Abstract
Low-grade extraskeletal osteosarcoma is an extremely rare neoplasm with only nine cases reported in the literature, in which only one case involved the retroperitoneum. Tendency to dedifferentiate as well as recur with high-grade variant is known and management principles are not well defined, necessitating a continuous review of literature. We report management of the largest such retroperitoneal tumour (18.0 cm× 18.3 cm×17.8 cm) in a 38-year-old man, which was treated with surgery followed by six cycles of cisplatin (90 mg/m2 days 1 and 2) and doxorubicin (75 mg/m2 day 3), cycled every 3 weeks (with granulocyte colony stimulating factor support as necessary). The patient is disease free after 3 years of follow-up.
Background
Low-grade extraskeletal osteosarcoma (LG-ESOS) is an extremely rare neoplasm with only nine cases reported in the literature since 1953, in which only one involved the retroperitoneum.1 Its biological behaviour is said to be better than its high-grade counterpart (high-grade extraskeletal osteosarcoma (HG-ESOS), which is also rare, with fewer than 300 cases reported)).2 3 Management principles are not well defined and tendency to dedifferentiate as well as recur with high-grade variant is known, thus necessitating a continuous review of literature. We present the case of the largest such retroperitoneal LG-ESOS; this is only the second such case reported so far.
Case presentation
A 38-year-old man presented to our hospital with a gradual onset, progressive distension of abdomen (mainly right sided), with dull pain, for 6 months, mildly interfering normal and increasingly significantly interfering vocational activity. There was no history of any surgical procedure, trauma, radiation exposure, any long-term medication or addictions. General physical examination was unremarkable and Karnofsky performance score (KPS) was 80. A large, hard, intra-abdominal, retroperitoneal lump of size 20 cm×20 cm was palpable, involving the right iliac, lumbar, hypochondrium and parts of umbilical and hypogastric regions crossing the midline. There was no clinical evidence of metastatic disease, and examinations of the spine, genitalia and rectum (examined by finger per rectally) were normal.
Investigations
Routine blood investigations were normal including levels of serum lactate dehydrogenase, α feto protein and β human chorionic gonadotropin. Contrast-enhanced CT (CECT) (with angiography of intra-abdominal vessels) of the abdomen, pelvis and chest revealed a large (18.0 cm×18.3 cm×17.8 cm), resectable, lobulated, retroperitoneal mass lesion in the right lumbar and iliac fosse regions (from L2 to S1 bodies), inseparable from the right psoas muscle with sunburst calcification, and minimal right hydrouretero nephrosis with displacement of the right ureter, and a normal chest scan (figure 1A,B). Blood supply of the tumour came from the right lumbar arteries, and major intra-abdominal vessels were uninvolved with the displacement of the inferior vena cava by the tumour (figure 2).
Figure 1.
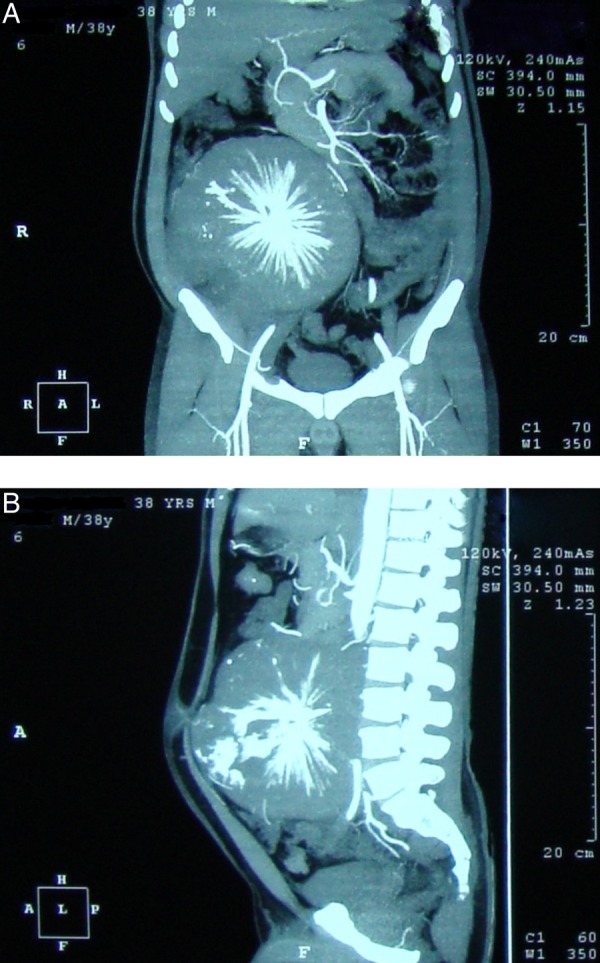
Contrast-enhanced CT: (A) coronal section showing the tumour displacing bowel loops and (B) sagittal section showing the tumour infiltrating psoas and stretching the anterior abdominal wall.
Figure 2.
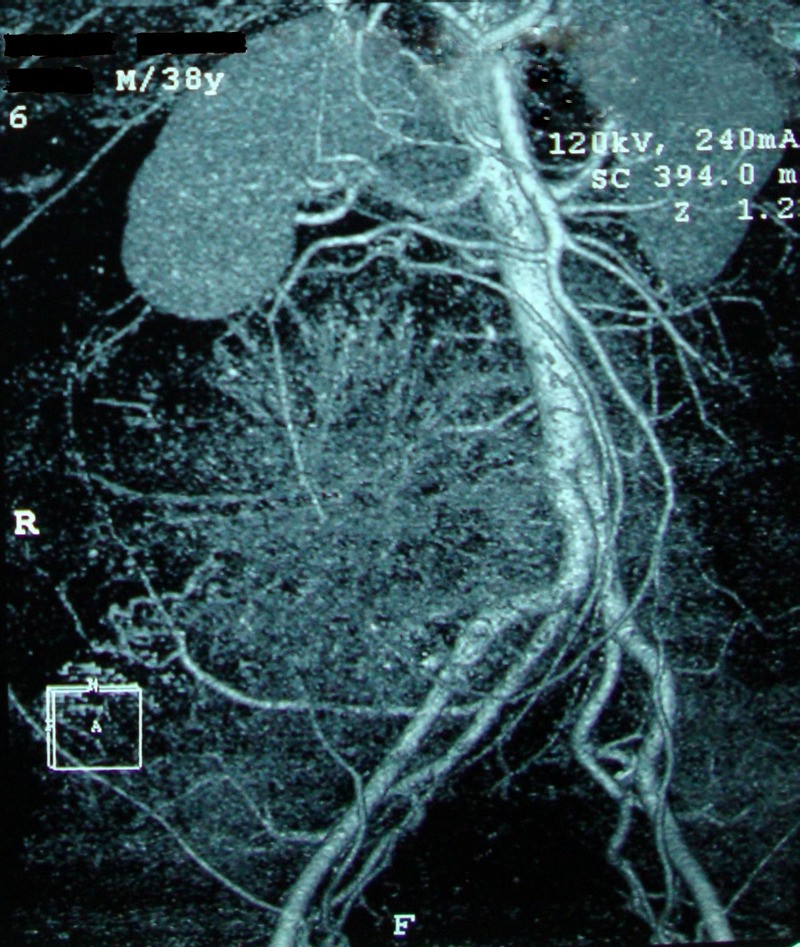
CT angiogram of intra-abdominal vessels showing displacement by the retroperitoneal tumour.
Differential diagnosis
A working diagnosis of soft tissue sarcoma (with radiological impression of ESOS) with possibility of its histological variants showing calcification (such as extraskeletal osteosarcoma, synovial sarcoma, parosteal osteosarcoma and mesenchymal chondrosarcoma) and benign lesions (such as myositis ossificans, ossifying lipoma, soft tissue chondroma or osteoma and ossifying fibromyxoid tumour) was made.
Treatment
The patient was offered upfront surgery, and on laparotomy, the tumour was found to have adhesions to the psoas, terminal ileum, mesentery of the ascending colon and right external iliac artery (<180° circumference) without lymphadenopathy. R1 resection (without lymph node dissection) with removal of the entire gross tumour preserving all major intra-abdominal solid organs with limited right colectomy and ileo-ascending anastomosis (with resection of 10 cm of terminal ileum) and shaving of margin of adventitia of external iliac artery was performed. Postoperative stay of the patient was uneventful and the patient was discharged on the seventh postoperative day.
Outcome and follow-up
On pathological examination, the tumour was hard, and cut with a grating sensation, and was partly bony; more so, in centre. Histological evaluation revealed areas of irregularly anastomosed woven bone (more mature at the centre than the periphery), with foci of atypical cartilage and fibrous tissue, and no fatty differentiation. A few giant cell osteoclasts were seen, with spindle cells in fibrous tissues showing hyperchromasia with low mitotic figures and mild nuclear atypia. A final pathological diagnosis of LG-ESOS was assigned. Four weeks later, the patient was started on six cycles of chemotherapy with cisplatin (90 mg/m2 days 1 and 2) and doxorubicin (75 mg/m2 day 3) every 3 weeks with granulocyte colony stimulating factor support as necessary. A close follow-up was continued with clinical examination every 3 months, and follow-up CECT of the abdomen, pelvis and chest every 6 months were performed for the first 2 years, and annually thereafter. The patient is disease free until date (completed 36 months follow-up) with KPS score of 100, and doing his occupational activities normally; figure 3 shows the absence of local recurrence in the past follow-up CECT of the abdomen in March 2013.
Figure 3.
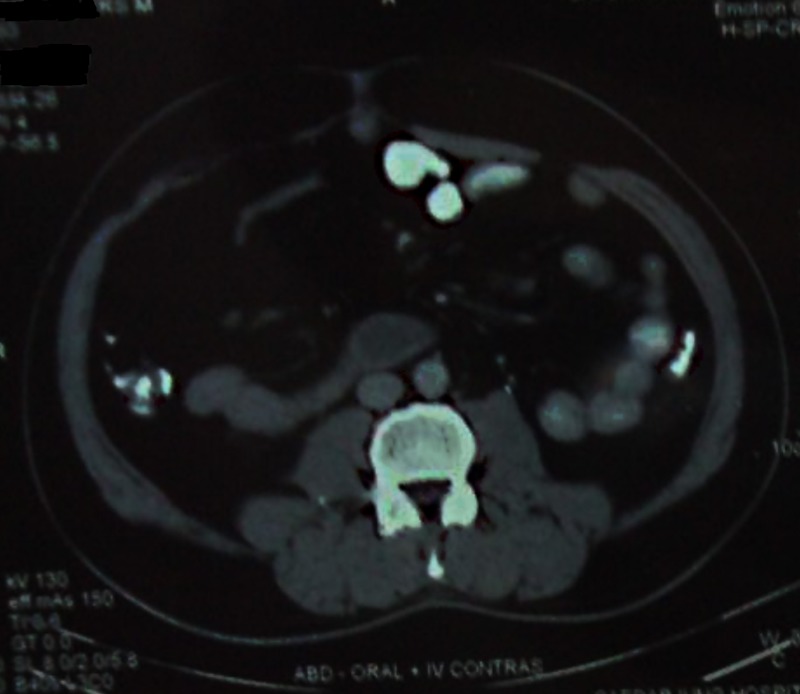
Follow-up contrast-enhanced CT at 3 years follow-up showing absence of local recurrence.
Discussion
ESOS, by definition, is said to arise from soft tissues without attachment to bone or periosteum, with malignant osteoid or bone (or both) formation and a uniform morphological sarcomatous tissue pattern, arising probably due to sarcomatous transformation of mesenchymal multipotent stem cells. A low-grade variant of ESOS has been rarely described (ESOS are usually of high grade). The present case, with only nine similar previously reported LG-ESOS cases, is summarised in table 1.1 3–9
Table 1.
Summary of cases reported in the literature with low-grade extraskeletal osteosarcoma, including the present case1 3–9
| Reference | Age/sex | Initial diagnosis | Location | Largest diameter | Treatment | Clinical outcome and follow-up period |
|---|---|---|---|---|---|---|
| 4 | 39/M | Myositis ossificans | Right thigh | 5 cm | Surgery | Did well for initial 5 years, but finally died with metastatic disease with high-grade histological picture |
| 5 | 57/F | LG-ESOS | Right popliteal fossa | 24 cm | Surgery | Disease free, 5 years |
| 6 | 74/F | Parosteal OS | Left axillae | 15 cm | Surgery | Disease free, 2 years |
| 7 | 3/F | LG-ESOS | Right temporal lobe of the brain | Not specified | R2 resection followed by chemotherapy (MTX, D, CP) and radio-surgery for residual mass | No distant or local relapse, 18 months |
| 8 | 35/F | Myositis ossificans | Left leg | 11 cm | Surgery | Disease free, 4 years |
| 3 | 40/F | Parosteal OS | Left upper back | 9 cm | Surgery | Lost to follow-up after 2 months |
| 3 | 32/M | LG-ESOS | Right thigh, and other areas | 16 cm | Inadequate surgery and chemotherapy (MTX) | Did not respond to chemotherapy given for 8 months, lung and retroperitoneum metastasis (high-grade histology), 4 years, death |
| 1 | 62/F | Differential diagnosis of OS, leiomyosarcoma, recurrent phaeochromocytoma | Retroperitoneum | 14 cm | Surgery | Died with a widespread metastasis (high-grade histology) after 33 months |
| 9 | 30/M | Myositis ossificans | Chest | 30 cm | Chemotherapy (D, CP, I) | Loco-regional extension, death 5 years after initial symptoms and 1 year after correct diagnosis |
| Present case | 38/M | LG-ESOS | Retroperitoneum | 18 cm | Surgery and chemotherapy (CP, D) | Disease free, 3 years |
CP, cisplatin; D, doxorubicin; I, ifosfamide; LG-ESOS, low-grade extraskeletal osteosarcoma; MTX, methotrexate; OS, osteosarcoma.
Median age at presentation is 39 years, with a gender ratio of six women/four men. Most LG-ESOS attain a large size prior to definite intervention with the delay in the diagnosis as a major contributing factor. Differential diagnosis of LG-ESOS includes many benign lesions such as myositis ossificans, ossifying lipoma, soft tissue chondroma or osteoma, and ossifying fibromyxoid tumour as well as malignant lesions such as HG-ESOS, parosteal OS, mesenchymal chondrosarcoma and synovial sarcoma.
Histology and clinical behaviour of ESOS is similar to skeletal OS with osteoblastic, chondroblastic, fibroblastic, and telangiectatic subtypes; with LG-ESOS being similar in histology and clinical behaviour to well-differentiated intraosseous and parosteal variants of skeletal OS (slow growing, but longstanding cases may dedifferentiate with increased mitotic activity, rapid loco-regional progression and metastasis). Reverse zoning phenomenon is also characteristic with the central deposition of osteoid/mature bone and peripheral atypical spindle cells/immature bone.
Recently, molecular analysis—amplification and/or over-expression of MDM2 and CDK4—has been suggested to help in diagnosis of early and/or ambiguous cases of LG-ESOS, exploiting the similarity to low-grade parosteal OS (in which amplification of genes in the 12q13–15 region, such as SAS, CDK4 and MDM2, have been found relatively frequent).9
The best local treatment for ESOS appears to be timely surgical resection (with limb salvage surgery as an acceptable option), but its effect on distant metastasis is not so clear. Thus, HG-ESOS is frequently also treated with aggressive multiagent chemotherapy (in addition to adequate surgical excision) similar to conventional osteosarcoma, although a few studies have found low response for cisplatin and doxorubicin-based regimen.10 Radiotherapy is similarly offered but is not well established. We extended adjuvant chemotherapy to our case as the tumour was very large and we were aware of its potential to recur with high-grade variety; moreover, only R1 resection was achieved. Also, at the time when we initially managed the case, no LG-ESOS had ever been reported to arise from the retroperitoneum, and thus there was a significant knowledge gap. The patient was kept under surveillance with frequent CECT evaluations as most recurrences of ESOS are observed within the first 3 years.
In conclusion, early diagnosis and timely adequate surgery with possible multiagent chemotherapy in selected cases may offer the best hope for this rare neoplasm.
Learning points.
Extraskeletal osteosarcomas (ESOS) are high-grade neoplasms; however, low-grade variants may exist extremely rarely.
Early diagnosis and timely adequate surgery may offer excellent prognosis in low-grade ESOS.
Diagnostic delay is the Achilles heel which worsens prognosis significantly.
Molecular methods may help to resolve diagnostic dilemma in early/equivocal cases.
Multiagent chemotherapy should be selectively offered.
Acknowledgments
The authors wish to acknowledge the help and support of Dr Ashwini Gupta and Dr Rajkumar Chejara in case management and preparation of this manuscript.
Footnotes
Contributors: JJ, AK, SPK and ASR were involved in conception and design of study. JJ was involved in acquisition, analyses/interpretation of data and preparation of draft manuscript. AK, SPK and ASR were involved in revising the manuscript critically for important intellectual content. All authors approved the final draft.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Arai H, Rino Y, Nishii T, et al. Well-differentiated extraskeletal osteosarcoma arising from the retroperitoneum that recurred as anaplastic spindle cell sarcoma. Case Rep Med 2010;2010:327591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tao SX, Tian GQ, Ge MH, et al. Primary extraskeletal osteosarcoma of omentum majus. World J Surg Oncol 2011;9:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abramovici LC, Hytiroglou P, Klein RM, et al. Well differentiated extraskeletal osteosarcoma: report of 2 cases, 1 with dedifferentiation. Hum Pathol 2005;36:439–43 [DOI] [PubMed] [Google Scholar]
- 4.Umiker W, Jaffe HL. Ossifying fibrosarcoma (extraskeletal osteogenic sarcoma) of thigh muscle; report of a case with recurrence and widespread metastases more than four and a half years after excision. Ann Surg 1953;138:795–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Present D, Bertoni F, Laus M, et al. Case report 565: low-grade osteosarcoma of soft tissues (popliteal fossa). Skeletal Radiol 1989;18:471–4 [DOI] [PubMed] [Google Scholar]
- 6.Yi ES, Shmookler BM, Malawer MM, et al. Well-differentiated extraskeletal osteosarcoma. A soft-tissue homologue of parosteal osteosarcoma. Arch Pathol Lab Med 1991;115:906–9 [PubMed] [Google Scholar]
- 7.Bauman GS, Wara WM, Ciricillo SF, et al. Primary intracerebral osteosarcoma: a case report. J Neurooncol 1997;32:209–13 [DOI] [PubMed] [Google Scholar]
- 8.Okada K, Ito H, Miyakoshi N, et al. A low-grade extraskeletal osteosarcoma. Skeletal Radiol 2003;32:165–9 [DOI] [PubMed] [Google Scholar]
- 9.Sabatier R, Bouvier C, de Pinieux G, et al. Low-grade extraskeletal osteosarcoma of the chest wall: case report and review of literature. BMC Cancer 2010;10:645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldstein-Jackson SY, Gosheger G, Delling G, et al. Extraskeletal osteosarcoma has a favourable prognosis when treated like conventional osteosarcoma. J Cancer Res Clin Oncol 2005;131:520–6 [DOI] [PMC free article] [PubMed] [Google Scholar]


