Abstract
OBJECTIVE:
To determine mortality and immune status improvement in HIV-infected pediatric patients on antiretroviral treatment (ART) in Malawi, Lesotho, and Swaziland.
METHODS:
We conducted a retrospective cohort study of patients aged <12 years at ART initiation at 3 sites in sub-Saharan Africa between 2004 and 2009. Twelve-month and overall mortality were estimated, and factors associated with mortality and immune status improvement were evaluated.
RESULTS:
Included in the study were 2306 patients with an average follow-up time on ART of 2.3 years (interquartile range 1.5–3.1 years). One hundred four patients (4.5%) died, 9.0% were lost to follow-up, and 1.3% discontinued ART. Of the 104 deaths, 77.9% occurred in the first year of treatment with a 12-month mortality rate of 3.5%. The overall mortality rate was 2.25 deaths/100 person-years (95% confidence interval [CI] 1.84–2.71). Increased 12-month mortality was associated with younger age; <6 months (hazard ratio [HR] = 8.11, CI 4.51–14.58), 6 to <12 months (HR = 3.43, CI 1.96–6.02), and 12 to <36 months (HR = 1.92, CI 1.16–3.19), and World Health Organization stage IV (HR = 4.35, CI 2.19–8.67). Immune status improvement at 12 months was less likely in patients with advanced disease and age <12 months.
CONCLUSIONS:
Despite challenges associated with pediatric ART in developing countries, low mortality and good treatment outcomes can be achieved. However, outcomes are worse in younger patients and those with advanced disease at the time of ART initiation, highlighting the importance of early diagnosis and treatment.
KEY WORDS: HIV, AIDS, pediatric, antiretroviral therapy, mortality, Africa south of the Sahara
What’s Known on This Subject:
There is evidence from both developed and developing countries that antiretroviral treatment significantly reduces mortality in HIV-infected children. However, in sub-Saharan Africa, numerous health system, financial, and human resource obstacles make delivering quality pediatric HIV care a challenge.
What This Study Adds:
We describe the experience of the Baylor International Pediatrics AIDS Initiative in Malawi, Lesotho, and Swaziland. Despite challenges delivering pediatric treatment in these countries, mortality and clinical outcomes approaching those from developed countries are feasible.
The global burden of HIV infection is high, with an estimated 34 million adults and children living with HIV by the end of 2010, the majority of whom reside in sub-Saharan Africa.1 Evidence from both developed and developing countries demonstrates that antiretroviral therapy (ART) can reduce mortality in HIV-infected adults and children.2–15 However, as of 2010, only 21% of eligible African children accessed ART despite scaling up of HIV services, and most national ART programs in the region are not meeting the World Health Organization (WHO) goal that infants and children represent at least 10% to 15% of all patients on ART.1,16
Although much work remains to improve ART access for HIV-infected African children, notable regional progress has been made in the past decade. Specifically, expanded prevention of mother-to-child transmission (PMTCT) strategies, greater availability of pediatric ART drug formulations, lower thresholds for child and infant ART eligibility including guidelines for universal treatment of HIV-infected infants, and developments in early infant HIV diagnostics such as dried blood spot methodology for virologic testing have contributed to advancing pediatric HIV services in the region.1,17
In this context, this study describes our experience providing care to HIV-infected children in Malawi, Lesotho, and Swaziland at Baylor International Pediatric AIDS Initiative (BIPAI) Centers of Excellence.18 We examine factors related to mortality and immune response to ART and compare our mortality rates with those reported from similar cohorts from both African and US settings.
Methods
BIPAI has created a network of Centers of Excellence and satellite clinics in 6 African countries in partnership with national governments beginning in 1999.19,20 The BIPAI clinics use a multidisciplinary staff including pediatricians, medical officers, clinical officers, nurses, pharmacists, and social workers to provide clinical care, medications, and psychosocial support free of charge to pediatric HIV-infected and exposed patients. BIPAI treatment programs began providing ART to children in 2004, 2005, and 2006 in Malawi, Lesotho, and Swaziland, respectively. The 3 BIPAI clinics had enrolled a combined total of >14 000 patients (∼30% were HIV-exposed and 70% HIV-infected at enrollment), and >5000 patients at the 3 sites had ever been started on ART by end of 2009.18
Children were initiated on ART per national guidelines which were based on WHO recommendations: clinical stage III or IV, severe immunosuppression per age-specific CD4 thresholds, and presumptive severe HIV disease in infants without virologic confirmation of infection.21–23 Additionally, in 2008–2009, each country implemented universal ART for HIV-infected infants <12 months regardless of CD4 or clinical stage per WHO recommendations.17 Standard pediatric first-line ART in Lesotho and Swaziland was zidovudine, lamivudine, and nevirapine or efavirenz, stavudine with lamivudine and nevirapine were first-line ART for children in Malawi. Each country used a nucleoside reverse transcriptase inhibitor backbone with boosted lopinavir/ritonavir for pediatric second-line therapy.
Baseline nutritional status was classified as normal or mild, moderate, or severe acute malnutrition by using weight for height (W/H) tables, z scores, mid-upper arm circumferences (MUAC), and edema assessments in accordance with national recommendations (mild malnutrition = W/H 80%–85%; moderate malnutrition = W/H 70%–79% or MUAC <12 cm; severe malnutrition: W/H <70% or MUAC <11 cm or edema). Chronic forms of malnutrition such as stunting were considered in patients’ WHO staging but were not reflected in the baseline assessments of acute malnutrition.
Provision of cotrimoxazole preventive therapy for Pneumocystis jiroveci pneumonia was routine for all HIV-exposed and infected children <5 years at each site. In Malawi, all HIV-infected children <15 years were eligible for cotrimoxazole preventive therapy, whereas in Swaziland and Lesotho, children > 5 years were eligible if their CD4 was below 350.21–23
Study Design
This was a retrospective cohort study for the time period October 2004 through October 2009. Patients were eligible for inclusion in the study if they were HIV-infected and ART was initiated before age 12 years. The cutoff age of 12 years was selected for inclusion to facilitate comparison with other study cohorts.
Routinely collected patient data stored in a standardized electronic medical record system in place at each site including past medical and social histories, WHO staging, laboratory results, medication history, and other clinical parameters including anthropomorphic measurements and nutritional assessments were used. Unique patient identifiers are used in the electronic medical record database to protect patient confidentiality.
Ethics approvals were obtained from the national review boards in Malawi, Lesotho, and Swaziland and also from the institutional review board at Baylor College of Medicine, Houston, Texas.
Statistical Analyses
SPSS version 18 (SPSS, Chicago, IL) was used for data analysis. The demographic and clinical features were described for the entire cohort and stratified by site using frequencies for categorical variables and medians and interquartile ranges (IQRs) for continuous variables (all non-Gaussian). Immune status classifications at baseline were done according to CD4 percentages for children <5 years and by absolute CD4 counts in children ≥5 years in accordance with WHO guidelines.24 For the purpose of this study, immune status improvement was defined as an improvement from baseline (ART initiation) in CD4% of >5 percentage points for children <5 years or absolute CD4 cell count >50 cells/mL for children 5 to 12 years. CD4 counts were measured as part of routine care; therefore, the laboratory values closest in time to 12 months after ART initiation were used. Children who died before 12 months on ART were excluded from the analysis for lack of a second CD4 data point.
Mortality rates were estimated for the entire study period (October 2004–October 2009) and for the first 12 months from ART initiation; patients lost to follow-up (LTFU) were assumed to be alive in the data analysis. Hazard ratios (HRs) and 95% confidence intervals (CIs) were used to estimate the unadjusted and adjusted (using Cox proportional hazards models) associations between overall mortality and each demographic and clinical subgroup. Follow-up time for each patient was calculated from the date of ART initiation to the date of file closure (death, transfer out, LTFU) or the last clinic visit before October 31, 2009. Differences in overall survival probability curves were displayed by using Kaplan-Meier plots for age and WHO clinical stage at ART initiation. Odds ratios and 95% CIs were used to estimate the unadjusted and adjusted (using logistic regression) association between the baseline features and immune status improvement within the first 12 months of ART initiation. For comparison with rates from other studies, mortality incidence rates and 95% CIs per 100 person-years were estimated under a Poisson distribution.
Results
Included in the study were 2306 HIV-infected infants and children. The median age at ART initiation was 3.1 (IQR 1.4–7.1) years, and median follow-up time on ART was 2.3 (IQR 1.5–3.1) years. At ART initiation, 69.7% of children had advanced HIV disease (WHO stage III or IV), 81.3% had advanced or severe immunosuppression, and 32.8% had some degree of malnutrition (Table 1). There was a consistent trend of higher rates of baseline immunosuppression in younger patients (Table 2).
TABLE 1.
Sociodemographic and Clinical Characteristics at ART Initiation
| Baseline Characteristics | Lesotho (n = 789) | Malawi (n = 880) | Swaziland (n = 637) | Total (N = 2306) | |
|---|---|---|---|---|---|
| Sociodemographic | |||||
| Age, median (IQR), y | 3.6 (1.4–7.4) | 2.4 (1.1–6.1) | 3.8 (1.5–7.6) | 3.1 (1.4–7.1) | |
| Gender, female | 399 (51.0%) | 411 (46.7%) | 299 (46.9%) | 1109 (48.2%) | |
| Clinical | |||||
| WHO Stage | |||||
| I | 175 (22.2%) | 86 (9.8%) | 62 (9.7%) | 323 (14.0%) | |
| II | 94 (11.9%) | 121 (13.7%) | 154 (24.2%) | 369 (16.0%) | |
| III | 340 (43.1%) | 540 (61.4%) | 277 (43.5%) | 1157 (50.2%) | |
| IV | 180 (22.8%) | 132 (15.0%) | 137 (21.5%) | 449 (19.5%) | |
| Missing | 0 (0.0%) | 1 (0.1%) | 7 (1.1%) | 8 (0.3%) | |
| Immunosuppression | |||||
| None | 50 (7.8%) | 76 (8.6%) | 49 (6.2%) | 175 (7.6%) | |
| Mild | 74 (11.6%) | 61 (6.9%) | 79 (10.0%) | 214 (9.3%) | |
| Advanced | 114 (17.9%) | 122 (13.9%) | 171 (21.7%) | 407 (17.6%) | |
| Severe | 389 (61.1%) | 595 (67.6%) | 486 (61.6%) | 1470 (63.7%) | |
| Missing | 10 (1.6%) | 26 (3.0%) | 4 (0.5%) | 40 (1.7%) | |
| CD4 Values | |||||
| Age <5 y: CD4%, median (IQR) | 15 (11–20) | 15 (10–19) | 14 (10–18) | 15 (10–19) | |
| Age ≥5 y: CD4 cell count, median (IQR) | 255 (111–351) | 235 (113–429) | 264 (108–416) | 253 (111–398) | |
| Nutritional status | |||||
| Normal | 399 (50.6%) | 639 (72.6%) | 387 (60.7%) | 1425 (61.8%) | |
| Mild malnutrition | 159 (20.2%) | 48 (5.5%) | 107 (16.8%) | 314 (13.6%) | |
| Moderate malnutrition | 140 (17.7%) | 74 (8.4%) | 75 (11.8%) | 289 (12.5%) | |
| Severe malnutrition | 71 (9.0%) | 36 (4.1%) | 47 (7.4%) | 154 (6.7%) | |
TABLE 2.
Degree of Immunosuppression at ART Initiation by Age
| Age | Immunosuppressiona | |||
|---|---|---|---|---|
| None | Mild | Advanced | Severe | |
| <12 mo (n = 407) | 14 (3.4%) | 15 (3.7%) | 40 (9.8%) | 338 (83.0%) |
| 12 to <36 mo; (n = 679) | 21 (3.1%) | 25 (3.7%) | 72 (10.6%) | 561 (82.6%) |
| 36 to <60 mo; (n = 328) | 14 (4.3%) | 27 (8.2%) | 59 (18.0%) | 228 (69.5%) |
| ≥60 mo; (n = 852) | 126 (14.8%) | 147 (17.3%) | 236 (27.7%) | 343 (40.3%) |
| Total (N = 2266) | 175 (7.7%) | 214 (9.4%) | 407 (18.0%) | 1470 (64.9%) |
Per WHO 2006 guidelines.
Mortality
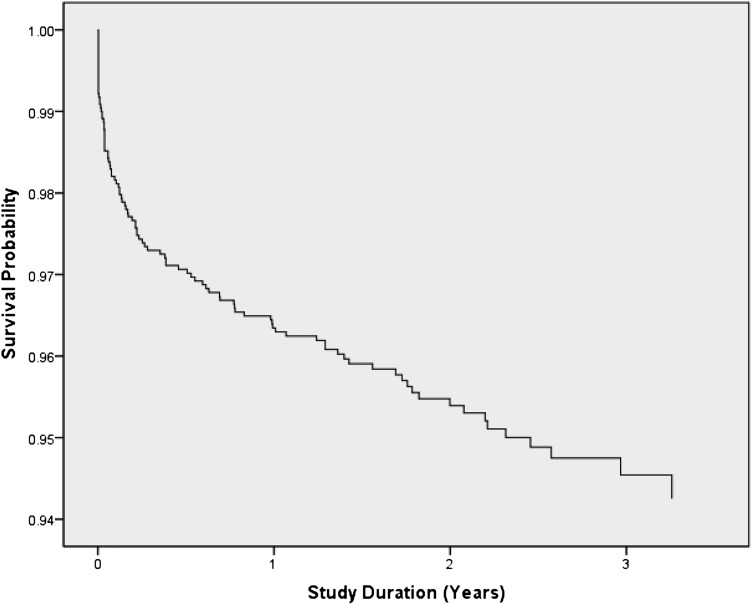
As of October 2009, 104 (4.5%) patients died, 208 (9.0%) were LTFU, and 30 (1.3%) discontinued ART before the end of the study period. The combined mortality rate was 2.25 deaths/100 person-years (95% CI 1.84–2.71). Of the 104 deaths, 81 (77.9%) occurred within a year of ART initiation, with a 12-month mortality rate of 3.5% (Fig 1). Of the 81 patients who died in the first 12 months, 35 (43.2%) were <1 year of age at ART initiation.
FIGURE 1.
Kaplan-Meier mortality curve for all study participants.
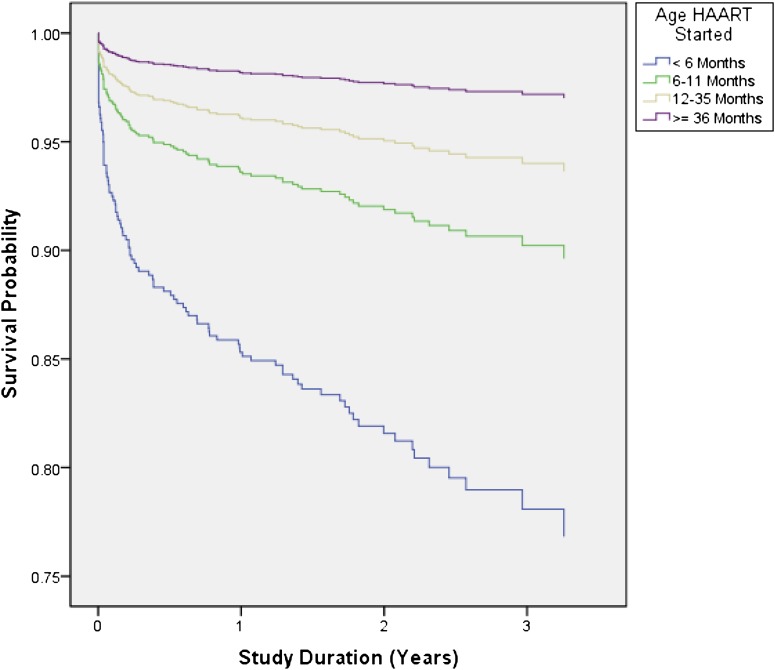
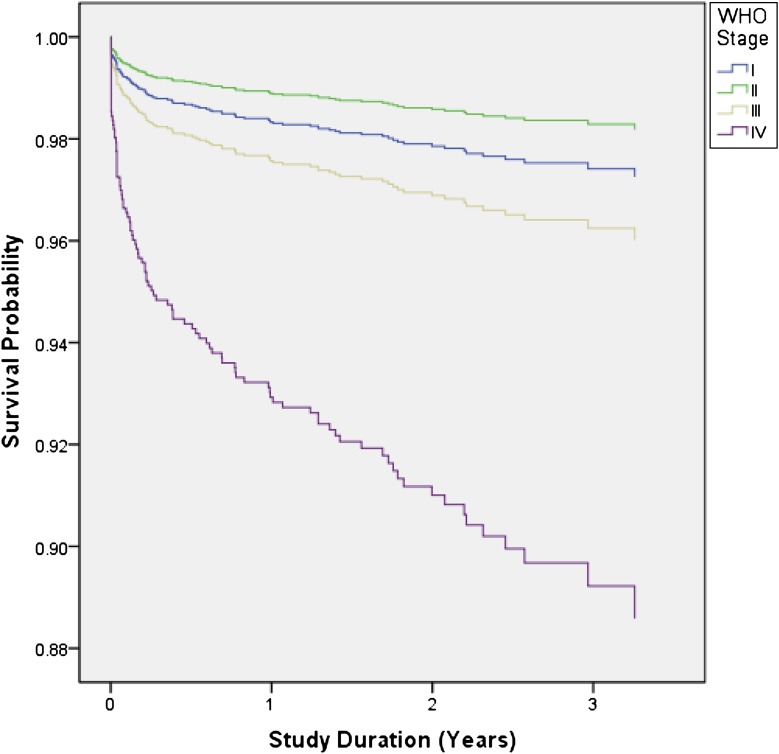
Mortality was strongly related to age at ART initiation (Table 3 and Fig 2). One hundred nineteen children initiated ART at <6 months of age, of whom 15.1% died within 12 months, compared with 5.7% of patients aged 6 to <12 months, 3.6% of patients aged 12 to <36 months, and 1.8% of patients ≥36 months. WHO stage at ART initiation was associated with mortality, although despite adjusting for age, stage II patients had the lowest mortality of all WHO stages (Table 3 and Fig 3).
TABLE 3.
Sociodemographic and Clinical Factors Associated With Mortality
| Baseline Characteristics | Total N = 2306 | Died n = 104, n (%) | Unadjusted Mortality: HR (95% CI) | Adjusted Mortality: HR (95% CI)a |
|---|---|---|---|---|
| Country | ||||
| Swaziland | 637 | 23 (3.6%) | Reference | |
| Malawi | 880 | 42 (4.8%) | 1.25 (0.75–2.08) | |
| Lesotho | 789 | 39 (4.9%) | 1.29 (0.77–2.17) | |
| Gender | ||||
| Male | 1191 | 54 (4.5%) | Reference | |
| Female | 1109 | 50 (4.5%) | 1.02 (0.69–1.49) | |
| Missing | 6 | 0 (0.0%) | ||
| Age at ART initiation | ||||
| <6 mo | 119 | 20(16.8%) | 8.78 (4.94–15.59) | 8.11 (4.51–14.58) |
| 6 to <12 mo | 299 | 22 (7.4%) | 3.73 (2.13–6.53) | 3.43 (1.96–6.02) |
| 12 to <36 mo | 699 | 34 (4.9%) | 2.22 (1.34–3.66) | 1.92 (1.16–3.19) |
| ≥36 mo | 1189 | 28 (2.4%) | Reference | Reference |
| PMTCT | ||||
| No PMTCT | 1422 | 50 (3.5%) | Reference | |
| PMTCT | 329 | 25 (7.6%) | 2.44 (1.51–3.96) | |
| Missing | 555 | 29 (5.2%) | ||
| WHO stage at ART initiation | ||||
| I | 323 | 10 (3.1%) | Reference | Reference |
| II | 369 | 5 (1.4%) | 0.40 (0.14–1.16) | 0.64 (0.22–1.90) |
| III | 1157 | 40 (3.5%) | 0.98 (0.49–1.95) | 1.43 (0.70–2.90) |
| IV | 449 | 48(10.7%) | 3.41 (1.72–6.75) | 4.35 (2.19–8.67) |
| Missing | 8 | 1 (12.5%) | ||
| Immunosuppression at ART initiation | ||||
| None | 175 | 5 (2.9%) | Reference | |
| Mild | 214 | 7 (3.3%) | 1.11 (0.35–3.49) | |
| Advanced | 407 | 9 (2.2%) | 0.79 (0.27–2.37) | |
| Severe | 1470 | 73 (5.0%) | 1.74 (0.70–4.31) | |
| Missing | 40 | 10 (25.0%) | ||
| Nutritional status at ART initiation | ||||
| Normal | 1425 | 41 (2.9%) | Reference | |
| Mild malnutrition | 314 | 11 (3.5%) | 1.20 (0.62–2.34) | |
| Moderate malnutrition | 289 | 16 (5.5%) | 2.04 (1.15–3.64) | |
| Severe malnutrition | 154 | 25(16.2%) | 7.14 (4.34–11.77) | |
| Missing data | 124 | 11 (8.9%) |
Adjusted for age at ART initiation and WHO stage at entry. Immune status and nutritional status were excluded from the model because they were collinear with WHO stage.
FIGURE 2.
Kaplan-Meier mortality curve for age at initiation of ART. Log-rank test (for equality of survival distributions over different age categorizations): P < .0001. HAART, highly active ART.
FIGURE 3.
Kaplan-Meier curve for baseline WHO clinical staging and mortality adjusting for age at ART initiation. Log-rank test (for equality of survival distributions over different WHO stages): P < .0001.
Unadjusted HRs demonstrated an increased risk of mortality associated with age ≤36 months at ART initiation, moderate and severe malnutrition, perinatal exposure to PMTCT antiretroviral prophylaxis, and WHO stage IV. In the multivariate analysis, age ≤36 months and WHO stage IV remained as statistically significant variables (Table 3).
Immune Status Improvement
The efficacy of ART as determined by immune status improvement was calculated for 1687 patients from the 3 sites (Malawi = 555; Lesotho = 648; Swaziland = 484) who had at least 2 CD4 cell counts; the first near the time of ART initiation and the second after ∼12 months on ART (range 5–18 months, median = 12 months); 90.5% of these patients experienced an improvement in immune status from ART initiation, with patients from Swaziland more likely to have improvement than those from Lesotho or Malawi (Table 4). Children <5 years exhibited a mean CD4% increase of 16.6% (SD 9.0%), and those aged ≥5 years had a mean absolute CD4 cell count increase of 399.9 cells/mL (SD 313.0). In the multivariate analysis, factors associated with less immune status improvement were being enrolled in Lesotho or Malawi, age <12 months, and WHO stage IV.
TABLE 4.
Immune Status Improvement at 12 Months (N = 1687)
| Immune Status Improvement | ||||
|---|---|---|---|---|
| n | Improved n (%), n = 1526 | Unadjusted OR (95% CI) | Adjusted OR (95% CI)a | |
| Country | ||||
| Swaziland | 484 | 452 (93.4%) | Reference | Reference |
| Malawi | 555 | 496 (89.4%) | 0.60 (0.38–0.93) | 0.61 (0.38–0.96) |
| Lesotho | 648 | 578 (89.2%) | 0.59 (0.38–0.90) | 0.59 (0.38–0.92) |
| Gender | ||||
| Male | 882 | 800 (90.7%) | Reference | |
| Female | 799 | 720 (90.1%) | 0.93 (0.68–1.29) | |
| Missing | 6 | 6 (100.0%) | ||
| Age | ||||
| <6 mo | 66 | 52 (78.8%) | 0.36 (0.19–0.68) | 0.34 (0.17–0.65) |
| 6 to <12 mo | 193 | 165 (85.5%) | 0.58 (0.36–0.91) | 0.58 (0.37–0.93) |
| 12 to <36 mo | 505 | 468 (92.7%) | 1.23 (0.82–1.85) | 1.26 (0.83–1.89) |
| ≥36 mo | 923 | 841 (91.1%) | Reference | Reference |
| PMTCT | ||||
| No PMTCT | 1086 | 995 (91.6%) | Reference | |
| PMTCT | 209 | 182 (87.1%) | 0.62 (0.39–0.97) | |
| Missing | 392 | 349 (89.0%) | ||
| WHO stage | ||||
| I | 227 | 210 (92.5%) | Reference | Reference |
| II | 282 | 257 (91.1%) | 0.83 (0.44–1.58) | 0.59 (0.30–1.15) |
| III | 872 | 791 (90.7%) | 0.79 (0.46–1.36) | 0.62 (0.35–1.10) |
| IV | 306 | 268 (87.6%) | 0.57 (0.31–1.04) | 0.46 (0.25–0.85) |
| Nutritional status at ART initiation | ||||
| Normal | 1076 | 981 (91.2%) | Reference | |
| Mild malnutrition | 260 | 236 (90.8%) | 0.95 (0.60–1.52) | |
| Moderate malnutrition | 210 | 187 (89.0%) | 0.79 (0.49–1.27) | |
| Severe malnutrition | 97 | 88 (90.7%) | 0.95 (0.46–1.94) | |
| Missing | 44 | 34 (77.3%) | ||
OR, odds ratio.
Adjusted for country, age, and WHO stage. Patients who died in the first 12 mo on ART excluded.
Discussion
This study presents data from a large, multicountry pediatric ART cohort. The study was retrospective in methodology using data from routine patient care. The operational nature of the information reflects on-the-ground conditions making the findings more applicable and facilitating comparisons to other programs in our setting.
The overall and 12-month mortality rates from our study (4.5% and 3.5%, respectively) are similar to the International Epidemiologic Databases to Evaluate AIDS in Southern Africa (IeDEA-SA) cohort for children remaining in care (4.8% and 4.5% respectively).7 The IeDEA-SA cohort which included 10 ART programs (7 in South Africa, and 1 each in Malawi, Mozambique, and Zimbabwe), had older patients (<16 vs <12 years), a higher median age at ART initiation (49 vs 37 months), and shorter duration of follow-up (17.3 vs 27.6 months). Despite our cohort including younger patients, with longer periods of follow-up on ART, no large difference in mortality was observed. A second IeDEA-SA study of South African children only reported a 3-year mortality rate of 7.7% and can be used as a benchmark for future analysis of our cohort.25 Like both IeDEA-SA studies, we found young age and advanced clinical stage at ART initiation to be associated with higher mortality rates.
We also determined an overall mortality rate for our cohort of 2.25 deaths per 100 person-years (95% CI, 1.84–2.71). This is lower than reported rates from another regional pediatric cohort from Kenya between August 2004 and November 2008 which had a mortality rate of 8.4 deaths/100 person-years (95% CI 5.21–12.68).8 This Kenyan cohort was older at ART initiation (4.9 vs 3.1 years), and the authors did not report age-specific mortality.
This study’s lower mortality rates compared with these other regional cohorts may relate to BIPAI’s capacity to deliver integrated, single-site comprehensive HIV care (ie, treatment of opportunistic infections, malaria, malnutrition, and referral for or treatment on site of tuberculosis). The concentration of financial, physical, and human resources, including pediatricians, in the BIPAI clinics is currently not feasible at most government ART sites. However, the BIPAI clinics also serve as referral centers within host countries for complicated and severe cases including children failing ART and those with Kaposi sarcoma, and this could potentially skew mortality rates higher.
Patients LTFU are an important consideration when discussing mortality because a certain percentage of these patients are customarily assumed to have died.26 LTFU patients were not included in our mortality calculations or in the comparison studies cited earlier. We had an 8.2% LTFU rate at 12 months after ART initiation and an overall LTFU rate of 9.0% over the entire study period. These are similar to LTFU rates reported in other cohorts of pediatric HIV patients in the region (8.4%–11.5%).7,27,28 If our reported 4.5% mortality is adjusted to assume that 50% of those LTFU had actually died, then our overall mortality would be 9.0%. This corrected mortality rate is similar to a family clinic model of care in South Africa with 0% LTFU and mortality of 9%.9 Both this pediatric cohort and the family-centered cohort demonstrate a better mortality rate than other regional pediatric cohorts with reported mortality of 8.6% to 13%, which did not account for LTFU.10,11,14,27
When compared with developed nations, our mortality rate on ART of 2.25 deaths per 100 person-years (95% CI 1.84–2.71) is higher than overall mortality rates reported for HIV-infected children in the United States through the Pediatric AIDS Clinical Trials Group (PATCG).6 PATCG 219/219C cited a mortality rate of 1.47 deaths per 100 person-years (95% CI 1.31–1.65) for children between April 1993 and December 2006 and an even lower mortality rate of 0.5 to 0.8 deaths per 100 person-years between 2000 and 2006 in the post-ART era.6 Direct comparisons are difficult because of the many variables that contribute to higher mortality in the developing world, but we are encouraged that our overall rates are approaching those reported from the United States and hope that we will see a similar trend toward lower, stabilized mortality rates in sub-Saharan Africa as national ART programs mature.
In our study, mortality was highest in the first year of ART, with 78% of all deaths occurring within that period, with decreasing mortality after 1 year on ART. This has been reported previously in both adult and pediatric populations.8,11,15,29,30 The advanced immunosuppression and clinical disease often associated with early mortality on ART reinforce the need for programmatic emphasis on earlier diagnosis, enrollment in care, and initiation of ART.
The higher mortality observed in our cohort among patients aged <1 year at ART initiation is not surprising because almost all infants in this group were enrolled before implementation of the WHO guidelines for the universal treatment of infants in late 2008 into 2009 at our sites. Ample evidence now shows that infants are at risk for rapid disease progression, CD4 counts and WHO staging are poorly predictive of outcomes, and delaying ART initiation until infants have advanced clinical disease or immunosuppression to initiate ART results in higher mortality rates.2,15,17 More than 90% of the infants in our study had advanced or severe immune suppression at ART initiation. Infants aged <12 months were also less likely to have improvement in immunologic status, which likely contributed to higher mortality.
Due to standard infant diagnostic protocols in the study countries, virologic testing is performed no earlier than 6 weeks of life, and thus the exact timing of infection in HIV-infected infants is not known. Infants with perinatal infection are at higher risk for early mortality than those infected later through breastfeeding.31 Late identification of perinatally infected infants likely also contributed to higher mortality in patients aged <12 months and contrasts with developed settings, where virologic testing of exposed infants begins at birth.
We also found that exposure to perinatal antiretrovirals for the purpose of PMTCT was associated with increased risk of mortality after ART initiation. Details of mortality by specific antiretroviral prophylaxis received are not available, but all 3 countries’ PMTCT regimens during the study period included single-dose nevirapine for infants.22,32–34 Relatively small studies have documented the risk of development of nonnucleoside reverse transcriptase inhibitor (NNRTI) resistance after failed single-dose nevirapine prophylaxis in HIV-infected infants, and a recent IeDEA-SA study demonstrated an increased probability of virologic failure after exposure to PMTCT regimens.35–38 During the time of this study, Malawi and Swaziland used an NNRTI-based first-line ART regimen for all infants and children regardless of PMTCT exposure, whereas, starting in 2008, Lesotho implemented a policy of using a protease inhibitor-based regimen for children with a history of maternal or infant NNRTI exposure. Given the retrospective design of the study and lack of resistance testing, causality cannot be determined, but possible development of NNRTI resistance should be considered. If resistance did develop, these infants would be at high risk for first-line ART failure and early mortality if they received an NNRTI-based first-line regimen.
Improvements in CD4 values were used as a proxy for ART efficacy as is commonly done in this setting where routine viral load testing is not possible.39,40 For children who survived at least 12 months on ART, there was good immunologic response to treatment with 90.5% of patients surpassing our definition for significant increase in absolute or percent CD4 with ART. It is not clear why patients from Malawi and Lesotho were less likely to experience immune recovery than those from Swaziland.
Conclusions
This study of a large cohort of HIV-infected children on ART at the BIPAI clinics in Malawi, Lesotho, and Swaziland adds to the growing body of literature demonstrating that infants and children in sub-Saharan Africa can achieve good treatment outcomes and low mortality rates on ART. Despite the challenges associated with the public health approach to implementation of comprehensive pediatric HIV care in developing countries, additional resources need to be directed toward children who are still underrepresented in many national ART programs in the region. Continued research to track long-term outcomes and mortality in children on ART should be a priority as care systems and patients mature, with continued focus on early identification and treatment of high-risk infants.
Acknowledgments
We acknowledge the valuable input received from Edith Q. Mohapi, Executive Director, Baylor College of Medicine Bristol-Myers Squibb Children’s Clinical Centre of Excellence (Maseru, Lesotho); Hailu N. Sarero, Executive Director, Baylor College of Medicine Bristol-Myers Squibb Children’s Clinical Centre of Excellence (Mbabane, Swaziland); Simona Ruta, Stefan S. Nicolau Institute of Virology (Bucharest, Romania); Heather R. Draper, Baylor College of Medicine, Department of Pediatrics and Texas Children’s Hospital (Houston, Texas); and Shaun Krog, Virtual Purple Professionals Services (Durban, South Africa).
Glossary
- ART
antiretroviral therapy
- BIPAI
Baylor College of Medicine International Pediatric AIDS Initiative
- CI
confidence interval
- HR
hazard ratio
- IeDEA-SA
International Epidemiologic Databases to Evaluate AIDS in Southern Africa
- IQR
interquartile range
- LTFU
lost to follow-up
- MUAC
mid-upper arm circumference
- NNRTI
nonnucleoside reverse transcriptase inhibitor
- PMTCT
prevention of mother-to-child transmission
- W/H
weight for height
- WHO
World Health Organization
Footnotes
Dr Kabue made substantial contributions to concept and design; acquisition, analysis, and interpretation of data; and drafting and critical revision of the article for important intellectual content. Dr Buck made substantial contributions to concept and design, interpretation of data, and drafting and critical revision of the article for important intellectual content. Dr Wanless made substantial contributions to concept and design, interpretation of data, and drafting and critical revision of the article for important intellectual content. Dr Cox contributed to concept and design, interpretation of data, and drafting and critical revision of the article for important intellectual content. Dr McCollum contributed to concept and design, interpretation of data, and drafting and critical revision of the article for important intellectual content. Dr Caviness contributed to concept and design, acquisition, analysis and interpretation of data, and critical revision of the article for important intellectual content. Dr Ahmed contributed to concept and design, interpretation of data, and drafting and critical revision of the article for important intellectual content. Dr Kim contributed to concept and design, interpretation of data, and drafting and critical revision of the article for important intellectual content. Dr Thahane contributed to concept and design, interpretation of data, and critical revision of the article for important intellectual content. Mr Devlin contributed to concept and design, interpretation of data, and critical revision of the article for important intellectual content. Mr Kochelani contributed to concept and design, interpretation of data, and critical revision of the article for important intellectual content. Mr Kazembe contributed to concept and design, interpretation of data, and drafting and critical revision of the article for important intellectual content. Ms Calles contributed to concept and design, interpretation of data, and critical revision of the article for important intellectual content. Mr Mizwa contributed to concept and design, interpretation of data, and critical revision of the article for important intellectual content. Dr Schutze contributed to concept and design, interpretation of data, and critical revision of the article for important intellectual content. Dr Kline contributed to concept and design, interpretation of data, and critical revision of the article for important intellectual content and final approval of version to be published.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Mark W. Kline and Mark M. Kabue were supported in part by grant D43 TW01036 from the Fogarty International Centre of the National Institutes of Health. The Children’s Clinical Center of Excellence in Malawi was funded by the Abbott Fund. The Children’s Clinical Centers of Excellence in Lesotho and Swaziland were funded in part by the Bristol-Myers Squibb Foundation. The Children’s Clinical Centers of Excellence in Lesotho and Swaziland receive ongoing funding from the governments of Lesotho and Swaziland, respectively.
References
- 1.World Health Organization. Global HIV/AIDS response: epidemic update and health sector progress towards universal access: progress report 2011; Geneva, Switzerland: World Health Organization HIV/AIDS Department; 2011
- 2.Violari A, Cotton MF, Gibb DM, et al. CHER Study Team . Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359(21):2233–2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wamalwa DC, Farquhar C, Obimbo EM, et al. Early response to highly active antiretroviral therapy in HIV-1-infected Kenyan children. J Acquir Immune Defic Syndr. 2007;45(3):311–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Brien DPSD, Sauvageot D, Zachariah R, Humblet P, Medecins Sans Frontieres . In resource-limited settings good early outcomes can be achieved in children using adult fixed-dose combination antiretroviral therapy. AIDS. 2006;20(15):1955–1960 [DOI] [PubMed] [Google Scholar]
- 5.Children and AIDS. Fifth stocktaking report 2010. New York, NY: UNICEF, UNAIDS, WHO, UNFPA, and UNESCO; 2010 [Google Scholar]
- 6.Brady MT, Oleske JM, Williams PL, et al. Pediatric AIDS Clinical Trials Group 219/219C Team . Declines in mortality rates and changes in causes of death in HIV-1-infected children during the HAART era. J Acquir Immune Defic Syndr. 2010;53(1):86–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fenner L, Brinkhof MW, Keiser O, et al. International Epidemiologic Databases to Evaluate AIDS in Southern Africa . Early mortality and loss to follow-up in HIV-infected children starting antiretroviral therapy in Southern Africa. J Acquir Immune Defic Syndr. 2010;54(5):524–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wamalwa DC, Obimbo EM, Farquhar C, et al. Predictors of mortality in HIV-1 infected children on antiretroviral therapy in Kenya: a prospective cohort. BMC Pediatr. 2010;10(33):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reddi A, Leeper SC, Grobler AC, et al. Preliminary outcomes of a paediatric highly active antiretroviral therapy cohort from KwaZulu-Natal, South Africa. BMC Pediatr. 2007;7(13):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rouet F, Fassinou P, Inwoley A, et al. ANRS 1244/1278 Programme Enfants Yopougon . Long-term survival and immuno-virological response of African HIV-1-infected children to highly active antiretroviral therapy regimens. AIDS. 2006;20(18):2315–2319 [DOI] [PubMed] [Google Scholar]
- 11.Leyenaar JK, Novosad PM, Ferrer KT, et al. Early clinical outcomes in children enrolled in human immunodeficiency virus infection care and treatment in lesotho. Pediatr Infect Dis J. 2010;29(4):340–345 [DOI] [PubMed] [Google Scholar]
- 12.de Martino M, Tovo PA, Balducci M, et al. Italian Register for HIV Infection in Children and the Italian National AIDS Registry . Reduction in mortality with availability of antiretroviral therapy for children with perinatal HIV-1 infection. JAMA. 2000;284(2):190–197 [DOI] [PubMed] [Google Scholar]
- 13.Sutcliffe CG, van Dijk JH, Bolton C, Persaud D, Moss WJ. Effectiveness of antiretroviral therapy among HIV-infected children in sub-Saharan Africa. Lancet Infect Dis. 2008;8(8):477–489 [DOI] [PubMed] [Google Scholar]
- 14.Malawi Paediatric Antiretroviral Treatment Group . Antiretroviral therapy for children in the routine setting in Malawi. Trans R Soc Trop Med Hyg. 2007;101(5):511–516 [DOI] [PubMed] [Google Scholar]
- 15.Davies MA, Egger M, Keiser O, Boulle A. Paediatric antiretroviral treatment programmes in sub-Saharan Africa: a review of published clinical studies. Afr J AIDS Res. 2009;8(3):329–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization. The 3 by 5 initiative: Treat three million people with HIV/AIDS by 2005. AIDS Treatment for Children. 2012. Available at: www.who.int/3by5/paediatric/en. Accessed January 20, 2012
- 17.World Health Organization. Antiretroviral Therapy for HIV Infection in Infants and Children: Towards Universal Access: Recommendations for a Public Health Approach—Revision. Geneva, Switzerland: World Health Organization; 2010 [PubMed] [Google Scholar]
- 18.Baylor College of Medicine International Pediatric AIDS Initiative. BIPAI Children’s Clinical Centres of Excellence Network. Available at http://bayloraids.org/programs. Accessed March 31, 2011
- 19.Kline MW, Ferris MG, Jones DC, et al. The Pediatric AIDS Corps: responding to the African HIV/AIDS health professional resource crisis. Pediatrics. 2009;123(1):134–136 [DOI] [PubMed] [Google Scholar]
- 20.BIPAI. BIPAI: Baylor International Pediatric AIDS Initiative at Texas Children’s Hospital. Annual Report. Houston, Texas: BIPAI; 2010
- 21.National Guidelines for HIV/AIDS Care and Treatment. Maseru, Lesotho: Government of Lesotho Ministry of Health and Social Welfare; 2010
- 22.Treatment of AIDS: Guidelines for the use of Antiretroviral Therapy in Malawi. Lilongwe, Malawi: Malawi Ministry of Health; April 2006
- 23.Paediatric HIV/AIDS Treatment Guidelines. Mbabane, Swaziland: Government of Swaziland; 2006
- 24.World Health Organization. Antiretroviral therapy of HIV infection in infants and children in resource-limited settings: towards universal access Recommendations for a public health approach—2006; Geneva, Switzerland: World Health Organization; 2006
- 25.Davies MA, Keiser O, Technau K, et al. International Epidemiologic Databases to Evaluate AIDS Southern Africa (IeDEA-SA) Collaboration . Outcomes of the South African National Antiretroviral Treatment Programme for children: the IeDEA Southern Africa collaboration. S Afr Med J. 2009;99(10):730–737 [PMC free article] [PubMed] [Google Scholar]
- 26.Yu JK, Chen SC, Wang KY, et al. True outcomes for patients on antiretroviral therapy who are “lost to follow-up” in Malawi. Bull World Health Organ. 2007;85(7):550–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bong CN, Yu JK, Chiang HC, et al. Risk factors for early mortality in children on adult fixed-dose combination antiretroviral treatment in a central hospital in Malawi. AIDS. 2007;21(13):1805–1810 [DOI] [PubMed] [Google Scholar]
- 28.Ellis J, Molyneux EM. Experience of anti-retroviral treatment for HIV-infected children in Malawi: the 1st 12 months. Ann Trop Paediatr. 2007;27(4):261–267 [DOI] [PubMed] [Google Scholar]
- 29.Bolton-Moore C, Mubiana-Mbewe M, Cantrell RA, et al. Clinical outcomes and CD4 cell response in children receiving antiretroviral therapy at primary health care facilities in Zambia. JAMA. 2007;298(16):1888–1899 [DOI] [PubMed] [Google Scholar]
- 30.Gupta A, Nadkarni G, Yang WT, et al. Early mortality in adults initiating antiretroviral therapy (ART) in low- and middle-income countries (LMIC): a systematic review and meta-analysis. PLoS ONE. 2011;6(12):e28691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marston M, Becquet R, Zaba B, et al. Net survival of perinatally and postnatally HIV-infected children: a pooled analysis of individual data from sub-Saharan Africa. Int J Epidemiol. 2011;40(2):385–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prevention of Mother to Child Transmission of HIV and Paediatric HIV Care Guidelines, 2nd ed. Lilongwe, Malawi: Malawi Ministry of Health , July 2008 [Google Scholar]
- 33.Prevention of Mother to Child Transmission of HIV Guideline 2006. Mbabane, Swaziland: The Kingdom of Swaziland Ministry of Health and Social Welfare; 2006
- 34.National Guidelines for the Prevention of Mother to Child Transmission of HIV. Maseru, Lesotho: Government of Lesotho Ministry of Health and Social Welfare; 2010
- 35.Eshleman SH, Hoover DR, Chen S, et al. Resistance after single-dose nevirapine prophylaxis emerges in a high proportion of Malawian newborns. AIDS. 2005;19(18):2167–2169 [DOI] [PubMed] [Google Scholar]
- 36.Arrivé E, Newell ML, Ekouevi DK, et al. Ghent Group on HIV in Women and Children . Prevalence of resistance to nevirapine in mothers and children after single-dose exposure to prevent vertical transmission of HIV-1: a meta-analysis. Int J Epidemiol. 2007;36(5):1009–1021 [DOI] [PubMed] [Google Scholar]
- 37.Chaix ML, Ekouevi DK, Peytavin G, et al. Impact of nevirapine (NVP) plasma concentration on selection of resistant virus in mothers who received single-dose NVP to prevent perinatal human immunodeficiency virus type 1 transmission and persistence of resistant virus in their infected children. Antimicrob Agents Chemother. 2007;51(3):896–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davies MA, Moultrie H, Eley B, et al. International Epidemiologic Databases to Evaluate AIDS Southern Africa (IeDEA-SA) Collaboration . Virologic failure and second-line antiretroviral therapy in children in South Africa—the IeDEA Southern Africa collaboration. J Acquir Immune Defic Syndr. 2011;56(3):270–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.WHO Case Definitions of HIV for Surveillance and Revised Clinical Staging and Immunological Classification of HIV-related Disease in Adults and Children. Geneva, Switzerland: World Health Organization ; 2007 [Google Scholar]
- 40.Battegay M, Nüesch R, Hirschel B, Kaufmann GR. Immunological recovery and antiretroviral therapy in HIV-1 infection. Lancet Infect Dis. 2006;6(5):280–287 [DOI] [PubMed] [Google Scholar]