Abstract
Objectives
We wanted to compare detection of a broad spectrum of human papillomavirus (HPV) types detected in cellular specimens from the vagina and cervix, which could provide information about the potential of each anatomical site for harboring infection. Previous studies have failed to present data on or detect a broad spectrum of HPV genotypes and/or have not carefully sampled the vagina, instead relying on self-collection that is likely contaminated with cervical cells.
Study Design
We conducted follow-up study of 353 women who had participated in study of HPV and cervical neoplasia in Costa Rica. We collected paired cervical and vaginal specimens; vaginal specimens were collected from the fornix to minimize cervical contamination. Specimens were tested in a masked fashion for >40 HPV types using a MY09/MY11 PCR method and type-specific dot blot hybridization.
Results
The prevalence for any carcinogenic HPV type in vaginal and cervical specimens was similar (P = 0.3). However, the prevalence for any HPV type in vaginal specimens was greater than in cervical specimens (P = 0.0002), primarily due to a twofold increased vaginal prevalence of HPV types of the α3/α15 phylogenetic species (e.g., HPV61) (P <0.00005).
Conclusions
Carcinogenic HPV types appeared to have a similar affinity for vaginal and cervical epithelium, but noncarcinogenic HPV types of the α3/α15 phylogenetic species may have a tropism for vaginal epithelium.
Both cervical and vaginal epithelia support HPV infections1,2 and the detection of vaginal HPV can precede detection of cervical HPV.3 However, vaginal cancer is exceedingly rare compared to cervical cancer,4 emphasizing the great importance of the metaplastic tissue of the cervical transformation zone for development of HPV-induced genital cancers.
Surprisingly few studies have compared the detection of a broad spectrum of HPV genotypes in paired specimens collected from the vagina and cervix of the same women. One study compared the detection of 27 HPV genotypes in self-collected cervicovaginal specimen to physician-collected cervical specimen.5 However, self-collected specimens represent an admixture of vaginal and cervical cells of an unknown ratio, which likely depends on the collection tool and training of the patients. Moreover, as in the aforementioned study,5 self-collection was often conducted immediately after collection of the cervical specimen, which could increase the percentage of exfoliative cells in the vagina where self-collection is occurring. Other studies have collected paired specimens, but have not presented a comparison of individual HPV types collected.
To further examine correlation of more than 40 individual HPV types and groups of types in the vagina and cervix, we collected paired specimens from women known to have been HPV-infected with carcinogenic HPV types. These women had participated in a population-based study of HPV and cervical neoplasia in Guanacaste, Costa Rica.
Materials and Methods
Study Population
This population-based cohort study included participants from Guanacaste, Costa Rica, enrolled between June 1993 and December 1994 with the approval of the National Cancer Institute and Costa Rican institutional review boards. The methods of cohort recruitment, screening, and follow-up have been previously published elsewhere.6
Three-hundred thirty-two women who tested positive for carcinogenic HPV genotypes (HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, or 68) at a follow-up visit 5–7 years after enrollment or at exit visit during follow-up but did not have concurrent cervical precancer or cancer were invited for a follow-up visit to participate in a study of HPV persistence and for patient safety. (At the time of selection, HPV66 had not yet been classified as carcinogenic7 and therefore was not included as a selection criterion.) As controls, we invited a random sample of 24 women without carcinogenic HPV infection to participate. We also invited 42 women who self-reported to be virgins at their 5–7 year follow-up visit. Of those 42 self-reported virgins, 26 (62%) were still not sexually active at the time of this ancillary, follow-up visit, and therefore were excluded from these analyses. Thus, 372 sexually active women were eligible for pelvic exams and HPV testing.
Specimen Collection
After a Pap specimen was collected using a Cervex brush directed at the cervical os and placed into PreservCyt (Cytyc Corporation, Marlborough, MA) for liquid-based cytology, paired cervical and vaginal HPV specimens were collected. The cervical HPV specimen was collected using a Dacron swab to sample the cervical os and stored in a transport medium (UCM; Digene Corporation, Gaithersburg, MD). Then, a vaginal HPV specimen was collected using a Dacron swab from the high end of the vaginal wall (vaginal fornix), targeting areas not touched by the speculum, and placed in UCM.
HPV DNA Testing
Masked PCR testing was conducted on the cervical and vaginal specimens as previously described.8 We used a MY09/M11 L1 consensus primer PCR (MY09/11 PCR) method to amplify the DNA and dot blot hybridization of PCR products using type-specific oligonucleotides for type-specific detection of HPV types 2, 6, 11, 13, 16, 18, 26, 31–35, 39, 40, 42–45, 51–59, 61, 62, 64, 66–74, 81–85, 82v, and 89. Of the 372 eligible women, 367 (99%) had valid HPV testing results for the vaginal specimens, 353 (95%) had valid HPV testing results for the cervical specimens, and 353 (95%) had valid HPV testing results for both specimens, which defined our analytic group and included 314 who had a previously documented carcinogenic HPV infection.
Statistical Analysis
We used contingency tables to compare the characteristics of women by their selection criterion. Fisher exact (categorical variables) and Kruskal–Wallis tests (continuous variable) were used to test for statistical differences between study groups.
We calculated the cervical and vaginal prevalence of each individual HPV type, any HPV type, phylogenetic species or group of species, and any carcinogenic HPV type (HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68), and any noncarcinogenic HPV type (all other types). Phylogenetic species or groups of species were defined as follows: α1/α8/α10: HPV6, 11, 40, 42, 32, 55, and 74; α3/α15: 61, 62, 71, 72, 81, 83, 84, 89; α5: HPV26, 51, 69, 82, and 82v; α6: HPV53, 56, and 66; α7: HPV18, 39, 45, 59, 68, 70, and 85; α9/α11: HPV16, 31, 33–35, 52, 58, 64, 67, and 73. We note that for multitype infections, each type contributed to the calculation of prevalence, i.e., a woman infected with HPV16 and HPV71 contributed to the prevalence for those types and the categories in which types are included. We also calculated binomial exact 95% confidence intervals (95% CI) for the prevalences of any HPV type and any carcinogenic HPV type. We tested for statistically significant differences (P < 0.05) in paired prevalences using an exact McNemar χ2 test.
We also compared the prevalence and calculated the agreement for α3/α15 types detected in vaginal and cervical specimens in women under the age of 50 versus in women 50 and older. We have previously observed significant differences in the prevalence of these types in vaginal specimens from hysterectomized women versus cervical specimens from nonhysterectomized women, restricted to women under the age of 50.1 For comparison, we calculated the prevalence and the agreement of α9/α11 types in cervical and vaginal specimens from women in both age groups. We also used a natural cubic regression spline to fit prevalence data to age, specifying interior knots at the ages 29 (5th percentile), 42 (50th percentile), and 72 (90th percentile),9 to provide a continuous functional relationship between age and HPV prevalence.
Finally, we used contingency tables to compare the prevalence of any HPV, α3/α15 types, and α9/α11 types between women with different selection criterion, with the Fisher exact test used to test for statistical significance.
Results
We examined the relationship of cervical and vaginal HPV types in a population mainly of women who had a carcinogenic HPV infection a median of ~4 years previously (median, mean, and range of 52, 48, and 21–111 months between visits, respectively). Women had a median, mean, and age range of 42, 44, and 28–85 years, respectively. Characteristics of women by their selection criteria are presented in Table 1. Women who were virgins at the year 5–7 visit but became sexually active before this follow-up visit were younger, less likely to ever have been pregnant, more likely to be using oral contraceptives, and less likely to be married.
TABLE 1.
Characteristics of Women Included in This Study by Their Selection Criteria
| All N = 353 |
Group 1 N = 314 |
Group 2 N = 24 |
Group 3 N = 15 |
P | |
|---|---|---|---|---|---|
| Median age (yr) | 42 | 42 | 46 | 29 | 0.0001 |
| Ever pregnant (%) | 93.5 | 96.2 | 100.0 | 26.7 | <0.0005 |
| Ever smoke (%) | 9.4 | 9.6 | 8.3 | 6.7 | 1 |
| Using oral contraceptives (%) | 16.4 | 15.9 | 8.3 | 40.0 | 0.04 |
| No sex partners in the last year (%) | 19.0 | 18.8 | 20.8 | 20.0 | 0.9 |
| Married (%) | 70.3 | 71.3 | 75.0 | 40.0 | 0.04 |
Group 1: Carcinogenic HPV positive at the year 5–7 visit; Group 2: Carcinogenic HPV negative at the year 5–7 visit; Group 3: Became sexually active between the year 5–7 visit and this follow-up visit. Kruskal-Wallis test was used to test for statistical differences in median age between groups.
Fisher exact was used to test for statistical differences in categorical variables between groups.
Table 2 shows the prevalence of individual HPV types and groups of HPV types. The most prevalent HPV types in the vaginal specimens were HPV71 (4.0%), HPV61 (3.7%), and HPV58 (3.4%). The most prevalent HPV types in the cervical specimens were HPV16 (4.0%), HPV58 (3.7%), and HPV71 (3.7%). HPV61 and HPV85 were significantly more common in vaginal specimens than in cervical specimens. (n.b., given the number of comparisons for types, these differences could be due to chance. Using Bonferroni’s correction for multiple comparisons, these differences would not be deemed significant.) The prevalence for any HPV type in vaginal specimens (42.5%, 95% CI = 37.2–47.8%) was greater than in cervical specimens (33.7%, 95% CI = 28.8–38.9%) (P = 0.0002). The prevalences for any carcinogenic HPV type in vaginal specimens (18.4%, 95% CI = 14.5–22.9%) and in cervical specimens (20.7%, 95% CI = 16.6–25.3%) were similar (P = 0.3).
TABLE 2.
Prevalence of HPV Types and HPV Groups in Paired Vaginal (V) and Cervical (C) Specimens, and Their Ratio (V/C), From 353 Women
| V | C | V/C | |
|---|---|---|---|
| HPV type | |||
| HPV6 | 1.4 | 0.9 | 1.7 |
| HPV11 | 0.3 | 0.0 | |
| HPV16 | 3.1 | 4.0 | 0.79 |
| HPV18 | 0.3 | 1.4 | 0.20 |
| HPV26 | 0.3 | 0.0 | |
| HPV31 | 3.1 | 2.8 | 1.1 |
| HPV32 | 1.4 | 0.9 | 1.7 |
| HPV33 | 0.6 | 0.9 | 0.67 |
| HPV35 | 1.4 | 1.7 | 0.83 |
| HPV39 | 0.9 | 1.7 | 0.75 |
| HPV40 | 0.6 | 0.3 | 2.0 |
| HPV45 | 0.9 | 0.9 | 1.0 |
| HPV51 | 2.6 | 2.0 | 1.3 |
| HPV52 | 2.0 | 2.0 | 1.0 |
| HPV53 | 2.3 | 2.6 | 0.89 |
| HPV54 | 0.6 | 0.3 | 2.0 |
| HPV55 | 0.3 | 0.3 | 1.0 |
| HPV56 | 2.0 | 1.4 | 1.4 |
| HPV58 | 3.4 | 3.7 | 0.92 |
| HPV59 | 0.9 | 1.4 | 0.60 |
| HPV61* | 3.7 | 1.4 | 2.6 |
| HPV62 | 1.4 | 0.3 | 5.0 |
| HPV66 | 0.0 | 0.9 | 0.0 |
| HPV67 | 0.0 | 0.3 | 0.0 |
| HPV68 | 0.0 | 0.3 | 0.0 |
| HPV70 | 3.1 | 1.4 | 2.2 |
| HPV71 | 4.0 | 3.7 | 1.1 |
| HPV72 | 1.4 | 0.9 | 1.7 |
| HPV73 | 0.6 | 0.6 | 1.0 |
| HPV81 | 1.7 | 0.9 | 2.0 |
| HPV82v | 0.3 | 0.6 | 0.5 |
| HPV83 | 1.4 | 0.3 | 5.0 |
| HPV84 | 2.3 | 1.1 | 2.0 |
| HPV85* | 2.3 | 0.6 | 4.0 |
| HPV group | |||
| Any HPV* | 42.5 | 33.7 | 1.3 |
| Carcinogenic HPV | 18.4 | 20.7 | 0.89 |
| Noncarcinogenic HPV* | 24.1 | 13.0 | 1.8 |
| α1/α8/α10 | 3.7 | 2.3 | 1.6 |
| α3/α15* | 14.2 | 7.7 | 1.9 |
| α5 | 3.1 | 2.6 | 1.2 |
| α6 | 4.5 | 4.0 | 1.1 |
| α7 | 8.2 | 6.8 | 1.2 |
| α9/α11 | 12.5 | 15.0 | 0.83 |
Definitions of the types included in carcinogenic HPV and in the different phylogenetic groups (e.g., α1/α8/α10) are presented in the methods. HPV13, 34, 42–44, 57, 64, 69, 82, and 89 were not detected in either specimen.
Statistically significant differences by an exact McNemar χ2 test.
Noncarcinogenic HPV types were more prevalent in vaginal specimens (24.1%) than in cervical specimens (13.0%; P < 0.0005). This difference was primarily due to types of the α3/α15 phylogenetic species, which do not include any carcinogenic HPV types but include highly-prevalent HPV61 and HPV71, being approximately twice as common in vaginal specimens (14.2%) than in cervical specimens (7.7%; P < 0.00005). By comparison, types of the α9/α11 phylogenetic species, which includes HPV16, the most common and most carcinogenic HPV type, were slightly less common in vaginal specimens (12.5%) than in cervical specimens (15.0%), although the difference did not reach statistical significance (P = 0.1).
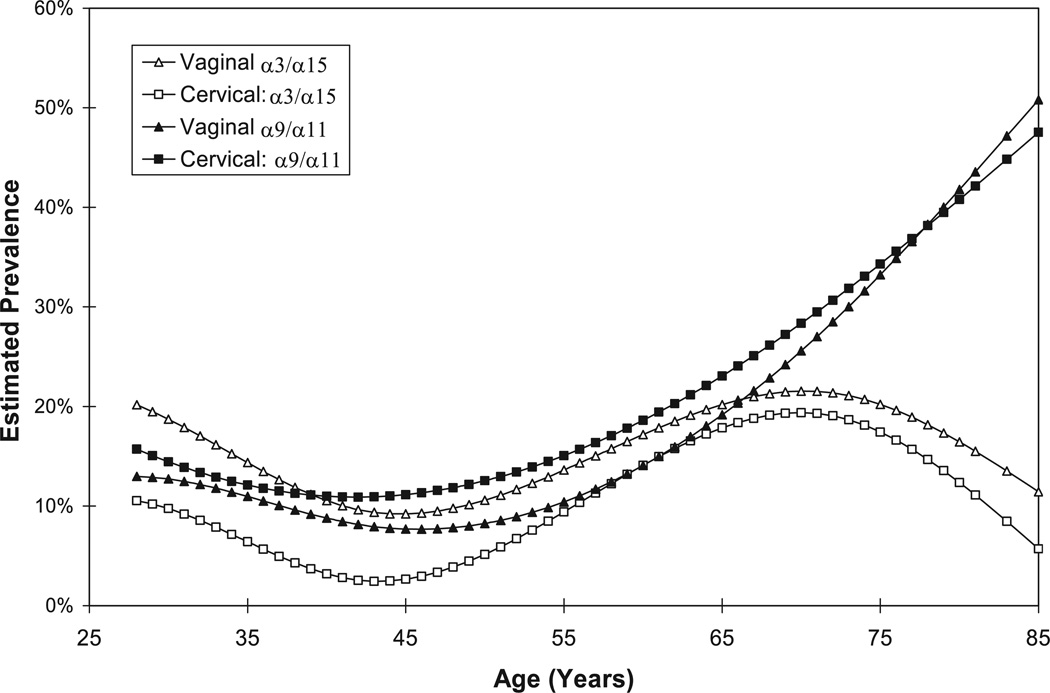
The greater prevalence of α3/α15 types in vaginal specimens versus cervical specimens was primarily restricted to women under the age of 50, in whom there was 13% prevalence in vaginal specimens and 5% prevalence in cervical specimens (P < 0.00005; Table 3). In women 50 and older, there was 16% prevalence in vaginal specimens and 13% prevalence in cervical specimens (P = 0.3). By comparison, there was nonsignificant lower prevalence of α9/α11 types in vaginal specimens than in cervical specimens in all women, women under 50, and women 50 and older. These patterns were borne out by fitting the prevalence data to age using a cubic regression spline (Fig. 1).
TABLE 3.
A Comparison of Detection of HPV Types in the α3/α15 Phylogenetic Species and α9/α11 Phylogenetic Species in the Vaginal (V) Specimens With the Cervical (C) Specimens for Women Under the Age of 50, 50 and Older, and All Women
| C+ | V+ | C−/V− | C+/V− | C−/V+ | C+/V+ | Total | P | κ | % Agree | |
|---|---|---|---|---|---|---|---|---|---|---|
| α3/α15 | ||||||||||
| 25–49 y | ||||||||||
| n | 14 | 34 | 219 | 2 | 22 | 12 | 255 | <0.00005 | 0.46 | 91 |
| % | 5 | 13 | 86 | 1 | 9 | 5 | ||||
| 50–85 y | ||||||||||
| n | 13 | 16 | 80 | 2 | 5 | 11 | 98 | 0.3 | 0.72 | 93 |
| % | 13 | 16 | 82 | 2 | 5 | 11 | ||||
| Total | ||||||||||
| n | 27 | 50 | 299 | 4 | 27 | 23 | 353 | <0.00005 | 0.55 | 91 |
| % | 8 | 14 | 85 | 1 | 8 | 7 | ||||
| α9/α11 | ||||||||||
| 25–49 y | ||||||||||
| n | 30 | 26 | 216 | 13 | 9 | 17 | 255 | 0.4 | 0.56 | 91 |
| % | 12 | 10 | 85 | 5 | 4 | 7 | ||||
| 50–85 y | ||||||||||
| n | 23 | 18 | 73 | 7 | 2 | 16 | 98 | 0.1 | 0.72 | 91 |
| % | 23 | 18 | 74 | 7 | 2 | 16 | ||||
| Total | ||||||||||
| n | 53 | 44 | 289 | 20 | 11 | 33 | 353 | 0.1 | 0.63 | 91 |
| % | 15 | 12 | 82 | 6 | 3 | 9 |
Kappa (κ) values and % agreement (% Agree) are presented. An exact McNemar’s χ2 test was used to test for statistically significant differences in prevalence.
Fig. 1.
A graph of the prevalence of cervical and vaginal α3/α15 and α9/α11 HPV types (adapted from reference 13) by age was fit using a cubic regression spline (see Methods) (cervical, square; vaginal, triangle; α3/α15, open symbol; α9/α11, solid symbol).
Finally, we considered the impact of our selection groups on HPV group prevalences (Table 4). Across different groups, vaginal HPV was more common than cervical HPV. This difference was most pronounced in α3/α15 types compared with that in α9/α11 types. The one exception is that women who were virgins and then became sexually active during follow-up had similar vaginal/cervical ratios for α3/α15 types and α9/α11 types, which could either reflect either a chance finding due to small numbers in this group or recent acquisitions that may first occur vaginally.3
TABLE 4.
HPV Group Prevalences for Women Included in This Study by Their Selection Criterion
| All N = 353 |
Group 1 N = 314 |
Group 2 N = 24 |
Group 3 N = 15 |
P | |
|---|---|---|---|---|---|
| Prevalence of vaginal HPV: | |||||
| All (%) | 42.5 | 44.0 | 12.5 | 60.0 | 0.02 |
| Prevalence of cervical HPV: | |||||
| All (%) | 33.7 | 36.0 | 8.3 | 26.7 | 0.06 |
| Ratio (V/C) | 1.3 | 1.2 | 1.5 | 2.2 | |
| Prevalence of vaginal HPV: | |||||
| α3/α15 (%) | 14.2 | 15.0 | 4.2 | 13.3 | 0.4 |
| Prevalence of cervical HPV: | |||||
| α3/α15 (%) | 7.7 | 8.3 | 0.0 | 6.7 | 0.4 |
| Ratio (V/C) | 1.8 | 1.8 | n/a | 2.0 | |
| Prevalence of vaginal HPV: | |||||
| α9/α11 (%) | 12.5 | 12.1 | 4.2 | 33.3 | 0.03 |
| Prevalence of cervical HPV: | |||||
| α9/α11 (%) | 15.0 | 15.9 | 4.2 | 13.3 | 0.4 |
| Ratio (V/C) | 0.8 | 0.8 | 1.0 | 2.5 |
Group 1: Carcinogenic HPV positive at the year 5–7 visit; Group 2: Carcinogenic HPV negative at the year 5–7 visit; Group 3: Became sexually active between the year 5–7 visit and this follow-up visit.
Discussion
We examined the relationship of vaginal and cervical HPV at the type-specific level. We confirmed that the prevalence of carcinogenic HPV was similar in vaginal and cervical specimens,1,2 suggesting that vaginal and cervical tissue are roughly equally capable of supporting carcinogenic HPV infections. However, the tissues have very have different potential to be transformed as evident from the relative occurrence of cervical cancer versus vaginal cancer.4
This study corroborated that HPV types of the α3/α15 phylogenetic species preferentially infect the keratinized tissue typically found in the vagina compared to nonkeratinized, metaplastic, or columnar tissue that typifies the cervix. This is illustrated by the difference in the prevalence patterns in women under the age of 50 versus those 50 and older, as seen previously when comparing vaginal specimens from hysterectomized women to cervical specimens from nonhysterectomized women.1 In the younger women, there is a greater prevalence of the α3/α15 types in the vaginal specimen versus the cervical specimen. In the older, perimenopausal or menopausal women, there was little difference in prevalence. In these older women, the cervical tissue atrophies due to hormonal changes and the transformation zone of the cervix typically migrates deeper into the canal. The os of the cervix where the cervical specimen is taken is covered with mature squamous epithelium, making it more like the vagina.10 We previously reported that 1) α3/α15 types were more common in vaginal specimens from hysterectomized women than in cervical specimens from nonhysterectomized women1; 2) a greater likelihood of these types being detected or persisting after excisional treatment compared with HPV16,11 the most persistent HPV type in the absence of treatment; and 3) the age-related shift in the milieu of HPV types in specimens from the os of the cervix.10 In toto these data provide strong evidence that types of α3/α15 phylogenetic species, which never cause cancer, preferentially infect and/or persistently infect vaginal and vaginal-like tissue.
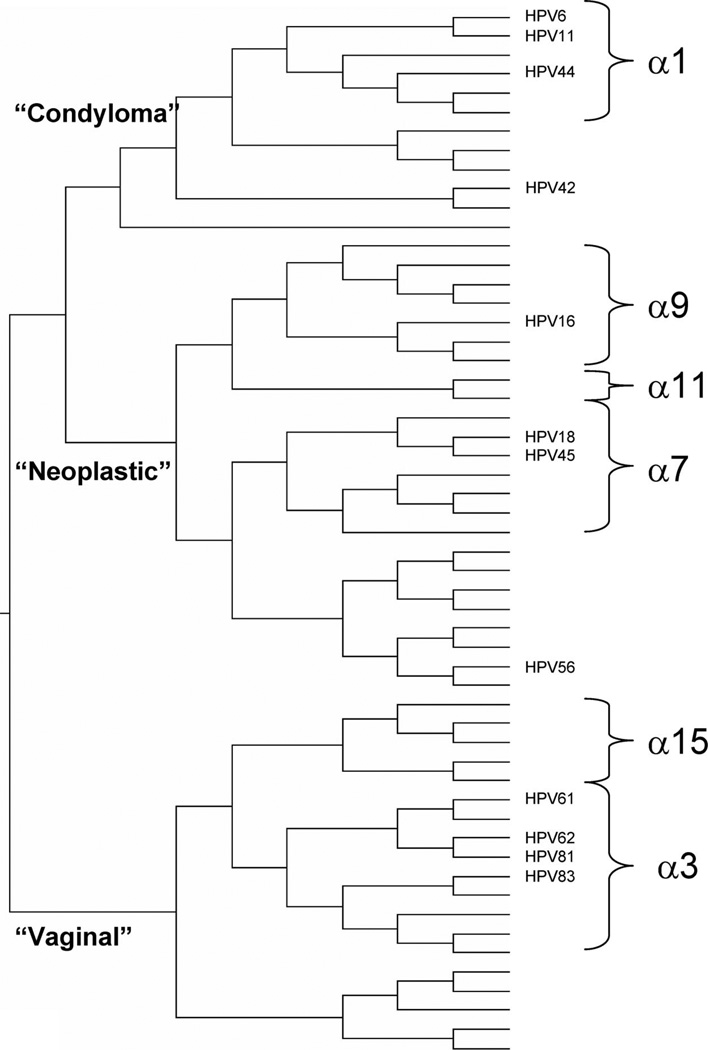
Therefore, these vaginal types have probably evolved a mechanism for viral survival and production that differs from the mechanisms employed by carcinogenic HPV types. Perhaps as a corollary, when these types are detected they are rarely accompanied by cytologic changes,12 perhaps because α3/α15 types infect mature squamous cells and/or when infecting any cell they are less likely to cause the abnormal cytology. Inspection of the evolutionary tree of the anogenital HPV genotypes13 (Fig. 2) shows that first branching separated the vaginal types from other α genus types, which later branched into separate lineages for genital warts (e.g., HPV6 and HPV11) and for neoplasia (e.g., HPV16 and HPV18). The molecular determinants of these different phenotypes are unknown.
Fig. 2.
An abbreviated version of the phylogenetic analysis (phylogenetic tree) for anogenital HPV types (adapted from reference 13).
We acknowledge that a major limitation of the study is that our study population was composed of women with a median age of 42 years and who primarily had a past carcinogenic HPV infection. Certainly, selection of this population of women led to a higher prevalence of carcinogenic HPV and α9/α11 types than is typically found in this age range in this population,14 especially in the older women who may have had long-term viral persistence as indicated by the spline analysis. (The median age of women with persistent carcinogenic HPV was 56 years; 45% and 32% of women 65 and older tested positive for carcinogenic HPV and α9/α11 types, respectively, all of which were persistent infections.) Specifically, our selection would seem to favor cervical infections by α9/α11 HPV types. However, we observe similar vaginal to cervical ratios for HPV group prevalence patterns for women who tested positive and negative for cervical carcinogenic HPV.
We also note that we did not select on vaginal HPV status, and therefore it seems unlikely that our selection would bias our results in the direction of an increased prevalence of α3/α15 HPV types in the vagina compared to the cervix. It is also noteworthy that most HPV infections are transient and that most of the infections detected at year 5–7 were no longer detectable 4 years later. Thus, while it is possible that our selection criteria favored the prevalence of cervical α9/α11 HPV types compared to vaginal α9/α11 HPV types, it is less obvious how it would favor the prevalence of vaginal α3/α15 HPV types compared with that of cervical α3/α15 HPV types. By collecting paired vaginal and cervical specimens from the same women, we have matched on biases and nuisance factors that potentially confounded a previous comparison of vaginal specimens from hysterectomized women and cervical specimens from nonhysterectomized women.1 Nevertheless, we cannot rule out that our selection criteria for this study has biased our findings and that in a random sample of the population, we would not observe these patterns. We therefore caution that our results may not generalize to all populations and further studies in true population samples are needed to confirm our observations.
Another consideration is that we evaluated prevalently detected HPV infections. It is unclear how these observed patterns would differ for incident infections. However, there is some evidence that vaginal infections precede cervical infections.3 If so, there would be even a greater difference between vaginal and cervical HPV detection for incidence infection although it is unclear whether there would be differences between the aforementioned phylogenetic groupings. The patterns of HPV prevalence among those women who recently became sexually active included in this study, despite small numbers, indicate that these differences by phylogenetic groups are less apparent for recently acquired infections.
A strength of our study is the choice of location for collecting the vaginal specimen. We collected the vaginal specimen from the vaginal fornix, and therefore from the recesses beyond the cervical os, as an attempt to minimize the possible contamination by sloughed cervical epithelial cells into the distal vagina that may partially obscure the relationship of HPV types with anatomical location. However, we cannot rule out that the vaginal specimen was “contaminated” with sloughed cervical cells and this could have partially obscured the true differences between HPV patterns for the two anatomical locations. Thus, differences for HPV types between vaginal and cervical epithelium could be greater than we could observe using exfoliative specimens following speculum insertion.
There are no other studies of which we are aware that present comprehensive data on HPV types as detected in the vagina and cervix. Some studies have collected paired specimens but relied on self-collected specimens for the vaginal specimen, which can be contaminated with cervical cells in unknown quantities due to an uncertain degree of sampling of the cervix and possibly sloughing of cervical cells into the vagina. Nearly all studies used for HPV testing either Hybrid Capture 2 (Digene Corporation), which collectively tests for 13 carcinogenic HPV types but does not provide information on the individual HPV genotypes present, or used a HPV genotyping assay but only presented data on overall HPV or carcinogenic HPV detection, rather than for individual HPV genotypes. One study comparing self-collected specimens to physician-collected cervical specimens tested specimens for 27 HPV genotypes but did not include many types of the α3/α15 phylogenetic species.5 A recent study found that HPV83 and HPV84, α3/α15 types, were in 2–3-fold greater abundance in self-collected specimens compared with physician-collected cervical specimens.15
In summary, we have found additional evidence to support the unique biologic interactions between genetically distinct but closely related HPV types and different anatomical sites of the lower genital tract: 1) Carcinogenic HPV types infect the entire lower genital tract equally but virtually require a transformation zone of metaplastic tissue for carcinogenic progression; and 2) types of α3/α15 specimens prefer the vaginal epithelium or the cervical epithelium when it becomes more mature, vaginal like. Broad phenotypic differences have been described for animal papillomaviruses, which exhibit exquisite host species specificity. Human papillomavirus types from different genera have different targets (e.g., sole of foot, nongenital skin, and anogenital skin) and infection is tied closely to the differentiation of their specific epithelial target.16 Here we provide strong additional evidence that there are yet even more subtle phenotypic differences between HPV types that are related to HPV species, akin to the increased importance of HPV18-related HPV types for causing adenocarcinoma of the cervix compared to squamous cell carcinoma.12 We suggest based on cumulative evidence that there may be unique HPV species-related biologic niches and survival strategies among the restricted HPV types that infect the lower female genital tract but more studies are needed to confirm these findings in more representative populations.
Acknowledgments
The authors acknowledge the enthusiastic work of the study staff in Guanacaste that made this effort possible, and the technical support of the Burk lab personnel. Dr. Rodriguez was supported by an appointment to the Senior Fellowship Program at the National Institutes of Health. The program is administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and the National Institutes of Health.
Supported by NCI contracts numbers NO1-CP-21081, NO1-CP-33061, NO1-CP-40542, NO1-CP-50535, NO1-CP-81023 with FUCODOCSA, Costa Rica, and CA78527 (R.D.B.) and by the Intramural Research Program of the NIH, NCI.
Footnotes
None of the authors has commercial or other associations that might pose a conflict of interest. NCI employees are under strict oversight by the NIH ethics office regarding any conflict of interest.
References
- 1.Castle PE, Schiffman M, Bratti MC, et al. A population-based study of vaginal human papillomavirus infection in hysterectomized women. J Infect Dis. 2004;190:458–467. doi: 10.1086/421916. [DOI] [PubMed] [Google Scholar]
- 2.Castle PE, Schiffman M, Glass AG, et al. Human papillomavirus prevalence in women who have and have not undergone hysterectomies. J Infect Dis. 2006;194:1702–1705. doi: 10.1086/509511. [DOI] [PubMed] [Google Scholar]
- 3.Winer RL, Lee SK, Hughes JP, et al. Genital human papillomavirus infection: Incidence and risk factors in a cohort of female university students. Am J Epidemiol. 2003;157:218–226. doi: 10.1093/aje/kwf180. [DOI] [PubMed] [Google Scholar]
- 4.Parkin DM, Bray F. Chapter 2: The burden of HPV-related cancers. Vaccine. 2006;24(suppl 3):S11–S25. doi: 10.1016/j.vaccine.2006.05.111. [DOI] [PubMed] [Google Scholar]
- 5.Gravitt PE, Lacey JV, Jr, Brinton LA, et al. Evaluation of self-collected cervicovaginal cell samples for human papillomavirus testing by polymerase chain reaction. Cancer Epidemiol Biomarkers Prev. 2001;10:95–100. [PubMed] [Google Scholar]
- 6.Bratti MC, Rodriguez AC, Schiffman M, et al. Description of a seven-year prospective study of human papillomavirus infection and cervical neoplasia among 10000 women in Guanacaste, Costa Rica. Rev Panam Salud Publica. 2004;15:75–89. doi: 10.1590/s1020-49892004000200002. [DOI] [PubMed] [Google Scholar]
- 7.Cogliano V, Baan R, Straif K, et al. Carcinogenicity of human papillomaviruses. Lancet Oncol. 2005;6:204. doi: 10.1016/s1470-2045(05)70086-3. [DOI] [PubMed] [Google Scholar]
- 8.Castle PE, Schiffman M, Gravitt PE, et al. Comparisons of HPV DNA detection by MY09/11 PCR methods. J Med Virol. 2002;68:417–423. doi: 10.1002/jmv.10220. [DOI] [PubMed] [Google Scholar]
- 9.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8:551–561. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 10.Castle PE, Jeronimo J, Schiffman M, et al. Age-related changes of the cervix influence human papillomavirus type distribution. Cancer Res. 2006;66:1218–1224. doi: 10.1158/0008-5472.CAN-05-3066. [DOI] [PubMed] [Google Scholar]
- 11.Kreimer AR, Katki H, Schiffman M, et al. Viral determinants of human papillomavirus persistence following loop electrical excision procedure treatment for cervical intraepithelial neoplasia grade 2 or 3. Cancer Epidemiol Biomark Prev. 2007;16:11–16. doi: 10.1158/1055-9965.EPI-06-0710. [DOI] [PubMed] [Google Scholar]
- 12.Kovacic MB, Castle PE, Herrero R, et al. Relationships of human papillomavirus type, qualitative viral load, and age with cytologic abnormality. Cancer Res. 2006;66:10112–10119. doi: 10.1158/0008-5472.CAN-06-1812. [DOI] [PubMed] [Google Scholar]
- 13.Schiffman M, Herrero R, Desalle R, et al. The carcinogenicity of human papillomavirus types reflects viral evolution. Virology. 2005;337:76–84. doi: 10.1016/j.virol.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Herrero R, Castle PE, Schiffman M, et al. Epidemiologic profile of type-specific human papillomavirus infection and cervical neoplasia in Guanacaste, Costa Rica. J Infect Dis. 2005;191:1796–1807. doi: 10.1086/428850. [DOI] [PubMed] [Google Scholar]
- 15.Jones HE, Allan BR, van de Wijgert JH, et al. Agreement between self- and clinician-collected specimen results for detection and typing of high-risk human papillomavirus in specimens from women in Gugulethu, South Africa. J Clin Microbiol. 2007;45:1679–1683. doi: 10.1128/JCM.02369-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Villiers EM, Fauquet C, Broker TR, et al. Classification of papillomaviruses. Virology. 2004;20:324, 17–27. doi: 10.1016/j.virol.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 17.Castellsague X, Diaz M, de Sanjose S, et al. Worldwide human papillomavirus etiology of cervical adenocarcinoma and its cofactors: Implications for screening and prevention. J Natl Cancer Inst. 2006;98:303–315. doi: 10.1093/jnci/djj067. [DOI] [PubMed] [Google Scholar]