BP (DBP) >90 mm Hg. Accordingly, uncontrolled hypertension was defined as participants with SR-HTN in whom A-HTN was present; similarly, those without SR-HTN in whom A-HTN was present were classified as unreported hypertension. All patients on antihypertensive medications were automatically classified as having a history of hypertension by nature of the fact that they had to have SR-HTN to be queried about medication use. Thus, only those with SR-HTN served as the denominator for delineating the prevalence of uncontrolled hypertension.
Table 1.
Self-reported Hypertension in the Southern Community Cohort Study: Baseline Characteristics and Prevalence Among Participants
| Characteristic | Overall, % (n=69 211) | Blacks, %
|
Whites, %
|
||
|---|---|---|---|---|---|
| Men (n=20 900) | Women (n=29 257) | Men (n=6647) | Women (n=12 407) | ||
| Hypertension | 57 | 51 | 64 | 51 | 52 |
| Age at interview, y | |||||
| 40–49 | 44 | 39 | 51 | 40 | 39 |
| 50–59 | 64 | 59 | 73 | 57 | 56 |
| 60–69 | 75 | 74 | 84 | 65 | 67 |
| 70–79 | 78 | 77 | 85 | 70 | 71 |
| Sex | |||||
| Women | 61 | ||||
| Men | 51 | ||||
| Race | |||||
| Black | 59 | ||||
| White | 52 | ||||
| Marital status | |||||
| Married | 58 | 59 | 63 | 57 | 51 |
| Single | 49 | 42 | 57 | 43 | 45 |
| Divorced | 57 | 51 | 65 | 48 | 51 |
| Widowed | 72 | 62 | 78 | 58 | 66 |
| Education | |||||
| Less than high school | 63 | 56 | 71 | 55 | 59 |
| High/vocational school | 55 | 47 | 63 | 49 | 53 |
| Some/junior (2-y) college | 54 | 48 | 60 | 51 | 48 |
| College | 52 | 49 | 57 | 50 | 43 |
| Graduate school | 49 | 54 | 57 | 48* P<0.05 | 35 |
| Annual household income, $ | |||||
| <15 000 | 59 | 52 | 67 | 52 | 56 |
| 15 000–24 999 | 55 | 48 | 62 | 50 | 52 |
| 25 000–49 999 | 54 | 51 | 59 | 52 | 47 |
| 50 000–99 999 | 47 | 48 | 54 | 48 | 39 |
| ≥100 000 | 41 | 49* | 48 | 41† P> 0.05 | 31 |
| Health insurance | |||||
| None | 50 | 43 | 60 | 44 | 49 |
| Any private insurance | 54 | 51 | 60 | 51 | 46 |
| Medicaid only | 61 | 57 | 65 | 57 | 56 |
| Medicare only | 73 | 71 | 80 | 66 | 65 |
| Military only | 51 | 48 | 55 | 56 | 54 |
| Other | 71 | 67 | 79 | 61 | 64 |
| BMI, kg/m2 | |||||
| Underweight, <18.5 | 36 | 41 | 37 | 29 | 29 |
| Normal, 18.5–24.9 | 38 | 36 | 46 | 32 | 33 |
| Overweight, 25–29.9 | 53 | 52 | 59 | 50 | 47 |
| Obese, 30–39.9 | 68 | 67 | 70 | 68 | 62 |
| Morbidly obese, >40 | 77 | 79 | 78 | 77 | 74 |
| Smoking | |||||
| Never | 63 | 57 | 67 | 56 | 56 |
| Former | 66 | 66 | 71 | 64 | 58 |
| Current <10 cpd | 50 | 44 | 57 | 48 | 46 |
| Current 10–19 cpd | 46 | 42 | 55 | 41 | 44 |
| Current ≥20 cpd | 48 | 46 | 57 | 43 | 46 |
| Alcohol drinking | |||||
| Heavy (>3 drinks/d) | 44 | 42 | 48 | 45 | 43 |
| Moderate (≤3 drinks/d) | 52 | 48 | 59 | 46 | 46 |
| None | 65 | 62 | 70 | 58 | 57 |
| Family history of heart disease | |||||
| No | 54 | 48 | 61 | 46 | 46 |
| Yes | 62 | 57 | 69 | 57 | 57 |
| MI/CABG | 55 | 49 | 63 | 47 | 50 |
| No | 83 | 84 | 89 | 78 | 78 |
| Yes | |||||
| Diabetes mellitus | |||||
| No | 50 | 44 | 58 | 45 | 46 |
| Yes | 82 | 81 | 84 | 79 | 77 |
| Stroke | |||||
| No | 55 | 49 | 63 | 49 | 51 |
| Yes | 81 | 84 | 85 | 77 | 72 |
| High cholesterol | |||||
| No | 48 | 42 | 56 | 39 | 41 |
| Yes | 76 | 78 | 81 | 73 | 67 |
| Depression | |||||
| No | 55 | 49 | 62 | 48 | 49 |
| Yes | 63 | 62 | 71 | 58 | 56 |
| Among those with hypertension, n (%) or mean (SD) | |||||
| Age at diagnosis of hypertension, mean (SD), y | 41.1 (12) | 41.0 (12) | 39.7 (12) | 43.0 (13) | 44.1 (12) |
| Duration of hypertension, mean (SD). y | 12.5 (11) | 11.5 (11) | 13.6 (12) | 11.3 (11) | 11.5 (11) |
| Antihypertensive medications‡ | |||||
| No | 3604 (18) | 1218 (24) | 1115 (13) | 551 (24) | 720 (17) |
| Yes | 16 603 (82) | 3776 (76) | 7489 (87) | 1747 (76) | 3591 (83) |
BMI indicates body mass index; CABG, coronary artery bypass surgery; cpd, cigarettes per day; and MI, myocardial infarction.
All P values for comparison of percentage distributions of persons with versus without hypertension, overall and within race subcategories, were <0.001 unless otherwise noted.
P<0.05.
P>0.05.
The use of antihypertensive medications was ascertained only for those who enrolled in the cohort in 2004 or later, so percentages are calculated on the basis of that subgroup.
Table 2.
Ascertained Hypertension in the Southern Community Cohort Study: Baseline Characteristics and Prevalence Among 5109 Participants Without Self-reported Hypertension
| Overall, % | Blacks, %
|
Whites, %
|
|||
|---|---|---|---|---|---|
| Men (n=1057) | Women (n=2214) | Men (n=474) | Women (n=1364) | ||
| Hypertension | 26 | 31 | 28 | 27 | 17 |
| Age at interview, y | |||||
| 40–49 | 23 | 27 | 25 | 26 | 13 |
| 50–59 | 30 | 35 | 33 | 27 | 22 |
| 60–69 | 29 | 35 | 34 | 29 | 21 |
| 70–79 | 33 | 38 | 36 | 24* | 29 |
| Sex | |||||
| Female | 24 | ||||
| Male | 29 | ||||
| Race | |||||
| Black | 29 | ||||
| White | 20 | ||||
| Marital status | |||||
| Married | 26 | 33 | 28 | 25 | 18 |
| Single | 25 | 25 | 27 | 27 | 17 |
| Divorced | 26 | 34 | 30 | 26 | 16 |
| Widowed | 26 | 28 | 30* | 42* | 17* |
| Education | |||||
| Less than 12th grade | 27 | 31 | 29 | 35 | 17 |
| High school/vocational school | 26 | 30 | 29 | 23 | 18 |
| Some/junior (2-y) college | 26 | 31 | 29 | 24 | 19 |
| College | 23 | 37 | 24 | 26 | 8 |
| Graduate school | 23* | 22* | 26* | 24* | 19* |
| Annual household income, $ | |||||
| <15 000 | 26 | 30 | 29 | 28 | 17 |
| 15 000–24 999 | 25 | 29 | 27 | 29 | 16 |
| 25 000–49 999 | 26 | 37 | 25 | 16 | 20 |
| 50 000–99 999 | 25 | 32 | 36 | 25 | 11 |
| ≥100 000 | 30* | 38* | 50* | 44* | 8* |
| Health insurance | |||||
| None | 26 | 30 | 30 | 26 | 18 |
| Any private insurance | 27 | 35 | 27 | 29 | 17 |
| Medicaid only | 22 | 32 | 24 | 26 | 12 |
| Medicare only | 28 | 36 | 31 | 28 | 17 |
| Military only | 28 | 33 | 56 | 21 | 15 |
| Other | 24* | 17* | 29* | 26* | 20* |
| BMI, kg/m2 | |||||
| Underweight, <18.5 | 16 | 14 | 27 | 18 | 9 |
| Normal, 18.5–24.9 | 20 | 26 | 21 | 19 | 12 |
| Overweight, 25–29.9 | 25 | 31 | 26 | 27 | 17 |
| Obese, 30–39.9 | 28 | 37 | 29 | 33 | 21 |
| Morbidly obese, >40 | 37 | 44 | 41 | 43 | 23 |
| Smoking | |||||
| Never | 27 | 36 | 28 | 21 | 19 |
| Former | 28 | 31 | 27 | 34 | 23 |
| Current <10 cpd | 24 | 22 | 30 | 12 | 15 |
| Current 10–19 cpd | 23 | 33 | 27 | 19 | 10 |
| Current ≥20 cpd | 23 | 29* | 30* | 28* | 15 |
| Alcohol drinking | |||||
| Heavy (>3 drinks/d) | 31 | 34 | 33 | 28 | 16 |
| Moderate (≤3 drinks/d) | 26 | 29 | 28 | 28 | 17 |
| None | 25* | 31* | 28* | 25* | 17* |
| Family history of heart disease | |||||
| No | 25 | 30 | 27 | 28 | 16 |
| Yes | 26* | 32* | 31* | 26* | 18 |
| MI/CABG | |||||
| No | 26 | 31 | 29 | 27 | 17 |
| Yes | 23* | 29* | 20* | 23* | 18* |
| Stroke | |||||
| No | 26 | 31 | 28 | 27 | 17 |
| Yes | 25* | 25* | 29* | 28* | 20* |
| Depression | |||||
| No | 27 | 31 | 28 | 26 | 19 |
| Yes | 22 | 27* | 29* | 28* | 16* |
| Diabetes mellitus | |||||
| No | 26 | 31 | 28 | 27 | 17 |
| Yes | 26* | 31* | 29* | 25* | 15* |
| High cholesterol | |||||
| No | 27 | 32 | 29 | 26 | 17 |
| Yes | 23* | 27* | 26* | 27* | 18* |
BMI indicates body mass index; CABG, coronary artery bypass surgery; cpd, cigarettes per day; and MI, myocardial infarction.
All P values for comparison of percentage distributions of persons with versus without hypertension, within race-sex subcategories, were <0.05 unless otherwise noted.
P≥0.05.
Table 3.
Ascertained Hypertension in the Southern Community Cohort Study: Baseline Characteristics and Prevalence Among 6183 Participants With Self-reported Hypertension*
| Overall, % | Blacks, %
|
Whites, %
|
|||
|---|---|---|---|---|---|
| Men (n=1370) | Women (n=2864) | Men (n=640) | Women (n=1309) | ||
| Hypertension | 54 | 61 | 56 | 47 | 44 |
| Use of antihypertensive medications† | 87 | 85 | 89 | 83 | 84 |
| Age at interview, y | |||||
| 40–49 | 54 | 60 | 57 | 47 | 42 |
| 50–59 | 55 | 62 | 57 | 44 | 45 |
| 60–69 | 51 | 59 | 53 | 51 | 43 |
| 70–79 | 54‡ | 56‡ | 56‡ | 53‡ | 49‡ |
| Sex | |||||
| Women | 53 | ||||
| Men | 56 | ||||
| Race | |||||
| Black | 58 | ||||
| White | 45 | ||||
| Marital status | |||||
| Married | 52 | 59 | 56 | 49 | 41 |
| Single | 56 | 62 | 56 | 41 | 45 |
| Divorced | 54 | 61 | 58 | 47 | 44 |
| Widowed | 54‡ | 61‡ | 55‡ | 40‡ | 51‡ |
| Education | |||||
| Less than 12th grade | 55 | 61 | 56 | 49 | 45 |
| High school/vocational school | 53 | 62 | 56 | 47 | 43 |
| Some/junior (2-y) college | 54 | 57 | 58 | 45 | 46 |
| College | 54 | 64 | 61 | 42 | 37 |
| Graduate school | 42‡ | 33‡ | 44‡ | 36‡ | 45‡ |
| Annual household income, $ | |||||
| <15 000 | 54 | 60 | 57 | 51 | 44 |
| 15 000–24 999 | 53 | 61 | 55 | 41 | 46 |
| 25 000–49 999 | 57 | 66 | 61 | 47 | 45 |
| 50 000–99 999 | 43 | 48 | 53 | 39 | 33 |
| ≥100 000 | 47 | 42‡ | 71‡ | 43‡ | 38‡ |
| Health insurance | |||||
| None | 58 | 66 | 61 | 50 | 48 |
| Any private insurance | 54 | 63 | 58 | 41 | 39 |
| Medicaid only | 50 | 57 | 52 | 47 | 40 |
| Medicare only | 49 | 49 | 53 | 47 | 40 |
| Military only | 55 | 58 | 43 | 56 | 56 |
| Other | 49 | 56 | 50 | 35‡ | 45‡ |
| BMI, kg/m2 | |||||
| Underweight, <18.5 | 45 | 38 | 63 | 0 | 35 |
| Normal, 18.5–24.9 | 51 | 59 | 51 | 56 | 35 |
| Overweight, 25–29.9 | 52 | 61 | 53 | 48 | 41 |
| Obese, 30–39.9 | 55 | 61 | 57 | 47 | 49 |
| Morbidly obese, >40 | 55 | 65‡ | 60‡ | 38‡ | 43 |
| Smoking | |||||
| Never | 56 | 62 | 58 | 50 | 45 |
| Former | 51 | 59 | 53 | 45 | 42 |
| Current <10 cpd | 55 | 59 | 54 | 64 | 47 |
| Current 10–19 cpd | 56 | 62 | 59 | 44 | 47 |
| Current ≥20 cpd | 50 | 61‡ | 58‡ | 45‡ | 43‡ |
| Alcohol drinking | |||||
| Heavy (>3 drinks/d) | 57 | 65 | 52 | 46 | 47 |
| Moderate (≤3 drinks/d) | 56 | 65 | 59 | 45 | 46 |
| None | 52 | 54 | 56 | 48‡ | 43‡ |
| Family history of heart disease | |||||
| No | 55 | 60 | 56 | 46 | 44 |
| Yes | 52‡ | 63‡ | 56‡ | 47‡ | 43‡ |
| MI/CABG | |||||
| No | 54 | 61 | 56 | 49 | 44 |
| Yes | 49 | 56‡ | 54‡ | 40‡ | 46‡ |
| Stroke | |||||
| No | 54 | 61 | 57 | 46 | 44 |
| Yes | 53‡ | 59‡ | 54‡ | 51‡ | 45‡ |
| Depression | |||||
| No | 56 | 61 | 57 | 50 | 48 |
| Yes | 48 | 60‡ | 54‡ | 42‡ | 41 |
| Diabetes mellitus | |||||
| No | 55 | 64 | 56 | 48 | 46 |
| Yes | 52 | 55 | 56‡ | 45‡ | 40‡ |
| High cholesterol | |||||
| No | 57 | 63 | 58 | 53 | 47 |
| Yes | 51 | 58‡ | 55‡ | 42 | 42‡ |
BMI indicates body mass index; CABG, coronary artery bypass surgery; cpd, cigarettes per day; and MI, myocardial infarction.
All P values for comparison of percentage distributions of persons with versus without hypertension, within race-sex subcategories, were <0.05 unless otherwise noted.
This analysis was restricted to those who enrolled in the cohort in 2004 or later and had information on use of antihypertensive medications.
Among those with ascertained hypertension.
P≥0.05.
In the analysis of SR-HTN and A-HTN, multivariable logistic regression models were used to estimate the adjusted prevalence odds ratios (ORs) and 95% confidence intervals (CIs) for factors associated with hypertension, overall and by race-sex. In addition, 3 fundamentally important questions were addressed using SR-HTN and A-HTN variables: (1) the epidemiological question about factors associated with the overall prevalence of hypertension in the SCCS population (number with SR-HTN or A-HTN/total number), (2) the health services question about factors associated with the probability of diagnosis among those with hypertension (number with SR-HTN/ number with SR-HTN or A-HTN), and (3) the clinical effectiveness question about determinants of the probability of control among those with diagnosed hypertension (number with SR-HTN but not A-HTN/number with SR-HTN). These sequences of nested probabilities were similarly modeled using multivariable logistic regression. Furthermore, through multivariable linear regression models, we evaluated the relationships between associated factors and SBP and DBP among participants with measured BP and analyzed pulse pressure (PP) and mean arterial pressure (MAP) to explore whether differences in arterial stiffness may account, in part, for racial differences in the prevalence of hypertension. PP was calculated as SBP minus DBP; MAP was calculated as follows: (DBP-1/3[PP]).
In all analyses, the candidate covariates were identified a priori and included race and sex (when appropriate), age, income, education, health insurance status, marital status, BMI, alcohol intake, cigarette smoking, caffeine intake (derived from responses to the SCCS dietary food frequency questionnaire), physical activity, and medical history (personal and family). A 2-tailed value of P<0.05 was accepted as the threshold for statistical significance. Analyses were conducted using SAS software, version 9.3 (SAS Institute, Inc, Cary, NC).
Results
Table 1 presents baseline characteristics and the distribution of SR-HTN among the 69 211 SCCS participants included in this analysis. Overall, 72% (n=50 157) were blacks and 28% (n=19 054) were whites, and ≈60% were women. At the baseline interview, 39 363 participants reported a previous diagnosis of hypertension, yielding an overall (crude) prevalence of 57%. The prevalence of SR-HTN was significantly higher among blacks than whites (59% versus 52%; P<0.001). The observed racial difference was, however, driven by the higher prevalence among black women (64%) compared with white women (52%), whereas black and white men had similar prevalences (51%). About 82% of those with SR-HTN reported the use of antihypertensive medications, with virtually identical proportions among black and white men (76%), whereas black women were somewhat more likely to use antihypertensive medications than white women (87% versus 83%; P<0.001). Overall, a diagnosis of hypertension was reported more frequently among participants who were older, had higher BMI, were not current smokers, had lower levels of education and income, had a family history of heart disease, and had a personal history of myocardial infarction/coronary bypass surgery, diabetes mellitus, high cholesterol, and depression. These findings were generally consistent across the 4 race and sex groups, with the prevalence of SR-HTN highest among black women across nearly all strata examined.
The distribution of A-HTN according to baseline characteristics among participants with and without SR-HTN is presented in Tables 2 and 3. Overall, there was a higher prevalence of A-HTN among blacks compared with whites. Among participants with measured BP but without SR-HTN, the prevalence of A-HTN in blacks and whites was 29% and 20%, respectively (P<0.001). Women accounted for most of the excess among blacks, with a prevalence of 28% for black women versus 17% for white women (P<0.001), as opposed to 31% for black men and 27% for white men (P=0.049). Among participants with measured BP and with SR-HTN, the prevalence of A-HTN was 58% and 45% in blacks and whites, respectively (P<0.001). The excess prevalence among blacks persisted regardless of sex: 61% versus 47% (P<0.001) for black versus white men and 56% versus 44% (P<0.001) for black versus white women. Table 4 provides details of the distribution of classes of antihypertensive medication among participants with A-HTN and SR-HTN. Notably, among those with A-HTN and SR-HTN, >94% were on at least 1 of the major classes of antihypertensive agents, whereas only 44% were on at least 2 classes and only 29% were on a diuretic agent. Compared with whites, blacks were less likely to be treated with angiotensin-converting enzyme inhibitors or β-blockers but more likely to be treated with calcium channel blockers or α2-agonists (P<0.01 for all comparisons).
Table 4.
Ascertained Hypertension in the Southern Community Cohort Study: Distribution of Classes of Antihypertensive Medications Among 2880 Participants With Self-reported Hypertension Who Acknowledged the Use of Antihypertensive Medications*
| Overall (n=2880) | Blacks, n
|
Whites, n
|
|||
|---|---|---|---|---|---|
| Men (n=708) | Women (n=1443) | Men (n=248) | Women (n=481) | ||
| ACE inhibitors† | 926 | 196 | 453 | 106 | 171 |
| ACE receptor blockers‡ | 423 | 92 | 227 | 30 | 74 |
| Calcium channel blockers§ | 873 | 196 | 500 | 51 | 126 |
| β-Blockers† | 487 | 94 | 215 | 52 | 126 |
| Any diuretic‡ | 835 | 164 | 468 | 62 | 141 |
| Types of diuretic | |||||
| Thiazide diuretics | 645 | 124 | 359 | 49 | 113 |
| Loop diuretics | 192 | 41 | 106 | 14 | 31 |
| Potassium-sparing diuretics: | 9 | 3 | 4 | 1 | 1 |
| aldosterone antagonists | |||||
| Potassium-sparing diuretics: | 74 | 17 | 45 | 1 | 11 |
| sodium channel blockers | |||||
| α1-Blockers‡ | 29 | 8 | 12 | 4 | 5 |
| α2-Agonists§ | 175 | 40 | 102 | 12 | 21 |
| Vasodilators‡ | 4 | 0 | 4 | 0 | 0 |
| Other‡ | 153 | 44 | 75 | 8 | 26 |
ACE indicates angiotensin-converting enzyme.
Data on type of antihypertensive medication used were missing for ≈18% of participants who reported medication use. More than 94% were on at least 1 of the major classes of agents (ACE, ACE receptor blockers, calcium channel blockers, β-blockers, and diuretic), 44% were on ≥2 classes, and 10% were on ≥3 classes.
Compared with whites, blacks were less likely to be on these classes of antihypertensive medications (P<0.01).
Compared with whites, blacks were more likely to be on these classes of antihypertensive medications (P<0.01).
Black vs white comparisons were not significant (P>0.05).
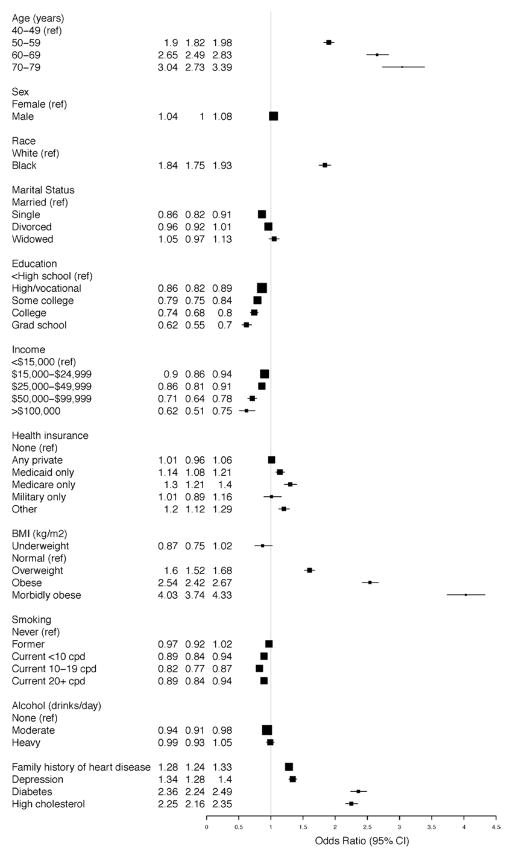
The adjusted model for SR-HTN in the overall cohort (Figure 1) demonstrates an increased OR for SR-HTN among blacks (OR, 1.84; 95% CI, 1.75–1.93), which was more pronounced in women (OR, 2.08; 95% CI, 1.95–2.21) than men (OR, 1.47; 95% CI, 1.36–1.60), and there was evidence of race-sex interaction (P<0.0001). Education and income were associated inversely with SR-HTN, with ORs of 0.62 (95% CI, 0.55–0.70) and 0.62 (95% CI, 0.51–0.75) among those with the highest levels of education (graduate school) and income (>$100 000), respectively. Other factors consistently positively associated with SR-HTN were age, BMI, history of depression, diabetes mellitus, high total cholesterol, and a family history of heart disease (Figure 1). The patterns of association were consistent across all race-sex groups (Figure IA–ID in the online-only Data Supplement). Of note, the association between morbid obesity and SR-HTN was most pronounced among white men (OR, 4.57; 95% CI, 3.43–6.10; Figure 1C) and white women (OR, 4.64; 95% CI, 3.97–5.43; Figure 1D).
Figure 1.
Self-reported hypertension in the Southern Community Cohort Study. Odds ratios for the associated factors in the overall population. BMI indicates body mass index; CI, confidence interval; and cpd, cigarettes per day.
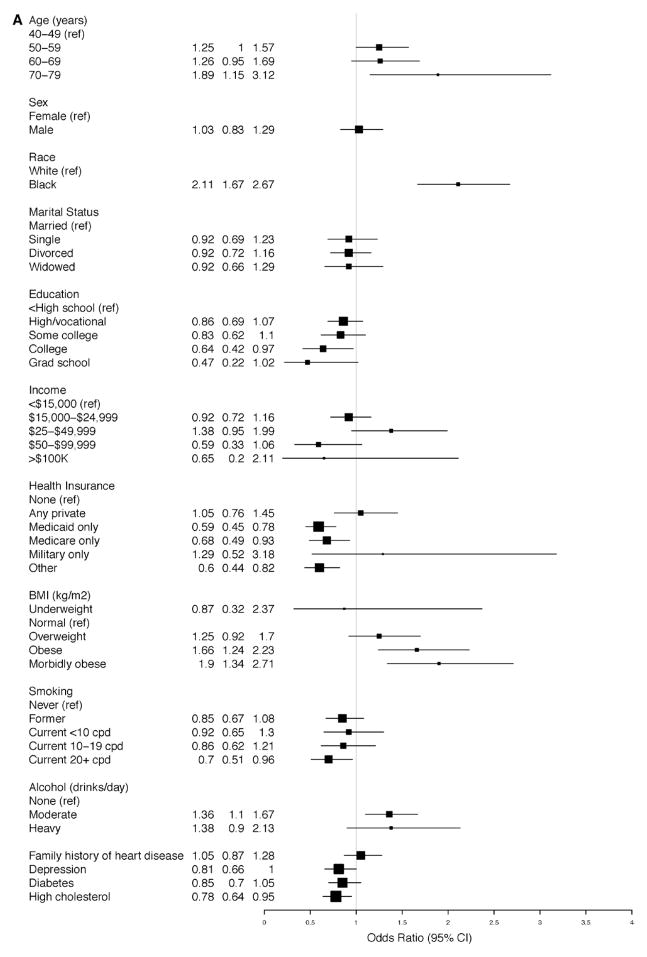
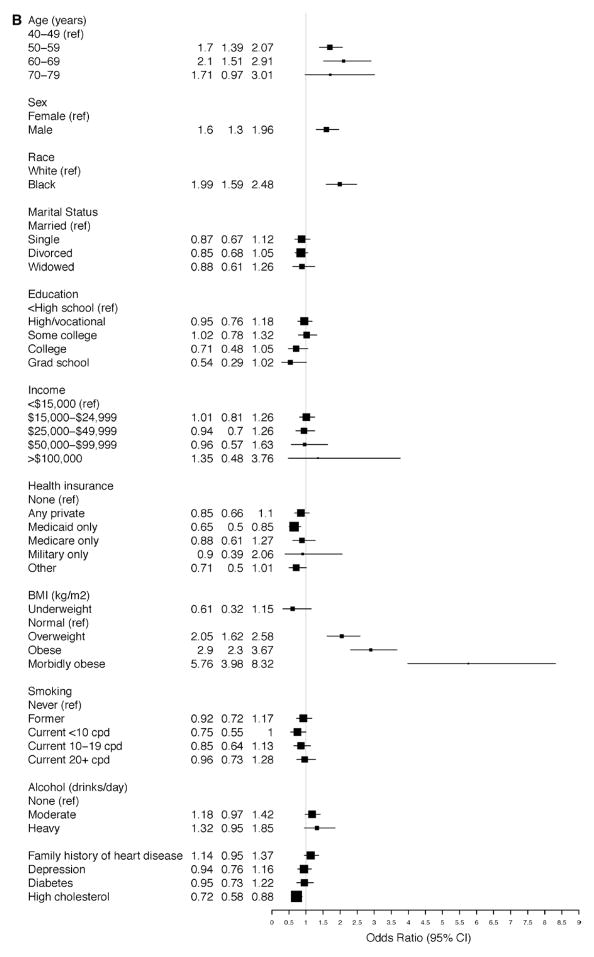
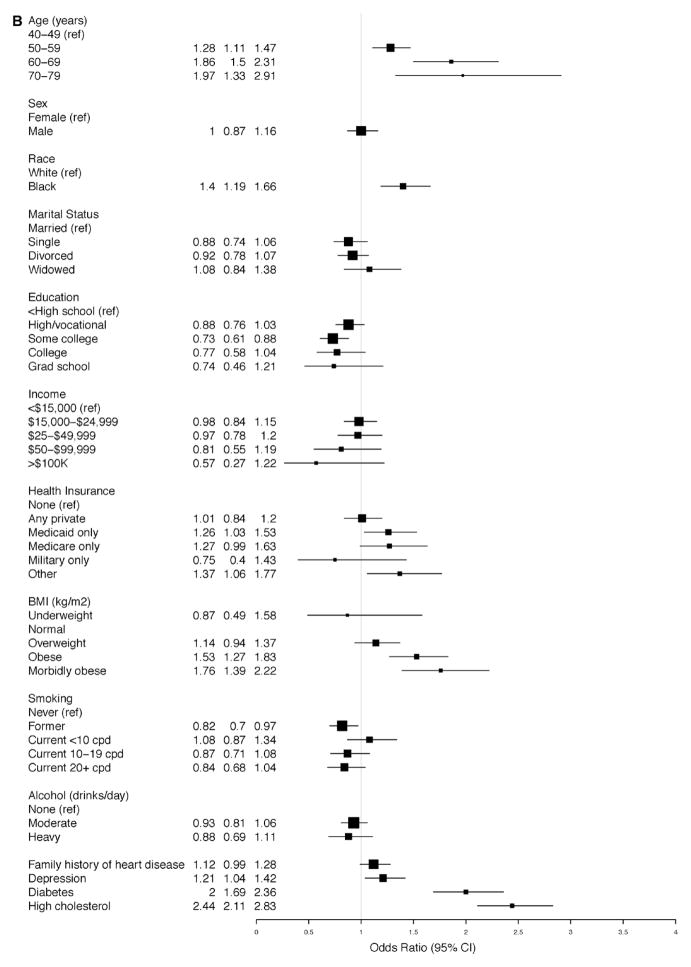
In the adjusted model for A-HTN among subjects with SR-HTN, blacks had increased OR for hypertension, that is, uncontrolled hypertension (OR, 2.11; 95% CI, 1.67–2.67; Figure 2A). Similarly, among those with A-HTN but without SR-HTN, blacks had increased OR for hypertension, that is, unreported hypertension (OR, 1.99; 95% CI, 1.59–2.48; Figure 2B). Morbid obesity was more strongly associated with unreported hypertension (OR, 5.76; 95% CI, 3.98–8.32; Figure 2B) than uncontrolled hypertension (OR, 1.90; 95% CI, 1.34–2.71; Figure 2A). Compared with women, men had increased OR for unreported hypertension (OR, 1.60; 95% CI, 1.30–1.96; Figure 2B). Elderly participants (70–79 years compared with 40–49 years of age) had an increased OR for uncontrolled hypertension (OR, 1.89; 95% CI, 1.15–3.12), whereas increased ORs for unreported hypertension were nearly twice as high among those at or >50 versus <50 years of age.
Figure 2.
Ascertained hypertension in the Southern Community Cohort Study. Odds ratios for the associated factors among participants (A) with self-reported hypertension and (B) without self-reported hypertension. BMI indicates body mass index; CI, confidence interval; and cpd, cigarettes per day.
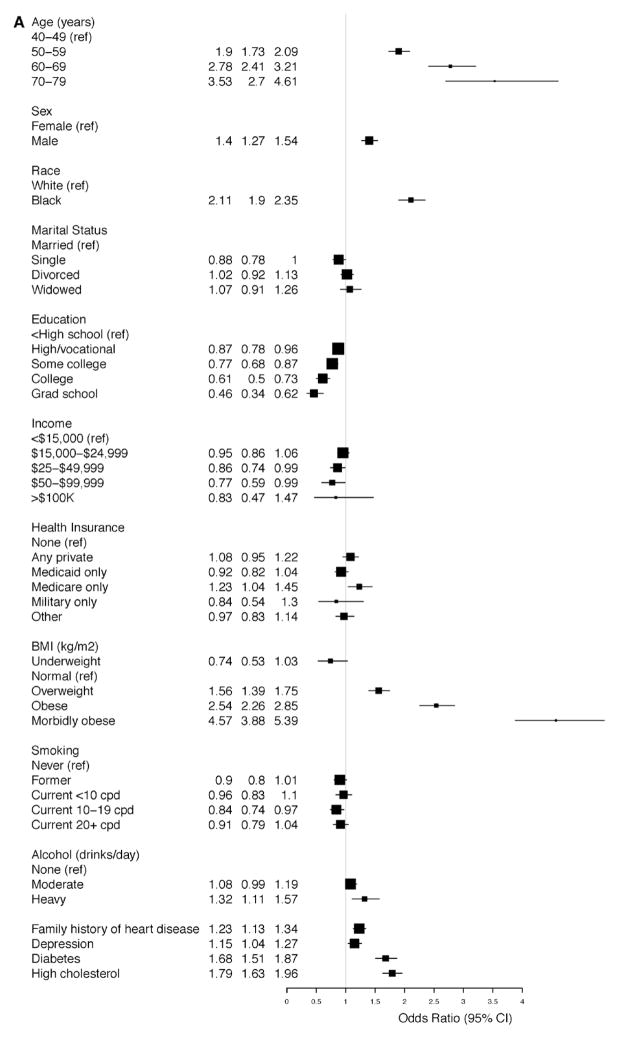
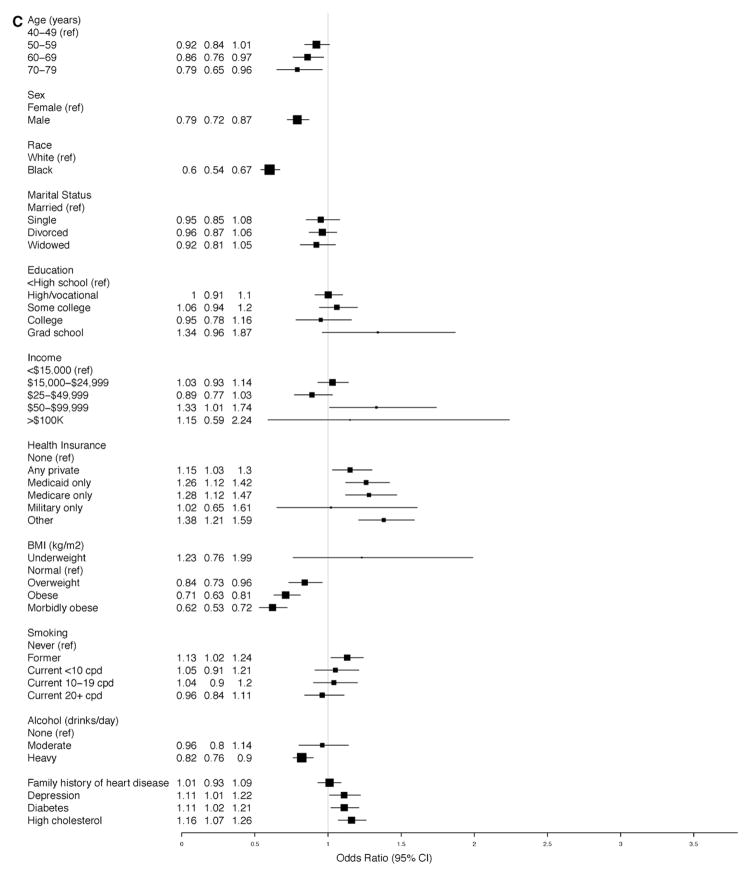
Consistent with the findings reported above, advanced age (elderly), black race, male sex, obesity, diabetes mellitus, and high cholesterol were strongly associated with increased odds for the presence of hypertension in the adjusted model for the overall prevalence of hypertension (Figure 3A). Similarly, the same factors except sex were strongly associated with increased odds for diagnosis among those with hypertension (Figure 3B). On the contrary, male sex (OR, 0.79; 95% CI, 0.72–0.87) and black race (OR, 0.6; 95% CI, 0.54–0.67) were both strongly associated with decreased odds for the control of BP among those with diagnosed hypertension (Figure 3C); other factors inversely associated with control of BP were overweight or obesity, age (≥60 years), and heavy alcohol use (Figure 3C).
Figure 3.
A, Overall prevalence of hypertension in the Southern Community Cohort Study (SCCS). Odds ratios for the associated factors. B, Probability of diagnosis among those with hypertension in the SCCS. Odds ratios for the associated factors. C, Probability of blood pressure control among those with diagnosed hypertension in the SCCS. Odds ratios for the associated factors. BMI indicates body mass index; CI, confidence interval; and cpd, cigarettes per day.
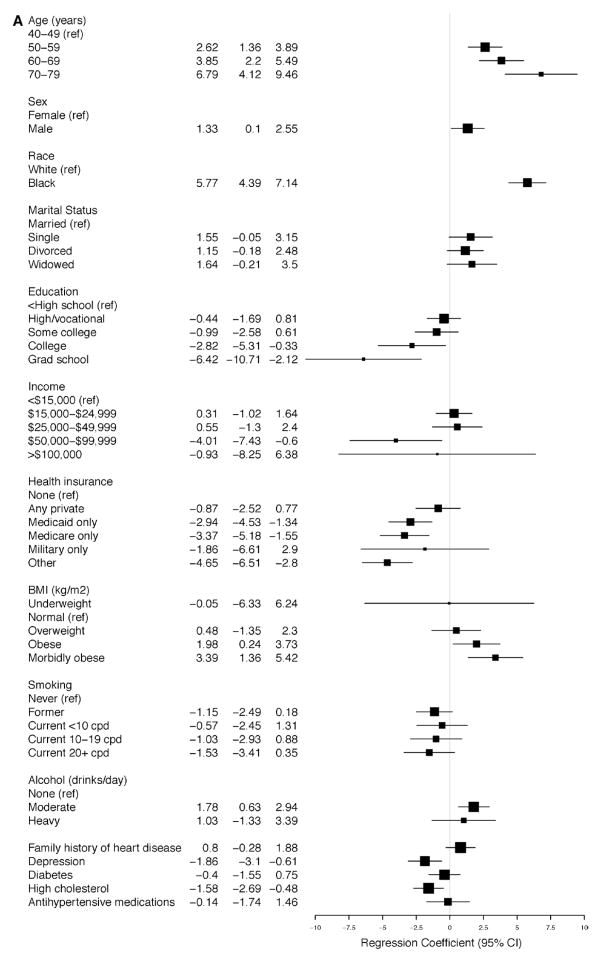
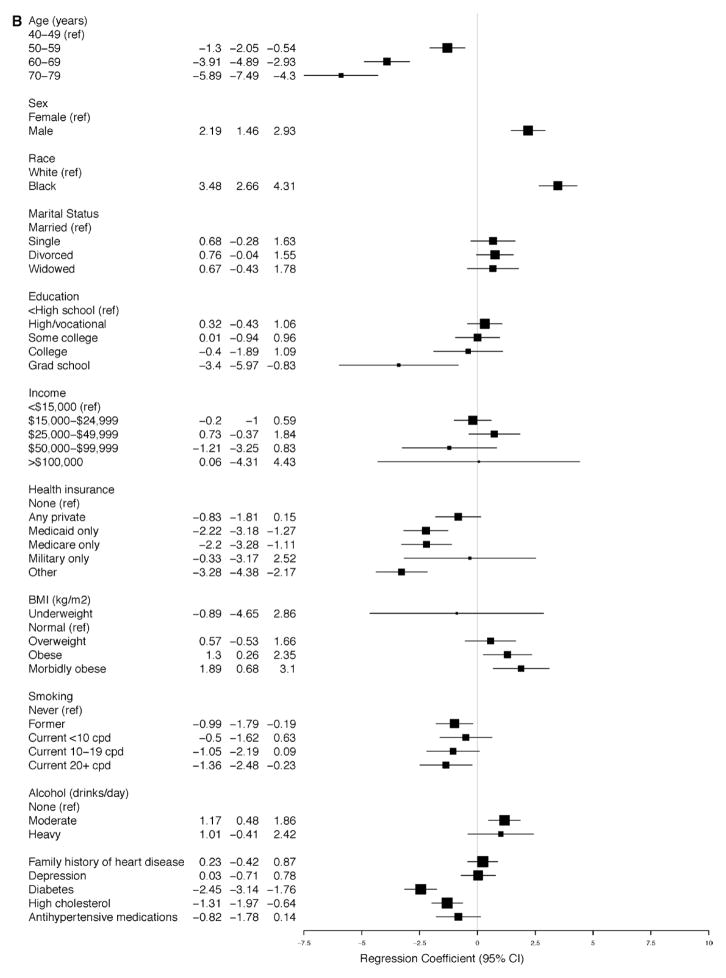
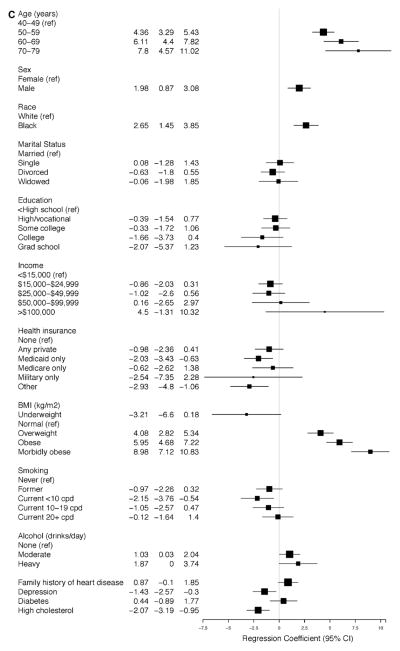
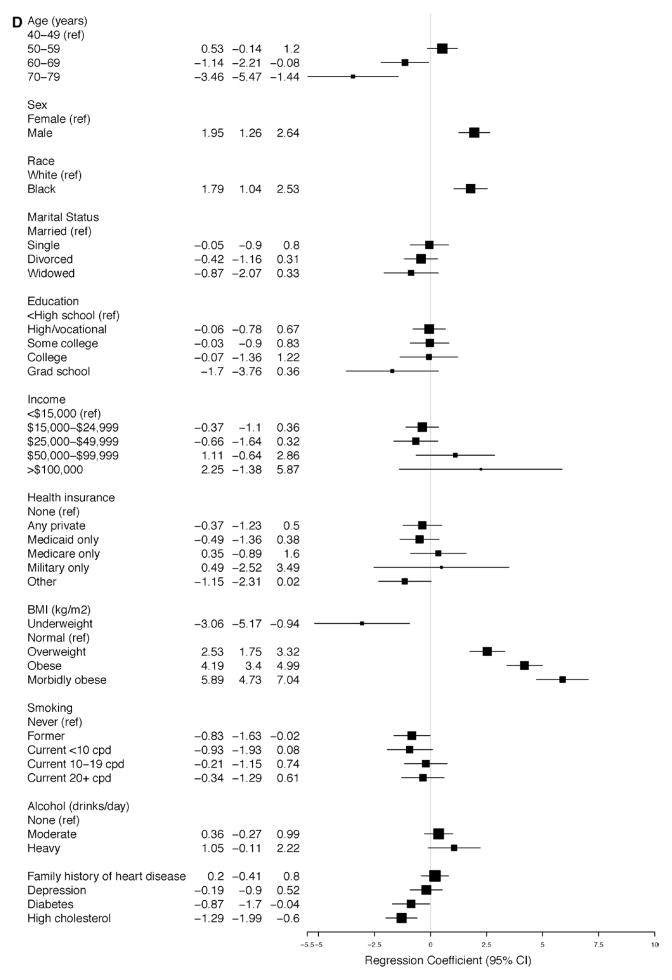
The linear regression models for SBP and DBP for participants with and without SR-HTN (Figure 4A–4D) demonstrate that the average SBP was higher and the average DBP was lower with increasing age, reaching a 6.8-mm Hg (95% CI, 4.12–9.46) increase in SBP and a 5.9-mm Hg (95% CI, −7.49 to −4.30) decrease in DBP among participants with ages 70 to 79 years versus 40 to 49 years of age with SR-HTN (Figure 3A and 3B). These findings were similar for participants without SR-HTN. Higher BMI was associated with higher average SBP and DBP measurements; however, the strength of association was strongest among participants without SR-HTN (Figure 4A and 4B). There was a positive association between male sex and SBP and DBP, although it was statistically significant only among participants with and without SR-HTN (Figures 3A, 3B, 4A, and 4B). In all adjusted analyses, the association between alcohol or smoking and hypertension was generally weak and inconsistent. Of note, physical activity was not associated with hypertension in this cohort.
Figure 4.
Relationship between baseline covariates and (A) systolic and (B) diastolic blood pressures in the Southern Community Cohort Study (SCCS) participants with self-reported hypertension (SR-HTN). Relationship between baseline covariates and (C) systolic and (D) diastolic blood pressures in the SCCS participants without SR-HTN.
In analyses of MAP and PP among participants with SR-HTN and measured BP (data not shown), black race was associated with a statistically significant 2.33-mm Hg increase in PP (95% CI, 1.27–3.39; P<0.001) in the adjusted linear model for PP. Relative to the reference age group (40–49 years), there was a significant association between PP and increasing age: for ages 50 to 59 years, 60 to 69 years, and 70 to 79 years, there was a 3.96-mm Hg (95% CI, 2.99–4.94; P<0.0001), a 7.89-mm Hg (95% CI, 6.62–9.16; P<0.0001), and a 12.69-mm Hg (95% CI, 10.63–14.75; P<0.0001) increase in PP, respectively. Of note, sex was not a predictor of PP among those with SR-HTN. Black race was associated with a 4.2-mm Hg increase in MAP (95% CI, 3.33–5.14; P<0.0001). In contrast to the pattern observed for PP, male sex was associated with 1.87-mm Hg increase (95% CI, 1.06–2.68; P<0.0001) in MAP, but the association with age was weak and inconsistent.
Among those with uncontrolled hypertension, male sex was associated with a 2.34-mm Hg decrease in PP (95% CI, −3.73 to −0.94; P=0.001) and black race was associated with a 2.04-mm Hg increase in PP (95% CI, 0.388–3.69; P=0.015). Also, there was strong association between age and PP; compared with those aged 40 to 49 years, there was a 5.17-mm Hg (95% CI, 3.74–6.61; P<0.0001), a 10.47-mm Hg (95% CI, 8.57–12.37; P<0.0001), and a 16.16-mm Hg increase (95% CI, 13.10–19.22; P<0.0001) in PP for those aged 50 to 59, 60 years, to 69 and 70 to 79 years of age, respectively. Male sex was associated with a 1.78-mm Hg (95% CI, 0.87–2.69; P=0.0001) increase in MAP, and black race associated with a 2.78-mm Hg (95% CI, 1.70–3.85; P<0.0001) increase in MAP.
Among those with unreported hypertension, male sex was associated with a PP decrease of 3.18 mm Hg (95% CI, −5.47 to −0.90; P=0.006), whereas black race was not associated with PP (P=0.87). Again, there was a strong association between age and PP, up to a 19.95-mm Hg increase (95% CI, 13.44–26.46; P<0.0001) for ages 70 to 79 years compared with 40 to 49 years. Male sex (P=0.18) and black race (P=0.74) were not associated with MAP in this group.
Discussion
In the SCCS cohort, race, age, and BMI were strongly associated with SR-HTN even after adjusting for potential confounders. In addition, education and income showed modest inverse associations with SR-HTN, whereas associations with smoking or alcohol were weak and inconsistent. Furthermore, among participants with SR-HTN, the high prevalence of A-HTN (58% of blacks versus 45% of whites; P<0.001) implies high prevalence of uncontrolled hypertension and is particularly concerning. Notably, in this subgroup, although >94% reported that they were on at least one of the major classes of antihypertensive agents, only 44% were on ≥2 classes and only 29% reported a diuretic, a recommended first-line agent. Collectively, the pattern of medication use in this cohort suggests suboptimal implementation of JNC 7 guidelines.3 Equally concerning is the high prevalence and racial disparity of A-HTN (29% of blacks versus 20% of whites; P<0.001) among participants without SR-HTN, consistent with unreported hypertension. Furthermore, it is interesting to note that black race, male sex, obesity, and heavy alcohol use were strongly associated with decreased odds for BP control in this cohort. These findings have important implications for future research and healthcare delivery related to the treatment of hypertension.
The fundamental mechanism for the well-established relationship between age and hypertension centers on changes in arterial integrity and function leading to less distensible vessels and an increase in PP, which result in a gradual rise in SBP through adult life but a slight decline in DBP after middle age.29 Thus, the noted associations in our cohort for both blacks and whites mirror established understanding. Similarly, the relationship between BMI and hypertension is well known,29 and likewise the underlying mechanisms, which include endothelial dysfunction and arteriosclerosis,30 increased sympathetic activity,31–33 activation of the renin-aldosterone- angiotensin system,34–36 and elevated stroke work because of BP-independent left ventricular remodeling—increase in wall thickness, mass, and cavity size.37
US morbidity and mortality statistics demonstrate that, compared with whites and other races, blacks have greater CVD and associated mortality. Arguably, hypertension is the most prominent beacon for CVD and disproportionately affects blacks.6,38,39 However, unlike BMI and age, the underlying mechanisms for the higher risk of hypertension in blacks are not entirely clear. The bulk of epidemiological studies in this arena have focused on SES, health insurance coverage, and access to care; it is generally established that SES, along with a parallel disproportionate burden of CVD risk factors, constitutes reasons for health disparities. Although we found inconsistent associations between SES indexes and A-HTN, others have noted inverse links between SES and hypertension,19 as we did for SR-HTN. However, in this cohort of blacks and whites with nearly identical SES characteristics,26–28 a higher risk of hypertension persisted in blacks. Furthermore, contrary to conventional wisdom, the association between BMI and hypertension was more pronounced in whites than in blacks. Thus, our findings raise additional questions and call for refinement or re-evaluation of the existing paradigm(s).
Overall, in the analysis of patients with SR-HTN in whom BP data were available, there was a strong association between age and PP that increased with advancing age and thus was consistent with the conventional understanding that PP increases with age because of increasing arterial stiffness. The association between age and PP was further accentuated in the subset of participants with uncontrolled or unreported hypertension. Of note, female sex was consistently associated with higher PP among participants with uncontrolled and unreported hypertension. This association was not apparent in the analysis of PP among those with SR-HTN. In summary, the results support the conclusions that black race and female sex were associated with a higher PP, which collectively suggest that greater arterial stiffness is a plausible underpinning for the race-sex disparities in the prevalence of hypertension. However, this finding does not account for the decreased odds of BP control associated with black race, male sex, obesity, and heavy alcohol use, all of which warrant further investigation.
Despite the results of this study and the unique characteristics of SCCS participants who are of similar and generally low SES regardless of race, SES cannot be ruled out as a contributor to racial disparities associated with hypertension. There are several potentially important social, economic, and environmental determinants that were not evaluated in this study. Most important, the interaction of SES and biology is likely relevant in precipitating biomolecular characteristics that could influence hypertension differentially by race. Credence for this suggestion stems from the fact that molecular markers, for example, plasma levels of fibrinogen, homocysteine, and intercellular adhesion molecule-1, are significantly increased in blacks compared with whites even after adjustment for traditional risk factors.40,41 Furthermore, blacks and whites with identical SES may still have significant differences in family history of hypertension, dietary salt intake, consumption of fruits and vegetables, and other biosocial determinants of hypertension; compounding these may be important differences in salt sensitivity, which modulates the incidence (and prevalence) of hypertension and hypertensive target-organ damage and overall CV mortality.42 In addition, recent evidence suggests that past SES factors—prenatal and childhood stress, neighborhood poverty and crime exposure, parental health and SES, and poor intergenerational economic mobility—may play much greater roles in the pathogenesis of racial health disparities than present SES conditions.43 The association between adult-onset disease and fetal and early-life exposures, the proposition of the Barker hypothesis, has been documented in many populations.44–51 Therefore, future research that seeks to assess the role of SES in hypertension should extend the evaluation beyond current SES characteristics to include family background and neighborhood conditions during childhood.
Limitations and Strengths
Some inconsistent associations may stem, in part, from random error because of misclassification and inaccuracies of self-reported data, which may have attenuated the power to observe potentially subtle associations. Another limitation is that this was a cross-sectional evaluation of cohort data, thus precluding any inferences on the temporal relationship between examined covariates and hypertension; this limitation may have also contributed to the inconsistencies in the association between hypertension and lifestyle factors, which are subject to change after a diagnosis of hypertension. As the cohort is followed up over time, future analyses will prospectively examine hypertension as a predictor of subsequent mortality, stroke, CVD, and other health outcomes. We emphasize that the similar and generally low income and education levels of SCCS participants regardless of race greatly minimized confounding by SES, generally an important mediator of racial differences in health care and burden of disease.
Despite the above limitations, this study has many strengths, including its large sample size, extensive data on lifestyle and SES indexes, and the internal validation from comparative analyses of A-HTN using measured BP. Furthermore, the observed relationships between age and BP are consistent with conventional understanding of pathophysiology and lend credence to the overall data and results. Finally, this study is the largest evaluation of the factors associated with hypertension in a cohort of blacks and whites of similar, predominantly low contemporaneous SES in the southeastern United States.
Conclusions
In this evaluation of hypertension in a large cohort of blacks and whites, race, age, and BMI were strongly associated with the risk of hypertension in all models before and after adjustment for potential confounders. However, the mechanisms underlying the higher risk of hypertension in blacks were not explained by differences in lifestyle, education, or income. The reasons for the suboptimal use of evidence-based treatment guidelines in this population need to be explored. In this context, analysis is currently underway to determine the characteristics and factors associated with the use of antihypertensive medications in the SCCS cohort. In terms of future research directions, we suggest that additional insight into the biomolecular milieu, prenatal and early-life exposures, historic SES conditions, and their interplay in patients with hypertension may improve our understanding of health disparities.
Supplementary Material
WHAT IS KNOWN
Hypertension is an established risk factor for cardiovascular disease and is the leading risk factor for death and disability worldwide.
The prevalence of cardiovascular disease risk factor is high in the Southeastern United States.
WHAT THE STUDY ADDS
The prevalence of unreported and uncontrolled hypertension across 12 states in the southeastern United States is high.
After controlling for socioeconomic status, black race, age, and body mass index are strongly associated with hypertension, including unreported and uncontrolled hypertension.
The pattern of medication use suggests suboptimal implementation of evidence-based hypertension treatment guidelines.
Acknowledgments
Sources of Funding
Dr Sampson’s effort was supported, in part, by the Harold Amos Medical Faculty Award of the Robert Wood Johnson Foundation. A grant award from PepsiCo, Inc, to Dr Sampson funded the analysis of data presented in this report. The SCCS is funded by grant R01 CA092447 from the National Cancer Institute, including American Recovery and Reinvestment Act Funding (3R01 CA029447-08 S1).
Footnotes
Disclosures
None.
References
- 1.World Health Organization. Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks. Geneva: World Health Organization; 2009. [Google Scholar]
- 2.Lawes CM, Vander Hoorn S, Rodgers A International Society of Hypertension. Global burden of blood-pressure-related disease, 2001. Lancet. 2008;371:1513–1518. doi: 10.1016/S0140-6736(08)60655-8. [DOI] [PubMed] [Google Scholar]
- 3.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 4.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, Amann M, Anderson HR, Andrews KG, Aryee M, Atkinson C, Bacchus LJ, Bahalim AN, Balakrishnan K, Balmes J, Barker-Collo S, Baxter A, Bell ML, Blore JD, Blyth F, Bonner C, Borges G, Bourne R, Boussinesq M, Brauer M, Brooks P, Bruce NG, Brunekreef B, Bryan-Hancock C, Bucello C, Buchbinder R, Bull F, Burnett RT, Byers TE, Calabria B, Carapetis J, Carnahan E, Chafe Z, Charlson F, Chen H, Chen JS, Cheng AT, Child JC, Cohen A, Colson KE, Cowie BC, Darby S, Darling S, Davis A, Degenhardt L, Dentener F, Des Jarlais DC, Devries K, Dherani M, Ding EL, Dorsey ER, Driscoll T, Edmond K, Ali SE, Engell RE, Erwin PJ, Fahimi S, Falder G, Farzadfar F, Ferrari A, Finucane MM, Flaxman S, Fowkes FG, Freedman G, Freeman MK, Gakidou E, Ghosh S, Giovannucci E, Gmel G, Graham K, Grainger R, Grant B, Gunnell D, Gutierrez HR, Hall W, Hoek HW, Hogan A, Hosgood HD, 3rd, Hoy D, Hu H, Hubbell BJ, Hutchings SJ, Ibeanusi SE, Jacklyn GL, Jasrasaria R, Jonas JB, Kan H, Kanis JA, Kassebaum N, Kawakami N, Khang YH, Khatibzadeh S, Khoo JP, Kok C, Laden F, Lalloo R, Lan Q, Lathlean T, Leasher JL, Leigh J, Li Y, Lin JK, Lipshultz SE, London S, Lozano R, Lu Y, Mak J, Malekzadeh R, Mallinger L, Marcenes W, March L, Marks R, Martin R, McGale P, McGrath J, Mehta S, Mensah GA, Merriman TR, Micha R, Michaud C, Mishra V, Mohd Hanafiah K, Mokdad AA, Morawska L, Mozaffarian D, Murphy T, Naghavi M, Neal B, Nelson PK, Nolla JM, Norman R, Olives C, Omer SB, Orchard J, Osborne R, Ostro B, Page A, Pandey KD, Parry CD, Passmore E, Patra J, Pearce N, Pelizzari PM, Petzold M, Phillips MR, Pope D, Pope CA, 3rd, Powles J, Rao M, Razavi H, Rehfuess EA, Rehm JT, Ritz B, Rivara FP, Roberts T, Robinson C, Rodriguez-Portales JA, Romieu I, Room R, Rosenfeld LC, Roy A, Rushton L, Salomon JA, Sampson U, Sanchez-Riera L, Sanman E, Sapkota A, Seedat S, Shi P, Shield K, Shivakoti R, Singh GM, Sleet DA, Smith E, Smith KR, Stapelberg NJ, Steenland K, Stöckl H, Stovner LJ, Straif K, Straney L, Thurston GD, Tran JH, Van Dingenen R, van Donkelaar A, Veerman JL, Vijayakumar L, Weintraub R, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams W, Wilson N, Woolf AD, Yip P, Zielinski JM, Lopez AD, Murray CJ, Ezzati M, AlMazroa MA, Memish ZA. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Executive summary: heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012;125:188–197. doi: 10.1161/CIR.0b013e3182456d46. [DOI] [PubMed] [Google Scholar]
- 6.Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA. 2010;303:2043–2050. doi: 10.1001/jama.2010.650. [DOI] [PubMed] [Google Scholar]
- 7.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper R, Rotimi C. Hypertension in blacks. Am J Hypertens. 1997;10(pt 1):804–812. doi: 10.1016/s0895-7061(97)00211-2. [DOI] [PubMed] [Google Scholar]
- 9.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER, 3rd, Simons-Morton DG, Karanja N, Lin PH DASH-Sodium Collaborative Research Group. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet.: DASH-Sodium Collaborative Research Group. N Engl J Med. 2001;344:3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 10.Jamerson K, DeQuattro V. The impact of ethnicity on response to antihypertensive therapy. Am J Med. 1996;101:22S–32S. doi: 10.1016/s0002-9343(96)00265-3. [DOI] [PubMed] [Google Scholar]
- 11.Saunders E, Weir MR, Kong BW, Hollifield J, Gray J, Vertes V, Sowers JR, Zemel MB, Curry C, Schoenberger J. A comparison of the efficacy and safety of a beta-blocker, a calcium channel blocker, and a converting enzyme inhibitor in hypertensive blacks. Arch Intern Med. 1990;150:1707–1713. [PubMed] [Google Scholar]
- 12.Cushman WC, Reda DJ, Perry HM, Williams D, Abdellatif M, Materson BJ. Regional and racial differences in response to antihypertensive medication use in a randomized controlled trial of men with hypertension in the United States: Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents. Arch Intern Med. 2000;160:825–831. doi: 10.1001/archinte.160.6.825. [DOI] [PubMed] [Google Scholar]
- 13.Gibbons GH. The pathophysiology of hypertension: the importance of angiotensin II in cardiovascular remodeling. Am J Hypertens. 1998;11(pt 2):177S–181S. doi: 10.1016/s0895-7061(98)00198-8. [DOI] [PubMed] [Google Scholar]
- 14.Gibbons GH. Physiology, genetics, and cardiovascular disease: focus on African Americans. J Clin Hypertens (Greenwich) 2004;6(suppl 1):11–18. doi: 10.1111/j.1524-6175.2004.03562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindhorst J, Alexander N, Blignaut J, Rayner B. Differences in hypertension between blacks and whites: an overview. Cardiovasc J Afr. 2007;18:241–247. [PMC free article] [PubMed] [Google Scholar]
- 16.Opie LH, Seedat YK. Hypertension in sub-Saharan African populations. Circulation. 2005;112:3562–3568. doi: 10.1161/CIRCULATIONAHA.105.539569. [DOI] [PubMed] [Google Scholar]
- 17.Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, White A, Cushman WC, White W, Sica D, Ferdinand K, Giles TD, Falkner B, Carey RM. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension. 2008;51:1403–1419. doi: 10.1161/HYPERTENSIONAHA.108.189141. [DOI] [PubMed] [Google Scholar]
- 18.Dragano N, Bobak M, Wege N, Peasey A, Verde PE, Kubinova R, Weyers S, Moebus S, Möhlenkamp S, Stang A, Erbel R, Jöckel KH, Siegrist J, Pikhart H. Neighbourhood socioeconomic status and cardiovascular risk factors: a multilevel analysis of nine cities in the Czech Republic and Germany. BMC Public Health. 2007;7:255. doi: 10.1186/1471-2458-7-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grotto I, Huerta M, Grossman E, Sharabi Y. Relative impact of socioeconomic status on blood pressure lessons from a large-scale survey of young adults. Am J Hypertens. 2007;20:1140–1145. doi: 10.1016/j.amjhyper.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 20.Hoang VM, Byass P, Dao LH, Nguyen TK, Wall S. Risk factors for chronic disease among rural Vietnamese adults and the association of these factors with sociodemographic variables: findings from the WHO STEPS survey in rural Vietnam, 2005. Prev Chronic Dis. 2007;4:A22. [PMC free article] [PubMed] [Google Scholar]
- 21.Le C, Chongsuvivatwong V, Geater A. Contextual socioeconomic determinants of cardiovascular risk factors in rural south-west China: a multilevel analysis. BMC Public Health. 2007;7:72. doi: 10.1186/1471-2458-7-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pickett KE, Wilkinson RG. People like us: ethnic group density effects on health. Ethn Health. 2008;13:321–334. doi: 10.1080/13557850701882928. [DOI] [PubMed] [Google Scholar]
- 23.Wilkinson RG, Pickett KE. Income inequality and socioeconomic gradients in mortality. Am J Public Health. 2008;98:699–704. doi: 10.2105/AJPH.2007.109637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jen KL, Brogan K, Washington OG, Flack JM, Artinian NT. Poor nutrient intake and high obese rate in an urban African American population with hypertension. J Am Coll Nutr. 2007;26:57–65. doi: 10.1080/07315724.2007.10719586. [DOI] [PubMed] [Google Scholar]
- 25.Blakely T, Tobias M, Atkinson J. Inequalities in mortality during and after restructuring of the New Zealand economy: repeated cohort studies. BMJ. 2008;336:371–375. doi: 10.1136/bmj.39455.596181.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Signorello LB, Schlundt DG, Cohen SS, Steinwandel MD, Buchowski MS, McLaughlin JK, Hargreaves MK, Blot WJ. Comparing diabetes prevalence between African Americans and whites of similar socioeconomic status. Am J Public Health. 2007;97:2260–2267. doi: 10.2105/AJPH.2006.094482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Signorello LB, Hargreaves MK, Steinwandel MD, Zheng W, Cai Q, Schlundt DG, Buchowski MS, Arnold CW, McLaughlin JK, Blot WJ. Southern Community Cohort Study: establishing a cohort to investigate health disparities. J Natl Med Assoc. 2005;97:972–979. [PMC free article] [PubMed] [Google Scholar]
- 28.Signorello LB, Hargreaves MK, Blot WJ. The Southern Community Cohort Study: investigating health disparities. J Health Care Poor Underserved. 2010;21(suppl):26–37. doi: 10.1353/hpu.0.0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aronow WS, Fleg JL, Pepine CJ, Artinian NT, Bakris G, Brown AS, Ferdinand KC, Forciea MA, Frishman WH, Jaigobin C, Kostis JB, Mancia G, Oparil S, Ortiz E, Reisin E, Rich MW, Schocken DD, Weber MA, Wesley DJ, Harrington RA ACCF Task Force. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. Circulation. 2011;123:2434–2506. doi: 10.1161/CIR.0b013e31821daaf6. [DOI] [PubMed] [Google Scholar]
- 30.Schiffrin EL. Vascular stiffening and arterial compliance: implications for systolic blood pressure. Am J Hypertens. 2004;17:39S–48S. doi: 10.1016/j.amjhyper.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 31.Messerli FH, Frohlich ED, Suarez DH, Reisin E, Dreslinski GR, Dunn FG, Cole FE. Borderline hypertension: relationship between age, hemodynamics and circulating catecholamines. Circulation. 1981;64:760–764. doi: 10.1161/01.cir.64.4.760. [DOI] [PubMed] [Google Scholar]
- 32.Morse SA, Zhang R, Thakur V, Reisin E. Hypertension and the metabolic syndrome. Am J Med Sci. 2005;330:303–310. doi: 10.1097/00000441-200512000-00008. [DOI] [PubMed] [Google Scholar]
- 33.Vaz M, Jennings G, Turner A, Cox H, Lambert G, Esler M. Regional sympathetic nervous activity and oxygen consumption in obese normotensive human subjects. Circulation. 1997;96:3423–3429. doi: 10.1161/01.cir.96.10.3423. [DOI] [PubMed] [Google Scholar]
- 34.Engeli S, Sharma AM. The renin-angiotensin system and natriuretic peptides in obesity-associated hypertension. J Mol Med (Berl) 2001;79:21–29. doi: 10.1007/s001090000144. [DOI] [PubMed] [Google Scholar]
- 35.Sharma AM, Engeli S, Pischon T. New developments in mechanisms of obesity-induced hypertension: role of adipose tissue. Curr Hypertens Rep. 2001;3:152–156. doi: 10.1007/s11906-001-0030-x. [DOI] [PubMed] [Google Scholar]
- 36.Sharma AM, Janke J, Gorzelniak K, Engeli S, Luft FC. Angiotensin blockade prevents type 2 diabetes by formation of fat cells. Hypertension. 2002;40:609–611. doi: 10.1161/01.hyp.0000036448.44066.53. [DOI] [PubMed] [Google Scholar]
- 37.Frohlich ED. The heart in hypertension: a 1991 overview. Hypertension. 1991;18(suppl):III62–III68. doi: 10.1161/01.hyp.18.5_suppl.iii62. [DOI] [PubMed] [Google Scholar]
- 38.Kshirsagar AV, Carpenter M, Bang H, Wyatt SB, Colindres RE. Blood pressure usually considered normal is associated with an elevated risk of cardiovascular disease. Am J Med. 2006;119:133–141. doi: 10.1016/j.amjmed.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 39.Selassie A, Wagner CS, Laken ML, Ferguson ML, Ferdinand KC, Egan BM. Progression is accelerated from prehypertension to hypertension in blacks. Hypertension. 2011;58:579–587. doi: 10.1161/HYPERTENSIONAHA.111.177410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Albert MA, Glynn RJ, Buring J, Ridker PM. C-reactive protein levels among women of various ethnic groups living in the United States (from the Women’s Health Study) Am J Cardiol. 2004;93:1238–1242. doi: 10.1016/j.amjcard.2004.01.067. [DOI] [PubMed] [Google Scholar]
- 41.Albert MA, Pare G, Morris A, Rose L, Buring J, Ridker PM, Zee RY. Candidate genetic variants in the fibrinogen, methylenetetrahydrofolate reductase, and intercellular adhesion molecule-1 genes and plasma levels of fibrinogen, homocysteine, and intercellular adhesion molecule-1 among various race/ethnic groups: data from the Women’s Genome Health Study. Am Heart J. 2009;157:777–783. e1. doi: 10.1016/j.ahj.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Calhoun DA, Oparil S. Racial differences in the pathogenesis of hypertension. Am J Med Sci. 1995;310(suppl 1):S86–S90. doi: 10.1097/00000441-199512000-00016. [DOI] [PubMed] [Google Scholar]
- 43.Johnson RC. The place of race in health disparities: how family background and neighborhood conditions in childhood impact later-life health. In: Newburger HB, Birch EL, Wachter SM, editors. Neighborhood and Life Chances: How Place Matters in Modern America. Philadelphia: University of Pennsylvania Press; 2010. [Google Scholar]
- 44.Barker DJ, Osmond C. Childhood respiratory infection and adult chronic bronchitis in England and Wales. BMJ (Clin Res Ed) 1986;293:1271–1275. doi: 10.1136/bmj.293.6557.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barker DJ, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet. 1986;1:1077–1081. doi: 10.1016/s0140-6736(86)91340-1. [DOI] [PubMed] [Google Scholar]
- 46.Barker DJ, Osmond C, Golding J, Kuh D, Wadsworth ME. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ. 1989;298:564–567. doi: 10.1136/bmj.298.6673.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barker DJ, Osmond C, Law CM. The intrauterine and early postnatal origins of cardiovascular disease and chronic bronchitis. J Epidemiol Community Health. 1989;43:237–240. doi: 10.1136/jech.43.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet. 1989;2:577–580. doi: 10.1016/s0140-6736(89)90710-1. [DOI] [PubMed] [Google Scholar]
- 49.Forsén T, Eriksson JG, Tuomilehto J, Osmond C, Barker DJ. Growth in utero and during childhood among women who develop coronary heart disease: longitudinal study. BMJ. 1999;319:1403–1407. doi: 10.1136/bmj.319.7222.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ravelli AC, van Der Meulen JH, Osmond C, Barker DJ, Bleker OP. Obesity at the age of 50 y in men and women exposed to famine prenatally. Am J Clin Nutr. 1999;70:811–816. doi: 10.1093/ajcn/70.5.811. [DOI] [PubMed] [Google Scholar]
- 51.Roseboom TJ, van der Meulen JH, Ravelli AC, van Montfrans GA, Osmond C, Barker DJ, Bleker OP. Blood pressure in adults after prenatal exposure to famine. J Hypertens. 1999;17:325–330. doi: 10.1097/00004872-199917030-00004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.