Summary
The role of the immune synapse, and in particular the role of the central region of the synapse (the central supramolecular activation cluster or cSMAC), is controversial. One model suggests that the sole function of the cSMAC is to downregulate receptors and turn off signaling and that TCR signaling occurs only in the pSMAC. A second model suggests that the role of the cSMAC depends on antigen quality and can both enhance signaling and receptor downregulation. Here, we provide evidence that while at early time points signaling occurs mainly outside the cSMAC, at later time points signaling does occur in the cSMAC. Additionally, we find that cSMAC formation can increase the stimulatory potency of weak agonists for the TCR. Combined with previous studies showing that cSMAC formation decreases the stimulatory potency of strong agonists, our data support a model that posits that signaling and receptor degradation are linked in the cSMAC and that the balance between signaling and degradation in the synapse is determined by antigen quality.
Introduction
The immunological synapse refers to the contact surface between the T cell and the antigen presenting cell (Grakoui et al., 1999; Monks et al., 1998). In some cases, there is a reorganization of membrane proteins at the contact surface with proteins such as the TCR, CD2, CD4 and CD8 moving to a central zone known as the central supramolecular activation complex (cSMAC) (Monks et al., 1998). The cSMAC is surrounded by the pSMAC, defined by the aggregation of the adhesion molecule LFA-1 and its ligand ICAM-1 (Monks et al., 1998). The functional significance of this highly specialized molecular patterning at the T cell-antigen presenting cell (APC) contact site has been controversial (O’Keefe et al., 2004; Varma et al., 2006; Yokosuka et al., 2005)
Originally, it was proposed that the concentration of receptors and ligands in the contact area would function to enhance receptor engagement and boost signaling (Grakoui et al., 1999). This idea is supported by the findings of many groups that a variety of signaling molecules (PKC-theta, Lck, ZAP70, Bcl-10 and PIP3) are also recruited to the cSMAC (Costello et al., 2002; Freiberg et al., 2002; Huppa et al., 2003; Monks et al., 1998; Schaefer et al., 2004; Stinchcombe et al., 2001). However, the finding that the peak of signaling occurs before cSMAC formation and the relative decrease in phosphorylated tyrosine residues in the cSMAC (Lee et al., 2002) suggested that the function of the cSMAC is mainly to facilitate receptor degradation. A purely degradative role for the cSMAC received support from single molecule studies using lipid bilayers that showed that TCRs first engage their ligands in the pSMAC, forming small signaling clusters that then move to the center of the contact where there is a paucity of active signaling molecules (Varma et al., 2006; Yokosuka et al., 2005). This has led to some confusion in the field about the exact role of the cSMAC.
We have tried to shed light on how the cSMAC regulates signaling and degradation by using computational methods to help interpret experimental results (Cemerski et al., 2007; Lee et al., 2003). These studies, using a Monte-Carlo simulation that did not make any a priori assumption about where signaling or degradation occurred, suggested that the function of the cSMAC would vary depending on the quality of the peptide-MHC ligand (Cemerski et al., 2007). For strongly stimulatory pMHC ligands, the model suggested that TCR triggering was not enhanced by cSMAC formation and signaling would occur primarily outside of the cSMAC; under these conditions, the model suggested that concentrating molecules in the cSMAC would primarily enhance degradation and we confirmed this experimentally (Cemerski et al., 2007). In the case of weak peptides, however, the model suggested that concentrating receptors and key signaling molecules in the cSMAC could enhance signaling and thus, enhance the stimulatory potency of weak agonists. When we simulated a model in which the cSMAC functions only to degrade TCRs we found that if that was the case, cSMAC formation should always inhibit signaling, regardless of pMHC ligand quality, (Cemerski et al., 2007).
Here we tried to distinguish between the two models focusing on two issues, whether signaling occurs in the cSMAC and whether cSMAC formation could enhance or inhibit the response to weak ligands. We have also tried to reconcile different studies by varying the concentration of antigen tested and analyzing our data at different time points. Our studies suggest that the role of the cSMAC changes depending on antigen quality, on antigen dose and depending on at what time the analysis is performed. These studies suggest that the regulation of cSMAC formation may play an important role in the immune response in both health and disease.
RESULTS
Detection of tyrosine phosphorylation in the cSMAC using lower doses of peptide
It has been suggested that TCRs do not signal in the cSMAC (Campi et al., 2005; Varma et al., 2006; Yokosuka et al., 2005). This idea is based on the finding that the intensity of signaling detected by staining with antibodies to phospho-tyrosine showed lower staining in the cSMAC as compared to the pSMAC (Lee et al., 2003). In addition, single-molecule tracking studies confirm that signaling intermediates are more prevalent in the pSMAC and are less abundant in the cSMAC (Varma et al., 2006; Yokosuka et al., 2005). Nevertheless, while the absence of TCR signaling in the cSMAC could explain this data, another explanation is that the low level of tyrosine phosphorylation detected in the cSMAC is because of the rate of downregulation of receptors and signaling components (Cemerski et al., 2007; Lee et al., 2003). We reasoned, therefore, that if we could slow down TCR downregulation, we might reveal whether signaling can occur in the cSMAC.
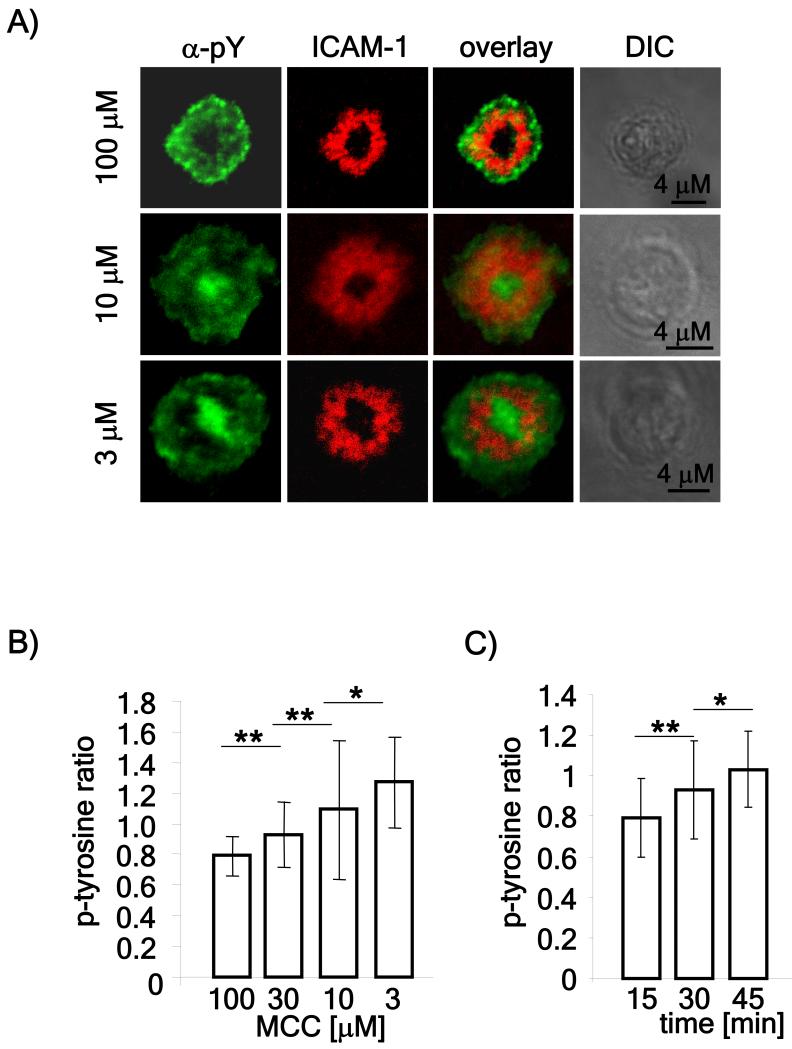
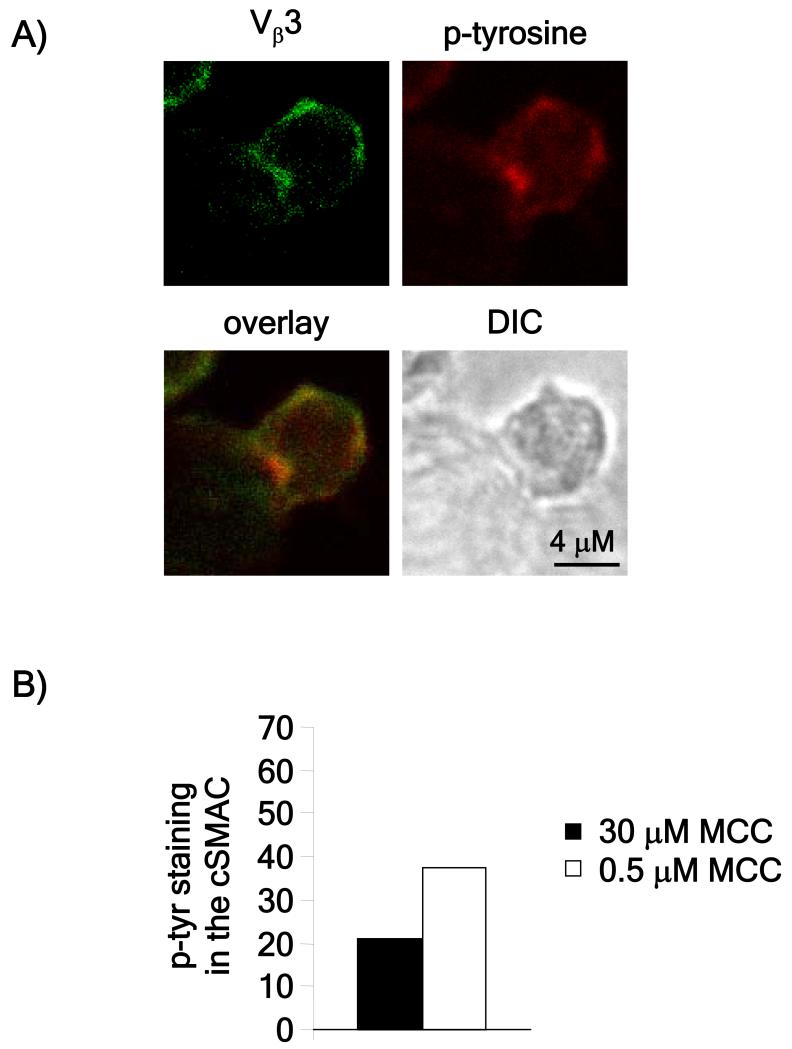
Since TCR downregulation is related to the dose of antigen (Hemmer et al., 1998; Itoh et al., 1999), we first tested whether signaling can be detected in the cSMAC under conditions where lower, but still stimulatory, doses of antigenic peptide are used to stimulate T cells from AND TCR transgenic mice that recognize a peptide from moth cytochrome c (Kaye et al., 1989). Previous studies showing low tyrosine phosphorylation in the cSMAC used planar lipid bilayers pulsed with 100 μM MCC (Lee et al., 2003), so this dose was used as the starting concentration, with decreasing amounts tested. Cells were flowed onto the lipid bilayers and after 60 minutes, fixed, permeabilized and stained for tyrosine phosphorylation. For each peptide concentration, tyrosine phosphorylation at the cSMAC was scored and presented as the ratio between phosphorylation in the cSMAC and the pSMAC. Consistent with previous (Lee et al., 2003), T cells stimulated on lipid bilayers loaded with 100 μM peptide showed less phosphotyrosine staining in the cSMAC, as compared to the pSMAC (Figure 1A and B). As the peptide dose was lowered, the ratio of phosphotyrosine staining between the cSMAC and the pSMAC gradually increased. At the 3 μM peptide dose, there was significantly more phosphotyrosine staining in the cSMAC as compared to the pSMAC (Figure 1A and B). We confirmed this data obtained with lipid bilayers by also stimulating T cells conjugated with peptide-pulsed APCs. We could easily detect staining for phosphorylated tyrosine in cSMACs and this appeared to be related to antigen dose (Figure 2).
Figure 1. Detection of tyrosine phosphorylation in the cSMAC using low doses of peptide.
A) Naïve CD4 T cells were isolated from spleens of AND mice, stimulated with MCC loaded, irradiated B10.BR splenocytes for 5 days and used on day 6. The rested AND T cells were flowed over lipid bilayers loaded with the indicated doses of MCC for one hour after which the bilayers were fixed, permeabilized and stained using an antibody to phosphotyrosine. The images are representative of over 50 cells obtained in three independent experiments. B) Phosphotyrosine staining in the cSMAC and pSMAC of more than 50 cells obtained in three independent experiments was measured using ImageJ software and represented as the ratio between phosphotyrosine staining in the cSMAC compared to the pSMAC. C) T cells were allowed to form mature synapses for 15, 30 or 45 minutes on lipid bilayers loaded with 3 μM MCC. The bilayers were stained and quantitated as described above. P values (* <0.05; ** < 0.01) were obtained using the Student’s t test.
Figure 2. Detection of tyrosine phosphorylation in T cell-APC conjugates.
A) Rested AND T cells were stimulated with CH27 cells loaded with the indicated amounts of peptide for one hour. Conjugates were then fixed, permeabilized and stained using antibodies to Vβ3 and phosphotyrosine. The images are representative of over 20 conjugates obtained in two independent experiments. B) Phosphotyrosine staining in the cSMAC was measured using ImageJ software and represented as the percentage of cells in which phospho-tyrosine staining in the cSMAC was at least 1.5 fold higher compared to the pSMAC (the area of the contact site excluding the cSMAC). Conjugates in which Vβ3 accumulation in the central third of the contact site was at least 1.5 times higher than at the rest of the contact site were considered to have cSMACs.
Since the 60 minute time point we chose, is much later than time points used by others (Campi et al., 2005; Yokosuka et al., 2005) we analyzed the ratio of phosphotyrosine between the cSMAC and pSMAC, after 15, 30 and 45 minutes upon stimulation. As shown in figure 1C, while the ratio of phosphotyrosine staining favored the pSMAC at early time points, over time, staining in the cSMAC increased.
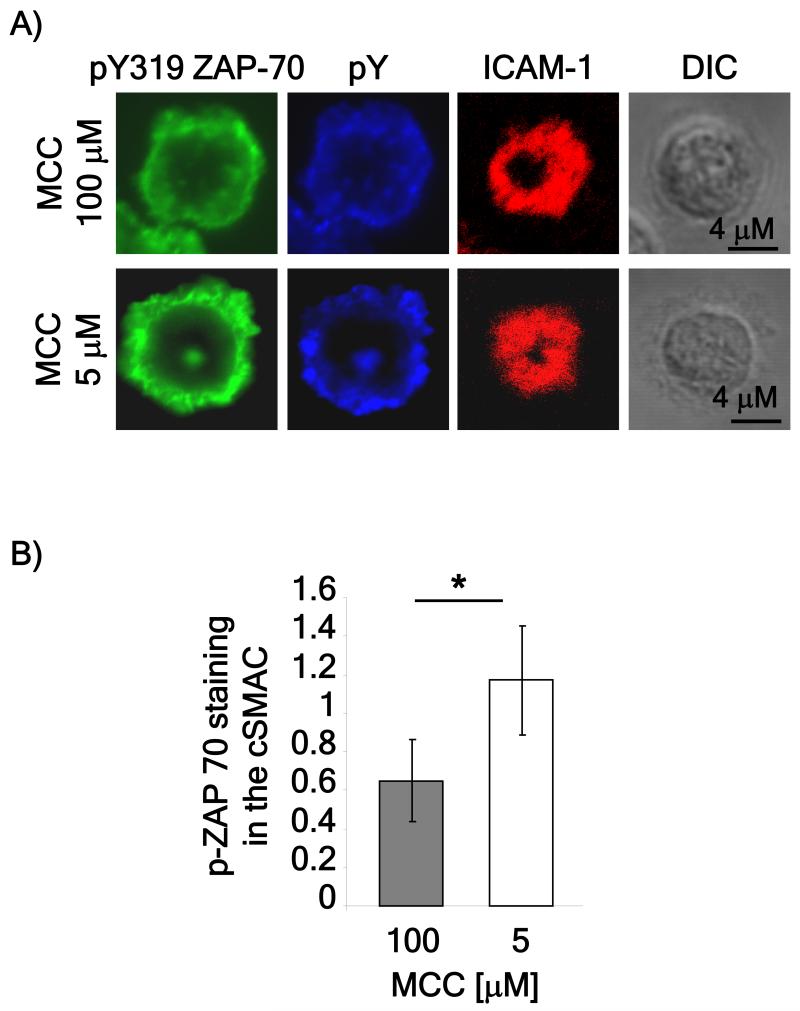
As tyrosine phosphorylation is potentially mediated by receptors other than the TCR, we validated our results using an antibody specific to phosphorylated ZAP-70, a signaling intermediate downstream of the TCR. Similar to our results obtained using the phosphotyrosine antibody, at low peptide doses, staining with an antibody to phosphorylated ZAP-70 revealed strong ZAP-70 phosphorylation in the cSMAC (Figure 3). We confirmed that this lower dose of peptide was still able to stimulate a strong proliferative response. In addition, calcium measurements of cells attached to the lipid bilayers showed that both peptide doses were able to stimulate similar levels of calcium mobilization. Interestingly, we did note that the calcium profile of cells stimulated with the lower peptide dose displayed a lower initial calcium spike and a more oscillatory nature of the sustained phase of calcium mobilization as compare to cells stimulated with a higher dose of peptide (figure S1).
Figure 3. Phosphorylated ZAP70 is detected in the cSMAC.
A) Naïve CD4 T cells were isolated from spleens of AND mice and prepared as described in Figure 2 except that anti-phospho-319 ZAP-70 antibodies were used. The images are representative of over 50 cells obtained in three independent experiments. B) The localization of anti-phospho-319 ZAP-70 staining of the bilayers in A was measured using ImageJ software and represented as the ratio between phospho-319 ZAP-70 staining in the cSMAC compared to the pSMAC. P value (* < 0.01) was obtained using the Student’s t test.
Pharmacological inhibition of TCR internalization enhances phosphotyrosine staining in the cSMAC
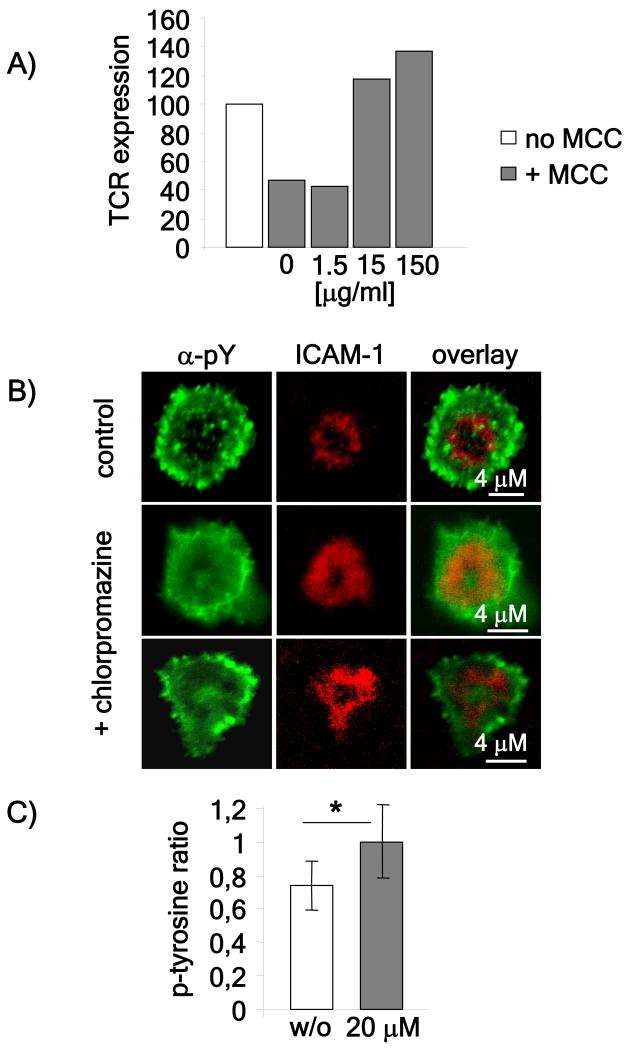
The results above are consistent with our previous study which suggested that the inability to detect signaling intermediates in the cSMAC might be due to rapid internalization and degradation of the TCR (Lee et al., 2003). Therefore, we wanted to test whether pharmacological inhibition of TCR internalization and downregulation would enhance phosphotyrosine staining in the cSMAC. We first confirmed that chlorpromazine, an inhibitor of clathrin-mediated internalization (Wang et al., 1993), was capable of inhibiting TCR downregulation in AND T cells conjugated with MCC pulsed-CH27 cells (figure 4A). We then tested the effects of chlorpromazine treatment on the pattern of phosphotyrosine staining of AND T cells incubated on bilayers pulsed with 100 μM MCC peptide. Cells were pre-treated for 20 minutes with chlorpromazine, then incubated on peptide-loaded lipid bilayers and stained for phosphotyrosine 60 minutes later. While cSMACs of untreated cells showed very little phosphotyrosine content, cells treated with chlorpromazine showed a significant increase in the phosphotyrosine signal in the cSMAC (Figure 4B and C). This is consistent with our hypothesis that, for strong ligands, enhanced TCR downregulation in the cSMAC limits the ability to detect phosphotyrosine there.
Figure 4. Pharmacological inhibition of TCR downregulation enhances phospho-tyrosine detection in the cSMAC.
A) Rested AND T cells were pre-incubated with the indicated doses of chlorpromazine for 20 minutes and stimulated with un-pulsed or 10 μM MCC-pulsed CH27 cells. To measure TCR downregulation, cell conjugates were disrupted using trypsin-EDTA, stained with anti-Vβ3 and analyzed by flow cytometry for surface TCR expression. TCR expression is shown as the percentage of TCR expression as compared to T cells stimulated with un-pulsed CH27 cells. The data is representative of three independent experiments. B) Rested untreated or chlorpromazine treated (20 μg/ml) AND T cells were flowed on lipid bilayers loaded with 100 μM MCC. After one hour the bilayers were fixed, permeabilized and stained using an antibody to phospho - tyrosine. The images shown are representative of over 50 cells analyzed in three independent experiments. C) Phosphotyrosine staining in the cSMAC and pSMAC of more than 50 cells obtained in three independent experiments was measured using ImageJ software and represented as the ratio between phosphotyrosine staining in the cSMAC as compared to the pSMAC. P values (* < 0.001) were obtained using the Student’s t test.
Accumulation of fully phosphorylated TCR-ζ at the cSMAC
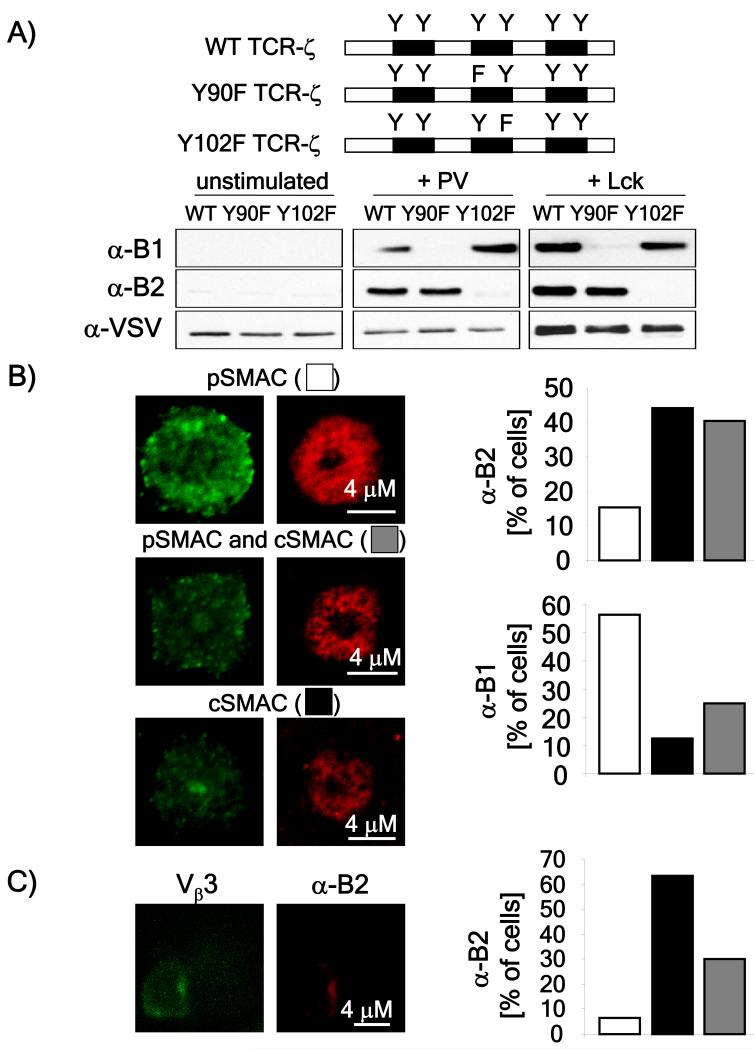
If concentrating TCRs in the cSMAC functions to both enhance signaling and subsequent downregulation, we reasoned that the intensity of tyrosine phosphorylated TCRs should be higher in the cSMAC as compared to the pSMAC. The TCR-ζ chain has six different tyrosines and it has been reported that the efficiency of ζ chain phosphorylation is related to the strength of receptor engagement with weak stimuli resulting in partially phosphorylated TCR-ζ chains and strong stimuli resulting in more fully phosphorylated TCR ζ chains (Madrenas et al., 1995; Sloan-Lancaster et al., 1994). We took advantage of previous data showing that the six tyrosines are phosphorylated in a specific order (Kersh et al., 1998a), to test the hypothesis that the cSMAC would function to enhance the efficiency of ζ chain phosphorylation. Specifically, it was shown that the second tyrosine of the second ITAM (the B2 tyrosine) is the last tyrosine to be phosphorylated when the fully phosphorylated (p23) TCR-ζ chain is generated (Kersh et al., 1998a) while the first tyrosine of the second ITAM (the B1 tyrosine) is phosphorylated in the partially phosphorylated (p21) TCR-ζ chain. We reasoned that the difference in the localization of partially and fully phosphrylated TCR-ζ chains in the immunological synapse could be used to determine where the strongest area of signaling occurs.
Phospho-specific antibodies were raised in rabbits using peptides containing the phosporylated B1 and B2 tyrosines, as previously described (Kersh et al., 1998a). To confirm the specificity of these antibodies, we tested whether they could detect tyrosine phosphorylation of a chimeric WT or a mutated TCR-ζ chain with the B1 and B2 tyrosines changed to phenylalanine expressed in HeLa cells (Figure 5A) (Timson Gauen et al., 1992). Treatment of cells with pervanadate (a tyrosine phosphatase inhibitor) or co-transfection with Lck, demonstrated that the antibodies detected phosphorylated ζ only if the specific B1 or B2, tyrosine was intact. (Figure 5A). This confirmed the specificity of the antibodies.
Figure 5. Localization of fully phosphorylated TCR-ζ at the cSMAC.
A) Schematic diagram showing the nomenclature of the six tyrosines in the ζ chain and the position of the B1 and B2 tyrosines that were mutated. HeLa cells, transfected with the indicated ζ chimeric constructs were lysed without stimulation (left panel) or after a 10 minute treatment with pervanadate (middle panel). In the right panel, cells lysates from cells co-expressing the indicated chimeric ζ construct with Lck. Lysates were resolved by SDS-PAGE transferred to nitrocellulose membranes and immunoblotted with the α-B1 and α-B2 rabbit antisera. B) Rested AND T cells were allowed to adhere for 40-60 minutes on planar lipid bilayers loaded with 100 μM of WT MCC peptide. Cells were fixed, permeabilized and stained with α-B1 and α-B2 antisera. Cells were scored and classified into three groups (predominant staining of the cSMAC, pSMAC or relatively even staining between cSMAC and pSMAC). Representative images of pSMAC-dominant; cSMAC-dominant; or both pSMAC and cSMAC dominant staining are shown. Images were quantitated using ImageJ software, in over 20 images obtained in at least two independent experiments, and represented as the percentage of cells showing one of the three patterns of staining” C) AND T cells were stimulated with CH27 cells loaded with the 20 μM MCC peptide for one hour then fixed, permeabilized and stained using anti-Vβ3 and the α-B2 antibody. The image shown is representative of over 40 cells obtained in three independent experiments. The localization on anti-B2 staining was quantified in the cells that formed cSMACs (based on anti-Vβ3 staining) and expressed as the percentage of cells having a pSMAC-enriched (empty bars), cSMAC enriched (black bars) or equally pSMAC and cSMAC enriched (grey bars) B2 staining.
The location of TCR-ζ chains phosphorylated on B1 and B2 was assessed by immunofluorescence staining in AND T cells incubated on planar lipid bilayers or with peptide-pulsed APCs loaded with 100 μM or 20 μM MCC. We scored synapses into three categories depending on whether staining was mainly in the cSMAC, the pSMAC or if it was evenly distributed between the two. In the majority of synapses, the B1 antibody stained mainly the pSMAC or was evenly distributed between the two (Figure 5B). In contrast, anti-B2 reactivity was found to be preferentially localized to the cSMAC in the synapses formed both between lipid bilayers and APCs (Figure 5B and 5C). The differential staining pattern between the two antibodies supports the idea that the level of phosphorylated TCR-ζ is different in the cSMAC versus the pSMAC and is consistent with the idea that the highest intensity signaling occurs in the cSMAC.
Accumulation of PIP3 at the cSMAC
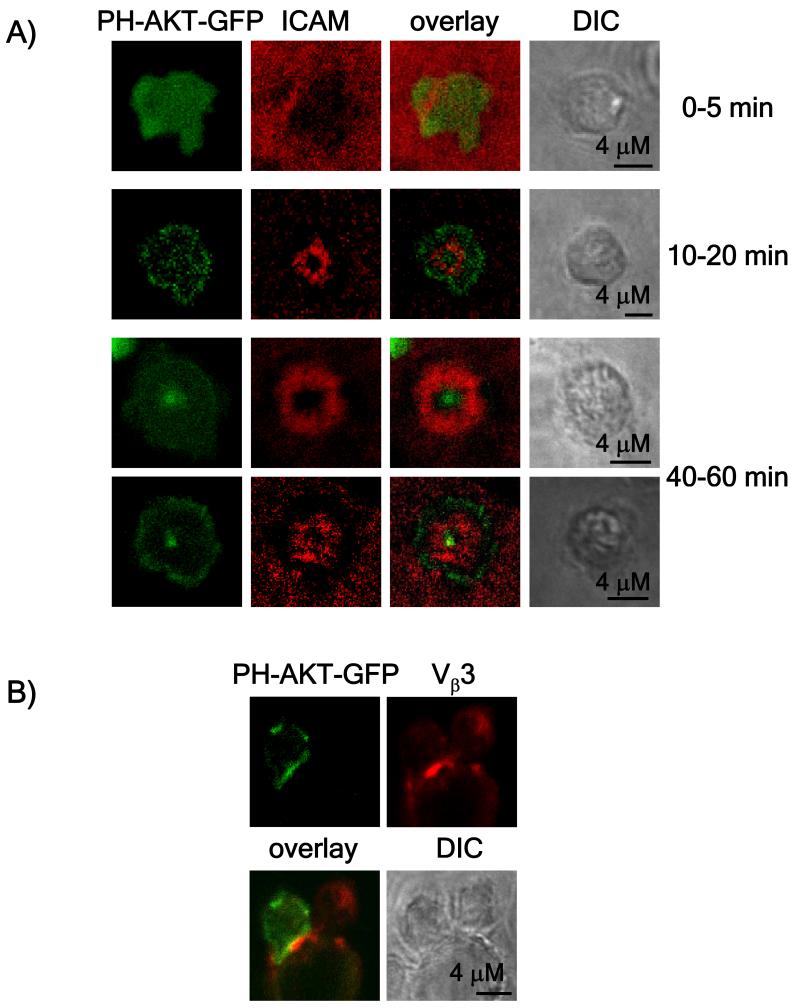
The production of the lipid PIP3 has been shown to be dependent on TCR signaling and, because it has a short half-life, should closely correlate with the location of active TCR signaling (Costello et al., 2002; Harriague and Bismuth, 2002; Huppa et al., 2003). We reasoned that if the cSMAC was an area of active signal transduction, we might expect it to also be a location where PIP3 is produced. To assess where in the immunological synapse PIP3 was being produced, a retrovirus expressing the PH domain of AKT fused to GFP (Giurisato et al., 2007) was used to follow the production of PIP3 in the synapse. At early time points, little to no enrichment of this marker was seen in the cSMAC (Figure 6A). However, after one hour, a clear enrichment of the probe in the cSMACs was seen (Figure 6A and 6B and Figure S2) a pattern consistent with previously published studies (Costello et al., 2002; Huppa et al., 2003). Because of the short half-life of PIP3 (Costello et al., 2002), this suggests that the major site of production, at later time points, is in the cSMAC.
Figure 6. PI3 kinase activity is enriched at the cSMAC.
CD4 T cells were purified from spleens from AND TCR transgenic mice and retrovirally transduced with the PH-AKT-GFP biosensor (Giurisato et al., 2007). GFP-positive cells were sorted and then incubated for one hour on lipid bilayers loaded with 100 μM MCC peptide (panel A) or with CH27 cells loaded with 20 μM MCC (panel B). Images shown here are representative of over 20 images obtained in at least two independent experiments.
Enforced cSMAC formation increases the stimulatory capacity of weak peptides
Our computational model of cSMAC function predicted that cSMAC formation has different effects depending on whether the peptide quality is strong versus weak (Cemerski et al., 2007). Previously, we took advantage of our finding that engagement of the receptor NKG2D can induce cSMAC formation regardless of whether antigen is present or not (Cemerski et al., 2007; Markiewicz et al., 2005). By transducing CD4+ AND T cells with NKG2D and APCs with a ligand for NKG2D, the response of T cells where cSMAC formation was strongly enhanced could be assessed. Using this system, we showed previously that NKG2D mediated cSMAC formation inhibits signaling mediated by strong agonists (Cemerski et al., 2007). We were, however, unable to assess the response to weak peptides in that study, because weak peptides are not known for the AND TCR. We therefore, turned to T cells from the 5C.C7 (Seder et al., 1992) and the 3L2 (Kersh et al., 1998b) transgenic mice because well characterized weak agonist peptides are defined for both of these TCRs (Kersh and Allen, 1996; Kersh et al., 1998c; Lyons et al., 1996; Reay et al., 1994; Wulfing et al., 1997).
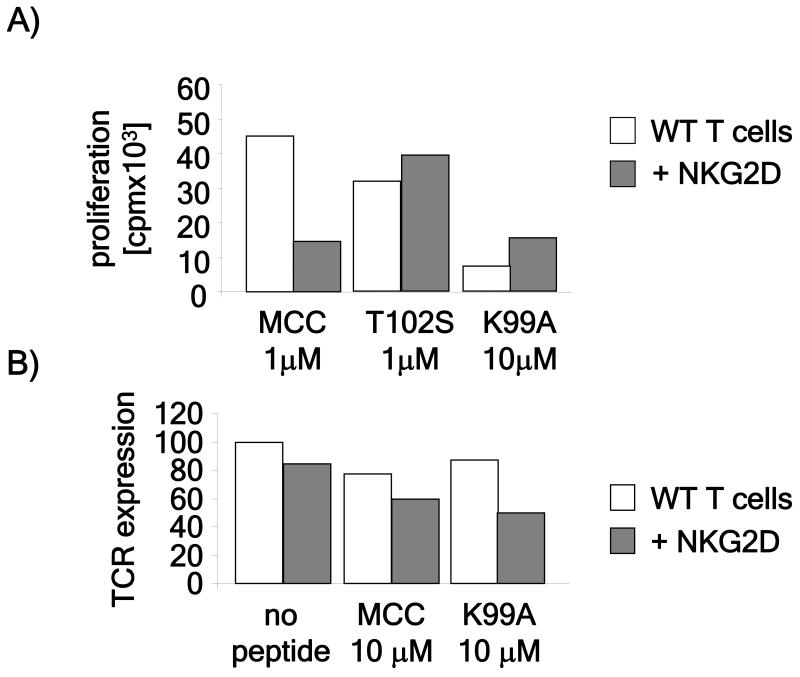
5C.C7 and 3L2 T cells were retrovirally transduced with cDNAs for NKG2D and a YFP-tagged form of the signaling adapter, DAP10 (Giurisato et al., 2007) and purified by cell sorting. Expression and co-assembly of NKG2D with DAP10 is required for it’s plasma membrane expression (Wu et al., 1999). The proliferative response of the transduced T cells to the wild-type as well as to a range of weak peptides was then tested. Consistent with our previous findings that enforced cSMAC formation inhibits strong peptides (Cemerski et al., 2007), the proliferative response of 5C.C7 T cells to the wild-type peptide was decreased in the presence of NKG2D (Figure 7A). Importantly, however, the proliferative response of transduced 5C.C7 T cells to weaker peptides was increased in the presence of NKG2D engagement and increased cSMAC formation (Figure 7A). Similar results were obtained using 3L2 T cells (Figure S3)
Figure 7. cSMAC formation increases the stimulatory potency of weak peptides.
A) NKG2D-induced cSMAC formation enhances the recognition of weak peptides. 5CC7 CD4+ T cells were retrovirally transduced with NKG2D and DAP10-YFP. Sorted NKG2D/DAP10 positive as well as negative cells were stimulated with CH27 cells transduced with Rae1ε loaded with the indicated peptides. Proliferative responses were assessed by [3H]-thymidine incorporation during the last 16 hours of a 72 hour stimulation and expressed in cpm The results shown are representative of three experiments. B) Increased NKG2D mediated TCR downregulation. WT and NKG2D/DAP10 transduced AND T cells were stimulated for 3 hours with Rae-1ε-expressing CH27 cells unpulsed or pulsed with 20 μM of the indicated peptides. After 3 hours, cells were stained for Vβ3 expression and TCR expression levels analyzed by flow cytometry. The result shown is a representative of two independent experiments.
We confirmed that NKG2D engagement enhanced TCR engagement and signaling by measuring TCR downregulation. The efficiency of TCR downregulation is thought to be directly related to agonist quality with better agonist quality resulting in more TCR downregulation (Hemmer et al., 1998; Itoh et al., 1999). TCR expression levels were measured by flow cytometry before and after stimulation with WT and K99A MCC peptides. NKG2D expression resulted in increased TCR downregulation in both WT and K99A MCC-stimulated AND T cells (Fig 7B). We also compared calcium mobilization of wild-type and NKG2D/DAP10-expressing T cells after stimulation with WT and weaker peptides. We found that the presence of NKG2D depressed the initial calcium spike but resulted in a more stable sustained phase of calcium mobilization (Fig S4 and S5). Thus, the presence of NKG2D does not appear to lead to a generalized enhancement of activation parameters, but rather, results in qualitative changes in signaling that effect the proliferative profile.
Discussion
The function of the cSMAC in the immunological synapse has been controversial (Cemerski and Shaw, 2006; Kupfer, 2006; Lin et al., 2005). While it was originally proposed that its purpose was to enhance signaling by the TCR (Grakoui et al., 1999), the finding that the cSMAC forms after the peak of measurable tyrosine phosphorylation (Lee et al., 2002) and the distinctly lower levels of tyrosine phosphorylation detected in the cSMAC (Lee et al., 2003) suggested that the cSMAC does not function to enhance TCR signaling. Supporting this idea is the finding that CD2AP deficient cells that cannot form cSMACs have hyperproliferative responses (Lee et al., 2003). In addition, studies using single molecule tracking showed that TCRs initiate signaling by forming microclusters in the pSMAC (Varma et al., 2006; Yokosuka et al., 2005). Thus, the current model is that the cSMAC has no signaling function, and most likely represents a structure that forms to enhance the internalization and degradation of phosphorylated proteins and receptors (Varma et al., 2006; Yokosuka et al., 2005).
Our previous work, however, suggested that a purely degradative role for the cSMAC might be dependent upon the quality of the peptide (Cemerski et al., 2007). Mathematical modeling suggested that when peptide quality is high (has a long half-life), receptors can engage and become phosphorylated without requiring clustering or requiring concentration in the cSMAC. However, because downregulation of receptors requires ubiquitination and because receptor ubiquitination involves the recruitment of enzymes from the cytosol to the membrane (Jang and Gu, 2003) (Is this the right reference), concentrating phosphorylated receptors in the cSMAC will enhance both enzyme recruitment and ubiquitination. The model predicted that in the case of weak peptides (short half-lives), receptor engagement and phosphorylation in the absence of the concentrating effect of cSMAC formation would be relatively inefficient and should be enhanced upon concentrating TCR and pMHC ligands in the cSMAC. Because these ligands are not strong enough to result in strong tyrosine phosphorylation of the receptor, the effect of cSMAC formation is less on receptor downregulation compared to its ability to enhance signaling. A compelling feature of this model is the ability to explain the effect of cSMAC formation on receptor degradation without invoking the assumption that the cSMAC is a specialized degradative structure.
The key distinguishing feature between a model where the cSMAC functions to only facilitate receptor degradation and a model where the cSMAC enhances signaling and degradation is whether formation of the cSMAC functions always inhibits signaling or whether the cSMAC inhibits or enhances signaling depending on whether the agonist is strong or weak, respectively (Cemerski et al., 2007). Inherent to the second model is the ability of signaling to occur in the cSMAC.
Using cells transduced with NKG2D and DAP10, we assessed the role of cSMAC formation on a range of weak peptides. Previously we found that engagement of NKG2D/DAP10 could stimulate the formation of immunological synapses with cSMACs independently of whether antigen was present or not (Markiewicz et al., 2005). We used this manipulation to show that enforced cSMAC formation could inhibit signaling by strong peptides in the AND TCR system (Cemerski et al., 2007). Here we extended these previous studies to show that expression of NKG2D on the 5C.C7 and 3L2 T cells resulted in enhanced recognition of weak peptides. The ability of NKG2D-induced enchancment of cSMAC formation to both inhibit signaling to strong peptides and enhance signaling to weak peptides supports the hypothesis that the cSMAC functions differently for peptides of different potencies. It also suggests that the effects of NKG2D are not related to a general effect of NKG2D signaling on T cell activation. We were surprised to find that the effect of NKG2D synapse formation on downstream pathways is not straightforward. We expected that downstream signals like the magnitude of calcium fluxes would reflect the enhanced or inhibited activation parameters but found instead that the changes induced by NKG2D mediated synapse formation were more complex with changes in the magnitude of the initial calcium flux as well as the slope of the sustained phase. Clearly, more work is needed to understand how synapse formation is related to both the magnitude of signaling and how it is sustained.
The observation that cSMAC formation enhances the stimulatory potency of weak ligands is predicated on the occurrence of signaling in the cSMAC. We tested this hypothesis directly. We found that T cell stimulation under conditions of reduced receptor internalization, achieved by using lower antigen dose as well as pharmacological inhibitors of internalization, resulted in enhanced detection of tyrosine phosphorylation in the cSMAC. More compelling was the data measuring PIP3 production in the synapse. Since PIP3 has a relatively short half-life in T cells, it is dependent on TCR engagement and is confined to the plasma membrane (Costello et al., 2002; Huppa et al., 2003). Therefore, the production of PIP3 can be used as a relatively good surrogate for TCR signaling. Our finding that PIP3 is largely confined to the cSMAC is consistent with the idea that at late time-points, TCR engagement in the cSMAC is largely responsible for PIP3 production. These findings also seem consistent with images published by others using the same AKT-PH domain probe (Costello et al., 2002; Huppa et al., 2003).
In addition, our finding that fully phosphorylated TCR ζ chains are preferentially found in the cSMAC also supports a model where the cSMAC enhances signaling. While it is well accepted that the six tyrosine’s in TCR-ζ are phosphorylated in a specific order, there has been some controversy about which tyrosine is phosphorylated last. This is important as a reagent recognizing this tyrosine would be a specific marker for fully phosphorylated TCR-ζ. Using phosphospecific antibodies, Kersh et al. (Kersh et al., 1998a) found that the B2 tyrosine was phosphorylated last while Van Oers et al (van Oers et al., 2000) found using mass spectrometry that it was the A2 tyrosine. Our results showing distinct staining of B1 versus B2 antibodies are consistent with the B2 being found mainly in fully phosphorylated TCR-ζ and support the findings of Kersh et al. We tested an A2 specific antibody defined by immunoblotting but it was unable to stain cells. Since the study by Van Oers et al. was performed using TCR-ζ purified from human thymus, it is possible that the difference is due to differences in developing T cells versus peripheral T cells or it could be due to differences between mouse and human T cells.
One important difference between our experiments and previous studies may be the late time-point that we used. In all of our experiments, we fixed cells after 60 minutes, a time point well after the cSMAC has formed (Lee et al., 2003). Indeed, even using the lower peptide dose, at earlier timepoints, we observed tyrosine phosphorylation predominantly in the pSMAC as reported by others (Mossman et al., 2005). As time passed we saw that gradually more and more signaling was detectable in the cSMAC (Figure 1C). While the formation of TCR microclusters in the periphery of the contact is likely in the early stages of synapse formation before the cSMAC has formed, how TCR signaling is maintained for hours after the cSMAC is not clear (Iezzi et al., 1998). Given the low abundance of antigen on APCs required to activate the T cell (Harding and Unanue, 1990; Irvine et al., 2002), it is difficult to imagine how enough agonist peptide could be present to continuously prime the formation of TCR microclusters in the pSMAC for the many hours that would be required if signaling could only occur in the pSMAC. A model that allows for signaling in the cSMAC can explain how signaling by a few antigenic peptides is sustained by positing that the few agonist peptides trapped in the cSMAC can repetitively trigger TCRs for hours (Valitutti et al., 1995). This model also suggests that concentrating endogenous pMHC complexes in the cSMAC could also contribute to the longevity of TCR engagement and signaling in combination with agonist peptides (Krogsgaard et al., 2005). Our in silico and in vitro studies suggest that the cSMAC is a versatile structure that can both enhance signaling and receptor degradation in a manner that depends upon antigen quality.
METHODS
Mice
AND TCR transgenic mice (B6;SJL-Tg(TcrAND)53Hed/J) and B10.BR mice (B10.BR-H2k H2-T18a/SgSnJ) were purchased from The Jackson Laboratory. 5C.C7 TCR transgenic mice (5C.C7 TCRtg RAG-2−/− H-2a) were obtained from Taconic Farms. 3L2 TCR transgenic mice were generated previously by Dr. Paul Allen (Kersh et al., 1998b). Mice were maintained under pathogen-free conditions in the Washington University animal facilities in accordance with institutional guidelines.
Peptides
Wild type and mutated moth cytochrome C (MCC) peptides 88-103 and hemoglobin peptides 65-74 were synthesized using standard F-Moc chemistry. The peptides were purified to homogeneity by reverse-phase HPLC, and their composition was confirmed by mass spectrometry and amino acid analysis (Washington University Mass Spectrometry Facility, St. Louis, MO).
Antiserum to phospho-TCR-ζ
Rabbit antisera were raised against the phosphotyrosine-containing peptides: MGGKQQRRRDPQEGV(PO4)YNALQKDKMAEA and NALQKDKMAEA(PO4)YSEIGTKGERRRGKGH, corresponding to the first and second tyrosines of the second ITAM motif of TCR-ζ (Biosource). The antisera was absorbed against the unphosphorylated peptide and then affinity purified using the phosphorylated peptide.
T cell purification
For purification of splenic T cells, splenocytes were depleted of CD8+, MHC class II-positive and immunoglobulin-positive cells by antibody-mediated nanoparticle negative selection (EasySep CD4+ negative selection cocktail, StemCell Technologies). The CD4+ T cells were stimulated for 4 days with irradiated B10.BR splenocytes and MCC peptide, rested for 2 days, and then used. For the experiments involving chlorpromazine the cells were incubated with 15 μg/ml of chlorpromazine for 20 minutes, washed and immediately used.
TCR downregulation
AND T cells were stimulated for 2 hours, at a 1:1 ratio, with peptide pulsed or unpulsed CH27 I-Ek cells. Upon stimulation, cells were lightly pelleted and treated with trypsin-EDTA to break cell conjugates, washed, and labeled with FITC-conjugated anti-CD4 and PE-conjugated anti-Vβ3 antibodies (BD PharMingen). Vβ3 surface expression was assessed by flow cytometry (FACScan, BD Biosciences) on CD4-positive gated cells. Data was analyzed by WinMDI 2.8 software (facs.scripps.edu/software.html) or CellQuest (BD PharMingen).
Transfection
The construct encoding Gζ a previously described ζ chimeric molecule (Timson Gauen et al., 1992), composed of the extracellular domain of the vesicular stomatitis virus glycoprotein (VSV G) and the transmembrane and cytoplasmic region of mouse TCR-ζ, was subjected to site-directed mutagenesis (Stratagene) to obtain mutated constructs of Gζ where the codons encoding Y90 and Y102 were changed to phenylalanine. HeLa cells were transfected with WT and mutant forms of Gζ using a transient expression system (Timson Gauen et al., 1992). Briefly, cells were infected with a recombinant vaccinia virus encoding a bacteriophage T7 RNA polymerase for 30 minutes, followed by CLONfectin (BD Biosciences Clontech)-mediated transfection with plasmids encoding the Gζ constructs.
Cell lysis, immunoprecipitation and western blotting
Transfected Hela cells were lysed for 10 min on ice in 1% NP-40 containing lysis buffer and cleared by centrifugation. Post-nuclear supernatants were resolved on SDS-PAGE under reducing conditions, transferred to nitrocellulose membranes and immunoblotted with the anti-B2 or anti-VSV antisera. Blots were visualized using ECL Western blotting kit (Pierce).
Retroviral transduction
The Plat E ecotropic packaging cell line (Morita et al., 2000) was transfected with a PH-AKT-GFP retroviral construct or a mixture of NKG2D and DAP10-YFP retroviral constructs. Purified CD4 T cells were stimulated with irradiated B10.BR splenocytes and the antigenic peptide. At 18 and 42 hours after stimulation, T cells were incubated for 4 hours with the viral supernatants and then centrifuged for 45 minutes in the presence of Lipofectamine™ 2000 (Invitrogen). On day 4 after stimulation, cells were sorted using a FACSVantage flow sorter (BD Biosciences) at the Washington University Pathology Cell Sorting Facility. For NKG2D/DAP10-YFP double transduction, cells were stained with a PE-labeled anti-NKG2D antibody and YFP-positive, PE-positive and negative cells were sorted and used for further experimentation.
T cell proliferation assays
NKG2D/DAP10 positive and negative CD4+ AND, 5C.C7 or 3L2 T cells were stimulated for 48 hours with mitomycin-treated CH27 cells expressing Rae-1ε and various amounts of peptides, pulsed with 1 μCi of [3H] thymidine/well, harvested 16 h later, and scintillation counted.
IS and cSMAC formation
Sorted retrovirally transduced AND T cells were incubated with peptide loaded or unloaded CH27 I-Ek cells for 30 minutes. Cells were then fixed, mounted on coverslips, and confocal images taken using a Zeiss LSM510 confocal microscope. Unprocessed images were scored for cSMAC formation and accumulation of tyrosine phosphorylated proteins at the cSMAC using ImageJ software (NIH, Bethesda, Maryland, http://rsb.info.nih.gov/ij/, 1997-2006). Conjugates were scored as cSMAC positive or phosphotyrosine at the cSMAC-positive if the central third of the contact area showed a 1.5 times enrichment of Vβ3 (for cSMAC scoring) or phosphotyrosine (scored in cSMAC positive conjugates) compared to the rest of the contact site.
Lipid bilayer preparation
Planar lipid bilayers were made by mixing Cy5-labbeled ICAM1-containing liposomes (generously provided by Dr Takashi Saito, RIKEN, Japan) and I-Ek containing liposomes, in a 1:1 ratio, on clean glass coverslips in a parallel plate flow cell (Bioptechs). I-Ek molecules were loaded overnight by flowing the indicated amount of WT MCC peptide over the bilayer. Rested CD4+ AND T cells were injected into the warmed (37°C) flow cells in HBS with 1% human serum albumin (Alpha Therapeutic). After 60 minutes, cells were fixed by flowing 2% paraformaldehyde over the bilayer, permeabilized using 0.05% Triton X-100, incubated in PBS containing 4% BSA and then stained with the indicated antibodies. Cells were imaged using a Zeiss LSM510 confocal system. Unprocessed images were scored, using ImageJ software (NIH, Bethesda, Maryland, http://rsb.info.nih.gov/ij/, 1997-2006).
Supplementary Material
Acknowledgments
We thank M. Dustin and T. Saito for fruitful discussions. This research is supported by the NIH (grants AI034094, AI057966, AI071195)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Campi G, Varma R, Dustin ML. Actin and agonist MHC-peptide complex-dependent T cell receptor microclusters as scaffolds for signaling. J Exp Med. 2005;202:1031–1036. doi: 10.1084/jem.20051182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cemerski S, Das J, Locasale J, Arnold P, Giurisato E, Markiewicz MA, Fremont D, Allen PM, Chakraborty AK, Shaw AS. The stimulatory potency of T cell antigens is influenced by the formation of the immunological synapse. Immunity. 2007;26:345–355. doi: 10.1016/j.immuni.2007.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cemerski S, Shaw A. Immune synapses in T-cell activation. Curr Opin Immunol. 2006;18:298–304. doi: 10.1016/j.coi.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Costello PS, Gallagher M, Cantrell DA. Sustained and dynamic inositol lipid metabolism inside and outside the immunological synapse. Nat Immunol. 2002;3:1082–1089. doi: 10.1038/ni848. [DOI] [PubMed] [Google Scholar]
- Freiberg BA, Kupfer H, Maslanik W, Delli J, Kappler J, Zaller DM, Kupfer A. Staging and resetting T cell activation in SMACs. Nat Immunol. 2002;3:911–917. doi: 10.1038/ni836. [DOI] [PubMed] [Google Scholar]
- Giurisato E, Cella M, Takai T, Kurosaki T, Feng Y, Longmore GD, Colonna M, Shaw AS. PI3-kinase activation is required to form the NKG2D immunological synapse. Mol Cell Biol. 2007 doi: 10.1128/MCB.01477-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–227. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- Harding CV, Unanue ER. Quantitation of antigen-presenting cell MHC class II/peptide complexes necessary for T-cell stimulation. Nature. 1990;346:574–576. doi: 10.1038/346574a0. [DOI] [PubMed] [Google Scholar]
- Harriague J, Bismuth G. Imaging antigen-induced PI3K activation in T cells. Nat Immunol. 2002;3:1090–1096. doi: 10.1038/ni847. [DOI] [PubMed] [Google Scholar]
- Hemmer B, Stefanova I, Vergelli M, Germain RN, Martin R. Relationships among TCR ligand potency, thresholds for effector function elicitation, and the quality of early signaling events in human T cells. J Immunol. 1998;160:5807–5814. [PubMed] [Google Scholar]
- Huppa JB, Gleimer M, Sumen C, Davis MM. Continuous T cell receptor signaling required for synapse maintenance and full effector potential. Nat Immunol. 2003;4:749–755. doi: 10.1038/ni951. [DOI] [PubMed] [Google Scholar]
- Iezzi G, Karjalainen K, Lanzavecchia A. The duration of antigenic stimulation determines the fate of naive and effector T cells. Immunity. 1998;8:89–95. doi: 10.1016/s1074-7613(00)80461-6. [DOI] [PubMed] [Google Scholar]
- Irvine DJ, Purbhoo MA, Krogsgaard M, Davis MM. Direct observation of ligand recognition by T cells. Nature. 2002;419:845–849. doi: 10.1038/nature01076. [DOI] [PubMed] [Google Scholar]
- Itoh Y, Hemmer B, Martin R, Germain RN. Serial TCR engagement and down-modulation by peptide:MHC molecule ligands: relationship to the quality of individual TCR signaling events. J Immunol. 1999;162:2073–2080. [PubMed] [Google Scholar]
- Jang IK, Gu H. Negative regulation of TCR signaling and T-cell activation by selective protein degradation. Curr Opin Immunol. 2003;15:315–320. doi: 10.1016/s0952-7915(03)00048-7. [DOI] [PubMed] [Google Scholar]
- Kaye J, Hsu ML, Sauron ME, Jameson SC, Gascoigne NR, Hedrick SM. Selective development of CD4+ T cells in transgenic mice expressing a class II MHC-restricted antigen receptor. Nature. 1989;341:746–749. doi: 10.1038/341746a0. [DOI] [PubMed] [Google Scholar]
- Kersh EN, Shaw AS, Allen PM. Fidelity of T cell activation through multistep T cell receptor zeta phosphorylation. Science. 1998a;281:572–575. doi: 10.1126/science.281.5376.572. [DOI] [PubMed] [Google Scholar]
- Kersh GJ, Allen PM. Structural basis for T cell recognition of altered peptide ligands: a single T cell receptor can productively recognize a large continuum of related ligands. J Exp Med. 1996;184:1259–1268. doi: 10.1084/jem.184.4.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersh GJ, Donermeyer DL, Frederick KE, White JM, Hsu BL, Allen PM. TCR transgenic mice in which usage of transgenic alpha- and beta-chains is highly dependent on the level of selecting ligand. J Immunol. 1998b;161:585–593. [PubMed] [Google Scholar]
- Kersh GJ, Kersh EN, Fremont DH, Allen PM. High- and low-potency ligands with similar affinities for the TCR: the importance of kinetics in TCR signaling. Immunity. 1998c;9:817–826. doi: 10.1016/s1074-7613(00)80647-0. [DOI] [PubMed] [Google Scholar]
- Krogsgaard M, Li QJ, Sumen C, Huppa JB, Huse M, Davis MM. Agonist/endogenous peptide-MHC heterodimers drive T cell activation and sensitivity. Nature. 2005;434:238–243. doi: 10.1038/nature03391. [DOI] [PubMed] [Google Scholar]
- Kupfer A. Signaling in the immunological synapse: defining the optimal size. Immunity. 2006;25:11–13. doi: 10.1016/j.immuni.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Lee KH, Dinner AR, Tu C, Campi G, Raychaudhuri S, Varma R, Sims TN, Burack WR, Wu H, Wang J, et al. The immunological synapse balances T cell receptor signaling and degradation. Science. 2003;302:1218–1222. doi: 10.1126/science.1086507. [DOI] [PubMed] [Google Scholar]
- Lee KH, Holdorf AD, Dustin ML, Chan AC, Allen PM, Shaw AS. T cell receptor signaling precedes immunological synapse formation. Science. 2002;295:1539–1542. doi: 10.1126/science.1067710. [DOI] [PubMed] [Google Scholar]
- Lin J, Miller MJ, Shaw AS. The c-SMAC: sorting it all out (or in) J Cell Biol. 2005;170:177–182. doi: 10.1083/jcb.200503032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons DS, Lieberman SA, Hampl J, Boniface JJ, Chien Y, Berg LJ, Davis MM. A TCR binds to antagonist ligands with lower affinities and faster dissociation rates than to agonists. Immunity. 1996;5:53–61. doi: 10.1016/s1074-7613(00)80309-x. [DOI] [PubMed] [Google Scholar]
- Madrenas J, Wange RL, Wang JL, Isakov N, Samelson LE, Germain RN. Zeta phosphorylation without ZAP-70 activation induced by TCR antagonists or partial agonists. Science. 1995;267:515–518. doi: 10.1126/science.7824949. [DOI] [PubMed] [Google Scholar]
- Markiewicz MA, Carayannopoulos LN, Naidenko OV, Matsui K, Burack WR, Wise EL, Fremont DH, Allen PM, Yokoyama WM, Colonna M, Shaw AS. Costimulation through NKG2D Enhances Murine CD8+ CTL Function: Similarities and Differences between NKG2D and CD28 Costimulation. J Immunol. 2005;175:2825–2833. doi: 10.4049/jimmunol.175.5.2825. [DOI] [PubMed] [Google Scholar]
- Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–86. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- Morita S, Kojima T, Kitamura T. Plat-E: an efficient and stable system for transient packaging of retroviruses. Gene Ther. 2000;7:1063–1066. doi: 10.1038/sj.gt.3301206. [DOI] [PubMed] [Google Scholar]
- Mossman KD, Campi G, Groves JT, Dustin ML. Altered TCR signaling from geometrically repatterned immunological synapses. Science. 2005;310:1191–1193. doi: 10.1126/science.1119238. [DOI] [PubMed] [Google Scholar]
- O’Keefe JP, Blaine K, Alegre ML, Gajewski TF. Formation of a central supramolecular activation cluster is not required for activation of naive CD8+ T cells. Proc Natl Acad Sci U S A. 2004;101:9351–9356. doi: 10.1073/pnas.0305965101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reay PA, Kantor RM, Davis MM. Use of global amino acid replacements to define the requirements for MHC binding and T cell recognition of moth cytochrome c (93-103) J Immunol. 1994;152:3946–3957. [PubMed] [Google Scholar]
- Schaefer BC, Kappler JW, Kupfer A, Marrack P. Complex and dynamic redistribution of NF-kappaB signaling intermediates in response to T cell receptor stimulation. Proc Natl Acad Sci U S A. 2004;101:1004–1009. doi: 10.1073/pnas.0307858100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seder RA, Paul WE, Davis MM, Fazekas de St Groth B. The presence of interleukin 4 during in vitro priming determines the lymphokine-producing potential of CD4+ T cells from T cell receptor transgenic mice. J Exp Med. 1992;176:1091–1098. doi: 10.1084/jem.176.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan-Lancaster J, Shaw AS, Rothbard JB, Allen PM. Partial T cell signaling: altered phospho-zeta and lack of zap70 recruitment in APL-induced T cell anergy. Cell. 1994;79:913–922. doi: 10.1016/0092-8674(94)90080-9. [DOI] [PubMed] [Google Scholar]
- Stinchcombe JC, Bossi G, Booth S, Griffiths GM. The immunological synapse of CTL contains a secretory domain and membrane bridges. Immunity. 2001;15:751–761. doi: 10.1016/s1074-7613(01)00234-5. [DOI] [PubMed] [Google Scholar]
- Timson Gauen LK, Kong AN, Samelson LE, Shaw AS. p59fyn tyrosine kinase associates with multiple T-cell receptor subunits through its unique amino-terminal domain. Mol Cell Biol. 1992;12:5438–5446. doi: 10.1128/mcb.12.12.5438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valitutti S, Muller S, Cella M, Padovan E, Lanzavecchia A. Serial triggering of many T-cell receptors by a few peptide-MHC complexes. Nature. 1995;375:148–151. doi: 10.1038/375148a0. [DOI] [PubMed] [Google Scholar]
- van Oers NS, Tohlen B, Malissen B, Moomaw CR, Afendis S, Slaughter CA. The 21- and 23-kD forms of TCR zeta are generated by specific ITAM phosphorylations. Nat Immunol. 2000;1:322–328. doi: 10.1038/79774. [DOI] [PubMed] [Google Scholar]
- Varma R, Campi G, Yokosuka T, Saito T, Dustin ML. T cell receptor-proximal signals are sustained in peripheral microclusters and terminated in the central supramolecular activation cluster. Immunity. 2006;25:117–127. doi: 10.1016/j.immuni.2006.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LH, Rothberg KG, Anderson RG. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J Cell Biol. 1993;123:1107–1117. doi: 10.1083/jcb.123.5.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Song Y, Bakker AB, Bauer S, Spies T, Lanier LL, Phillips JH. An activating immunoreceptor complex formed by NKG2D and DAP10. Science. 1999;285:730–732. doi: 10.1126/science.285.5428.730. [DOI] [PubMed] [Google Scholar]
- Wulfing C, Rabinowitz JD, Beeson C, Sjaastad MD, McConnell HM, Davis MM. Kinetics and extent of T cell activation as measured with the calcium signal. J Exp Med. 1997;185:1815–1825. doi: 10.1084/jem.185.10.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokosuka T, Sakata-Sogawa K, Kobayashi W, Hiroshima M, Hashimoto-Tane A, Tokunaga M, Dustin ML, Saito T. Newly generated T cell receptor microclusters initiate and sustain T cell activation by recruitment of Zap70 and SLP-76. Nat Immunol. 2005;6:1253–1262. doi: 10.1038/ni1272. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.