Summary
Objective
To be used in diagnostic studies, it must be demonstrated that biomarkers can differentiate between diseased and non-diseased patients. Therefore, the purpose of this study was to answer the following questions: (1) is serum cartilage oligomeric matrix protein (sCOMP) elevated in patients with radiographically diagnosed knee osteoarthritis (OA) compared to controls? (2) Are there differences in sCOMP levels when comparing differing radiographic OA severities to controls?
Methods
Systematic review and meta-analysis. Data Sources: A systematic search of CINAHL, PEDro, Medline, and SportsDiscus was completed in March 2010. Keywords: knee, osteoarthritis, sCOMP, radiography. Study inclusion criteria: Studies were written in English, compared healthy adults with knee OA patients, used the Kellgren Lawrence (K/L) classification, measured sCOMP, and reported means and standard deviations for sCOMP.
Results
For question 1, seven studies were included resulting in seven comparisons. A moderate overall effect size (ES) indicated sCOMP was consistently elevated in those with radiographically diagnosed knee OA when compared to controls (ES = 0.60, P < 0.001). For question 2, four studies were included resulting in 13 comparisons between radiographic OA severity levels and controls. Strong ESs were calculated for K/L-1 (ES = 1.43, P = 0.28), K/L-3 (ES = 1.05, P = 0.04), and K/L-4 (ES = 1.40, P = 0.003). A moderate ES was calculated for K/L-2 (ES = 0.60, P = 0.01).
Conclusions
These results indicate sCOMP is elevated in patients with knee OA and is sensitive to OA disease progression. Future research studies with a higher level of evidence should be conducted to investigate the use of this biomarker as an indicator for OA development and progression.
Keywords: Biomarkers, Kellgren Lawrence, Radiography, Degenerative joint disease
1. Introduction
Characterized by irreversible joint destruction such as cartilage degradation, osteophyte development and joint space narrowing, osteoarthritis (OA) affects millions of individuals each year1, 2, 3 & 4. Knee OA, either affecting the patellofemoral or the tibiofemoral joint, is the most common cause of disability in the United States2 and 5, causing pain and loss of function1, 3, 6, 7 & 8. Currently, there are few diagnostic tools used to identify individuals with knee OA. The diagnosis of OA is based on patient reports of pain and stiffness, and the presence of osteophytes and joint space narrowing as viewed on radiographs. Although many patients will demonstrate both symptomatic and visual indicators of OA, there is not a direct correlation between clinical indicators and actual joint damage2 & 9. Given the limitation of current diagnostic tools and that early osteoarthritic changes such as articular cartilage abnormalities are silent10, OA is often unrecognized until it has reached an irremediable and disabling level2. The ability to develop intervention strategies with the hope of delaying irreversible joint damage remains difficult due to the lack of sensitive and valid preradiographic diagnostic tools2. Identifications of sensitive diagnostic tools to recognize pre-radiographic OA are necessary in order to develop and implement intervention strategies aimed at delaying irreversible joint damage2.
Several serum and/or synovial fluid biomarkers have been identified in the literature to diagnose preradiographic OA3, 4, 11, 12, 13 & 14. For a biomarker to be useful in diagnosing early joint damage, it must be sensitive to differences between healthy individuals and those with OA, and also among varying degrees of severity of joint disease4, 15 & 16. Examples of these biomarkers include keratan sulfate12 and pentosidine11, both which tend to be elevated in patients with OA. Another biomarker that is theorized to have significant diagnostic value for beginning OA, is serum cartilage oligomeric matrix protein (sCOMP)2.
Serum COMP is a non-collagen biomarker for cartilage degradation present in articular cartilage, and other tissues such as ligament, meniscus, synovial membrane, and tendon1, 17, 18, 19, 20 & 21. Numerous studies have investigated the relationship of sCOMP in patients with and without knee OA3, 4, 12, 14, 22 & 23. Validation of this relationship will provide scientists and physicians with a prospective pre-radiographic diagnostic indicator that may be clinically applicable and may assist in the development of treatment interventions for early stage OA.
The purpose of this systematic review was to answer the following questions: (1) is sCOMP elevated in patients with radiographically diagnosed knee OA compared to controls? (2) Are there differences in sCOMP levels when comparing differing radiographic OA severities to controls?
2. Methods
Search strategy
A computerized literature search was completed in March of 2010 utilizing: CINAHL (from 1981), PEDro (from 1929) Medline (from 1966), and SportDiscus (from 1985). The search terms used were, knee, osteoarthritis, sCOMP, and radiography. All abstracts from the search results were reviewed. If the abstracts did not contain enough information to include or exclude the study from the review, the study was reviewed in its entirety. In addition, all reference lists were cross-referenced for relevant studies not included in the original searches.
Criteria for study selection
The inclusion criteria for the studies used in this systematic review were:
Subjects with radiographically diagnosed knee OA and disease free control groups.
Studies using the Kellgren Lawrence (K/L) scale to classify knee OA.
Studies that measured sCOMP or used sCOMP as an outcome.
Studies reporting means and associated measures of variability.
Studies using human adults (18+ years or older).
Studies published in the English language.
Assessment of publication bias
A funnel plot was used to provide an illustrative assessment of publication bias. In addition, Duval and Tweedie’s Trim and Fill method and Orwin’s Fail-Safe N were used to further interpret possible publication bias. The Duvall and Tweedie’s Trim and Fill method looks for missing studies on the left side of the mean effect using a fixed effects model24. The asymmetric studies from the right hand side of the mean effect are trimmed, the unbiased effect is located, then the studies to left of the mean are then filled in24. This method results in an adjusted cumulative effect, and provides a conservative estimate of the total number of studies that are “missing”. Orwin’s Fail-Safe N test was employed to assess the robustness of the observed overall effects of the moderators on sCOMP25.
Sensitivity analysis
The “one-study removed method” was used to test the stability of the cumulative effect across the included studies by determining if the results of one particular study substantially influenced the overall effect24. The analysis systematically removes each study and replaces it so that the influence of each study can be individually evaluated. If the removal of any given study results in little change, it can be concluded that the pooled result is robust24. For the second question, we performed an additional sensitivity analysis to determine the influence of sample size on the overall effect for each of the individual K/L comparisons. Study comparisons were dichotomized into “large (>10 subjects per group) or “small” (<10 subjects per group). As a group, “large” studies and then “small” studies were selectively removed in order to assess for changes in the overall result based on sample size.
Assessment of study quality
The study quality was assessed independently by two authors using a quality index for non-randomized studies26. This index was adapted from a previously published version by Downs and Black27. Based on the study designs for the included studies, the quality index assessment tool26 was selected in order to compare case-control and retrospective-cohort studies.
A total of 16 items were used to assess study quality for each study. The quality index assessment tool addressed areas such as: clarity of objectives, main outcomes, subject characteristics and main findings, as well as, external validity and internal validity concerning bias and confounding26. Any discrepancies in scores between authors were discussed and a mutual score was reached. Using previously published criteria26, those studies achieving ≥75% of the criteria were considered high quality, 60–74% were considered moderate quality, and ≤60% were considered low quality.
Data extraction and statistical analysis
The variable of interest for this study was sCOMP. The reported unit of measure is typically ng/ml, but sCOMP levels have been reported using μg/ml and U/L. For meta-analysis, all sCOMP units of measure were used for data extraction and statistical analysis. Furthermore, in some cases sCOMP levels are not normally distributed. Recognition of this will allow for the data to be transformed using a logarithmic transformation, assuring the assumptions of the general linear model23 & 28. For the purposes of this meta-analysis, we recognize that a normal distribution might not have been present before data analysis; however, we did not or could not modify the data to control for this.
For this systematic review of the literature, K/L severity classification system for OA was used as an inclusion criterion. This classification system was chosen as it is a common classification system used to grade OA29. Studies using other forms of OA severity classification systems were excluded to ensure consistent comparisons across all studies.
3. Level of Evidence
The level of evidence for the included studies was assessed using the Oxford Centre for Evidence-Based Medicine (CEBM)-Levels of Evidence32. The levels of evidence range from 1a to 5, with 1a representing a systematic review of prospective cohort studies, and level 5 representing expert opinion without critical appraisal, bench research or “first principles”. The CEBM strength-of recommendation grades are A, B, C, and D. Grade A represents consistent level 1 studies; grade B represents consistent level 2 or 3 studies or extrapolations from level 1 studies; grade C represents level 4 studies or extrapolations from level 2 or 3 studies; and grade D represents level 5 evidence, troublingly consistent or inconclusive studies of any level32.
4. Results
Study selection
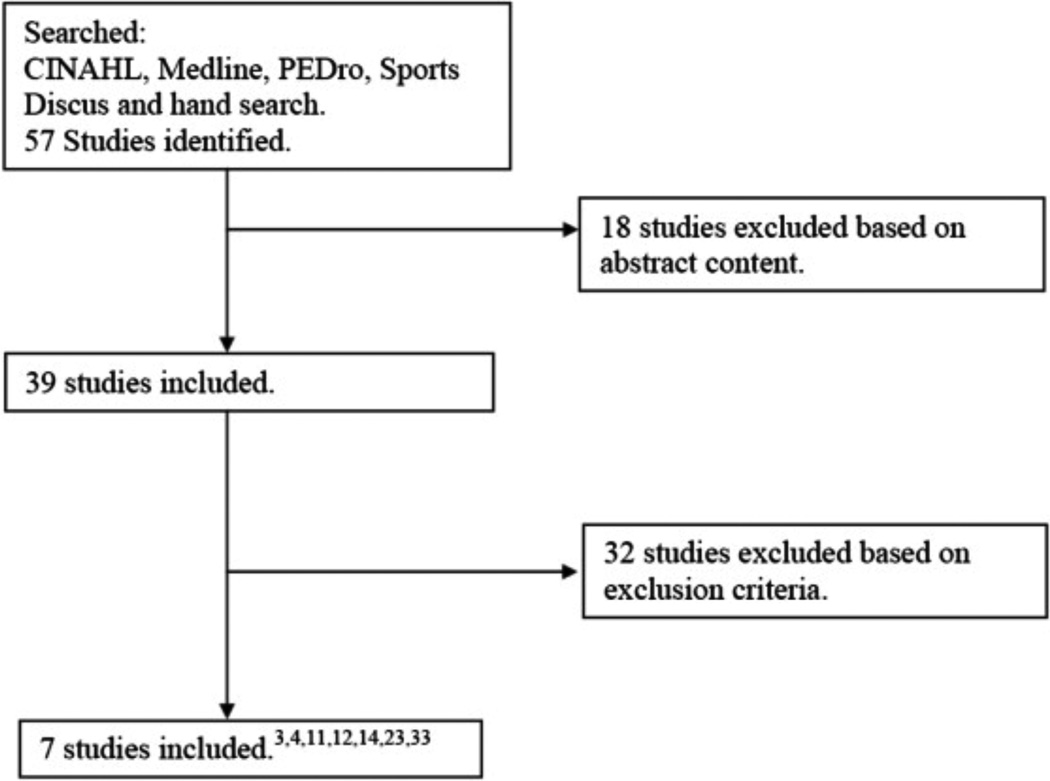
Computerized and hand searches yielded 57 studies that were included in the initial review (Fig. 1). Based on the inclusion criteria and presentation of necessary data, a total of seven studies were included in this review (Table I)3, 4, 11, 12, 14, 23 & 33. The reason(s) for exclusion for the remaining 50 studies can be found in the online Appendix Table I.
Fig. 1.
Summary of search history and included studies.
Table I.
Studies systematically reviewed to determine if sCOMP is elevated in patients with radiographically diagnosed knee OA
| Authors | Level of evidence (CEBM) |
Quality index score (%) |
Study design | OA group inclusion criteria |
No. control patients |
No. OA patients |
Dependent variables measured |
|---|---|---|---|---|---|---|---|
| Cibere et al. (2009)14, * | 2b | 81.25% | Exploratory cohort | Aged 40–79 years; pain, aching, or discomfort in or around the knee on most days of the month at any time in the past; any pain, aching, or discomfort in or around the knee in the past 12 months | 16 | Pre- ROA = 105 ROA = 80† Total = 185 |
C2C; C1, 2C; CPII; CS846; NTX-I; CTX-II; HA; sCOMP; WOMAC |
| Clark et al. (2007)4 | 2b | 81.25% | Retrospective cohort | Caucasian, knee OA K/L ≥ 2 | 148 | K/L 2 = 109 K/L 3,4 = 34 Total = 143 |
sCOMP |
| Fernandeset al. (2007)3, ‡ | 4 | 68.75% | Case control | Aged 40–70 years, mechanical pain in one or both knees for minimum 3 months, and knee crepitus upon clinical evaluation | 86 | SOA = 75** Pain = 11 NOA = 18** Total = 104 |
WOMAC; VAS and sCOMP |
| Jordan et al. (2003)23 | 2b | 87.5% | Retrospective cohort | Radiographs and serum COMP samples in database | 302 | K/L 2 = 313 K/L 3 = 110 K/L 4 = 44 Total = 467 |
sCOMP |
| Mundermann et al. (2009)33 | 4 | 43.75% | Case control | Definite osteophyte presence in the medial or lateral tibiofemoral compartment; a narrowest point inter-bone distance of the medial compartment less than the lateral compartment; pain in and around at least one knee for most of the days in the past months; at least some difficulty with two or more items in the WOMAC physical function scale | 41 | K/L 1 = 11 K/L 2 = 7 K/L 3 = 12 K/L 4 = 12 Total = 42 |
Gait analysis; ambulatory load and sCOMP |
| Senolt et al. (2004)11 | 4 | 25% | Case control | Undergoing arthrocentesis, no history of renal disease of diabetes mellitus and had normal levels of creatinine | 38 | K/L 1 = 2 K/L 2 = 18 K/L 3 = 14 K/L 4 = 4 Total = 38 |
sCOMP and Pentosidine |
| Wakitani et al. (2007)12,†† | 4 | 25% | Case control | Patients undergoing knee surgery | 24 | K/L 1 = 7 K/L 2 = 4 K/L 3 = 6 K/L 4 = 7 Total = 24 |
Articular cartilage assessment, keratan sulfate, CS6, CS846, HA and sCOMP and synovial COMP |
Pre-ROA = pre-radiographic OA (K/L < 2); ROA = radiographic OA (K/L ≥ 2); C2C = Type II collagen cleavage neopeptide; C1, 2C = Types I and II collagen cleavage neoepitope; CS846 = cartilage proteoglycan aggrecan turnover epitope; NTX-I = N-telopeptide of type I collagen; CTX-II = C-telopeptide of type II collagen; HA = hyaluronan acid; WOMAC = Western Ontario McMaster University Index.
Only the ROA group was used for this review.
SOA (symptomatic OA) = K/L grades 2, 3, 4; NOA (non-symptomatic OA) = K/L 2 or higher; Pain = K/L 0 or 1; VAS = visual analog scale.
Only the SOA and NOA groups were used for this review.
C6S = chondroitin 6 sulfate.
Is sCOMP elevated in patients with radiographic knee OA compared to controls?
Seven studies met the inclusion criteria to answer this question (Table I)3, 4, 11, 12, 14, 23 & 33. The mean quality index assessment for the included studies was 59% (25–87.5%). Three studies4, 14 & 23 were considered high quality, one3 was considered moderate quality, and three studies11, 12 & 33 were considered low quality. Level C evidence exists that sCOMP is elevated in patients with knee OA. This recommendation was reached based on consistent level 4 studies with extrapolations from level 2 or 3 studies32.
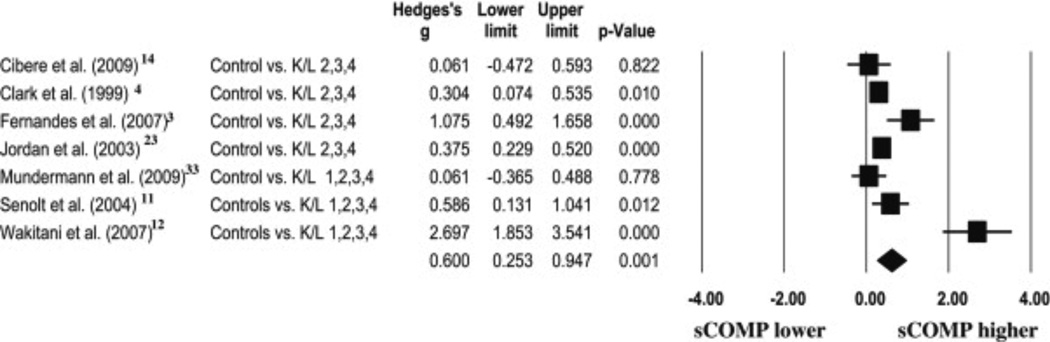
A total of seven ESs and 95% CIs were calculated (Fig. 2). Calculated ESs ranged from 0.06 [(CI) – 0.47–0.60] to 2.70 (1.85–3.54). A total of four ESs were weak, one ES was moderate and two ESs were strong. The results of the random effects meta-analysis revealed a moderate overall ES of 0.60 (0.25–0.94,P = 0.001), indicating sCOMP was consistently elevated in patients with radiographically diagnosed knee OA compared to controls.
Fig. 2.
A Forest plot depicting the calculated ESs of sCOMP in patients with radiographically diagnosed knee OA when compared to controls. The diamond at the bottom of the plot represents the overall ES.
Publication Bias
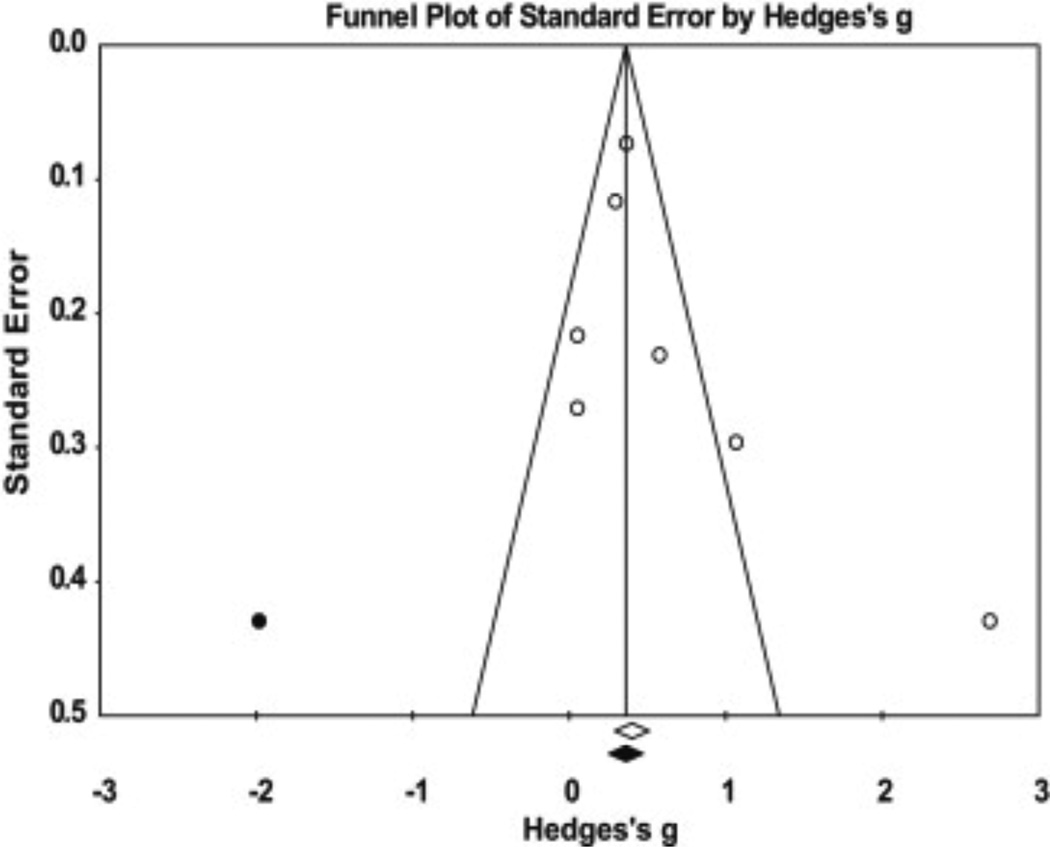
The trim and fill24 analysis indicated one study is missing, and the addition of this study would result in an insignificant weak ES of 0.39 (−0.03–0.81, Fig. 3). In addition, the Orwin’s Fail-Safe N indicated a range of 20–50 additional studies (based on the trivial ES range of 0.05–0.10) would be needed to nullify the overall effect. Therefore, the effect of publication bias introduced across the studies is likely trivial. If all relevant studies beyond those analyzed in this meta-analysis were included, the ES would probably remain unchanged.
Fig. 3.
A funnel plot of standard error by Hedges’ g using Duval and Tweedie’s Trim and Fill method to assess publication bias of the studies used to determine if sCOMP is elevated in patients with radiographically diagnosed knee OA when compared to controls.
Sensitivity analysis
Following the one-study removed method, the ESs ranged from 0.37 to 0.71. The lowest lower confidence limit was 0.17 and the highest upper confidence limit was 1.20. All P-values were P < 0.01. This indicated that there was not one particular study which substantially influenced the overall effect.
Are there differences in sCOMP levels when comparing differing radiographic OA severities to controls?
To be included in data analysis, a study must have presented data for the K/L severities in order to make control comparisons. Using this additional inclusion criteria, four studies4, 11, 12 & 23 were included and 13 comparisons were made (Table I). The mean quality index assessment for the included studies used to answer this question was 56% (25–87.5%). Two studies4 & 23 were considered high quality and two studies11 & 12 were considered low quality. Level C evidence exists that sCOMP levels are elevated according to OA severity when compared to healthy controls. This recommendation was reached based on consistent level 4 studies with extrapolations from level 2 or 3 studies32.
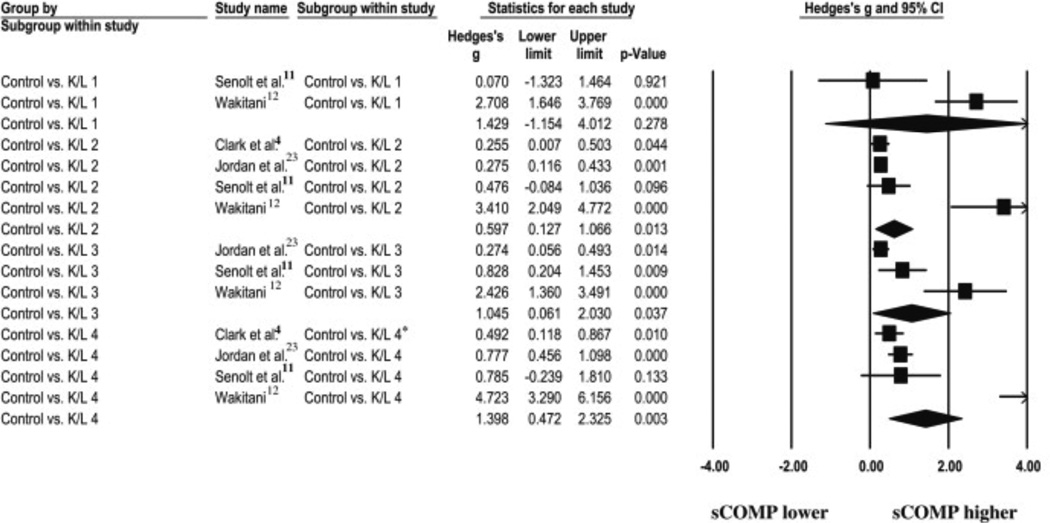
A total of 13 comparisons were used, with a calculated overall effect of 1.00 (0.65–1.35, P < 0.001). To answer question 2, subgroup ESs were calculated for each of the OA severities (K/L-1,-2, etc.) compared to controls. Strong ESs were observed for K/L-1, K/L-3, and K/L-4 while a moderate ES was observed for K/L-2 ( Fig. 4). The subgroup ES for the K/L-1 comparison 11 & 12 was 1.43 (−1.15–4.02, P = 0.28). The subgroup ES for the K/L-2 comparison 4, 11, 12 & 23 was 0.60 (0.13–1.06, P = 0.01). The subgroup ES for the K/L-3 comparison 11, 12 & 23 was 1.05 (0.06–2.03, P = 0.04). The subgroup ES for the K/L-4 comparison 4, 11, 12 & 23 was 1.40 (0.47–2.36, P = 0.003) 4, 11, 12 & 23
Fig. 4.
A Forest plot depicting the calculated ESs of sCOMP exist when comparing patients with differing radiographic knee OA severities compared to controls. Each of the diamonds represents the overall ES for each of the comparisons made for each (K/L) classification.
Our results indicate a significant moderate effect for K/L-2, and a significant strong effect for K/L-3 and K/L-4 when compared to controls. Therefore, the subgroup meta-analysis revealed strong trends in elevated sCOMP levels as OA severity levels increase. However, the CIs for the ESs of each subgroup do overlap, therefore caution must be used when interpreting these results.
Publication bias
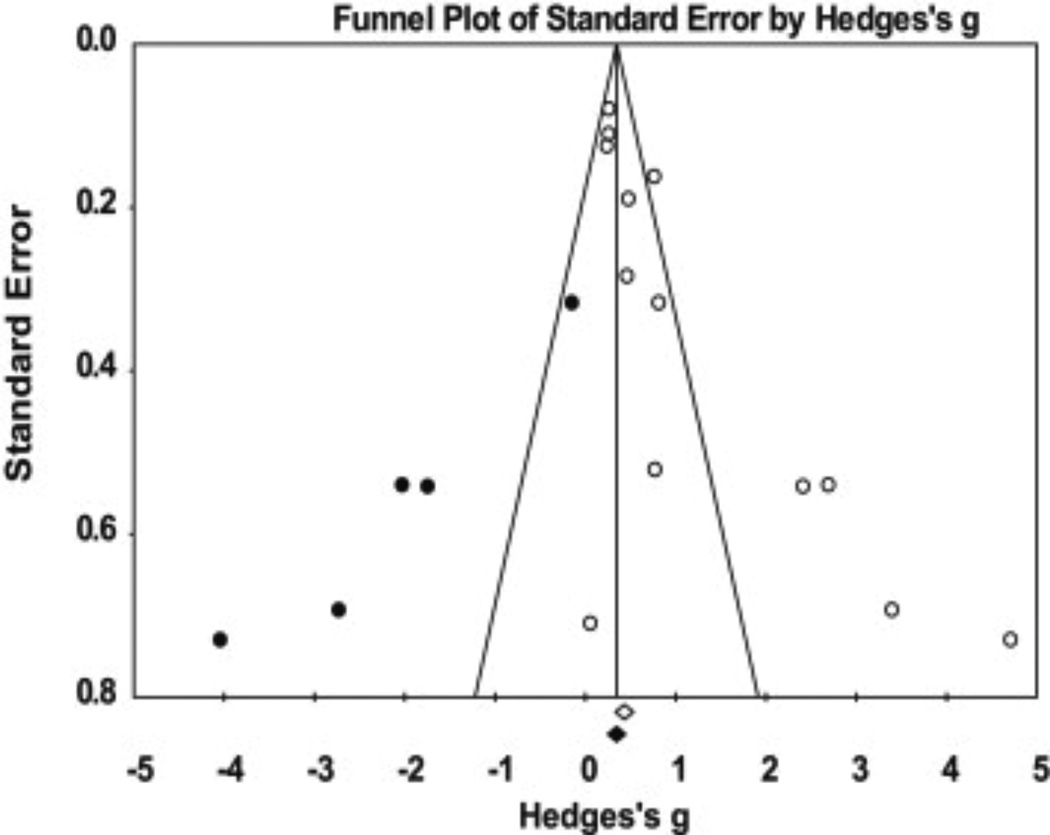
A publication bias assessment using the trim and fill method24 was performed for all 13 comparisons. The results indicated publication bias is likely and there are five comparisons missing (Fig. 5). The addition of these five comparisons to the left of the overall mean effect would result in an insignificant weak overall effect of 0.39 (−0.002–0.79), with the CIs crossing zero. Additionally, the results of Orwin’s Fail-Safe N indicated a range of 44–100 additional studies (based on the trivial ES range of 0.05–0.10) would be needed to nullify the overall effect. Therefore, the effect of publication bias introduced across the studies is trivial. If all relevant studies beyond those analyzed in this meta-analysis were included, the ES would probably remain unchanged.
Fig. 5.
A funnel plot of standard error by Hedges’s g using Duval and Tweedie’s Trim and Fill method to assess publication bias of the comparisons used to determine if differences in sCOMP levels exist when comparing differing knee OA severities when compared to healthy controls.
Sensitivity analysis
Following the one-study removed method; the ESs remained strong and ranged from 0.78 to 1.30. The lowest lower confidence limit was 0.48, and the highest upper confidence limit was 1.60. All P-values were P < 0.001. This indicated that there was not one particular comparison that substantially influenced the overall effect calculated for the question.
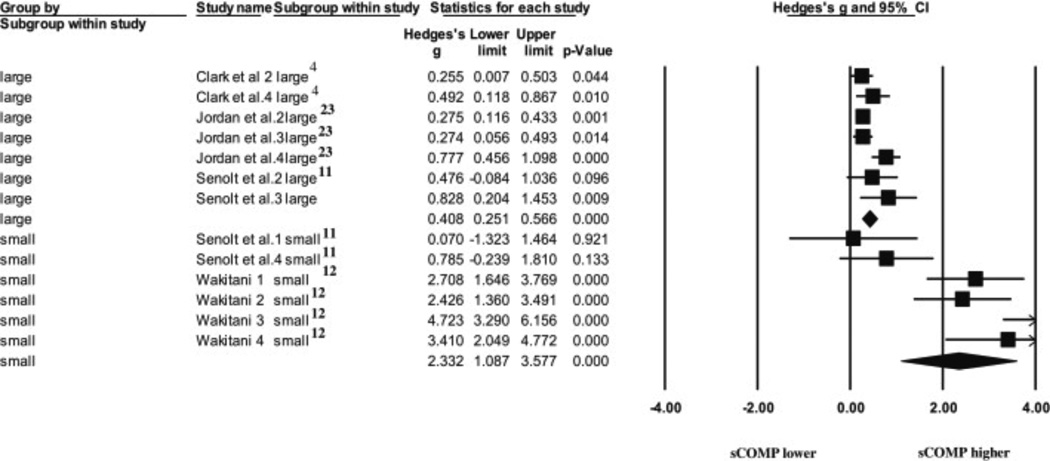
A sense of caution must be employed when interpreting the K/L-1 vs control ES, as the two comparisons used to calculate this ES had a combined total of nine subjects in the group, and the CI encompassed zero. Given the recent results of a meta-epidemiological study34, small comparisons such as those exhibited in the K/L-1 comparison, can inflate the overall effect (see K/L-1 diamond, Fig. 4). In order to determine the effect of the smaller subgroups (n < 10) on the overall ESs, we performed a sensitivity analysis comparing the overall effect of large vs. small subgroups. The results of this analysis ( Fig. 6) indicated small groups had a much larger overall effect (ES = 2.33, CI: 1.09–3.60), compared to larger groups (ES = 0.41, CI: 0.25–0.60), demonstrating small groups significantly influenced the overall effect for each subgroup. However, if we were to remove the comparisons with <10 subjects per group, no comparisons would be available for the K/L-1 severity.
Fig. 6.
A Forest plot depicting the calculated ESs for large groups ( n > 10) and small groups ( n < 10) used to answer the second question.
5. Discussion
To be a useful biomarker for the diagnosis of pre-radiographic knee OA, the biomarker must have a strong correlation with the disease, thereby having levels that are distinguishable between patients with and without the condition4, 15 & 16. Based on the results of this systematic review, we concluded that sCOMP is consistently elevated in patients with radiographic knee OA compared to healthy controls (Fig. 2). Furthermore, higher levels of sCOMP are associated with a trend toward greater radiographic OA severity when compared to controls, as indicated by the ESs (Fig. 3). Further investigation is warranted to determine if this marker can be utilized to assess the presence of pre-radiographic OA, and in evaluating OA interventions in order to delay permanent joint degradation.
Study quality assessment
The mean quality index assessment for these studies was 59%. Three4, 14 & 23 of the studies were considered high quality, one was considered moderate quality3 and three of the studies11, 12 & 33 were considered low quality. For the studies that were considered low quality, a majority of the criteria examining external and internal bias were either unable to be determined or not represented in the manuscript. These criteria included subject recruitment and representation of the population being sampled, blinding of investigators, appropriateness of statistical tests used and validity and reliability of the measurement tools used. Also, missing were criteria that were specific to internal validity. For example, a majority of the internal validity (confounding) criteria were not represented in most manuscripts considered moderate and low quality. These criteria addressed whether subjects were recruited from the same population, during the same period of time and if there was adequate adjustment for confounding in the analyses.
K/L classification and assessment
As part of the inclusion criteria, the K/L classification for knee OA must have been included and reported. This classification system was chosen because it is the most commonly used radiological classification system used to grade OA29. Two studies reviewed did not report the number of radiologists used to determine K/L classification12 & 33. Senolt et al.11 reported the use of two radiologists for the review of subject radiographs and K/L classification assignment. One of the studies reported using a blinded rheumatologist to assign K/L classifications3. Cibere et al. 14 reported two blinded investigators with good interrater reliability (ICC = 0.79) read the radiographs and independently classified the patients. Consensus was reached between readers if they disagreed on K/L classification for the patients14. Finally, two studies reported the use of a single radiologist assessing the radiographs for K/L classification assignment and reported the inter- and intrarater reliability4 & 23. It is important to report the number of individuals that are involved in reviewing radiographs, as this information will allow the readers to understand that bias was adequately controlled for. Also, reporting the inter- and intrarater reliability allows for interpretation of the generalizability of these measures among different clinicians.
Only three studies used in this review provided definitions for each of the K/L classifications used to assess OA severity3, 4 and 23. A recent review of K/L classifications used in current published research, specifically in reference to a grade of 2, reported grade 2 definitions are different throughout the reported literature29. Therefore it is essential the K/L grades be defined in each of the studies35. Knowing which definition the authors used to identify K/L-2 subjects will allow better comparisons to be made across the studies.
Serum COMP ELISAs and associated coefficients of variation (CV)
It has been previously reported that certain ELISAs are more appropriate for detecting human sCOMP than others36. For the purposes of this systematic review we did not exclude studies based on the ELISA used to detect human sCOMP levels. Therefore, multiple ELISAs were used to detect human sCOMP for the studies that were included in our review (Table II). Three studies used an ELISA manufactured by AnaMar Medical3, 14 & 33, three studies4, 11 & 23 used an in house ELISA as previously reported by Vilim et al. 37, and one study used an ELISA manufactured by Kamiya Biomedical Company12. The study using the Kamiya Biomedical Company ELISA 12 did have the largest calculated ES. However, the authors cannot conclude whether or not this ELISA was more sensitive than the others used in this review. It must be noted that Jordan et al. 23 and Clark et al. 4 did report modifications to the Vilim et al. 37 ELISA protocol in that an alkaline phosphatase-conjugated avidin was used rather than the previously reported peroxidase.
Table II.
ELISA manufacturer information and associated CVs for each of the studies
| Study | ELISA manufacture information |
Type of ELISA |
Specific antibodies used |
Interassay CV | Intraassay CV |
|---|---|---|---|---|---|
| Cibere et al.14 | AnaMar Medical, Lund, Sweden | Sandwich | Two monoclonal antibodies (not specified) | <5% | <5% |
| Clark et al.4 | In house method* | Competitive | Monoclonal antibody 17-C10 | Not reported | Not reported |
| Fernandeset al.3 | Anamar Medical, Uppsala, Sweden | Sandwich | Two monoclonal antibodies (not specified) | Not reported | Not reported |
| Jordan et al.23 | In house method* | Sandwich | Monoclonal antibodies 16-F12 and 17-C10 | 9.7% for “normal” controls 8.7% for “high” controls | 5.8% for “normal” controls 6.6% for “high” controls |
| Mundermannet al.33 | AnaMar Medical AB, Lund, Sweden | Sandwich | Mouse monoclonal antibodies (not specified) | <1.9% | <2.7% |
| Senolt et al.11 | In house method* | Sandwich | Monoclonal antibodies 17-C10 and 16-F12 | Not reported | Not reported |
| Wakitani et al.12 | Kamiya Biomedical Company, Seattle, WA. | Sandwich | Not specified | Not reported | Not reported |
The same in house method was used for each of these studies, and the information regarding this method can be found in Vilim V, Voburka Z, Vytasek R, Senolt L, Tchetverikoc I, Kraus VB, et al. Monoclonal antibodies to human cartilage oligomeric matrix protein: epitope mapping and characterization of sandwich ELISA. Clin Chim Acta 2003;328:59–69. However, it must be noted that Clark et al.4 and Jordan et al.23 reported slight modifications to the in house method used for their studies.
Previous research reported a weak correlation (R2 = 0.210) between the AnaMar Medical ELISA and the in house ELISA reported by Vilim et al. 36 & 37 for measuring sCOMP levels in the same subjects 36. For the purposes of this review, readers must be aware that there are differences in detecting sCOMP levels for each of the different ELISA manufactures. The only way to ascertain which ELISA kit is the best at detecting sCOMP levels can only be accomplished by comparing each technique with serum samples from the same subjects, which cannot be done using the available data for this meta-analysis. One advantage of the data contained within this systematic review and meta-analysis is that the ESs provide a unitless measure of the magnitude of change between groups which allow standardization for comparison between studies.
CVs are reported in order to determine the assay variability, and the interassay and intraassay CVs are most commonly reported38. Four studies did not report the inter- or intraassay CVs for the ELISAs used to determine human sCOMP3, 4, 11 & 12. Three studies did report inter- and intraassay CVs; with Mundermannet al. 33 reporting the lowest CVs (<1.9% for interassay, <2.7% for intraassay). Table II reports the inter- and intraassay CVs for each of the studies used in this systematic review and meta-analysis. The acceptable range of reportable CVs are often laboratory specific, therefore it is important that this information be presented in order to ascertain the variability of the reported sCOMP levels in each of the ELISAs performed.
Sensitivity analysis and publication bias assessments
We conducted two separate sensitivity analyses. For the first question, the lowest pooled ES was interpreted as weak (ES = 0.37, CI: 0.18–0.60). For the second analysis, the lowest pooled ES was interpreted as strong (ES = 0.78, CI: 0.50–1.01). Based on these findings, we concluded that our reported results were stable, and that no individual study substantially influenced the overall pooled effects calculated for either meta-analysis.
For the second question, we performed a sensitivity analysis investigating the effects of the small groups (n < 10) on the overall effects for each of the subgroups (Fig. 6). The results of this sensitivity analysis revealed the small groups have a much larger overall effect (ES = 2.33, CI: 1.1–3.6) compared to the large groups (ES = 0.41, CI: 0.25–0.60). However, the smaller groups also demonstrated greater imprecision, indicating potentially inflated results of the smaller groups, which will directly influence the overall effect.
We also assessed publication bias using the Orwin’s Fail-Safe N25 and Duval and Tweedie’s Trim and Fill method24. Although the initial funnel plot indicated there was a possibility of publication bias for each of our questions (Fig. 3 and Fig. 5), our secondary publication bias analysis indicated that more than 20 studies for the first question and 44 studies for the second question, with non-significant results, would need to be included in the analysis in order to make the pooled results become trivial and nullify our overall effects.
6. Limitations
This review is not without limitations. First, it must be noted that only studies using the K/L classification scale for OA were included. This classification system was chosen as it is a common classification system used to grade OA29. There are other systems for classifying OA; however, we chose to use only the K/L classification scale in order to ensure consistent comparisons across studies.
Second, a recent meta-epidemiological study associated with randomized control trials for OA treatment determined smaller studies (n < 100) can have a deleterious effect on the interpretation of the overall meta-analysis results 34. For the purposes of our meta-analysis, we considered a group small if they contained <10 subjects. For question 2, we had six groups that were considered small. In order to determine whether or not these groups had a harmful effect on the overall ESs for each subgroup, we conducted a secondary analysis of small vs. large comparisons (Fig. 6). Based on this analysis, we determined that the small group comparisons might inflate the overall ESs. Therefore caution is necessary when interpreting our results for the K/L-1 comparison as this subgroup only had nine subjects. In addition, it was not possible to determine if several of the studies were prospective or retrospective in nature. Inclusion of this information in the future will allow more accurate synthesis of the available data.
7. Conclusions
The usefulness of sCOMP as a diagnostic biomarker for identification of knee OA prior to the occurrence of permanent joint degradation is contingent upon the sensitivity to detect differences in patients with and without the disease and between differing severities of the disease4, 15 & 16. For both questions posed in this review, level C evidence is currently available in the literature. This recommendation was reached based on consistent level 4 studies or extrapolations from level 2 studies32. To strengthen this recommendation, future rigorous prospective investigations should be conducted. However, based on the available reported data, our results indicate sCOMP is elevated in patients with diagnosed knee OA compared to controls (Fig. 2). Furthermore, clear trends were identified based on disease severity with larger ESs for K/L-3 and K/L-4 comparisons when compared to K/L-2 comparisons (Fig. 4).
Acknowledgments
There are no sources of funding for this study.
Footnotes
8. Author contributions
All authors made substantial contributions to all three sections: (1) the conception and design of the study, or acquisition of data, or analysis and interpretation of the data, (2) the drafting of the article or revising it critically for important intellectual content and, (3) final approval of the version submitted.
9. Conflict of interest
There are no competing interests to disclose for any of the authors on this manuscript.
References
- 1.Garnero P, Delmas PD. Biomarkers in osteoarthritis. Curr Opin Rheumatol. 2003;15:641–646. doi: 10.1097/00002281-200309000-00020. [DOI] [PubMed] [Google Scholar]
- 2.Tseng S, Reddi AH, Di Cesare PE. Cartilage oligomeric matrix protein (COMP): a biomarker of arthritis. Biomark Insights. 2009;4:33–44. doi: 10.4137/bmi.s645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fernandes FA, Pucinelli MLC, da Silva NP, Feldman D. Serum cartilage oligomeric matrix protein (COMP) levels in knee osteoarthritis in a Brazilian population: clinical and radiological correlation. Scand J Rheumatol. 2007;36(3):211–215. doi: 10.1080/03009740601154186. [DOI] [PubMed] [Google Scholar]
- 4.Clark G, Jordan JM, Vilim V, Renner JB, Dragomir AD, Luta G, et al. Serum cartilage oligomeric matrix protein reflects osteoarthritis presence and severity: the Johnston County Osteoarthritis Project. Arthritis Rheum. 1999;42(11):2356–2364. doi: 10.1002/1529-0131(199911)42:11<2356::AID-ANR14>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 5.Palmieri-Smith RM, Thomas AC. A neuromuscular mechanism of posttraumatic osteoarthritis associated with ACL injury. Exerc Sport Sci Rev. 2009;37(3):147–153. doi: 10.1097/JES.0b013e3181aa6669. [DOI] [PubMed] [Google Scholar]
- 6.Vilim V, Vytasek R, Olejarova M, Machacek S, Gatterova J, Prochazka B, et al. Serum cartilage oligomeric matrix protein reflects the presence of clinically diagnosed synovitis in patients with knee osteoarthritis. Osteoarthritis Cartilage. 2001;9(7):612–618. doi: 10.1053/joca.2001.0434. [DOI] [PubMed] [Google Scholar]
- 7.Andersson MLE, Thorstensson CA, Roos EM, Petersson IF, Heinegayrd D, Saxne T. Serum levels of cartilage oligomeric matrix protein (COMP) increase temporarily after physical exercise in patients with knee osteoarthritis. BMC. 2006;7(98) doi: 10.1186/1471-2474-7-98. http://dx.doi.org.ezproxy.uky.edu/10.1186/1471-2474-7-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruyere O, Collette JC, Ethgen O. Biochemical markers of bone and cartilage remodeling in prediction of longterm progression of knee osteoarthritis. J Rheumatol. 2002;30:1043–1050. [PubMed] [Google Scholar]
- 9.Garnero P, Piperno M, Gineyts E, Christgau S, Delmas PD, Vignon E. Cross sectional evaluation of biochemical markers of bone, cartilage, and synovial tissue metabolism in patients with knee osteoarthritis: relations with disease activity and joint damage. Ann Rheum Dis. 2001;60(6):619–626. doi: 10.1136/ard.60.6.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wislowska M, Jablonska B. Serum cartilage oligomeric matrix protein (COMP) in rheumatoid arthritis and knee osteoarthritis. Clin Rheumatol. 2005;2:278–284. doi: 10.1007/s10067-004-1000-x. [DOI] [PubMed] [Google Scholar]
- 11.Senolt L, Braun M, Olejarova M, Forejtova S, Gatterova J, Pavelka K. Increased pentosidine, an advanced glycation end product, in serum and synovial fluid from patients with knee osteoarthritis and its relation with cartilage oligomeric matrix protein. Ann Rheum Dis. 2005;64(6):886–890. doi: 10.1136/ard.2004.029140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wakitani S, Nawata M, Kawaguchi A, Okabe T, Takaoka K, Tsuchiya T, et al. Serum keratan sulfate is a promising marker of early articular cartilage breakdown. Rheumatology. 2007;46(11):1652–1656. doi: 10.1093/rheumatology/kem220. [DOI] [PubMed] [Google Scholar]
- 13.Pavelka K, Forejtova S, Olejarova M, Gatterova J, Senolt L, Spacek P, et al. Hyaluronic acid levels may have predictive value for the progression of knee osteoarthritis. Osteoarthritis Cartilage. 2004;12(4):277–283. doi: 10.1016/j.joca.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 14.Cibere J, Zhang H, Garnero P, Poole AR, Lobanok T, Saxne T, et al. Association of biomarkers with pre-radiographically defined and radiographically defined knee osteoarthritis in a population-based study. Arthritis Rheum. 2009;60(5):1372–1380. doi: 10.1002/art.24473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bauer DC, Hunter DJ, Abramson SB, Attur M, Corr M, Felson D, et al. Classification of osteoarthritis biomarkers: a proposed approach. Osteoarthritis Cartilage. 2006;14(8):723–727. doi: 10.1016/j.joca.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Otterness IG, Swindell AC, Zimmerer RO, Poole AR, Ionescu M, Weiner E. An analysis of 14 molecular markers for monitoring osteoarthritis: segregation of the markers into clusters and distinguishing osteoarthritis at baseline. Osteoarthritis Cartilage. 2000;8(3):180–185. doi: 10.1053/joca.1999.0288. [DOI] [PubMed] [Google Scholar]
- 17.Hedbom E, Antonsson P, Hjerpe A, Aeschlimann D, Paulsson M, Rosa-Pimentel E, et al. Cartilage matrix proteins. An acidic oligomeric protein (COMP) detected only in cartilage. J Biol Chem. 1992;267(9):6132–6136. [PubMed] [Google Scholar]
- 18.Muller G, Michel A, Altenburg E. COMP (cartilage oligomeric matrix protein) is synthesized in ligament, tendon, meniscus, and articular cartilage. Connect Tissue Res. 1998;39(4):233–244. doi: 10.3109/03008209809021499. [DOI] [PubMed] [Google Scholar]
- 19.Neidhart M, Hauser N, Paulsson M, DiCesare PE, Michel BA, Hauselmann HJ. Small fragments of cartilage oligomeric matrix protein in synovial fluid and serum as markers for cartilage degradation. Br J Rheumatol. 1997;36(11):1151–1160. doi: 10.1093/rheumatology/36.11.1151. [DOI] [PubMed] [Google Scholar]
- 20.DiCesare P, Hauser N, Lehman D, Pasumarti S, Paulsson M. Cartilage oligomeric matrix protein (COMP) is an abundant component of tendon. FEBS Lett. 1994;354(2):237–240. doi: 10.1016/0014-5793(94)01134-6. [DOI] [PubMed] [Google Scholar]
- 21.Recklies AD, Baillargeon L, White C. Regulation of cartilage oligomeric matrix protein synthesis in human synovial cells and articular chondrocytes. Arthritis Rheum. 1998;41(6):997–1006. doi: 10.1002/1529-0131(199806)41:6<997::AID-ART6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 22.Bleasel F, Poole AR, Heinegard D, Saxne T, Holderbaum D, Ionescu M, et al. Changes in serum cartilage marker levels indicate altered cartilage metabolism in families with the osteoarthritis-related type II collagen gene COL2A1 mutation. Arthritis Rheum. 1999;42(1):39–45. doi: 10.1002/1529-0131(199901)42:1<39::AID-ANR5>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 23.Jordan JM, Luta G, Stabler M, Renner JB, Dragomir AD, Vilim V, et al. Ethnic and sex differences in serum levels of cartilage oligomeric matrix protein: the Johnston County Osteoarthritis Project. Arthritis Rheum. 2003;48(3):675–681. doi: 10.1002/art.10822. [DOI] [PubMed] [Google Scholar]
- 24.Borenstein M, Hedges L, Higgins J, et al. Introduction to Meta-analysis. West Sussex, UK: John Wiley & sons, Ltd.; 2009. [Google Scholar]
- 25.Orwin RG. A fail-Safe N for effect size in meta-analysis. J Educ Stat. 1983;8(2):157–159. [Google Scholar]
- 26.Munn J, Sullivan SJ, Schneiders AG. Evidence of sensorimotor deficits in functional ankle instability: a systematic review with meta-analysis. J Sci Sports Med. 2010;13(1):2–12. doi: 10.1016/j.jsams.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 27.Downs S, Black N. The feasibility of creating a check list for the assessment of methodological quality of both randomised and non-randomised trials. J Epidemiol Community Health. 1998;52(6):377–384. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dragomir AD, Kraus VB, Renner JB, Luta G, Clark A, Vilim V, et al. Serum cartilage oligomeric matrix protein and clinical signs and symptoms of potential pre-radiographic hip and knee pathology. Osteoarthritis Cartilage. 2002;10(9):687–691. doi: 10.1053/joca.2002.0816. [DOI] [PubMed] [Google Scholar]
- 29.Schiphof D, Boers M, Bierma-Zeinstra SMA. Differences in descriptions of Kellgren and Lawrence grades of knee osteoarthritis. Ann Rheum Dis. 2008;67:1034–1036. doi: 10.1136/ard.2007.079020. [DOI] [PubMed] [Google Scholar]
- 30.Hedges LV, Olkin I. Statistical Methods for Meta-analysis. Academic Press Inc; 1985. [Google Scholar]
- 31.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd edn. Hillsdale, NJ: Lawrence Erlbuam Assoc.; 1988. [Google Scholar]
- 32.Centre for Evidence Based Medicine. Levels of evidence and grades of recommendation. [cited 2010 May 15]; Available from http://www.cebm.net/index.aspx?o=1025.
- 33.Mundermann KB, King RL, Smith TP, Andriacchi Change in serum COMP concentration due to ambulatory load is not related to knee OA status. J Orthop Res. 2009;27(11):1408–1413. doi: 10.1002/jor.20908. [DOI] [PubMed] [Google Scholar]
- 34.Nuesch E, Trelle S, Reichenbach S, Rutjes A, Tschannen B, Altman D, et al. Small study effects in meta-analyses of osteoarthritis trials: meta-epidemiological study. BMJ. 2010;341:c3515. doi: 10.1136/bmj.c3515. http://dx.doi.org.ezproxy.uky.edu/10.1136/bmj.c3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oiestad BE, Engebretsen L, Storheim K, Risberg MA. Knee osteoarthritis after anterior cruciate ligament injury: a systematic review. Am J Sports Med. 2009;37(7):1434–1144. doi: 10.1177/0363546509338827. [DOI] [PubMed] [Google Scholar]
- 36.Stabler T, Fang F, Jordan J, Vilim V, Kraus VB. A comparison of methods for measuring cartilage oligomeric matrix protein (COMP) in human subjects with knee OA. Osteoarthritis Cartilage. 2007;15(Suppl C):C81–C82. [Google Scholar]
- 37.Vilim V, Voburka Z, Vytasek R, Senolt L, Tchetverikov I, Kraus VB, et al. Monoclonal antibodies to human cartilage oligomeric matrix protein: epitope mapping and characterization of sandwich ELISA. Clin Chim Acta. 2003;328:59–69. doi: 10.1016/s0009-8981(02)00375-3. [DOI] [PubMed] [Google Scholar]
- 38.Reed GF, Lynn F, Meade BD. Use of coefficient of variation in assessing variability of quantitative assays. Clin Diagn Lab Immunol. 2002;9(6):1235–1239. doi: 10.1128/CDLI.9.6.1235-1239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]