Abstract
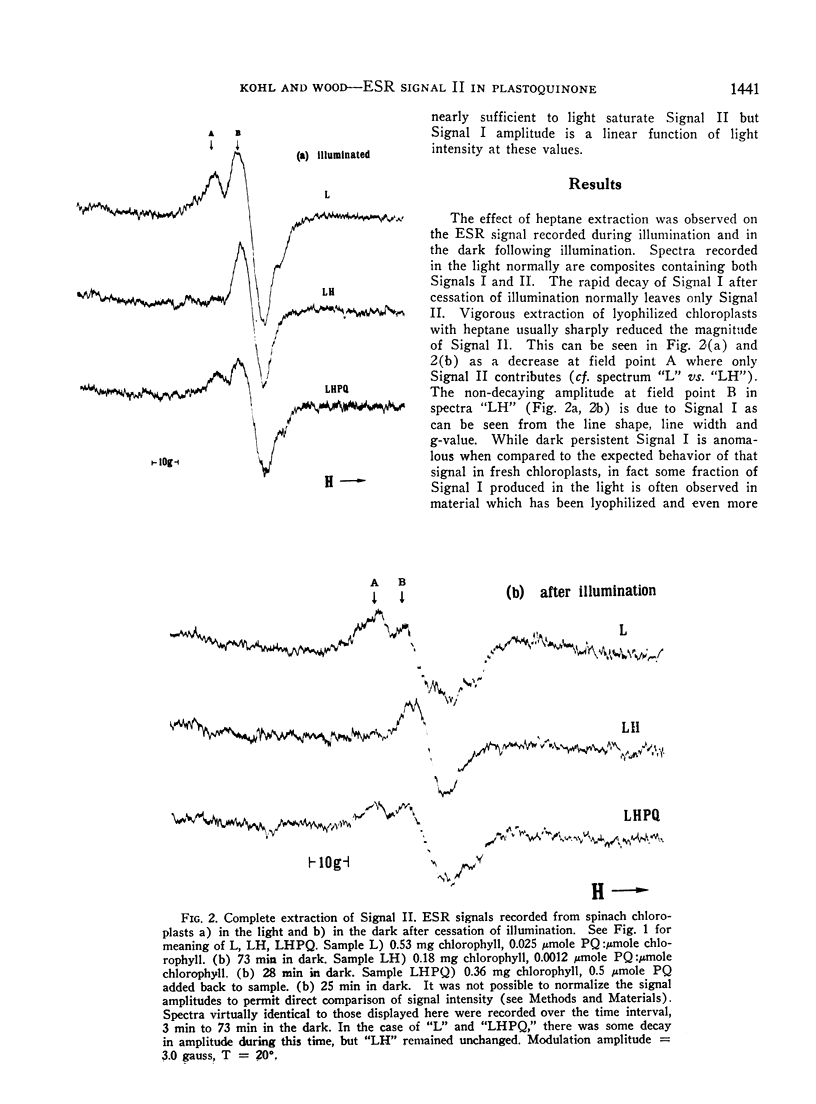
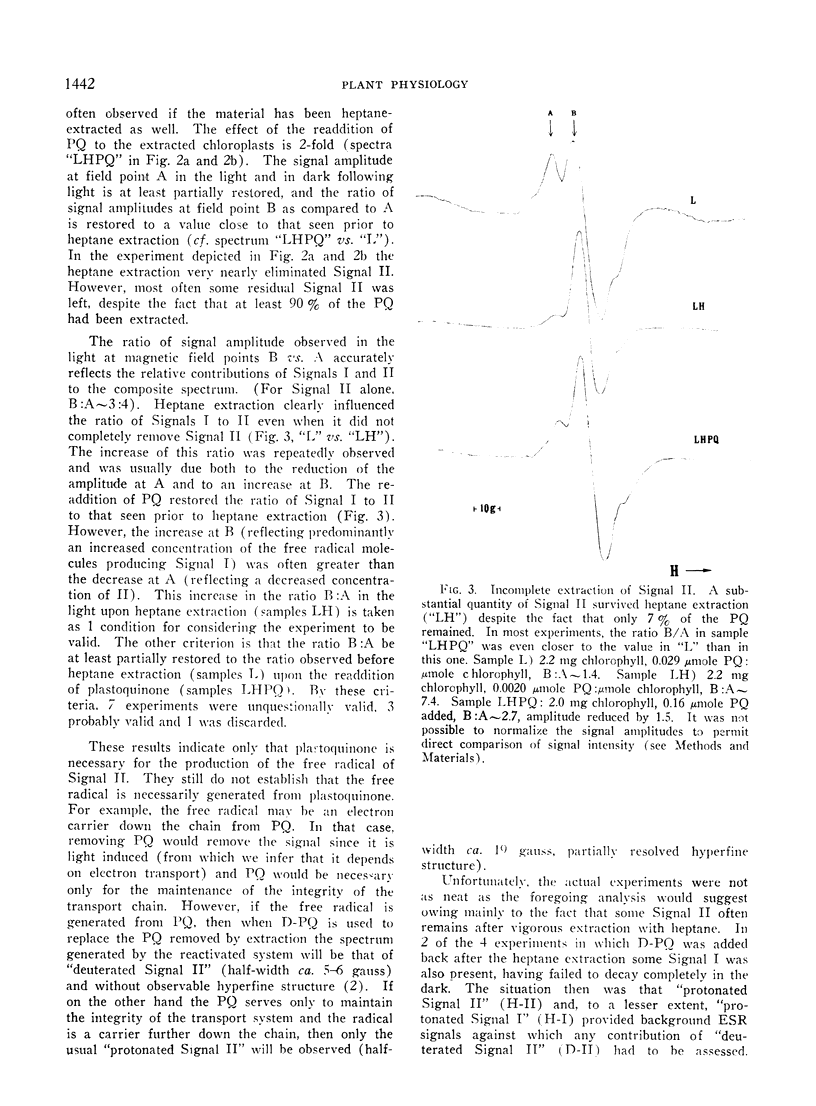
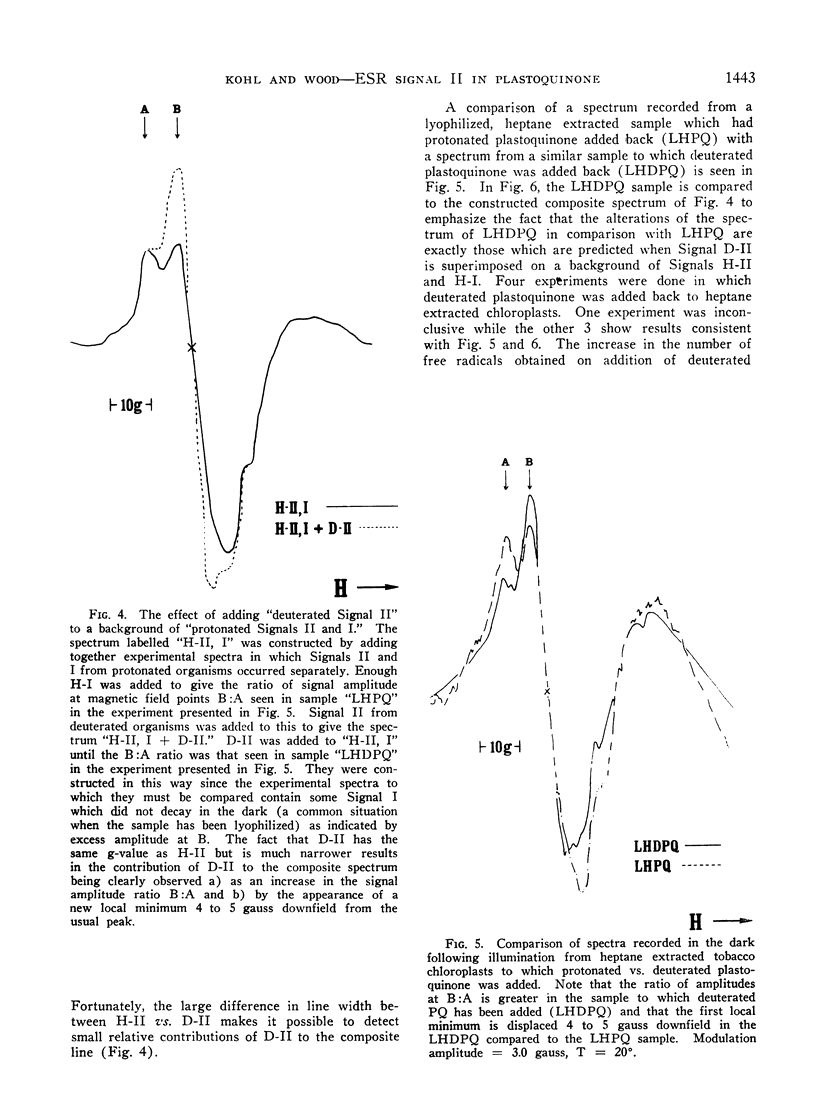
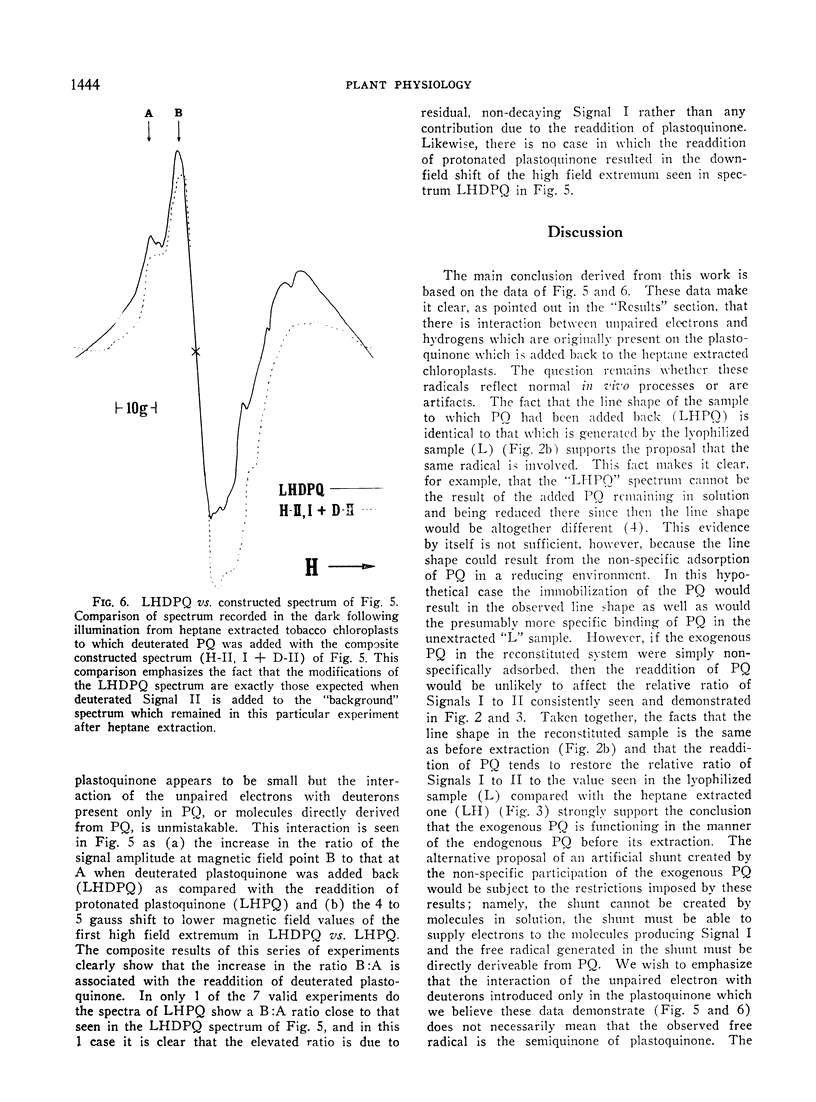
Speculation as to the identity of Signal II, the light-induced, broad, slow decaying electron spin resonance signal with hyperfine structure observed in photosynthetic materials, has tended to center on the semiquinone of plastoquinone. Experiments reported here were designed to give direct evidence bearing on that speculation. Heptane extraction of lipids from lyophilized spinach and tobacco chloroplast fragments reduced the amplitude of Signal II and increased the ratio of Signal I:Signal II. Reconstitution of the system by the addition of plastoquinone partially restored Signal II as well as the ratio of Signal I:Signal II to its pre-extraction condition. Addition of totally deuterated plastoquinone to extracted chloroplasts in which considerable Signal II had survived heptane extraction resulted in a spectrum which showed the characteristic hallmarks of Signal II observed in totally deuterated organisms. These results establish that a free radical immediately derived from plastoquinone contributes to Signal II. The data taken by themselves are consistent with plastochromanoxyl as well as plastosemiquinone free radicals giving rise to Signal II. Other contributors to Signal II are not ruled out.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton J. R., Clayton R. K., Reed D. W. An identification of the radical giving rise to the light-induced electron spin resonance signal in photosynthetic bacteria. Photochem Photobiol. 1969 Mar;9(3):209–218. doi: 10.1111/j.1751-1097.1969.tb07285.x. [DOI] [PubMed] [Google Scholar]
- COMMONER B., HEISE J. J., LIPPINCOTT B. B., NORBERG R. E., PASSONNEAU J. V., TOWNSEND J. Biological activity of free radicals. Science. 1957 Jul 12;126(3263):57–63. doi: 10.1126/science.126.3263.57. [DOI] [PubMed] [Google Scholar]
- Kohl D. H., Townsend J., Commoner B., Crespi H. L., Dougherty R. C., Katz J. J. Effects of isotopic substitution on electron spin resonance signals in photosynthetic organisms. Nature. 1965 Jun 12;206(989):1105–1110. doi: 10.1038/2061105a0. [DOI] [PubMed] [Google Scholar]
- Kohl D. H., Wright J. R., Weissman M. Electron spin resonance studies of free radicals derived from plastoquinone, alpha- and gamma-tocopherol and their relation to free radicals observed in photosynthetic materials. Biochim Biophys Acta. 1969 Aug 5;180(3):536–544. doi: 10.1016/0005-2728(69)90032-2. [DOI] [PubMed] [Google Scholar]
- McElroy J. D., Feher G., Mauzerall D. C. On the nature of the free radical formed during the primary process of bacterial photosynthesis. Biochim Biophys Acta. 1969 Jan 14;172(1):180–183. doi: 10.1016/0005-2728(69)90105-4. [DOI] [PubMed] [Google Scholar]



