Abstract
A fundamental limitation in devising new therapeutic strategies for killing cancer cells with DNA damaging agents is the need to identify synthetic lethal interactions between tumor-specific mutations and components of the DNA damage response (DDR) in vivo. The stress-activated p38MAPK/MK2 pathway is a critical component of the DDR network in p53-deficient tumor cells in vitro. To explore the relevance of this pathway for cancer therapy in vivo, we developed a specific gene targeting strategy in which Cre-mediated recombination simultaneously creates isogenic MK2-proficient and MK2-deficient tumors within a single animal. This allows direct identification of MK2 synthetic lethality with mutations that promote tumor development or control response to genotoxic treatment. In an autochthonous model of NSCLC, we demonstrate that MK2 is responsible for resistance of p53-deficient tumors to cisplatin, indicating synthetic lethality between p53 and MK2 can successfully be exploited for enhanced sensitization of tumors to DNA damaging chemotherapeutics in vivo.
Introduction
DNA damage signaling and checkpoint control pathways are among the most commonly mutated networks in human tumors (Negrini et al., 2010). Although dampening of DNA repair pathways and suppression of DNA damage signaling networks that arrest the cell cycle after genotoxic stress enhances genomic instability during tumor development, it also furnishes an ‘Achill’s heel’ for anti-cancer therapy. Emerging data suggest that synthetic lethal interactions between mutated oncogenes or tumor suppressor genes with molecules involved in the DNA damage response could be used to preferentially kill cancer cells, by exploiting dependencies that are not shared by normal tissue (Lord and Ashworth, 2012; Morandell and Yaffe, 2012; Reinhardt et al., 2009).
In response to DNA damage, cells activate complex signaling networks that mediate DNA repair and cell cycle arrest, or if the damage is extensive, trigger apoptosis (Ciccia and Elledge, 2010). Two canonical protein kinase pathways in both normal and cancer cells arrest the cell cycle in response to damaged DNA: the ATR-Chk1 and the ATM-Chk2 pathway. We previously identified a third cell cycle checkpoint pathway mediated by the stress-activated protein kinases p38 MAPK and its substrate MAPKAP Kinase-2 (MK2). This MK2 pathway is critical for arresting the cell cycle after genotoxic stress, including cisplatin-induced DNA crosslinks and topoisomerase inhibitor-induced DNA strand breaks only in tumor cells that lack functional p53, while MK2 is dispensable for checkpoint function in p53-proficient non-tumor cells (Manke et al., 2005, Reinhardt et al., 2007). Importantly, both the ATR-Chk1 pathway and the p38-MK2 pathway are required for effective cell cycle checkpoint function in the absence of p53 (Reinhardt et al., 2010). Under this condition, cytoplasmic MK2 orchestrates a cell cycle checkpoint through the post-transcriptional regulation of gene expression by modulating the function of RNA-binding proteins (RBP). MK2 phosphorylates the RNA binding protein hnRNPA0, inducing its association with, and stabilization of the mRNA of Gadd45α, a known CDK inhibitor (Reinhardt et al., 2010). In addition, MK2 induces miR-34c in response to DNA damage in cells that lack p53. MiR-34c then represses the translation of c-Myc to promote S-phase arrest (Cannell et al., 2010).
Our previous observations based on immortalized tumor cell lines and xenograft tumors in nude mice (Reinhardt et al., 2007; Reinhardt et al., 2010) suggest that therapeutic targeting of MK2 might be a useful strategy to enhance killing of p53-defective tumors by DNA-damaging chemotherapy in situ. Normal host tissues are expected to be protected from the enhanced genotoxicity of MK2 inhibition due to the presence of functional p53.
To directly test this hypothesis and study the role of MK2 in tumor development and chemotherapeutic treatment in vivo, we generated a novel type of conditional knock out mouse in which we can simultaneously generate MK2-expressing and MK2-null tumors within a single animal. Here we use this approach to study the role of MK2 in a well-established autochthonous model of non-small-cell lung cancer (NSCLC) (Jackson et al., 2005; Jackson et al., 2001; Johnson et al., 2001) that closely recapitulates the histopathology and therapeutic response of the human disease. In this model, the expression of oncogenic KrasG12D (found in ~30% of human NSCLCs) initiates the formation of lung adenomas in mice that are either wild type or deficient for p53 (mutated in ~50% of human NSCLCs) and differ only in MK2 expression status. This ability to generate otherwise genetically identical tumors in individual mice that differ in only a single genetic locus, and monitor their response to treatment allows a direct in vivo analysis of synthetic lethal interactions in a solid tumor model.
Results
A Cre-versible strategy for comparing wild type and knockout tumors in a single animal
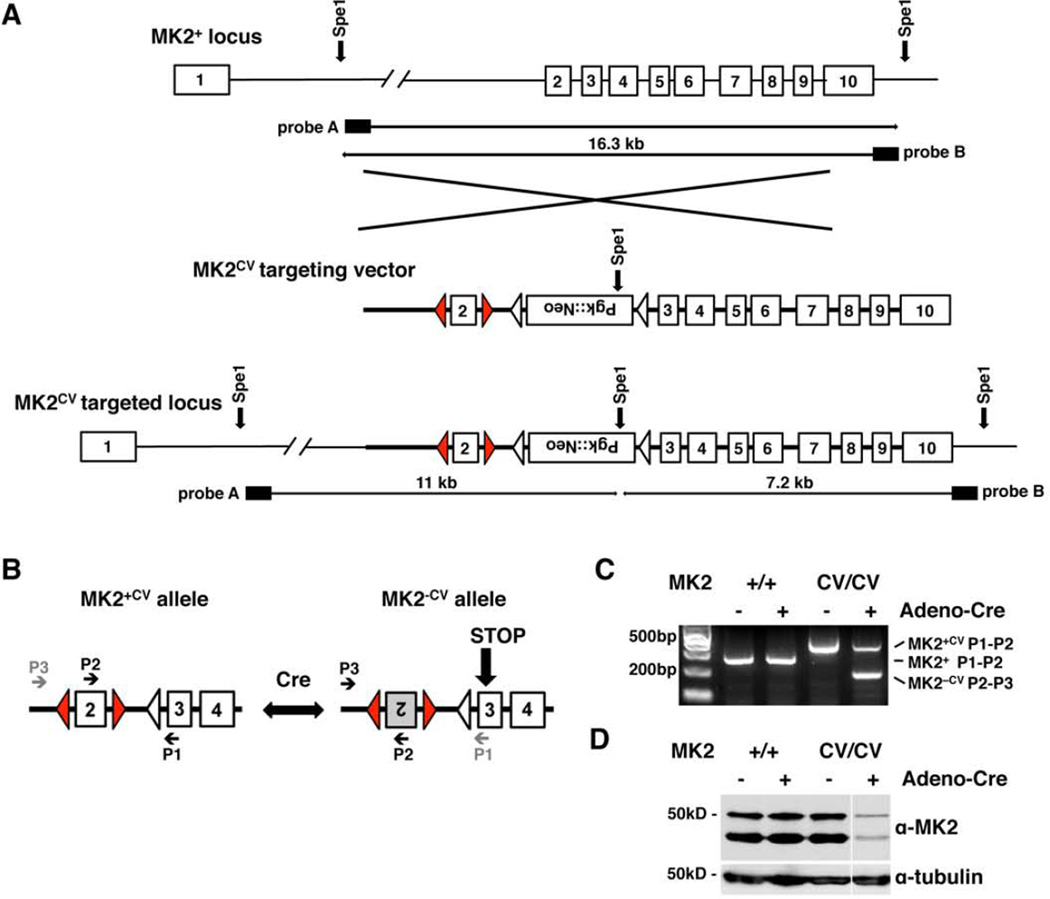
To simultaneously study MK2-proficient and MK2-deficient tumors within a single animal we generated a new mouse model with a stochastic reversible MK2 knockout phenotype. In this inducible model, Cre-mediated recombination switches exon 2 reversibly from an MK2 expressing state to an MK2 inactive state and back. To generate mice carrying “Cre-versible” alleles of MK2 (MK2CV), we constructed a targeting vector with LoxP sites, the recognition sites for Cre-recombinase, flanking exon 2 in opposing orientations (Figures 1A, S1A). Upon Cre-mediated recombination the region flanked by the LoxP sites is inverted rather than excised as in classical conditional alleles, where the LoxP sites are oriented in the same direction (Tronche et al., 2002). Consequently, exon 2 can invert reversibly from an MK2-expressing orientation (MK2+CV) to an MK2-null orientation (MK2−CV) and back as long as Cre is active in the cell (Figure 1B). Inversion of exon 2 disrupts the splice donor and acceptor sites, resulting in an mRNA product with exon 1 directly fused to exon 3. This leads to a frame shift and a STOP codon in the beginning of exon 3, creating a highly truncated reading frame that is not translated into functional MK2 proteins (i.e. loss of both the 46 KDa and 42 KDa isoforms corresponding to Uniprot P49137-1 and P49137-2 for human MK2).
Figure 1. A Cre-versible strategy for comparing wild type and knockout tumors in a single animal.
(A) Schematic representation of the wild type MK2 (MK2+) genomic locus (top), the MK2 Cre-versible (MK2CV) targeting vector (middle) and the targeted MK2CV genomic locus (bottom). MK2 exons 1 to 10: white boxes. LoxP sites: red triangles, FRT sites: white triangles. The final targeting construct consists of 2.7kB of the 5’ flanking sequence before exon 2, the first LoxP site, exon 2 followed by a second LoxP site in reverse complement sequence as the first LoxP site, a FRT-Pgk::Neo-FRT cassette and exons 3 to 10. Positions of SpeI restriction sites and probes A and B for Southern Blot detection of targeted ES cell are highlighted.
(B) Diagram of exons 2 to 4 of the MK2CV allele after FlPe-mediated excision of the Pgk::Neo-cassette: Upon Cre-mediated recombination, exon 2 can invert reversibly between the MK2-expressing (MK2+CV) and the MK2-negative orientation (MK2−CV). Inversion of exon 2 results in a “STOP” codon early in exon 3. P1, P2 and P3 indicate primers for genotyping.
(C) PCR analysis of MK2+/+ and MK2CV/CV MEFs infected with adenoviral Cre-recombinase (Adeno-Cre) or control, positions for primers P1, P2 and P3: Figure 1B. MK2+ or MK2+CV alleles yield 241bp or 333bp PCR products with primers P1 and P2, respectively. The inverted MK2−CV allele yields a 190bp PCR product with primers P2 and P3, the distance between primers P1 and P3 is too long to form a product.
(D) Western Blot of MK2+/+ and MK2CV/CV MEFs infected with Adeno-Cre or control: MK2+ and MK2+CV alleles encode for two MK2 protein isoforms with 46kD and 42kD, respectively. No protein product is formed from the MK2−CV allele, γ-tubulin: loading control.
In vitro infection of mouse embryonic fibroblasts (MEFs) from homozygous mice carrying two copies of the MK2Cre-versible allele (MK2CV/CV) with adenoviral Cre-recombinase (Adeno-Cre) confirmed the Cre-mediated inversion of exon 2, shown by PCR (Figure 1C) and sequencing of cDNA fragments obtained from RNA of Adeno-Cre-infected MK2CV/CV MEFs (Figures S1B, C). MK2 protein levels were strongly decreased in MK2CV/CV MEFs upon Adeno-Cre as a result of the inversion of exon2 in a significant subset of infected cells (Figure 1D). This Cre-versible allele, when used in combination with murine tumor models, allows altered MK2 expression in a tissue specific manner, and at the same time, generates a mixture of MK2-proficient and MK2-deficient cells in any tissue where Cre-recombinase is active. As a consequence, tumors that are genetically identical except for MK2 expression can be directly compared in vivo in single animals.
MK2-expressing and MK2-deficient tumors develop in a murine autochthonous model of Non-Small Cell Lung Cancer
To explore the role of MK2 in cancer development, progression and response to DNA damaging chemotherapy in an epithelial tumor type in vivo we focused on a well-established autochthonous mouse model of NSCLC. MK2CV/CV mice were crossed against mice containing either wild type TP53 (p53+/+) or biallelic floxed TP53 (p53flox/flox) in combination with a KrasG12D allele preceded by a LoxP-STOP-LoxP cassette (Jackson et al., 2005; Jackson et al., 2001; Johnson et al., 2001) in the endogenous Kras locus (Figure S2A). After intra-tracheal administration of a Cre-recombinase expressing adenovirus (Adeno-Cre), the expression of oncogenic KrasG12D initiates the development of adenomas at 100% penetrance even in a wildtype p53 background (Jackson et al., 2001; Johnson et al., 2001), while concomitant loss of p53 shortens latency and leads to advanced histopathology (Jackson et al., 2005) including adenocarcinomas. Importantly, because Adeno-Cre does not integrate into the genome of infected cells, the MK2 expression state that is established by transient Cre expression in the tumor-initiating cell is maintained throughout further tumor evolution. Thus, this novel Cre-versible mouse model allows comparison of the initiation and/or progression of otherwise genetically identical MK2-expressing and MK2-deficient lung tumors within the same animal and, simultaneously, allows the effects of DNA damaging chemotherapy to be directly studied in each tumor type.
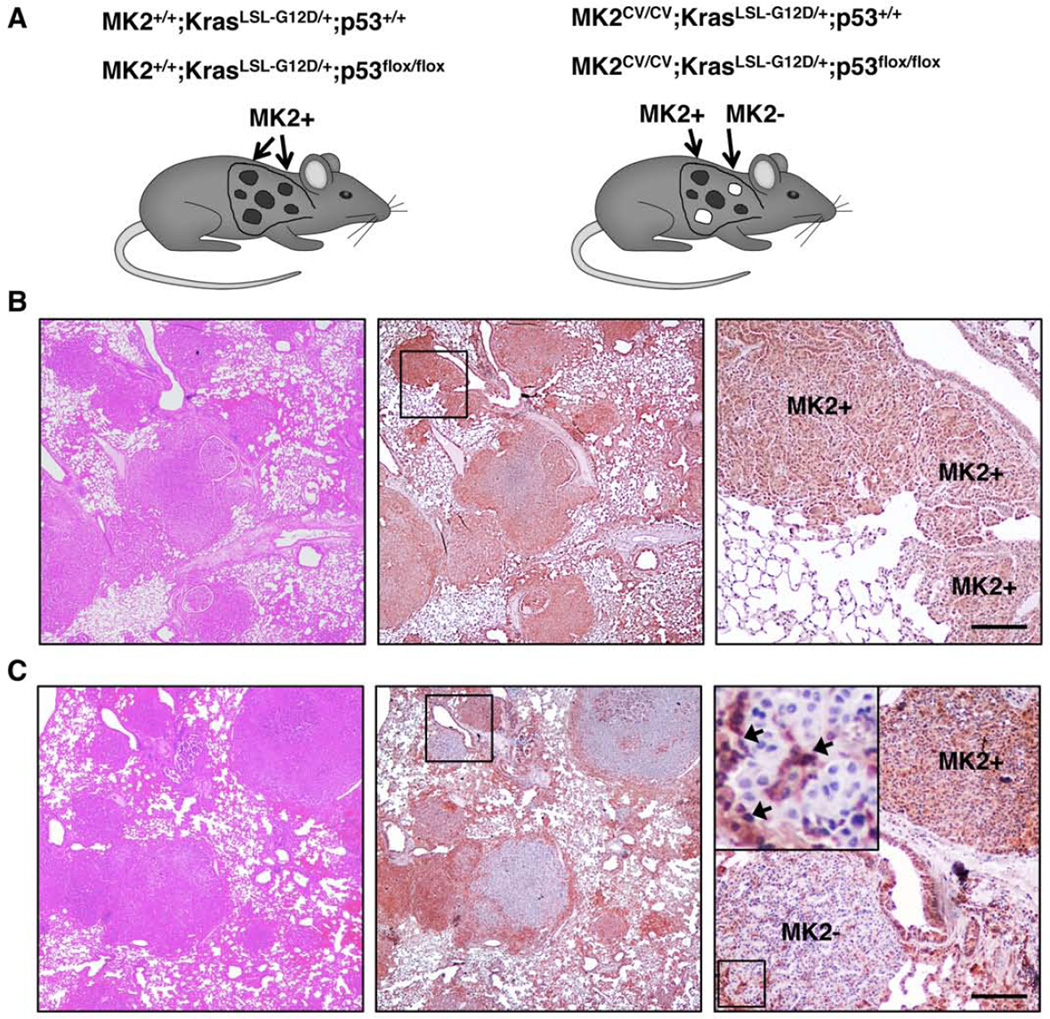
Figure 2A gives an overview over the different mouse lines generated for this study. In MK2+/+ mice both the normal lung tissue and all tumors express MK2 (MK2+) as shown by immunohistochemistry for MK2 expression (Figure 2B for p53flox/floxmice, Figure S2B for p53+/+ mice). In contrast, a subset of MK2CV/CV tumor-initiating cells lose expression of MK2 upon Cre-mediated inversion of exon 2 on both MK2CV alleles, leading to a mixture of MK2-positive (MK2+) and MK2-negative tumors (MK2−) within the same animal (Figures 2C for p53flox/floxmice, Figure S2C for p53+/+ mice). (Since homozygous MK2+CV/+CV and heterozygous MK2+CV/−CV tumors both stain positive for MK2, they are grouped together as MK2+ tumors.) In addition, we observed intense MK2 staining in stromal cells surrounding and infiltrating the tumors (Figure 2C, insert). The histological appearance of both MK2-expressing and MK2-null tumors was comparable to tumors described for the original KrasLSL-G12D;p53+/+ (Jackson et al., 2001; Johnson et al., 2001) and KrasLSL-G12D; p53flox/flox models (Jackson et al., 2005).
Figure 2. MK2-expressing and MK2-deficient tumors develop in a murine autochthonous model of Non-Small Cell Lung Cancer.
(A) Combinations of MK2, Kras and p53 alleles used in this study: MK2+/+ or MK2CV/CV mice harbour one copy of the K-rasLSL-G12D allele in a wildtype p53 background (p53+/+), or they contain two copies of the p53flox allele (p53flox/flox). After Cre-mediated recombination, the respective tumor MK2 status in MK2+/+ and MK2CV/CV mice is indicated as “MK2+” for MK2-expressing tumors, and “MK2-“ for MK2-negative tumors, exclusively found in MK2CV/CV mice.
(B) Tumors from a MK2+/+;KrasLSL-G12D/+;p53fl/fl mouse at the experimental endpoint were stained with haematoxilin and eosin (H&E, left) or by IHC for MK2 (brown staining, middle). Right: close-up of three MK2+ tumors. Scale bar = 50µm
(C) Tumors from a MK2CV/CV;KrasLSL-G12D/+;p53fl/fl mouse at the experimental endpoint, stained as in panel B. Right: close-up of MK2+ tumor (brown staining) and MK2− tumor (blue counterstain only). MK2 expressing stroma cells infiltrate and surround an MK2-negative tumor (arrows). In B and C, the experimental endpoint corresponds to 20% basal weight loss of tumor bearing animals.
When we compared the initial onset of tumors, we observed that MK2CV/CV mice develop tumors at the same latency and frequency as MK2+/+ mice (Figure S2D). While the MK2 expression status has no influence on tumor initiation, tumors in p53flox/flox mice had progressed further at earlier timepoints than tumors with wildtype p53, as the loss of p53 is known to promote the progression of KrasG12D-induced lung adenocarcinomas (Jackson et al., 2005). Therefore, for p53flox/flox mice, 6 weeks was chosen as the earliest time point for quantification of tumor areas, while 9 weeks was chosen for mice with wild type p53. At these early time points, the total tumor burden in both models was similar with 11% of the lung area occupied by tumor for both MK2+/+ and MK2CV/CV in the p53-deficient model (KrasG12D/+;p53Δ/Δ) (Figure S2D). In a p53 wildtype background (KrasG12D/+;p53+/+), 11% of lung area in MK2+/+ mice and 9% in MK2CV/CV mice were occupied by tumors (Figure S2D). Thus, tumor initiation in this autochthonous model of NSCLC is independent of the tumor MK2 status.
MK2-deficient tumors dominate the total tumor burden over time in a manner that is dependent on the loss of p53
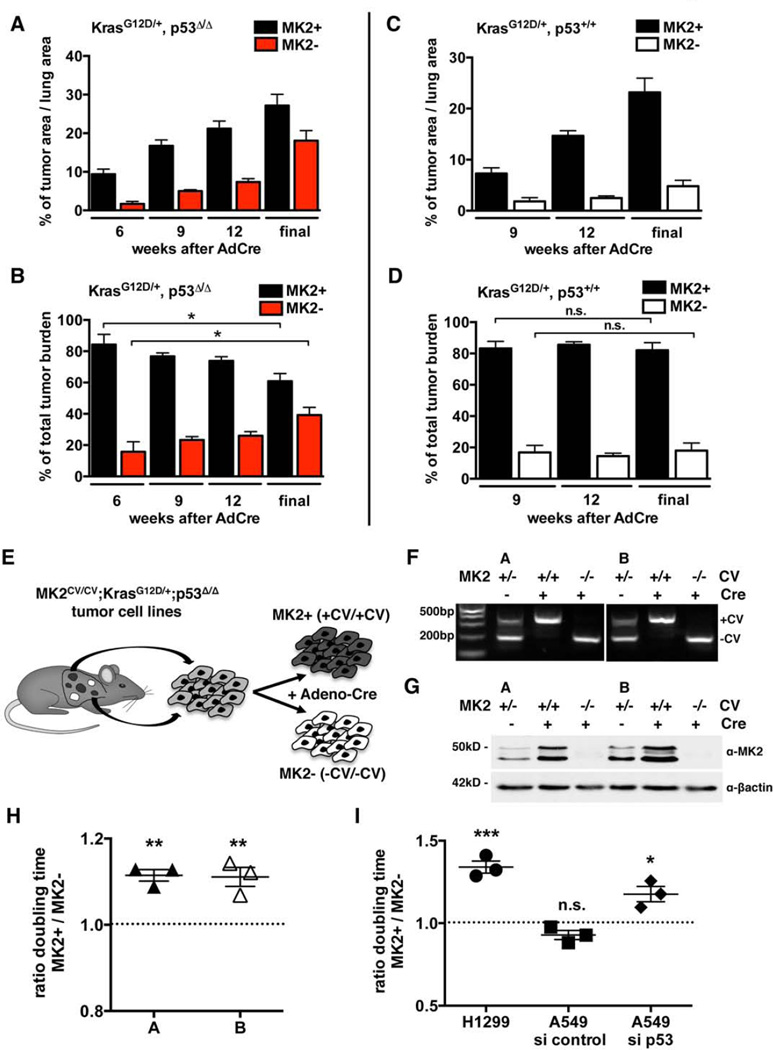
While the presence or absence of MK2 expression has no effect on tumor initiation, it did appear to affect tumor progression. For p53-null tumors, the total tumor burden of MK2+ and MK2− tumors combined progressed over time from 11% at 6 weeks to 45% of total lung area (Figure 3A). In p53-proficient tumors, the tumor burden increased from 9% after 9 weeks to 28% of total lung area (Figure 3C). When we separated the tumors according to MK2-expression, we observed that, over time, the increase of MK2/p53 double knockout (DKO) tumor areas (MK2−) outpaced that of MK2-expressing tumors (MK2+) (Figure 3A). This became more obvious, when we quantified the relative lung area occupied by MK2+ or MK2− tumors as percentage of total tumor burden. At the earliest time points MK2+ tumors account for 84% of the total tumor area in both p53fl/fl and p53+/+ mice, while 16% of tumors are MK2− (Figures 3B, D). Interestingly, MK2/p53 DKO tumors subsequently accounted for 23% of total tumor burden after 9 weeks, 26% after 12 weeks and 39% of the total tumor burden at the experimental endpoint. In striking contrast, in the presence of wild-type p53, the proportion of MK2− tumors remained constant over time (Figures 3B, D).
Figure 3. MK2-deficient tumors dominate the total tumor progression over time in a manner that is dependent on the loss of p53.
(A, B) Quantification of MK2CV/CV;KrasG12D/+;p53Δ/Δ tumors at weeks 6, 9 and 12 after tumor induction and at experimental endpoint (final): MK2+ and MK2− tumor areas are shown as percentage of total lung area (A) and relative percentages of total tumor burden (B). (A–D): n = 4–5 mice per time point, *: p ≤ 0.02, error bars indicate SEM.
(C–D) Quantification of MK2CV/CV;K-rasG12D/+;p53+/+ tumors at weeks 9 and 12 after tumor induction and at experimental endpoint (final): MK2+ and MK2− tumor areas shown as percentage of total lung area (C) and relative percentages of total tumor burden (D).
(E) Generation of isogenic MK2+ and MK2− KrasG12D/+;p53Δ/Δ murine NSCLC cell lines.
(F) PCR analysis of two isogenic MK2+ and MK2− murine NSCLC cell line pairs, A and B, before (−Cre) and after infection with Adeno-Cre (+Cre). Initial cell lines harbored one MK2 expressing (+CV) and one MK2 negative (–CV) allele. After Cre infection, selected clones inverted one allele, resulting in homocygous MK2+CV/+CV (MK2+) or MK2−CV/−CV (MK2−) cells.
(G) Western Blot of isogenic MK2+ and MK2− murine NSCLC cell line pairs. β-actin: loading control.
(H) Ratio of doubling times between MK2+ and MK2− isogenic cell lines A and B. (G–H): n = 3 independent experiments, *: p<0.02, **: p<0.008, *** p<0.001, error bars = SEM.
(I) Ratio of doubling times between H1299 cells stably expressing a control hairpin (MK2+) or hairpin against MK2 (MK2−) and A549 cells with or without siRNA against MK2 in combination with or without siRNA against 53.
This increase in MK2/p53 DKO tumor burden during tumor progression could be caused by enhanced proliferation, by reduced cell death, or by a combination of these two mechanisms. To examine this, we generated tumor cell lines from MK2CV/CV;KrasLSLG12D/+; p53flox/flox mice. To avoid heterogeneity in cell lines from different tumors, we used the Cre-versible MK2 allele to create isogenic MK2+ and MK2− clones from the same parental cell lines by re-infecting single cell clones in culture with Adeno-Cre (Figure 3E). Two pairs of NSCLC cell lines, A and B, were chosen for further study. For both cell lines we generated one MK2+ clone with both alleles oriented in the MK2-expressing orientation (MK2+CV/+CV), and one MK2− clone with both alleles inverted to the MK2-negative orientation (MK2−CV/−CV) (Figure 3F, G).
Comparison of proliferation rates revealed that MK2/p53-DKO murine NSCLC cells doubled on average 11% faster then their isogenic MK2-proficient counterparts (Figure 3H). To investigate whether this MK2-dependent suppression of proliferation in p53-deficient cells was unique to murine lung tumors, we knocked down MK2 in two human NSCLC cell lines. In p53-deficient H1299 cells, shRNA knock down of MK2 shortened their doubling time by 34% (Figure 3I). In contrast, in p53-proficient A549 cells the doubling time was largely independent on MK2 expression (Figure 3I). However, combined knock-down of p53 and MK2 in these cells shortened their doubling time by 18% relative to p53-knock-down/MK2 expressing A549 cells (Figure 3I).
These data clearly indicate, that the combined loss of MK2 and p53 accelerates proliferation both in vitro in murine and human NSCLC cell lines and in vivo in a murine autochthonous model of NSCLC. When integrated over time, these moderate differences in cell proliferation result in a progressive increase in MK2-negative tumor burden over MK2-expressing tumors. In contrast, no such difference exists between MK2+ and MK2− tumors that retain p53 function.
MK2 is required for survival after DNA damage in p53-deficient lung tumor cells
We have previously shown in vitro that U2OS and HeLa tumor cell lines with a defective p53-pathway are critically dependent upon a cytoplasmic p38/MK2 pathway for prolonged G2/M and G1/S checkpoint maintenance in response to chemotherapy-induced DNA damage (Reinhardt et al., 2007; Reinhardt et al., 2010). Platinum-based compounds are widely used as frontline chemotherapeutic agents for the treatment of NSCLC patients (Azzoli et al., 2009). We therefore investigated if targeting of MK2 could be a useful strategy to preferentially enhance DNA damaged-induced killing of p53-defective NSCLC cells derived from primary tumors.
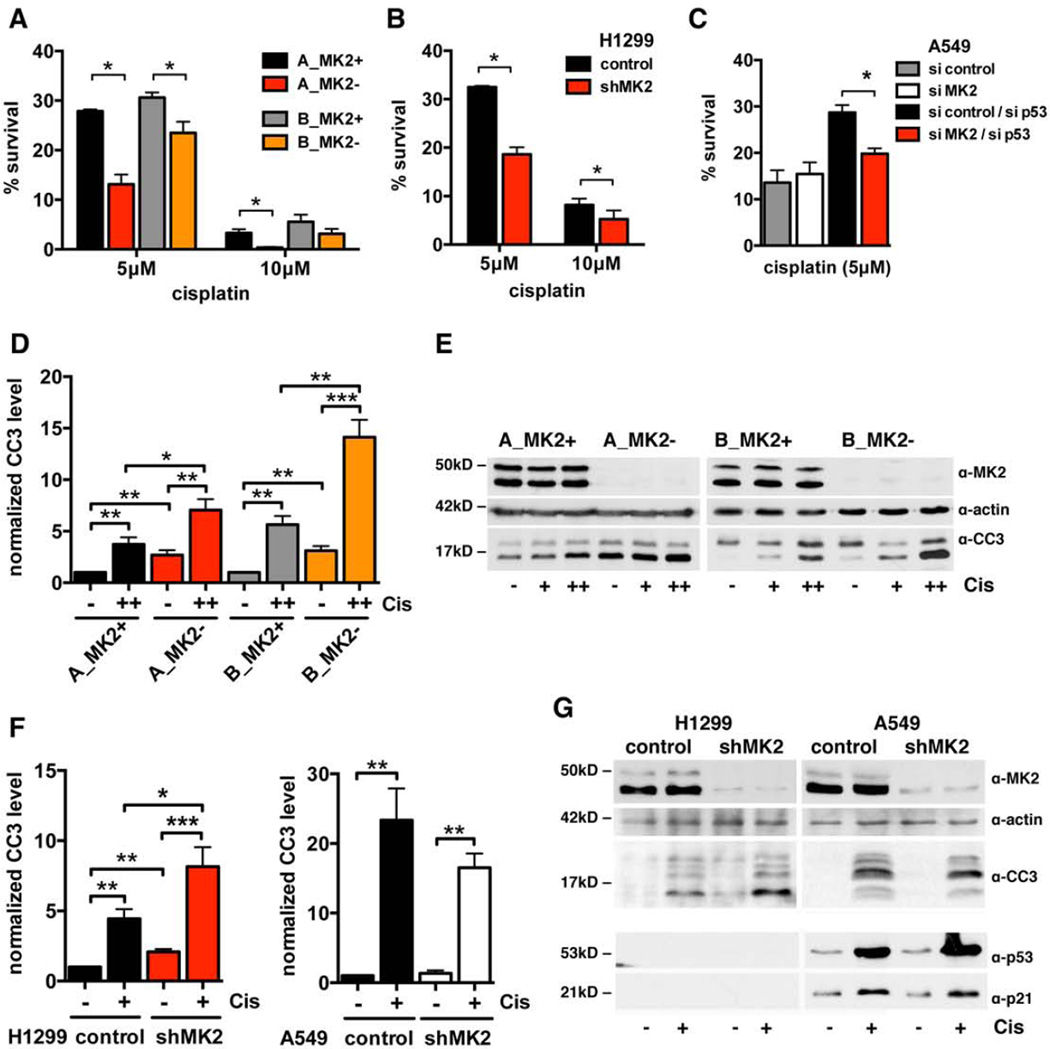
Isogenic MK2+ and MK2− murine NSCLC tumor cells were treated with cisplatin and assayed for clonogenic survival. MK2− cells showed a strong increase in cisplatin-sensitivity (Figure 4A). While on average 30% of MK2+ cells survive treatment with 5µM cisplatin, loss of MK2 reduced the survival to between 13% (cell line A) and 24% (cell line B) survival. Treatment with 10µM cisplatin resulted in similar differences in survival between the MK2+ and MK2− cell lines. This same chemo-sensitizing effect upon loss of MK2 is observed in the human p53-deficient cell line H1299 (Figure 4B). Knock down of MK2 in this cell line reduced survival following treatment with 5µM cisplatin to 19% compared to 33% of cells with intact MK2 levels. Significant differences in survival were also observed following treatment with 10µM cisplatin. In striking contrast, in A549 cells with functional p53 no significant increase in chemo-sensitivity was observed following MK2-knockdown. Knock down of p53 in these cells actually improved survival in the presence of MK2, while a combined knock down of MK2 and p53 rendered this cell line more sensitive to cisplatin treatment, leading to a decrease in survival from 29% to 20% (Figure 4C).
Figure 4. Loss of MK2 results in increased chemo-sensitivity in p53-deficient tumor cells in vitro.
(A) Quantification of clonogenic survival assays for isogenic MK2+ and MK2− murine NSCLC cell line pairs A and B, treated with vehicle, 5µM or 10µM cisplatin. Assays were performed in triplicate for each condition and normalized to control-treated cells. (A–C): n = 3 independent experiments, *: p<0.05, error bars = SEM.
(B,C) Quantification of clonogenic survival assays for H1299 cells (B) stably expressing a control hairpin or hairpin against MK2 and for A549 cells (C) with and without siRNA against MK2 in combination with or without siRNA against p53.
(D) Quantification of Western blots for Cleaved Caspase 3 (CC3) levels in isogenic MK2+ and MK2− murine NSCLC cell line pairs A and B. Cells were treated with vehicle or cisplatin (+: 5µM, ++ 10µM) for 24h. (D–F): n = 5 independent experiments, A549: n = 3, *: p<0.05, **: p<0.09 ***: p<0.0008, error bars = SEM. CC3 levels were normalized to vehicle-treated MK2+ cells.
(E) Representative Western Blots for CC3 and MK2 levels in murine NSCLC cell lines after cisplatin treatment. β-actin is a loading control.
(F) Quantification of Western blots for CC3 in human NSCLC cell lines H1299 and A549 stably expressing a control hairpin or hairpin against MK2. Cells were treated with cisplatin (+: 10µM) or vehicle for 24h.
(G) Representative Western Blot for CC3 and MK2 levels in human NSCLC cell lines in response to cisplatin treatment. The induction of p53 and p21 in A549 cells in response to cisplatin is shown.
To further probe the mechanism of differential chemo-sensitivity in more detail, we monitored the extent of apoptosis in MK2+ and MK2− cells in response to cisplatin treatment by measuring cleaved caspase 3 (CC3) levels. Interestingly, murine MK2/p53-DKO NSCLC cells (Figures 4D, E) as well as human H1299 cells stably knocked down for MK2 (Figures 4F, G) displayed a twofold increase in CC3 levels even in the absence of cisplatin-induced DNA-damage. Despite this, we consistently observed a net increase in the proliferation of these cells (Figure 3, Figure S4, see Discussion). Following cisplatin treatment, both MK2− and MK2+ cells showed enhanced cleavage of caspase 3. However, cells lacking both MK2 and p53 displayed much higher levels of CC3 in both murine NSCLC cell lines A and B (Figures 4D, E) and human H1299 cells (Figures 4F, G). In contrast, the extent of caspase-3 cleavage in cisplatin-treated p53-proficient A549 cells was independent of MK2 status (Figures 4F, G). These data extend our previous findings in other cell types (Reinhardt et al., 2007; Reinhardt et al., 2010) and implicate MK2-inhibition as a mechanism to sensitize p53-deficient NSCLC tumor cell lines to clinically relevant chemotherapeutic treatments.
The enhanced basal rate of proliferation that we observed when murine and human NSCLC cells were depleted of both MK2 and p53 was completely lost following 5µM cisplatin treatment (Figure S4A, B). After DNA damage, one of the MK2− murine NSCLC lines (cell line A) actually proliferated slower than its MK2+ counterpart, while for all other cell lines (cell line B, H1299, A549), there was no statistically significant difference in relative proliferation rates after damage between MK2 + and MK2− cells. In all cell lines the extent of cell proliferation was markedly reduced following cisplatin treatment (Figures S4C – G).
MK2 and p53 co-deficiency in an autochthonous model of NSCLC results in dramatic chemo-sensitivity
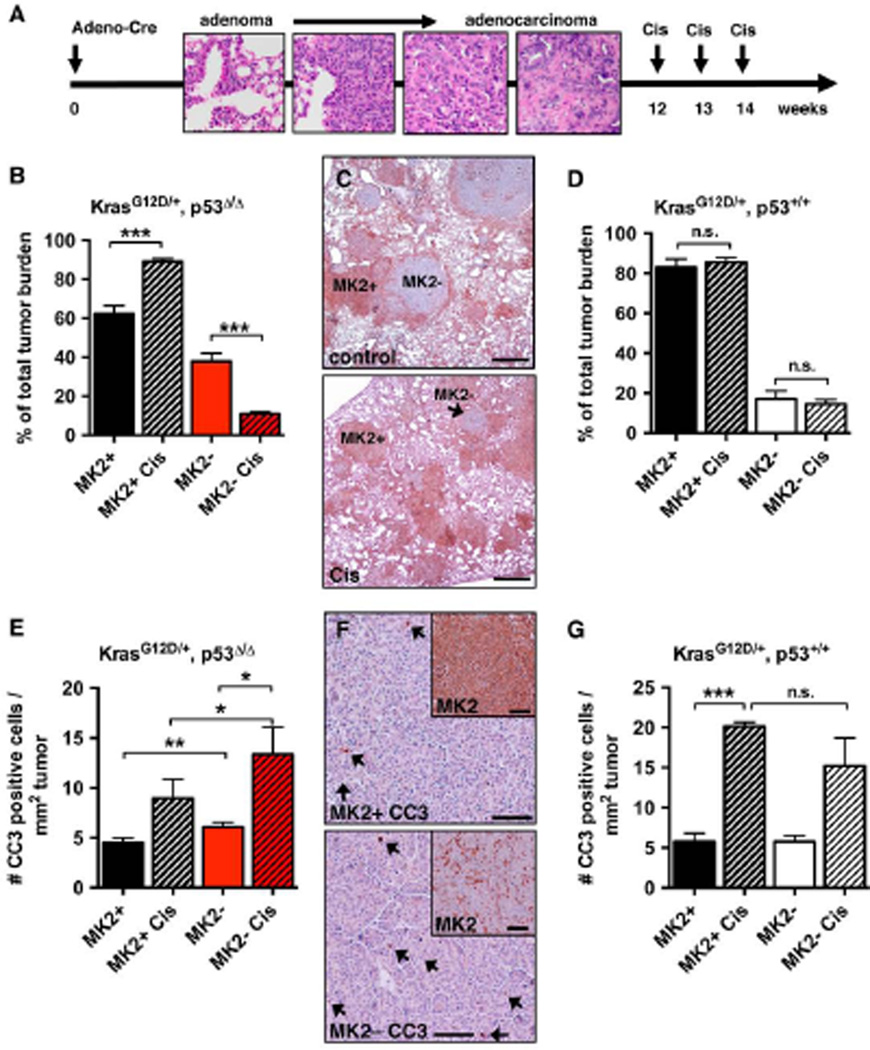
To investigate whether these in vitro observations can be extended to the response of autochthonous p53-proficient and p53-deficient lung tumors in vivo, individual animals bearing a mixture of MK2+ and MK2− lung tumors were treated with 3 doses of cisplatin (Cis, 5mg/kg, i.p.) at 12, 13 and 14 weeks after tumor induction (Figure 5A). The relative ratios of MK2+ versus MK2− tumor areas in cisplatin treated mice and vehicle-treated control mice were then compared at the experimental endpoint. In mice with p53-deficient tumors (KrasG12D/+;p53Δ/Δ) the percentage of the total lung tumor burden composed of MK2− tumors was strongly reduced from 39% to 11% following cisplatin treatment (Figures 5B and 5C). In contrast, in a wild type p53 tumor background (KrasG12D/+;p53+/+), there was no difference in the ratio of MK2+ to MK2− lung tumor burden in response to cisplatin treatment (Figure 5D).
Figure 5. MK2 and p53 co-deficiency in an autochthonous model of NSCLC results in dramatic chemo-sensitivity.
(A) Timeline for chemotherapy treatments: Mice were infected with Adeno-Cre at time 0. Cisplatin (5mg/kg, i.p.) was given at 12, 13 and 14 weeks after tumor initiation.
(B) Relative percentages of MK2+ and MK2− KrasG12D/+;p53Δ/Δ lung tumor areas per total tumor burden in mice vehicle-treated or treated with cisplatin at the experimental endpoint. (B–D): n = 6 mice / condition, ***: p=0.0001, error bars = SEM.
(C) IHC of tumor bearing lungs from MK2CV/CV;KrasLSL-G12D/+;p53flox/flox mice at the experimental endpoint. Top: Lung from an untreated mouse. Bottom: Lung from a cisplatin-treated mouse. MK2+ tumors: brown staining, MK2− tumors: blue counterstain only, one example for each tumor type labeled, scale bar = 0.25mm.
(D) Relative percentages of MK2+ and MK2− KrasG12D/+;p53+/+ lung tumor areas per total tumor burden at the experimental endpoint in mice treated with vehicle or cisplatin.
(E) Number of cleaved caspase-3 (CC3) positive cells per mm2 of tumor area in MK2+ and MK2− K-rasG12D/+;p53Δ/Δ lung tumors. Mice were vehicle-treated or treated with cisplatin (10mg/kg, i.p.) 12 weeks after tumor induction. Lung tissue was harvested 48h later and analyzed by IHC for MK2 and CC3, n = 5 mice / condition, *: p<0.05, **: p<0.004, error bars = SEM. n (tumors) = 222 (MK2+), 232 (MK2+ Cis), 57 (MK2−), 87 (MK2− Cis).
(F) Immunohistochemistry of one representative MK2+ (top) and one MK2− (bottom) tumor from the same MK2CV/CV;KrasLSL-G12D/+;p53flox/floxmouse after cisplatin treatment as described in Figure 5E. CC3 positive cells: brown staining (arrows), scale bar = 50µm. Insert: Serial section with MK2 staining of the same tumor region. MK2 positive stromal cells cause brown staining in the MK2− tumor.
(G) Number of CC3 positive cells/mm2 of tumor area in KrasG12D/+;p53+/+ lung tumors. n = 3 mice / condition, error bars = SEM. n (tumors) = 129 (MK2+), 116 (MK2+ Cis), 55 (MK2−), 27 (MK2− Cis).
To investigate if this shift in in vivo tumor ratio resulted from increased apoptosis in MK2/p53 DKO tumor cells, we stained tumors for CC3 in the absence or presence of cisplatin treatment. Similar to our observations in vitro, a small but statistically significant increase in CC3 positive cells in MK2/p53 DKO tumors was seen even in the absence of exogenous DNA damage. Importantly, however, DNA damaging chemotherapy further enhanced this difference (Figures 5E, F). In contrast, cisplatin treatment of autochthonous tumors containing wild type p53 resulted in a strong increase in CC3 positive cells irrespective of the tumor MK2 status (Figure 5G).
Discussion
Deficiency in p53 represents a difficult clinical challenge as it is generally thought to be associated with resistance to genotoxic anti-cancer therapies (Rusch et al., 1995; Viktorsson et al., 2005). Therefore, novel therapeutic concepts to overcome the resistance of p53-defective neoplastic disease are urgently needed. In the present study we identified a molecular liability of p53-defective tumors that could be therapeutically exploited. We show that in response to genotoxic chemotherapy MK2 is essential for the survival of NSCLC tumor cells that lack functional p53, but is dispensable in p53-proficient cells. Importantly, findings from murine and human cell lines could be extended to a mouse model of NSCLC and demonstrate for the first time in an autochthonous cancer model in vivo, that loss of MK2 specifically sensitizes p53-deficient tumors to the DNA damaging agent cisplatin. In stark contrast, the MK2 expression status has no effect on the treatment-response of p53-proficient cancer cells. This suggests a potential for enhanced chemo-sensitization of p53-deficient tumors to DNA damaging chemotherapy in vivo through synthetic lethality between p53 and MK2. Importantly, because adjacent non-tumor tissue has intact p53-function, this approach could potentially increase the therapeutic window for DNA damaging chemotherapy, and can likely be applied to different tumor types.
In order to directly compare MK2-proficient and MK2-deficient tumor cells within a single animal, we created a new genetically engineered mouse model in which the gene of interest could be reversibly deactivated in vivo. By combining the MK2 ‘Cre-versible’ or MK2CV allele with an autochthonous model of NSCLC, we are able to study MK2 synthetic lethality with genetic mutations that promote tumor progression and with the response of tumor cells to genotoxic treatment in a novel internally controlled setting in vivo. This method should be generally applicable to study other genes in the context of synthetic lethality with anti-cancer agents in otherwise genetically identical solid tumors in situ within individual animals.
We found that MK2-deficient tumors were detected at the expected rate early after tumor induction and formed at the same latency as MK2-proficient tumors independent of their p53 status. In p53-deficient tumor cells we paradoxically observed both an increase in basal apoptosis and an enhancement of proliferation in the absence of exogenous DNA damage. The increased tumor burden that was seen in MK2/p53− DKO autochthonous tumors over time indicates that enhanced proliferation rather then apoptosis dominates the phenotype. Similar results were also seen in murine xenografts from H-RasG12V-transformed p53-deficient MEFs when progressively monitored over time (Reinhardt et al., 2007). Faster cell doubling times seen in both murine and human MK2/p53-DKO NSCLC cell lines in vitro are likely caused by an MK2-dependent loss of cell cycle delay following oncogenic stress. However, in contrast to what is observed for other checkpoint kinases like ATM or Chk2 (Negrini et al., 2010), there is no data suggesting that MK2 expression is frequently lost in human tumors, suggesting that its function as a regulator of proliferation is incompletely understood in this context. In this regard, the absence of MK2 mutations in human tumors and the lack of a correlation between expression of MK2 and p53 is similar to that of Chk1 (Figure S5).
In recent years, there has been increasing effort to target MK2 as a promising candidate for several inflammatory diseases (Fyhrquist et al., 2010; Gaestel et al., 2009). Different inhibitors for MK2 have been developed and are currently under investigation in animal models (Rao et al., 2012; Xiao et al., 2013). Emerging data about the role of MK2 in the DNA damage response, and particularly its synthetic lethal interaction with the loss of p53 after genotoxic stress, strongly suggest that testing of these compounds should be extended to include combination therapies with DNA damaging agents in anti-cancer treatments.
Experimental procedures
Generation of MK2 ‘Cre-versible’ (CV) mice
To generate mice carrying ‘Cre-versible’ alleles of MK2 we constructed the targeting vector (Figure 1A) using standard cloning approaches, see Suppl. Exp. Procedures. Homocygous MK2CV/CV and MK2+/+ mice were crossed with KrasLSLG12D/+; p53fl/fl mice (Jackson et al., 2005) from the laboratory of Tyler Jacks (MIT) to obtain MK2CV/CV or MK2+/+;KrasLSL-G12D/+;p53flox/flox and MK2CV/CV or MK2+/+;KrasLSL-G12D/+;p53+/+ mutant mice.
Tumor initiation and cisplatin treatment
Mice were infected with 2.5 × 107 PFU of Adenovirus expressing Crerecombinase (Ad5CMVCre, University of Iowa) by intra-tracheal administration (DuPage et al., 2009). Mice were given freshly prepared cisplatin in PBS at 5mg/kg bodyweight i.p. at weeks 12, 13 and 14 after tumor initiation. The experimental endpoint was determined by weight loss of tumor bearing animals (20% of starting body weight). For measurement of CC3 positive cells, mice were given a single dose of 10mg/kg bodyweight i.p. 48h prior to sacrifice.
Immunohistochemical analysis and quantification
For IHC: see Suppl. Exp. Procedures. Tumor areas and CC3 positives cells per mm2 were analyzed using BioQuant software. For the quantification of tumor areas 10 fields of view (FOV) in different lobes of each mouse were analyzed at 10× magnification.
Doubling times
After cisplatin (5µM) or vehicle treatment for 4.5h cells were plated at in duplicates at 50 000 cell per well in a 6-well dish, cells numbers were counted every 24h for a total of 72h. Doubling times were calculated by exponential regression.
Clonogenic Survival Assay
After treatment with vehicle, 5µM or 10µM cisplatin for 4.5h, cells were plated in triplicates at a concentration of 2000 cells for mock treated and 5000 or 10 000 cells for cisplatin treated per well in a 6-well dish. After 8 days cells were fixed and stained with modified Wright-stain (Sigma-Aldrich). Colonies consisting of >50 cells were counted; surviving fractions were determined by normalization against untreated cells.
For additional methods, primer sequences used in Southern Blots, genotyping and cDNA sequencing, and antibodies and chemicals see Suppl. Exp. Procedures.
Supplementary Material
A novel genetic switch maps synthetic lethality within tumors in single animals
MK2/p53-codeficiency enhances tumor growth in untreated NSCLC
MK2/p53-codeficiency markedly sensitizes lung tumors to chemotherapy in vivo
This gene/switch approach can identify effective combination anti-cancer therapies.
Acknowledgements
We thank members of the Yaffe and Jacks labs for helpful advice and discussions, and R.T. Bronson (Tufts University) for histopathology. We thank M. Gaestel (Medical School Hannover), M.A. Kerenyi (TCH Harvard) and the Swanson Biotechnology Center, especially the Ripple ES/Transgenics Facility, the Hope Babette Tang (1983) Histology Facility and the Biopolymers & Proteomics Core Facility at the Koch Institute / MIT. This work was supported by the Austrian Science Fund (FWF) (J 2900-B21) to S.M., NIH grants (ES015339, GM60594, GM59281, CA112967), Janssen Pharmaceutical Inc. (Transcend), the Koch Institute and Center for Environmental Health Sciences Core Grants (P30-CA14051, ES-002109) to M.B.Y. the Volkswagenstiftung (Lichtenberg Program), the Deutsche Forschungsgemeinschaft (SFB-832/A21, KFO-286/RP2, RE2246/2-1), the Ministry for Science and Technology, NRW (313-005-0910-0102), Deutsche Jose Carreras Leukämie Stiftung (DJCLS R12/26 to H.C.R., and the Anna Fuller Fund (New Haven, CT) to I.G.C. The authors wish to dedicate this paper to the memory of Officer Sean Collier for his caring service to the MIT.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Azzoli CG, Baker S, Jr, Temin S, Pao W, Aliff T, Brahmer J, Johnson DH, Laskin JL, Masters G, Milton D, et al. American Society of Clinical Oncology Clinical Practice Guideline update on chemotherapy for stage IV non-small-cell lung cancer. J Clin Oncol. 2009;27:6251–6266. doi: 10.1200/JCO.2009.23.5622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell IG, Kong YW, Johnston SJ, Chen ML, Collins HM, Dobbyn HC, Elia A, Kress TR, Dickens M, Clemens MJ, et al. p38 MAPK/MK2-mediated induction of miR-34c following DNA damage prevents Myc-dependent DNA replication. Proc Natl Acad Sci U S A. 2010;107:5375–5380. doi: 10.1073/pnas.0910015107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccia A, Elledge SJ. The DNA damage response: making it safe to play with knives. Molecular cell. 2010;40:179–204. doi: 10.1016/j.molcel.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPage M, Dooley AL, Jacks T. Conditional mouse lung cancer models using adenoviral or lentiviral delivery of Cre recombinase. Nat Protoc. 2009;4:1064–1072. doi: 10.1038/nprot.2009.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyhrquist N, Matikainen S, Lauerma A. MK2 signaling: lessons on tissue specificity in modulation of inflammation. J Invest Dermatol. 2010;130:342–344. doi: 10.1038/jid.2009.372. [DOI] [PubMed] [Google Scholar]
- Gaestel M, Kotlyarov A, Kracht M. Targeting innate immunity protein kinase signalling in inflammation. Nat Rev Drug Discov. 2009;8:480–499. doi: 10.1038/nrd2829. [DOI] [PubMed] [Google Scholar]
- Jackson EL, Olive KP, Tuveson DA, Bronson R, Crowley D, Brown M, Jacks T. The differential effects of mutant p53 alleles on advanced murine lung cancer. Cancer Res. 2005;65:10280–10288. doi: 10.1158/0008-5472.CAN-05-2193. [DOI] [PubMed] [Google Scholar]
- Jackson EL, Willis N, Mercer K, Bronson RT, Crowley D, Montoya R, Jacks T, Tuveson DA. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev. 2001;15:3243–3248. doi: 10.1101/gad.943001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L, Mercer K, Greenbaum D, Bronson RT, Crowley D, Tuveson DA, Jacks T. Somatic activation of the K-ras oncogene causes early onset lung cancer in mice. Nature. 2001;410:1111–1116. doi: 10.1038/35074129. [DOI] [PubMed] [Google Scholar]
- Lord CJ, Ashworth A. The DNA damage response and cancer therapy. Nature. 2012;481:287–294. doi: 10.1038/nature10760. [DOI] [PubMed] [Google Scholar]
- Manke IA, Nguyen A, Lim D, Stewart MQ, Elia AE, Yaffe MB. MAPKAP kinase-2 is a cell cycle checkpoint kinase that regulates the G2/M transition and S phase progression in response to UV irradiation. Mol Cell. 2005;17:37–48. doi: 10.1016/j.molcel.2004.11.021. [DOI] [PubMed] [Google Scholar]
- Morandell S, Yaffe MB. Exploiting synthetic lethal interactions between DNA damage signaling, checkpoint control, and p53 for targeted cancer therapy. Prog Mol Biol Transl Sci. 2012;110:289–314. doi: 10.1016/B978-0-12-387665-2.00011-0. [DOI] [PubMed] [Google Scholar]
- Negrini S, Gorgoulis VG, Halazonetis TD. Genomic instability--an evolving hallmark of cancer. Nat Rev Mol Cell Biol. 2010;11:220–228. doi: 10.1038/nrm2858. [DOI] [PubMed] [Google Scholar]
- Rao AU, Xiao D, Huang X, Zhou W, Fossetta J, Lundell D, Tian F, Trivedi P, Aslanian R, Palani A. Facile synthesis of tetracyclic azepine and oxazocine derivatives and their potential as MAPKAP-K2 (MK2) inhibitors. Bioorg Med Chem Lett. 2012;22:1068–1072. doi: 10.1016/j.bmcl.2011.11.113. [DOI] [PubMed] [Google Scholar]
- Reinhardt HC, Aslanian AS, Lees JA, Yaffe MB. p53-deficient cells rely on ATM- and ATR-mediated checkpoint signaling through the p38MAPK/MK2 pathway for survival after DNA damage. Cancer Cell. 2007;11:175–189. doi: 10.1016/j.ccr.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt HC, Hasskamp P, Schmedding I, Morandell S, van Vugt MA, Wang X, Linding R, Ong SE, Weaver D, Carr SA, Yaffe MB. DNA damage activates a spatially distinct late cytoplasmic cell-cycle checkpoint network controlled by MK2-mediated RNA stabilization. Mol Cell. 2010;40:34–49. doi: 10.1016/j.molcel.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt HC, Jiang H, Hemann MT, Yaffe MB. Exploiting synthetic lethal interactions for targeted cancer therapy. Cell Cycle. 2009;8:3112–3119. doi: 10.4161/cc.8.19.9626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronche F, Casanova E, Turiault M, Sahly I, Kellendonk C. When reverse genetics meets physiology: the use of site-specific recombinases in mice. FEBS Lett. 2002;529:116–121. doi: 10.1016/s0014-5793(02)03266-0. [DOI] [PubMed] [Google Scholar]
- Viktorsson K, De Petris L, Lewensohn R. The role of p53 in treatment responses of lung cancer. Biochem Biophys Res Commun. 2005;331:868–880. doi: 10.1016/j.bbrc.2005.03.192. [DOI] [PubMed] [Google Scholar]
- Xiao D, Palani A, Huang X, Sofolarides M, Zhou W, Chen X, Aslanian R, Guo Z, Fossetta J, Tian F, et al. Conformation constraint of anilides enabling the discovery of tricyclic lactams as potent MK2 non-ATP competitive inhibitors. Bioorg Med Chem Lett. 2013;23:3262–3266. doi: 10.1016/j.bmcl.2013.03.109. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.