Abstract
The non-human primate MPTP model of Parkinson’s disease is an essential tool for translational studies. However, the currently used methodologies to produce parkinsonian monkeys do not follow unified criteria, and the applied models may often fall short of reproducing the characteristics of patients in clinical trials. Pooling of data from the parkinsonian monkeys produced in our Centers provided the opportunity to evaluate thoroughly the behavioral outcomes that may be considered for appropriate modeling in preclinical studies. We reviewed records from 108 macaques including rhesus and cynomolgus species used to model moderate to advanced parkinsonism with systemic MPTP treatment. The attained motor disability and the development of levodopa-induced dyskinesias, as primary outcomes, and the occurrence of clinical complications and instability of symptoms were all analyzed for correlations with the parameters of MPTP administration and for estimation of sample sizes. Results showed that frequently the MPTP-treated macaque can recapitulate the phenotype of patients entering clinical trials, but to produce this model consistently it is important to adapt the MPTP exposure tightly according to individual animal responses. For studies of reduced animal numbers it is also important to produce stable models, and stability of parkinsonism in macaques critically depends on reaching “marked” motor disability. The analyzed data also led to put forward recommendations for successfully producing the primate MPTP model of Parkinson’s disease for translational studies.
Keywords: Parkinson’s disease, MPTP, non-human primate, model, dyskinesia
Introduction
New therapies for Parkinson’s disease (PD) are assessed for efficacy and safety in preclinical studies using various animal models including primates. Currently, the primate model produced with the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) is considered the gold standard animal model because of its close resemblance to PD with the exception of only two characteristics: damage to other monoaminerigc systems and classic Lewy body formation in the brain (Dauer and Przedborski, 2003). MPTP has high affinity for dopaminergic neurons, but lesions of adrenergic, serotoninergic, and other systems have been reported inconsistently (Forno et al., 1986; Jenner, 2008). Regarding the pathologic hallmark of PD, definite Lewy bodies have not been confirmed following MPTP toxicity in human or monkey (Forno et al., 1993). Nevertheless, this model, particularly with systemic MPTP treatment in large monkeys (macaques), characteristically replicates marked cellular loss in the substantia nigra pars compacta, the cardinal motor symptoms of PD including abnormalities in axial and appendicular movements and postures, and the full extent of motor complications associated with chronic dopaminergic treatment (Jenner, 2000, 2003). Furthermore the primate MPTP model can reproduce non-motor symptoms of PD including cognitive, sleep and gastrointentinal dysfunction (Barraud et al., 2009; Chaumette et al., 2009; Schneider et al., 2010). These exceptional characteristics of the primate MPTP model generated high expectations for its application since its original descriptions (Bankiewicz et al., 1986; Bloem et al., 1990; Burns et al., 1983). For three decades, parkinsonian monkeys have been used extensively and successfully in a variety of translational studies (Bibbiani et al., 2005; Eden et al., 1991; Liang et al., 2008; Luquin et al., 1999; Stockwell et al., 2009), and the model is recognized to have “fantastic translational potential” (Bezard and Przedborski, 2011).
The large experience in the use of MPTP-treated primates led to the publication of several reviews where the model pros and cons and its comparisons with other animal models of PD were discussed (Collier et al., 2003; Fox and Brotchie, 2010; Marin et al., 2006; Morin et al., 2013; Olanow and Kordower, 2009). However, commonly applied methodologies for MPTP treatment and the characteristic features of the produced parkinsonian monkeys have never been formally surveyed. The information obtained from these studies is key for adequately modeling PD in monkeys, and can be used to establish guidelines for application of the model. This is particularly important for translational studies, which rely on reproducibility and consistency of parkinsonian behaviors in the monkeys. The MPTP lesion is largely dependent on individual features of the monkeys (species, gender, age, health conditions, etc.) and the toxin bioavailability determined by dosages, schedules, etc. As a result, the phenotypes in the primate MPTP model may bear considerable variability, and despite the lack of systematic comparisons, it can be predicted that the groups of parkinsonian monkeys currently in use are quite heterogeneous. For instance, a large number of interventional treatments target the middle/advanced stages of PD that develop after years of disease progression and antiparkinsonian drug therapy. Presumably, this long-standing parkinsonism has not always been reproduced in the primate model within the context of modern, rapid-pace research and a lack of common inclusion criteria. Taking advantage of a large data sample from 108 monkeys (macaques) produced in two Institutions (Emory University, Center 1 and University of Navarra, Center 2), we retrospectively reviewed the clinical monkey records to analyze differences in commonly used primate models using consistent assessment of motor behavior and thorough statistical processing. These analyses of behavioral outcomes in relation to the MPTP treatments led to discuss the key methodological points and provide guidelines that may help avoid shortcomings so that the application of MPTP-treated monkeys can be optimally reliable and useful.
Production of the primate MPTP Model
Animals
Data (n=108) included two species of macaques, Rhesus and Cynomolgus (RM and CM; Macaca mulatta and fascicularis) with bilateral parkinsonism following intravenous (i.v.) MPTP injections for use in a variety of studies for clinical translation; therefore, models were prepared similarly in Centers 1 and 2 (see Table 1 for demographic details). Because of similar animal characteristics and procedures between the two Centers, monkeys could be grouped by the species. All studies were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals or the European Union guidelines, and approved by the Institutional Animal Care and Use Committees of respective Universities. The animals were maintained in similar living conditions in both Centers. While the EU and US regulations differ regarding housing size, they are similar in all other aspects (nutrition, environmental conditions, social housing, enrichment, etc.). The housing differences between the two Centers were minimized because of the guidelines for cage size adjustment according to animal size/weight at the US Center 1 (Yerkes National Primate Research Center).
Table 1.
Characteristics of Parkinsonian Monkeys
| Species (Macaca) | Rhesus | Cynomolgus | ||
|---|---|---|---|---|
| MPTP administration | i.v. | i.v. | ||
| Number of animals | N=23 | N=85 | Comparison | |
| Centera | Emory | 23 | 10 | |
| Navarra | 0 | 75 | ||
| Gender | Female | 12 | 7 | p < 0.0001b |
| Male | 11 | 78 | ||
| Age | ||||
| 4.6 ± 0.5 | 4.3 ± 0.2 | p = 0.6 | ||
| (years) | ||||
| (2–10) | (1.7–7.0) | |||
| Weight | ||||
| 5.8 ± 0.4 | 3.7 ± 0.1 | p < 0.0001c | ||
| (kg) | ||||
| (2.9–9.9) | (2–7.3) | |||
Each Center, Emory University (Atlanta, GA, USA) and University of Navarra (Pamplona, Spain) with the corresponding number of monkeys. The distribution of gender, age and weight is shown in each species (RM and CM). Most CM were males selected for pharmacological studies to avoid cyclic hormonal effects of females. Weight difference between species is expected as RM are typically larger than CM. Data are mean ± SEM and the ranges are given in parenthesis.
Fisher’s exact test.
Two sample t-test
Methodological considerations in the production of the model for the analyzed dataset are primarily concerned with differences in the included macaques and the applied systemic MPTP treatments. The macaques used in Centers 1 and 2 studies originated from non-selective breeding and had similar characteristics (Table 1). Males and females of each macaque species were included, but in the CM group gender was skewed by a higher number of males (78 males and 7 females) to avoid the behavioral effects of cyclic female hormones. It is unlikely that such hormonal effects impacted the behavioral outcomes in the RM group that had an equivalent number of males and females. All monkeys were adults (1.7–10 years; aging animals were not included) with a similar age distribution between species (p=0.6). Weight averages differed between species (5.8 kg in RM and 3.7 kg in CM) because of the constitutional larger size of rhesus rather than differences in health conditions (all animals were in good health upon study entry). Altogether, the particular characteristics of the used macaques do not appear to bias results for the produced parkinsonian monkeys in these studies.
MPTP Treatment
The methods of systemic MPTP administration included variable intravenous doses given repeatedly until inducing parkinsonian motor disability of moderate degree and symptoms stabilized (Liang et al., 2008; Luquin et al., 1992; Vazquez-Claverie et al., 2009). MPTP administration was withdrawn earlier in 7 CM for study of mild parkinsonism, and data from these monkeys were excluded from relevant analyses. Most parameters of i.v. MPTP administration (single doses, total cumulative doses (TCD), number of injections, and treatment duration) varied widely in both species in spite of targeting similar levels of parkinsonism (Table 2). This variability has been attributed to different susceptibility to systemic MPTP administration across macaque species and individual animals (Eidelberg et al., 1986; Fornai et al., 1997; Jakowec and Petzinger, 2004). The total amount (TCD) of MPTP ranged from < 1 to 27.7/18.5 mg/kg in RM/CM, while the single dose (MPTP dose administered per injection) had a limited range (from 0.5/0.3 to 1/1.5 mg/kg in RM/CM) depending on individual responses and sensitivity to develop acute complications. Congruent with TCD variation, the number of injections and treatment duration (1–53/42 injections and 0.1–29/27 months, RM/CM) were also largely variable throughout the dataset. The MPTP injection frequency was not computed for analysis because intervals between injections were 1–2 weeks in most cases. The comparison between species showed significantly larger single doses, TCD and treatment duration in RM indicating the lower susceptibility to MPTP in this species (Table 2).
Table 2.
Differences in MPTP Administration
| Rhesus | Cynomolgus | Comparison | |
|---|---|---|---|
| Single dose (mg/kg) | 0.8 ± 0.03 (0.5–1) |
0.6 ± 0.03 (0.3–1.5) |
p = 0.0008 |
| Number of injections | 17.5 ± 2.7 (1–53) |
13.8 ± 0.9 (1–42) |
p = 0.2 |
| TCD (mg/kg) | 10.6 ± 1.8 (0.8–27.7) |
5.9 ± 0.4 (0.8–18.5) |
p = 0.02 |
| Duration (months) | 11.2 ± 1.6 (0.1–29) |
6.8 ± 0.6 (0.1–27) |
p = 0.02 |
Systemic MPTP administration in each species (RM and CM). Each parameter of intravenous MPTP administration was compared between species. Single dose: dose per injection (in cases of varying single doses the highest dose was computed). TCD: total cumulative dose given over the whole treatment. The duration of MPTP treatment was taken from the first to the last MPTP injection, which could have some variability in the intervals between injections, explaining the lack of exact correlation between treatment duration and number of injections (temporary discontinuation of MPTP administration beyond the usual range of interval variability was subtracted from the total duration of MPTP treatment). Data are presented as mean ± SEM, and the ranges are given in parenthesis. Two sample t-test.
Assessment of Acute Effects
The systemic administration of MPTP may produce unwanted effects in the acute and subacute phases that are followed by parkinsonian motor disability in the chronic phase (modeled behavioral outcomes). These acute toxic effects of MPTP are due to marked catecholamine depletion (Ambrosio et al., 1988; Przedborski et al., 2001; Waters et al., 1987; and can lead to severe clinical complications (reduced appetite, weight loss, compromise of mobility and ability to self-feed, dehydration, lethargy, etc.) and death. It was important to analyze these unintended outcomes because they may influence not only the index of success producing the model in the required number of animals, but also the behavioral phenotype in the produced models. Acute complications were rated as mild, moderate, or severe (scores 1 to 3) depending on their degree and the amount of intervention required to stabilize the animal (i.e. mild, reduced food intake and weight loss requiring hand-feeding; moderate, markedly reduced mobility requiring hand-feeding and antiparkinsonian treatment; and severe, immobility, dehydration, lethargy etc. requiring rehydration, nasogastric tube-feeding, and antiparkinsonian treatment). Acute complications developed in 42% of the MPTP-treated macaques with relevant records (n=89, Table 3A), the majority being moderate to severe. The presence of acute complications and their severity computing the highest scores after any given MPTP injection were used for analysis.
Table 3.
Acute Complications and Association with Parameters of MPTP Administration
| A. | Rhesus | Cynomolgus | |||
|---|---|---|---|---|---|
| Complications | Yes | 21 (91%) | 16 (24%) | ||
| No | 2 | 50a | |||
| Severity of complications | 1 | 5 | 6 | ||
| 2 | 4 | 6 | |||
| 3 | 12 | 4 | |||
| Drug treatmentb | Yes | 16 | 8 | ||
| No | 5 | 8 | |||
| Deathc | Yes | 3 | 3 | ||
| No | 18 | 13 | |||
|
| |||||
| B. | Acute Complications | ||||
|
MPTP Treatment “Predictors” |
Occurrence | Severity | Occurrence | Severity | |
|
| |||||
| Single dose | – | – | Positive ß = 2.3 (1) p = 0.04 |
Positive ß = 2.4 (1.0) p = 0.02 |
|
| Number of Injections | Negative Infinity |
Negative ß = −0.12 (0.04 p = 0.009 |
– | – | |
| TCD | Negative Infinity |
Negative ß = −0.2 (0.07) p = 0.004 |
– | – | |
| Duration | – | – | – | – | |
A. Complete data regarding the development of acute complications were available in the whole group of RM (n=23) and 66 cases of CM (Total monkeys n=89).
The lower number of complications in CM was likely the result of the reduced single doses of MPTP in this species. The severity of complications was scored as 1, mild; 2, moderate; and 3, severe according to pre-established criteria (see “Production of the model: methods and data analyses” section).
Antiparkinsonian drug treatment was given to recover from marked acute immobility.
Death due to severe acute complications indicates animals euthanized because of poor response to therapeutic measures.
Data are presented as mean ± SEM, and the ranges are given in parenthesis. Fisher’s exact test. B. The predictive value of MPTP treatment parameters for occurrence or severity of acute complications controlling for effects of gender, age and weight, was analyzed. Severity was rated as 0 for monkeys with no complications. The number of injections and TCD were strong negative predictors for complications in RM (treatment duration showed a trend for negative predictor, p ≥ 0.05). There was a trend for the number of injections to be a negative predictor for complications in CM. In contrast, the single dose was a positive predictor for complications in CM indicating species differences in MPTP sensitivity. Positive and negative ß values (SEM) are shown when p < 0.05, or denoted as infinity meaning that the outcome can be perfectly predicted by the parameter of MPTP treatment (multiple binary or ordinal logistic regressions).
Assessment of Chronic Effects
The modeled parkinsonism in MPTP-treated monkeys was assessed by two primary outcomes, (1) parkinsonian motor disability in all monkeys (n=108) and (2) levodopa-induced dyskinesias in a subgroup with systematic induction of this outcome (n=39). Both were measured using a standardized motor disability scale for MPTP-treated nonhuman primates (Papa and Chase, 1996), which is similar to the UPDRS used for PD patients. This rating scale for monkeys has higher specificity for parkinsonian motor deficits than tests of locomotion or motor tasks, which are influenced by attention, motivation, etc. Motor disability is rated in Part I of the scale, and total motor disability score (MDS) ranges from 0 to 39. Levodopa-induced dyskinesias are rated in Part II of the scale, and total score ranges from 0 to 24 (motor fluctuations induced by chronic levodopa therapy were not analyzed here). The scales used in Center 2 had slight differences, and thus, scores were converted to the range of the scale originally used in Center 1 for comparison. The targeted level of motor impairment had to remain stable after withdrawal of MPTP treatment for the model to be considered complete because spontaneous recovery could occur (Collier et al., 2003; Taylor et al., 1997). In both species, recovery of motor deficits was observed most frequently between 3 and 8 weeks after single MPTP injections. Thus, stability of symptoms (MDS) was assessed weekly for a minimum of 8 weeks after the last MPTP injection. The occurrence of spontaneous recovery was analyzed in animals with documented records (recovery yes or no; n=41). After reaching a stable MDS, 16 RM and 23 CM received chronic dopaminergic treatment to develop dyskinesias. Monkeys were treated with daily oral carbidopa/levodopa (Sinemet 25/100 mg) at variable doses depending on the individual motor impairment (50–150 mg 2 to 4 times per day) with the exception of 4 monkeys initially given SKF82958 1 mg/kg s.c. per day instead of levodopa. Dyskinesias usually appeared within 2–3 weeks to 4 months of regular oral levodopa treatment. The chronic development of stable dyskinesias (persistent over months) can be considered parallel to that observed in PD patients, and thus, they were taken as the homologous levodopa-induced dyskinesias (LID) in monkeys.
Statististics
The statistical analyses included: bivariate comparisons for demographics, MPTP treatments or outcome measures1 between species; a probit model for analysis of recovery from MPTP lesion; multiple linear or logistic regressions for relationships between MPTP treatment and primary outcomes; and multiple binary and ordinal logistic regressions for associations between acute complications and MPTP treatments or the primary outcomes. Cumulative logit link models were used in the ordinal logistic regression for analyzing severity of complications. Age, gender and weight were included as predictors in all regressions (the estimated parameters from the logistic models were log odds ratio; R statistical programming language (R, 2011)). The impact of acute complications in the success to produce the model was analyzed for sample size estimation as follows. Assuming N be the number of monkeys included in the study and X the number of parkinsonian monkeys alive or without severe complications, and X follows binomial distribution:
Here p is the probability of monkeys alive or without severe complications, and it is assumed that p follows normal distribution with model parameters estimated from the data:
Let C be the number of desired parkinsonian monkeys for the planned study. The probability of the number of parkinsonian monkeys alive or without severe complications to be above or equal to the number of desired parkinsonian monkeys was obtained through simulations:
This probability represents the power to obtain the desired number of animals. Similar power analyses were also applied to estimate the achievement of critical levels of motor disability. Assuming the obtained MDS (Y) follows normal distribution, for a desired MDS threshold T:
was computed from the normal distribution. Next, assume X is the number of monkeys with MDS equal to or greater than T, and follows binomial distribution:
The probability Pr (X ≥ C), where C is the number of required monkeys with the desired MDS equal to or greated than T, was computed from the binomial distribution function.
Severe acute complications of MPTP depend on doses and macaque species
The development of acute complications that compromise the successful production of the model was analyzed to determine their relationship to the parameters of MPTP administration. The presence and severity of acute complications were recorded in all RM (n=23) but not in all CM (n=66). The different incidence of complications between RM (91%) and CM (24%) can be attributed to the lower MPTP doses often injected to the CM due to their higher sensitivity to the toxin (Table 3A). Despite the adjustments of MPTP doses in CM leading to fewer complications, the single dose of MPTP was the risk factor identified in this species (strong correlation with the occurrence and severity of complications; Table 3B). In fact, the single dose of MPTP in CM was the only risk factor for acute complications. No positive correlations of any factors were found in RM, and the probability of RM to develop severe complications was 0.52 (95% confidence interval: 0.32, 0.72). Contrarily, the number of MPTP injections and TCD had very strong inverse correlations (ß=infinity in RM) or a trend for inverse correlations (non-significant ß values in CM) with complications (the occurrence and severity). These results demonstrate that lower sensitivity to MPTP in resistant animals (more predominantly seen in RM) led to extended MPTP treatments, i.e: higher number of injections and TCD with reduced incidence of acute complications. No clear association of MPTP treatment duration with acute complications was found in either species. These analyses show that the predictive value of the MPTP single dose for complications in CM is of critical importance to achieve high survival and success producing the model in this species with small sample sizes. In contrast, the number of injections or TCD that are robust negative predictors of complications in RM strongly indicate MPTP resistance, and may help predict animals with instability of symptoms (see spontaneous recovery below).
Stable parkinsonian models require individual adjustments of MPTP treatment
To determine methodological factors that influence the production of stable parkinsonian monkeys, firstly the attained MDS was analyzed in relation to the parameters of MPTP administration in all monkeys (n=108). The stabilized MDS were on average higher in CM (22.1 ± 0.9 in CM and 18.1 ± 1.3 in RM; p = 0.01; Figure 1A) demonstrating higher sensitivity to MPTP in CM in spite of lower single doses, TCD and duration of MPTP treatment (Table 2). The sensitivity to MPTP differed not only between macaque species, but also among monkeys of the same species. Different responses to MPTP across animals resulted in a wide range of TCD, number of injections, and duration of MPTP treatment in each species. Moreover, in spite of targeting a similar level of parkinsonism in all RM and 78 CM (7 CM had milder parkinsonism due to early suspension of MPTP treatment and were excluded from this analysis), the stabilized MDS varied by 20 points in both RM and CM (Figure 1A), and was not positively correlated with any MPTP administration parameter in either species (Figure 2). MDS had only a “negative” association with the number of MPTP injections (RM, p = 0.0088; CM, p = 0.03), due to the frequent development of resistance. The large variability in MDS even applying similar systemic MPTP treatments suggests that the produced models for any given study may express marked phenotype differences.
Figure 1.
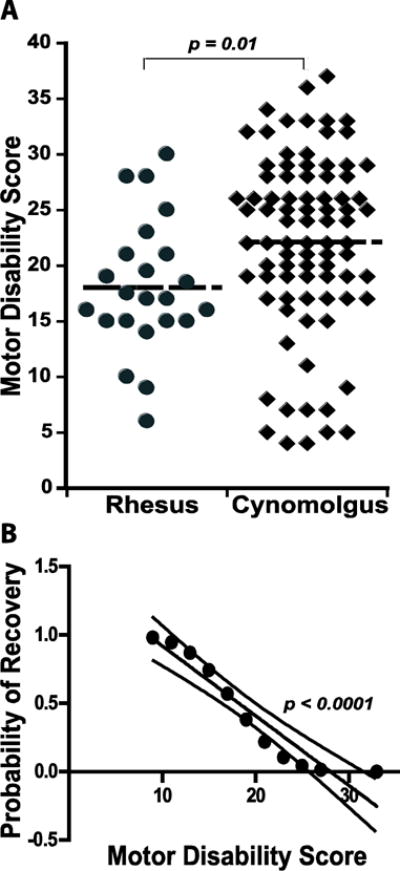
A. Parkinsonian motor disability induced by systemic MPTP treatment in macaques. MDS were widely distributed in both species indicating variability of outcomes following systemic MPTP treatment. MDS was measured with the standardized motor disability scale for MPTP-treated nonhuman primates scoring animals in the morning for baseline parkinsonian state (no antiparkinsonian medication). The horizontal line in each group (RM and CM) denotes the average MDS. Two-sample t-tests. B. Probability of spontaneous functional recovery following MPTP administration. The computation of all recovery episodes (yes/no) clustered according to the monkey’s MDS ranging from 9 to 33 was used in a Probit model to calculate the significance of MDS for recovery (p = 0.02). The probabilities calculated in the analysis were ploted for linear regression (R2 = 0.9, p < 0.0001, dashed lines are the 95% confidence intervals for the line interpolation). The probability for recovery is reduced to 0.3 with MDS ≥ 20.
Figure 2.
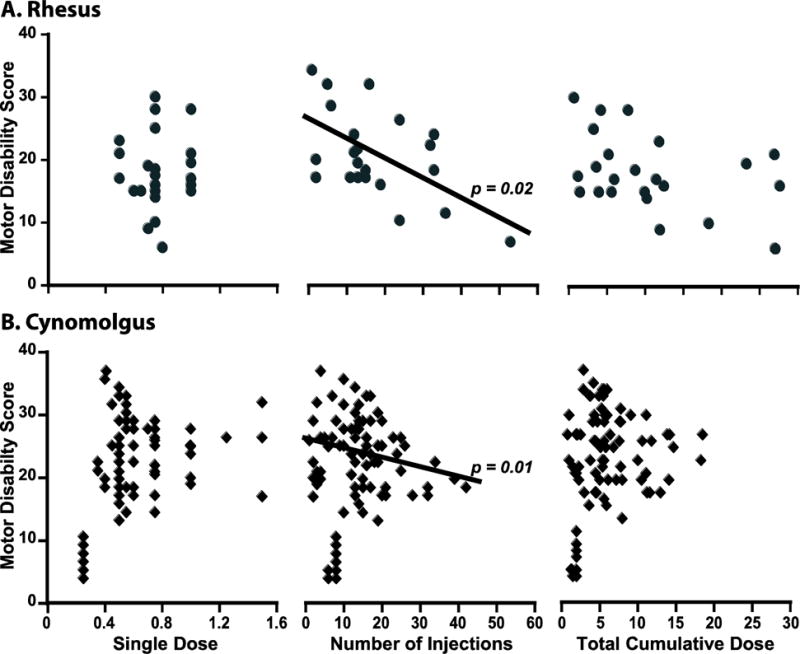
Influences of the method used in systemic MPTP treatment. In RM (A) and CM (B), the correlation of single doses, number of injections, and TCD of systemic (i.v.) MPTP with the stable motor disability scores (baseline scores taken in the morning without medication) are depicted. The MPTP treatment was intentionally withdrawn after reaching mild MDS in 7 CM as needed for the studies (some of MDS below 10 in panel B; these data were subtracted from the analyses). Motor disability was inversely correlated with the number of MPTP injections in both species indicating the significant development of resistance to MPTP in macaques. The lack of positive association indicates the independence of MDS from the parameters of MPTP treatment. Multiple linear regressions with gender, age and weight included in the model.
Secondly, because stability of symptoms is also critical for application of the model, the occurrence of spontaneous recovery was analyzed. Relevant records of recovery (yes/no) were available from 41 monkeys (RM and CM together), and showed spontaneous recovery in 46% (19 monkeys). Moreover, the total of observed recoveries was 27 because some of the 19 animals recovered repeatedly following successive MPTP injections. Additionally, the level of recovery averaged 38% reduction of MDS (from 17 to 87%), and could develop gradually over weeks or months. Recovery was more frequent in RM (55%) than CM (38%), which translated into more extensive MPTP treatment in the former due to higher resistance to MPTP. Because recovery decreased with extended MPTP administration, the association between the occurrence of recovery and MDS was also examined. The probability of recovery correlated inversely with MDS (Figure 1B) demonstrating higher instability in models of mild parkinsonism. The level of motor disability required to attain a reduced probability of recovery (Pr = 0.3) was at scores ≥ 20; hence this MDS was used in power analysis for estimation of sample sizes (see below). Altogether these analyses show that although the MPTP levels or regimens used for systemic administration may have an impact on the acute toxic effects, they do not correlate with the chronic establishment of motor deficits, and stability of the model is favored with higher MDS. Therefore, individual adjustments of the MPTP treatment seem to be key to effectively producing stable parkinsonian monkeys.
Stable LID depend critically on the level of parkinsonism
The development of stable LID in monkeys with chronic dopaminergic drug treatment (n=39) was also analyzed as a modeled outcome because of its frequent use for studies with intended translation into therapeutics for advanced parkinsonian patients. LID overtly developed in monkeys of both species following chronic exposure to dopaminergic drug treatment (15 out of 16 RM and 19 out of 23 CM). As for all monkeys, MDS of the monkeys under chronic dopaminergic treatment also differed between species (17.7 ± 0.9 in RM and 21.7 ± 1.7 in CM; p = 0.04; Figure 3A). Congruent with differences in MDS, LID scores were slightly higher in CM (11.9 ± 0.7) than RM (8.4 ± 1.1; p = 0.01) (Figure 3B). In both species, the development of dyskinesias and their intensity strongly correlated with MDS, and thus, all monkeys were grouped for regression analysis (p < 0.0001; Figure 3C). The large effect size of MDS on LID development precluded its use as a controlling factor in the analysis of independent effects of the MPTP administration parameters on LID. Noteworthy, chronically stable dyskinesias did not develop in 5 monkeys with MDS below 14 or 16 in RM or CM, respectively, and the majority of dyskinetic monkeys (62%) had severe parkinsonism with MDS of 20 or higher (Figure 3C). Although there were only 5 levodopa-treated monkeys with mild MDS (< 14/16), it was recorded frequently in the other 34 monkeys that LID did not develop earlier when they had lower MDS in spite of prolonged chronic levodopa exposure. Those monkeys required additional MPTP administration to reach higher MDS in order to express LID. Further analysis of the relationship between MDS and dyskinesias including potential confounding factors associated with the animal characteristics (gender, age and weight) showed that the development of LID and their severity (dyskinesia scores) were strongly predicted by MDS (predictive value reaching “infinity”) in either species (Table 4). Therefore, the development of stable LID in parkinsonian monkeys is clearly dependent on reaching marked levels of motor disability.
Figure 3.
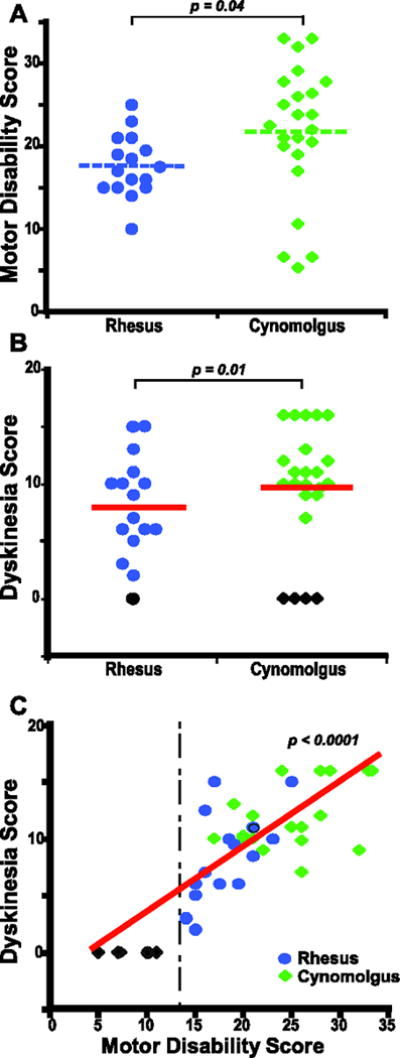
Development of dyskinesias in primate MPTP models. A. Distribution of MDS in RM and CM chronically treated with dopaminergic drugs to develop LID (levodopa in all but 4 monkeys that received SKF82958). B. LID scores reached after chronic dopaminergic treatment in each species. All monkeys chronically treated with dopaminergic drugs were included in the analysis independently of the development of LID. One rhesus and 4 cynomolgus monkeys chronically treated with levodopa did not develop LID (black symbols). A and B: Horizontal lines denote the mean. Two-sample t-tests. C. The association between LID scores and MDS are depicted (all monkeys together because the development of dyskinesias followed the same pattern in RM and CM). The vertical line denotes the lower limit of MDS for the development of LID, and indicates the tight dependence of dyskinesias on the degree of parkinsonism. Multiple linear regressions with gender, age and weight included in the model.
Table 4.
Predictors of Modeled Outcomes with Systemic MPTP Treatment
| Motor Disability Score |
Dyskinesias (Yes/No) |
Dyskinesias Score |
||
|---|---|---|---|---|
| Predictor | Rhesus | Cynomolgus | Rhesus and Cynomolgus Combined |
|
|
MPTP Treatment Number of Injections |
Negative ß = −0.24 (0.09) p = 0.02 |
Negative ß = −0.23 (0.09) p = 0.01 |
||
| Complications | Positive ß = 4.1 (1) p = 0.001 |
Positive ß = 2.7 (0.9) p = 0.004 |
Positive ß = 1.5 (0.7) p = 0.04 |
|
| MDS | Positive Infinity |
Positive ß = 0.54 (0.09) p < 0.0001 |
||
The predictive value of the parameters of MPTP administration (single dose, number of injections, and TCD) and the severity of acute complications for the intended behavioral outcomes (MDS, the development [Yes/No] and severity of LID [score]) were analyzed. The predictive value of MDS for LID was also analyzed. Because of similar patterns for the development of LID between RM and CM, data were combined for analysis of predictability by MDS. The analyses highlight the importance of complications as positive predictors of outcomes, and the direct, perfect (infinity) relationship between dyskinesias and MDS. Positive and negative ß values (SEM) are shown starting at p < 0.05; the significance of predictors was taken after correction for effects of gender, age and weight (multiple linear or logistic regressions depending on the type of data, binary or continuous).
Acute complications of MPTP administration predict MDS and LID
To determine predictors of MDS and LID, we analyzed the parameters of MPTP treatment (RM, n=23; CM, n=78) and the development of acute complications (RM, n=23; CM, n=66) controlling for the potential confounding effects of animal differences related to gender, age and weight in each species. The only positive predictor of MDS and LID scores was the severity of acute complications, which had high positive predictive value in both RM and CM (Table 4; severity of complications graded as a continuous variable was used instead of occurrence of complications). In both species, the number of MPTP injections had negative predictive value for MDS, confirming the frequent appearance of resistance (Table 4). The age and gender distributions had no effects on the intended (primary) or unintended outcomes (acute complications) in either species. Weight had a tendency to affect outcomes, although larger samples are needed for an accurate assessment of its role. In summary, developing significant acute complications appears to be an indicator for achieving marked levels of parkinsonism and LID.
Model consistency requires adjustment of sample sizes or MPTP treatment length
The preceding analyses point to key features that may affect the success in producing the required number of modeled parkinsonian monkeys according to the study design. These features are: (1) the development of acute complications, (2) the resulting MDS at levels of frequent spontaneous recovery, and (3) the resulting MDS below the levels necessary for LID development. Analysis of acute complications leading to death in RM (n=23) showed that estimated sample sizes need to be between 20% and 30% larger than the required number of animals for the study to achieve the desired number of “alive” parkinsonian monkeys (power = 0.8; Figure 4A). However, the incidence of severe complications still risks results with such sample sizes, and to avoid severe complications, estimations need to increase considerably more (Figure 4B). Considering that largely increased animal numbers may not be feasible in many studies, planned investigations should consider the high incidence of serious complications and prepare for increased clinical support to stabilize the animals. This analysis was done in the RM group where there was no positive correlation between any MPTP treatment parameter and acute complications. In CM, complications are strongly associated with the single dose of MPTP, and thus, they could be reduced by administering lower MPTP doses per injection.
Figure 4.
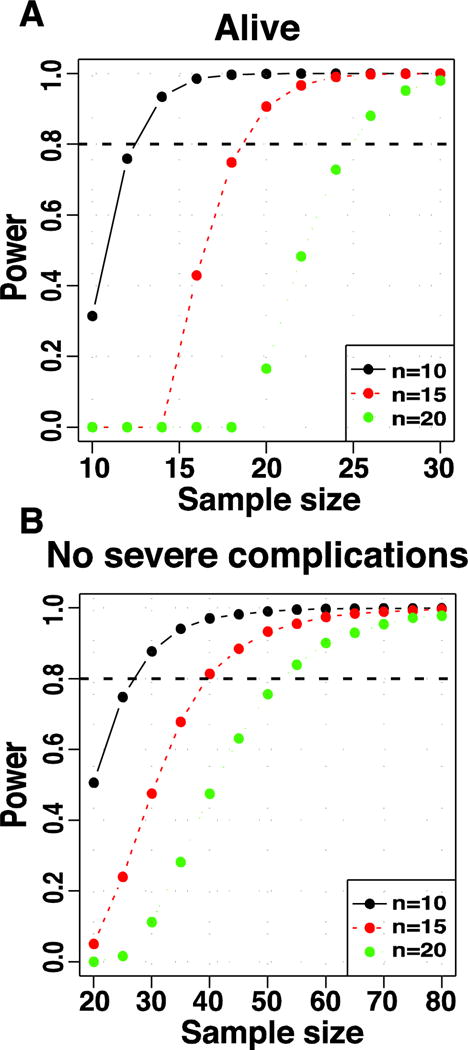
Sample size estimation based upon acute complications and death with systemic MPTP treatment. In A, the power curves show the estimated sample sizes to obtain 10 (black), 15 (red) and 20 (green) parkinsonian monkeys surviving (Alive) the acute toxicity of MPTP. In B, the curves show the corresponding estimations to produce parkinsonian monkeys without developing severe acute complications. The analysis was performed in the group of RM in which the development of acute complications was not correlated positively with any parameter of MPTP administration. All RM had records of complications (yes/no and severity) and the total number (n=23) was computed in the analysis. The horizontal line denotes power = 0.8 (see analysis details in “Production of the model: methods and data analyses”).
Regarding the common appearance of spontaneous recovery, the analyses in RM (n=23) and CM (n=78 with exclusion of 7 animals with early termination of MPTP treatment) showed that sample sizes need to be increased substantially to achieve MDS ≥ 20 thereby reducing the probability of recovery to 0.3 (Figure 1B). Estimated samples need to increase by 50–75% to obtain the desired number of monkeys with MDS ≥ 20 in the CM species, and much more in the RM that usually reach lower MDS (power = 0.5–0.8; Figure 5A). In addition, to obtain the desired animal number with moderate to severe MDS that can lead to the development of LID (i.e.: ≥ 14 in RM and ≥ 16 in CM), it is necessary to estimate samples ~30 and 50% larger than needed for CM and RM, respectively (power = 0.8; Figure 5B).
Figure 5.
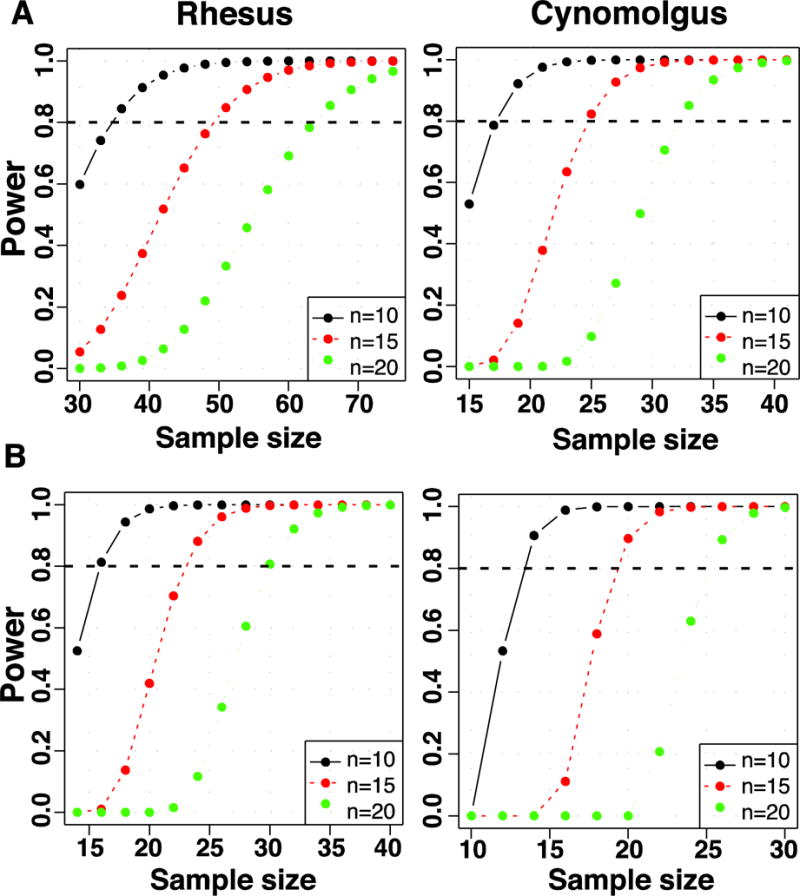
Sample size estimation based upon the obtained levels of parkinsonism. In A, the power curves show the estimated sample sizes to obtain 10 (black), 15 (red) and 20 (green) parkinsonian RM (left) and CM (right) with a MDS ≥ 20 that is associated with a reduced probability (pr) of recovery (pr = 0.3; Figure 1B). In B, the curves show the corresponding estimations to obtain parkinsonian RM and CM with a MDS ≥ 14 and 16, respectively, as needed for the development of dyskinesias. All RM (n=23) were included in the analyses. Seven CM that had milder parkinsonism due to early suspension of MPTP treatment were excluded from these power analyses (CM, n=78). The horizontal lines denotes power = 0.8 (see analysis details in “Production of the model: methods and data analyses”).
These findings indicate that sample size estimates need to be enlarged significantly to be at the desired power level to produce the required parkinsonian models in both species, particularly in RM. However, this premise is in conflict with general primate research criteria of keeping the animal number as minimal as possible. Alternatively, power analysis data can be construed as underscoring the need to plan extended MPTP treatments with frequent repetition of injections to overcome spontaneous recovery and achieve critical levels of motor disability while reducing the animal number.
Discussion
Variable MPTP sensitivity
Most relevant technical considerations in these data analyses are related to the methods of MPTP administration, the selected species, and the type of animal breeding. The large amount of data analyzed here included the use of different MPTP protocols, and thus, data are likely applicable to most methods of systemic MPTP administration (Bezard et al., 2001; Collier et al., 2003; Hadj Tahar et al., 2001; Johnston et al., 2010; Samadi et al., 2008). Radically different regimens of systemic MPTP administration such as the continuous infusion that have not been used in these studies would need to be analyzed for alternative results in further studies. Similarly, the results obtained in these analyses cannot be applied to other species used to model PD in primates (marmosets, squirrel monkeys, etc.). In addition, all data analyzed here originated from not selectivily bred animals. Thus, if in the future selectively bred macaques become of common use for the MPTP primate model of PD, the behavioral outcomes of the model would need to be reappraised. Finally, continuation studies of biochemical and histological changes are important to determine the correlation with patterns of MPTP lesion, and may help understand the substrate of the observed behavioral results.
One of the salient findings in this study was the variable sensitivity to MPTP that involves differential responses between primate species and across individual monkeys. RM required more MPTP despite reaching lower MDS than CM, indicating the importance of species selection. The difference in response between macaque species is aligned with variable sensitivities to the toxin across rodents, non-human and human primates (Duty and Jenner, 2011; Johannessen et al., 1985). This may be explained by metabolic differences including the conversion or clearance of MPP+, the toxic metabolite of MPTP (Capitanio and Emborg, 2008; Johannessen et al., 1985). Additionally, a wide range of MPTP levels was administered to the monkeys of the same species, although similar (moderate) parkinsonism was targeted. By contrast, standard protocols for MPTP treatment have been promoted in the previous literature, particularly the early reports (Burns et al., 1983; Schneider and Roeltgen, 1993; Schneider et al., 1999; Taylor et al., 1997). The present data indicate that studies guided by the MPTP exposure may result in different motor disabilities across monkeys of the same species and produce inconsistent models.
Acute complications
The systemic administration of MPTP that chronically induced moderate to severe parkinsonism was also associated with significant acute complications due to widespread toxic effects (Przedborski et al., 2001). The sensitivity to MPTP clearly played a role in the development of acute complications (Emborg, 2007). Monkeys with resistance to MPTP also had fewer and milder complications (high MPTP injection number and TCD were negative predictors for acute complications in RM). As its counterpart, monkeys exhibiting higher sensitivity to MPTP also had more acute complications (MPTP single dose was a positive predictor for acute complications in CM). Altogether these data show that the species sensitivity to MPTP correlates with the development of acute complications, which have strong predictive value for the intended outcomes. Acute complications of MPTP administration were the only positive predictor of the intended outcomes, MDS and LID, in either species of macaques. This association indicates that marked catecholamine depletion acutely (Algeri et al., 1987; Ambrosio et al., 1988; Gibb et al., 1989; Przedborski et al., 2001; Waters et al., 1987) results in a more extended lesion chronically. Thus, the avoidance of acute complications may result in the production of incomplete models particularly in more sensitive macaque species.
Spontaneous recovery
An important finding of these analyses was the common appearance of recovery from MPTP-induced motor deficits leading to extended MPTP treatments in parallel with the high resistance shown in monkeys of both species. This occurred after any given MPTP injection (Taylor et al., 1997), but it was more likely to appear with mild parkinsonism. Studies of pathological correlates in MPTP-treated monkeys have shown that motor deficits can be compensated even with extensive nigrostriatal lesions (Mounayar et al., 2007). It is also possible that neurons may be dysfunctional with down-regulation of TH but not dead after a given MPTP injection, and subsequently their function may be completely restored. Additionally, sprouting from ventral and medial striatal areas, which are typically less affected by MPTP, may compensate the more denervated dorsal and lateral striatum (Song and Haber, 2000). The compensatory mechanisms underlying spontaneous recovery are uncertain, but this feature poses an important shortcoming compromising the successful production of the model.
The correlation analyses between the four parameters of MPTP administration and the primary outcome MDS demonstrated that the development of parkinsonism is independent of the method of MPTP treatment. MDS could not be predicted by the MPTP administration parameters, and thus, these data suggest that changing the applied protocols may not result in achieving the targeted disability more consistently. MDS was only associated “negatively” with the number of MPTP injections due to the common development of resistance. In addition, the analyses showed that a significant level of disability is important for (1) stability of the model and (2) development of LID. Furthermore, it was demonstrated that there is a strong, direct relationship between LID development and MDS in both macaque species. In parallel, levodopa-induced motor fluctuations and abnormal involuntary movements in rodents with 6-hydroxydopamine lesion have been reported to correlate with the extent of the lesion (Cenci et al., 1998; Iravani and Jenner, 2011; Papa et al., 1994; Winkler et al., 2002). The present data are also congruent with LID development in patients that correlates with drug exposure and disease progression (Jenner, 2008). Thus, studies designed in mildly parkinsonian monkeys incur a higher risk of spontaneous recovery and model inconsistency (Elsworth et al., 2000). In addition, models of mild parkinsonism may be inadequate for studies targeting LID and other motor complications of chronic antiparkinsonian therapy.
Sample sizes
Power analyses showed that the high frequency of severe acute complications and the variability in behavioral phenotypes (not attaining the required MDS) have a large impact in the estimation of sample sizes in MPTP primate studies. In particular, low MDS leading to frequent functional recovery may compromise the attained parkinsonism or LID development in the animal groups prepared for any given study. This situation is further complicated by the common use of small animal numbers increasing the probability of pronounced differences in the produced phenotypes across subjects. If limitations in the timelines to produce the model are also considered, it can be predicted that often the applied models could not have matched the features of advanced patients entering clinical trials. As a result, the translatability of data from some applications of the model may be questionable, and the reproduction of primate results in clinical trials has not always succeeded (Armentero et al., 2011; Fernandez et al., 2010; Kordower et al., 2000; Lang et al., 2006; Palfi et al., 2002). Therefore, it is useful to establish a set of guidelines for optimal production of parkinsonian monkeys, particularly for their most significant use in translational studies (Bezard and Przedborski, 2011; Olanow and Kordower, 2009), and the discussed data provide the basis for it.
Guidelines for systemic MPTP treatment in macaques
Species selection should be based on the differential sensitivity to MPTP and the timeline of the study. If rapid production of stable parkinsonism (RM have shown higher resistance than CM) or higher levels of disability and LID are needed, the selection may favor the cynomolgus species. In contrast, when the production of the models can be extended for longer periods, as in electrophysiology or multidisciplinary studies with prolonged use of each animal, it may be appropriate to choose the rhesus species.
The range of single MPTP doses should be carefully selected particularly in species highly sensitive such as the CM. The MPTP dose given at once is a high risk factor for severe acute complications in CM and should be adjusted to avoid significant animal losses. Also, within the same macaque species, the MPTP exposure should be adapted according to individual sensitivity, which ultimately results in variable time courses of MPTP across animals for any given study. Yet, the key to effectively producing a monkey model of moderate to severe parkinsonism relies on variable MPTP exposures that, for some monkeys, may be safer over longer treatments.
Protocols of systemic MPTP administration should consider the variability of individual responses to MPTP. Variable outcomes, their instability due to spontaneous recovery, and independence on parameters of MPTP administration strongly support that protocols should remain open to adjustments and extensions of the MPTP treatment to ensure that all monkeys reach an adequate and permanent level of parkinsonism.
Recovery from MPTP-induced motor deficits should be monitored for extended periods with standardized measurements. It is critical to avoid the use of monkeys that at the time of testing have recovered to a certain extent from the disability shown at the end of MPTP administration. Serial controls of stability of symptoms after the last MPTP injection should be performed for a minimum of 4 to 8 weeks depending on the level of motor disability since the probability of recovery decreases as the MDS increases.
Studies should be designed to attain the appropriate level of parkinsonian disability. A marked degree of parkinsonism favors stability of the model, and is critical to reproduce chronic, stable LID. Considering the large increases in sample estimates (more pronounced for RM) needed to overcome the variability of MDS, it is necessary to select species according to the study timeline, and plan on extended MPTP treatments customizing its administration to individual requirements as a strategy for using a reduced number of animals.
Estimation of sample sizes should consider the potential loss due to severe acute complications. In addition to animal deaths leading to sample estimates 20–30% larger than the required animal number, it should be considered that sever, acute complications may occur frequently requiring intensive care of the animals to avoid further losses.
Conclusions
Overall, data show that systemic MPTP treatment in large monkeys may well reproduce marked parkinsonism and overt complicated responses to dopaminergic drugs as seen in patients with moderate/advanced PD. However, the intricate primate response to MPTP may result in the production of monkeys with mild, variable, or unstable parkinsonism that do not meet the requisite features of a PD model for use in preclinical trials of new drugs or other therapeutic interventions. Therefore, modeling PD in monkeys for translational studies relies critically on customized systemic MPTP treatment for which the availability of methodological guidelines can be useful. The set of recommendations provided here are based on a large data sample analyzing the most prominent features and are intended to unify criteria, but are not exclusive. The state of the art for this model underscores the importance of tailoring the MPTP exposure to the sensitivity of individual animals for consistency of stable parkinsonian features. It also shows that assiduous MPTP treatment is necessary to succeed in producing adequate models while minimizing the number of used primates.
Highlights.
We review records of 108 MPTP treated macaques
We analyze parameters of MPTP treatment in relation to model outcomes
Stable parkinsonian models require individual adjustment of MPTP treatment
L-dopa induced dyskinesia depends on the degree of parkinsonism
We provide guidelines for systemic MPTP treatment in macaques
Acknowledgments
The authors would like to thank Dr. Serge Przedborski for thoughtful insight and comments in the preparation of this manuscript. This work was supported by NIH grants NS045962 and NS073994 (S.M.P.); Fondo de Investigaciones Sanitarias (FIS 00/0321) and the agreement between Foundation for Applied Medical Research (FIMA) and the U.T.E. project CIMA (M.R.L.), NIH grants RR025008 and ACTSI award UL1TR000454 (H.W.); and NCRR RR000165 and ORIP/OD OD011132 (Yerkes National Primate Research Center). We thank Jessica S. Whithear for her valuable assistance in data processing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
MDS and dyskinesia scores were widely graded and composed of continuous variables. Fisher’s exact test was applied for comparisons of categorical data related to the development of acute complications of MPTP treatment.
References
- Algeri S, Ambrosio S, Garofalo P, Gerli P. Peripheral effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and its main metabolite 1-methyl-4-phenylpyridinium ion (MPP+) in the rat. Eur J Pharmacol. 1987;141:309–312. doi: 10.1016/0014-2999(87)90277-9. [DOI] [PubMed] [Google Scholar]
- Ambrosio S, Blesa R, Mintenig GM, Palacios-Araus L, Mahy N, Gual A. Acute effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) on catecholamines in heart, adrenal gland, retina and caudate nucleus of the cat. Toxicol Lett. 1988;44:1–6. doi: 10.1016/0378-4274(88)90122-1. [DOI] [PubMed] [Google Scholar]
- Armentero MT, Pinna A, Ferre S, Lanciego JL, Muller CE, Franco R. Past, present and future of A(2A) adenosine receptor antagonists in the therapy of Parkinson’s disease. Pharmacol Ther. 2011 doi: 10.1016/j.pharmthera.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankiewicz KS, Oldfield EH, Chiueh CC, Doppman JL, Jacobowitz DM, Kopin IJ. Hemiparkinsonism in monkeys after unilateral internal carotid artery infusion of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) Life Sci. 1986;39:7–16. doi: 10.1016/0024-3205(86)90431-5. [DOI] [PubMed] [Google Scholar]
- Barraud Q, Lambrecq V, Forni C, McGuire S, Hill M, Bioulac B, Balzamo E, Bezard E, Tison F, Ghorayeb I. Sleep disorders in Parkinson’s disease: the contribution of the MPTP nonhuman primate model. Exp Neurol. 2009;219:574–582. doi: 10.1016/j.expneurol.2009.07.019. [DOI] [PubMed] [Google Scholar]
- Bezard E, Dovero S, Prunier C, Ravenscroft P, Chalon S, Guilloteau D, Crossman AR, Bioulac B, Brotchie JM, Gross CE. Relationship between the appearance of symptoms and the level of nigrostriatal degeneration in a progressive 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned macaque model of Parkinson’s disease. J Neurosci. 2001;21:6853–6861. doi: 10.1523/JNEUROSCI.21-17-06853.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezard E, Przedborski S. A tale on animal models of Parkinson’s disease. Mov Disord. 2011;26:993–1002. doi: 10.1002/mds.23696. [DOI] [PubMed] [Google Scholar]
- Bibbiani F, Costantini LC, Patel R, Chase TN. Continuous dopaminergic stimulation reduces risk of motor complications in parkinsonian primates. Experimental neurology. 2005;192:73–78. doi: 10.1016/j.expneurol.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Bloem BR, Irwin I, Buruma OJ, Haan J, Roos RA, Tetrud JW, Langston JW. The MPTP model: versatile contributions to the treatment of idiopathic Parkinson’s disease. J Neurol Sci. 1990;97:273–293. doi: 10.1016/0022-510x(90)90225-c. [DOI] [PubMed] [Google Scholar]
- Burns RS, Chiueh CC, Markey SP, Ebert MH, Jacobowitz DM, Kopin IJ. A primate model of parkinsonism: selective destruction of dopaminergic neurons in the pars compacta of the substantia nigra by N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Proc Natl Acad Sci U S A. 1983;80:4546–4550. doi: 10.1073/pnas.80.14.4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capitanio JP, Emborg ME. Contributions of non-human primates to neuroscience research. Lancet. 2008;371:1126–1135. doi: 10.1016/S0140-6736(08)60489-4. [DOI] [PubMed] [Google Scholar]
- Cenci MA, Lee CS, Bjorklund A. L-DOPA-induced dyskinesia in the rat is associated with striatal overexpression of prodynorphin- and glutamic acid decarboxylase mRNA. Eur J Neurosci. 1998;10:2694–2706. [PubMed] [Google Scholar]
- Chaumette T, Lebouvier T, Aubert P, Lardeux B, Qin C, Li Q, Accary D, Bezard E, Bruley des Varannes S, Derkinderen P, Neunlist M. Neurochemical plasticity in the enteric nervous system of a primate animal model of experimental Parkinsonism. Neurogastroenterology and motility. 2009;21:215–222. doi: 10.1111/j.1365-2982.2008.01226.x. [DOI] [PubMed] [Google Scholar]
- Collier TJ, Steece-Collier K, Kordower JH. Primate models of Parkinson’s disease. Exp Neurol. 2003;183:258–262. doi: 10.1016/s0014-4886(03)00246-2. [DOI] [PubMed] [Google Scholar]
- Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- Duty S, Jenner P. Animal models of Parkinson’s disease: a source of novel treatments and clues to the cause of the disease. Br J Pharmacol. 2011 doi: 10.1111/j.1476-5381.2011.01426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden RJ, Costall B, Domeney AM, Gerrard PA, Harvey CA, Kelly ME, Naylor RJ, Owen DA, Wright A. Preclinical pharmacology of ropinirole (SK&F 101468-A) a novel dopamine D2 agonist. Pharmacology, biochemistry, and behavior. 1991;38:147–154. doi: 10.1016/0091-3057(91)90603-y. [DOI] [PubMed] [Google Scholar]
- Eidelberg E, Brooks BA, Morgan WW, Walden JG, Kokemoor RH. Variability and functional recovery in the N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of parkinsonism in monkeys. Neuroscience. 1986;18:817–822. doi: 10.1016/0306-4522(86)90102-8. [DOI] [PubMed] [Google Scholar]
- Elsworth JD, Taylor JR, Sladek JR, Jr, Collier TJ, Redmond DE, Jr, Roth RH. Striatal dopaminergic correlates of stable parkinsonism and degree of recovery in old-world primates one year after MPTP treatment. Neuroscience. 2000;95:399–408. doi: 10.1016/s0306-4522(99)00437-6. [DOI] [PubMed] [Google Scholar]
- Emborg ME. Nonhuman primate models of Parkinson’s disease. ILAR journal / National Research Council, Institute of Laboratory Animal Resources. 2007;48:339–355. doi: 10.1093/ilar.48.4.339. [DOI] [PubMed] [Google Scholar]
- Fernandez HH, Greeley DR, Zweig RM, Wojcieszek J, Mori A, Sussman NM. Istradefylline as monotherapy for Parkinson disease: results of the 6002-US-051 trial. Parkinsonism Relat Disord. 2010;16:16–20. doi: 10.1016/j.parkreldis.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Fornai F, Vaglini F, Maggio R, Bonuccelli U, Corsini GU. Species differences in the role of excitatory amino acids in experimental parkinsonism. Neuroscience and biobehavioral reviews. 1997;21:401–415. doi: 10.1016/s0149-7634(96)00042-5. [DOI] [PubMed] [Google Scholar]
- Forno LS, DeLanney LE, Irwin I, Langston JW. Similarities and differences between MPTP-induced parkinsonsim and Parkinson’s disease. Neuropathologic considerations. Adv Neurol. 1993;60:600–608. [PubMed] [Google Scholar]
- Forno LS, Langston JW, DeLanney LE, Irwin I, Ricaurte GA. Locus ceruleus lesions and eosinophilic inclusions in MPTP-treated monkeys. Ann Neurol. 1986;20:449–455. doi: 10.1002/ana.410200403. [DOI] [PubMed] [Google Scholar]
- Fox SH, Brotchie JM. The MPTP-lesioned non-human primate models of Parkinson’s disease. Past, present, and future. Prog Brain Res. 2010;184:133–157. doi: 10.1016/S0079-6123(10)84007-5. [DOI] [PubMed] [Google Scholar]
- Gibb WR, Terruli M, Lees AJ, Jenner P, Marsden CD. The evolution and distribution of morphological changes in the nervous system of the common marmoset following the acute administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Mov Disord. 1989;4:53–74. doi: 10.1002/mds.870040109. [DOI] [PubMed] [Google Scholar]
- Hadj Tahar A, Ekesbo A, Gregoire L, Bangassoro E, Svensson KA, Tedroff J, Bedard PJ. Effects of acute and repeated treatment with a novel dopamine D2 receptor ligand on L-DOPA-induced dyskinesias in MPTP monkeys. Eur J Pharmacol. 2001;412:247–254. doi: 10.1016/s0014-2999(01)00737-3. [DOI] [PubMed] [Google Scholar]
- Iravani MM, Jenner P. Mechanisms underlying the onset and expression of levodopa-induced dyskinesia and their pharmacological manipulation. J Neural Transm. 2011;118:1661–1690. doi: 10.1007/s00702-011-0698-2. [DOI] [PubMed] [Google Scholar]
- Jakowec MW, Petzinger GM. 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned model of parkinson’s disease, with emphasis on mice and nonhuman primates. Comparative medicine. 2004;54:497–513. [PubMed] [Google Scholar]
- Jenner P. Factors influencing the onset and persistence of dyskinesia in MPTP-treated primates. Ann Neurol. 2000;47:S90–104. [PubMed] [Google Scholar]
- Jenner P. The MPTP-treated primate as a model of motor complications in PD: primate model of motor complications. Neurology. 2003;61:S4–11. doi: 10.1212/wnl.61.6_suppl_3.s4. [DOI] [PubMed] [Google Scholar]
- Jenner P. Molecular mechanisms of L-DOPA-induced dyskinesia. Nat Rev Neurosci. 2008;9:665–677. doi: 10.1038/nrn2471. [DOI] [PubMed] [Google Scholar]
- Johannessen JN, Chiueh CC, Burns RS, Markey SP. Differences in the metabolism of MPTP in the rodent and primate parallel differences in sensitivity to its neurotoxic effects. Life Sci. 1985;36:219–224. doi: 10.1016/0024-3205(85)90062-1. [DOI] [PubMed] [Google Scholar]
- Johnston TH, Fox SH, McIldowie MJ, Piggott MJ, Brotchie JM. Reduction of L-DOPA-induced dyskinesia by the selective metabotropic glutamate receptor 5 antagonist 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned macaque model of Parkinson’s disease. J Pharmacol Exp Ther. 2010;333:865–873. doi: 10.1124/jpet.110.166629. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Emborg ME, Bloch J, Ma SY, Chu Y, Leventhal L, McBride J, Chen EY, Palfi S, Roitberg BZ, Brown WD, Holden JE, Pyzalski R, Taylor MD, Carvey P, Ling Z, Trono D, Hantraye P, Deglon N, Aebischer P. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson’s disease. Science. 2000;290:767–773. doi: 10.1126/science.290.5492.767. [DOI] [PubMed] [Google Scholar]
- Lang AE, Gill S, Patel NK, Lozano A, Nutt JG, Penn R, Brooks DJ, Hotton G, Moro E, Heywood P, Brodsky MA, Burchiel K, Kelly P, Dalvi A, Scott B, Stacy M, Turner D, Wooten VG, Elias WJ, Laws ER, Dhawan V, Stoessl AJ, Matcham J, Coffey RJ, Traub M. Randomized controlled trial of intraputamenal glial cell line-derived neurotrophic factor infusion in Parkinson disease. Ann Neurol. 2006;59:459–466. doi: 10.1002/ana.20737. [DOI] [PubMed] [Google Scholar]
- Liang L, DeLong MR, Papa SM. Inversion of dopamine responses in striatal medium spiny neurons and involuntary movements. J Neurosci. 2008;28:7537–7547. doi: 10.1523/JNEUROSCI.1176-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luquin MR, Laguna J, Obeso JA. Selective D2 receptor stimulation induces dyskinesia in parkinsonian monkeys. Ann Neurol. 1992;31:551–554. doi: 10.1002/ana.410310514. [DOI] [PubMed] [Google Scholar]
- Luquin MR, Montoro RJ, Guillen J, Saldise L, Insausti R, Del Rio J, Lopez-Barneo J. Recovery of chronic parkinsonian monkeys by autotransplants of carotid body cell aggregates into putamen. Neuron. 1999;22:743–750. doi: 10.1016/s0896-6273(00)80733-3. [DOI] [PubMed] [Google Scholar]
- Marin C, Rodriguez-Oroz MC, Obeso JA. Motor complications in Parkinson’s disease and the clinical significance of rotational behavior in the rat: have we wasted our time? Exp Neurol. 2006;197:269–274. doi: 10.1016/j.expneurol.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Morin N, Jourdain VA, Di Paolo T. Modeling dyskinesia in animal models of Parkinson disease. Exp Neurol. 2013 doi: 10.1016/j.expneurol.2013.01.024. [DOI] [PubMed] [Google Scholar]
- Mounayar S, Boulet S, Tande D, Jan C, Pessiglione M, Hirsch EC, Feger J, Savasta M, Francois C, Tremblay L. A new model to study compensatory mechanisms in MPTP-treated monkeys exhibiting recovery. Brain. 2007;130:2898–2914. doi: 10.1093/brain/awm208. [DOI] [PubMed] [Google Scholar]
- Olanow CW, Kordower JH. Modeling Parkinson’s disease. Ann Neurol. 2009;66:432–436. doi: 10.1002/ana.21832. [DOI] [PubMed] [Google Scholar]
- Palfi S, Leventhal L, Chu Y, Ma SY, Emborg M, Bakay R, Deglon N, Hantraye P, Aebischer P, Kordower JH. Lentivirally delivered glial cell line-derived neurotrophic factor increases the number of striatal dopaminergic neurons in primate models of nigrostriatal degeneration. J Neurosci. 2002;22:4942–4954. doi: 10.1523/JNEUROSCI.22-12-04942.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papa SM, Chase TN. Levodopa-induced dyskinesias improved by a glutamate antagonist in Parkinsonian monkeys. Ann Neurol. 1996;39:574–578. doi: 10.1002/ana.410390505. [DOI] [PubMed] [Google Scholar]
- Papa SM, Engber TM, Kask AM, Chase TN. Motor fluctuations in levodopa treated parkinsonian rats: relation to lesion extent and treatment duration. Brain Res. 1994;662:69–74. doi: 10.1016/0006-8993(94)90796-x. [DOI] [PubMed] [Google Scholar]
- Przedborski S, Jackson-Lewis V, Naini AB, Jakowec M, Petzinger G, Miller R, Akram M. The parkinsonian toxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): a technical review of its utility and safety. J Neurochem. 2001;76:1265–1274. doi: 10.1046/j.1471-4159.2001.00183.x. [DOI] [PubMed] [Google Scholar]
- R, D.C.T. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Viena, Austria: 2011. [Google Scholar]
- Samadi P, Gregoire L, Morissette M, Calon F, Hadj Tahar A, Dridi M, Belanger N, Meltzer LT, Bedard PJ, Di Paolo T. mGluR5 metabotropic glutamate receptors and dyskinesias in MPTP monkeys. Neurobiol Aging. 2008;29:1040–1051. doi: 10.1016/j.neurobiolaging.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Schneider JS, Roeltgen DP. Delayed matching-to-sample, object retrieval, and discrimination reversal deficits in chronic low dose MPTP-treated monkeys. Brain Res. 1993;615:351–354. doi: 10.1016/0006-8993(93)90049-s. [DOI] [PubMed] [Google Scholar]
- Schneider JS, Tinker JP, Decamp E. Clonidine improves attentional and memory components of delayed response performance in a model of early Parkinsonism. Behavioural brain research. 2010;211:236–239. doi: 10.1016/j.bbr.2010.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JS, Tinker JP, Van Velson M, Menzaghi F, Lloyd GK. Nicotinic acetylcholine receptor agonist SIB-1508Y improves cognitive functioning in chronic low-dose MPTP-treated monkeys. The J Pharmacol Exp Ther. 1999;290:731–739. [PubMed] [Google Scholar]
- Song DD, Haber SN. Striatal responses to partial dopaminergic lesion: evidence for compensatory sprouting. J Neurosci. 2000;20:5102–5114. doi: 10.1523/JNEUROSCI.20-13-05102.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockwell KA, Scheller D, Rose S, Jackson MJ, Tayarani-Binazir K, Iravani MM, Smith LA, Olanow CW, Jenner P. Continuous administration of rotigotine to MPTP-treated common marmosets enhances anti-parkinsonian activity and reduces dyskinesia induction. Exp Neurol. 2009;219:533–542. doi: 10.1016/j.expneurol.2009.07.011. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Elsworth JD, Roth RH, Sladek JR, Jr, Redmond DE., Jr Severe long-term 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced parkinsonism in the vervet monkey (Cercopithecus aethiops sabaeus) Neuroscience. 1997;81:745–755. doi: 10.1016/s0306-4522(97)00214-5. [DOI] [PubMed] [Google Scholar]
- Vazquez-Claverie M, Garrido-Gil P, San Sebastian W, Izal-Azcarate A, Belzunegui S, Marcilla I, Lopez B, Luquin MR. Acute and chronic 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine administrations elicit similar microglial activation in the substantia nigra of monkeys. J Neuropathol Exp Neurol. 2009;68:977–984. doi: 10.1097/NEN.0b013e3181b35e41. [DOI] [PubMed] [Google Scholar]
- Waters CM, Hunt SP, Jenner P, Marsden CD. An immunohistochemical study of the acute and long-term effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in the marmoset. Neuroscience. 1987;23:1025–1039. doi: 10.1016/0306-4522(87)90178-3. [DOI] [PubMed] [Google Scholar]
- Winkler C, Kirik D, Bjorklund A, Cenci MA. L-DOPA-induced dyskinesia in the intrastriatal 6-hydroxydopamine model of parkinson’s disease: relation to motor and cellular parameters of nigrostriatal function. Neurobiol Dis. 2002;10:165–186. doi: 10.1006/nbdi.2002.0499. [DOI] [PubMed] [Google Scholar]


