Abstract
Chronic conditions are the most important cause of morbidity, mortality and health expense in the USA. Comparative effectiveness research (CER) seeks to provide evidence supporting the relative value of alternative courses of action. This research often concludes with estimates of the likelihood of desirable and undesirable outcomes associated with each option. Patients with chronic conditions should engage with their clinicians in deciding which of these options best fits their goals and context. In practicing shared decision-making (SDM), clinicians and patients should make use of CER to inform their deliberations. In these ways, SDM and CER are interrelated. SDM translates CER into patient-centered practice, while CER provides the backbone evidence about options and outcomes in SDM interventions. In this review, we explore the potential for a SDM–CER synergy in improving healthcare for patients with chronic conditions.
Keywords: chronic condition, comparative effectiveness research, shared decision-making
Critical definitions
Shared decision-making (SDM) describes the work that patients and clinicians do together to consider the relative merits of available management options and decide on a plan that fits the patient goals, preferences and context.
Comparative effectiveness research (CER) seeks to determine the benefits and harms of alternative options for prevention, diagnosis and treatment to enable decision-makers (e.g., patients and clinicians) to make informed clinical and policy decisions [1].
Legaré and Witteman recently proposed a concise definition of SDM that illustrates the alignment in objectives between SDM and CER well [2]. This definition characterizes SDM as an ‘interpersonal and interdependent’ process composed of three essential elements: recognition of the need for a decision; understanding of the best evidence for risks and benefits; and consideration of the provider’s guidance and the patient’s values and preferences.
SDM and CER relate to each other in two ways: first, CER offers estimates of the benefits and harms associated with each option. Clinicians and patients should make use of this CER evidence to inform their deliberations to enable patient-centered decision-making. Thus, SDM is a way to translate CER into patient-centered practice. SDM requires patients and clinicians to consider the best evidence about the relative effectiveness of alternative courses of action. SDM must therefore judiciously apply the results of CER for it to avoid being fraudulent. Second, interventions that promote SDM can be delivered using various approaches (e.g. within the clinical encounter or with a health coach outside the clinical encounter; electronic or paper-based, among others), but evidence-based best practices are sparse. Research comparing the effectiveness of different SDM interventions may improve their implementation in routine care.
In this paper, we will explore these SDM–CER relationships in the setting of healthcare for patients with chronic conditions. Nontransmissible chronic conditions are the most important causes of morbidity and expense in the USA [3,101]. Care for these patients offers an urgent and exciting opportunity for both SDM and CER. We will provide an overview of the origins of SDM, review the CER of SDM as an intervention in this context and consider future challenges and opportunities for SDM and CER.
Brief history of SDM
The term ‘shared decision-making’ in healthcare might have appeared in the medical literature over 30 years ago [4], but its underlying model of mutual participation was first described in 1956 [5]. The mutual participation model represented a departure from medical practice solely as a clinician-driven activity and introduced it as a give-and-take relationship, based on equality and respect. Even from its earliest stages, this model was felt to be particularly useful in the management of chronic conditions [5]. This recognition that behavioral, psychosocial and lifestyle interventions, in the hands of patients, could affect health beyond the effect of biomedical interventions, furthered the interest in engaging patients in healthcare [6]. It was in this context of changing ethical and clinical thought that SDM began to develop an identity: in 1982, a Presidential Commission Report concluded that “shared decision-making is the appropriate ideal for patient–professional relationships” [4].
At first, the practice of this ‘appropriate ideal’ manifested primitively as informed consent and patient education [7]. It was not until the late 1990s that Charles et al. provided a formal framework for the application of SDM in clinical practice [8]. They highlighted the need for bidirectional information exchange, participation of both parties in deliberation and agreement about the resulting plan. This framework, developed in the context of one-time decisions, is the most commonly cited framework for SDM interventions [9].
Since then, the understanding and application of SDM has grown into an attitude or overarching approach to care delivery. In this way, SDM represents a meaningful approach to improve the quality of care by promoting patient-centered care [10]. In moving from specific actions at the point of care to an attitude or stance, SDM has lost some specificity while enhancing its reach. SDM is now considered a keystone of not only individual patient–clinician consultations, but also of the practices and culture of health systems, and even national health policy [9,11–13].
The Salzburg Statement on Shared Decision-making [14], the product of international collaboration in formulating SDM’s core goals, was released in early 2011 and, although it called on patients and clinicians to “work together to be coproducers of health” through the appropriate provision of two-way communication, it did not establish a firm definition for what SDM ought to be and was silent with respect to patients with chronic conditions.
SDM in chronic conditions
Although SDM, as a concept, has been particularly connected to the clinical context of chronic conditions since its inception, the exact reasons for this logical fit have only been described in recent years. Chronic conditions are characterized by cumulative consequences of management decisions, and are thus optimal opportunities to consider long-term risks and benefits in the context of patient values and preferences. Chronic conditions, as described in the US Department of Health and Human Services strategic framework for multiple chronic conditions, last more than 1 year and require ongoing management and/or limit activities of daily living (e.g., diabetes, hypertension and chronic back pain) [15]. These sorts of clinical contexts are different in important ways from those that involve acute conditions (e.g., acute pancreatitis and acute-myocardial infarction) and one-time, irreversible management options (e.g., screening tests and total knee arthroplasty).
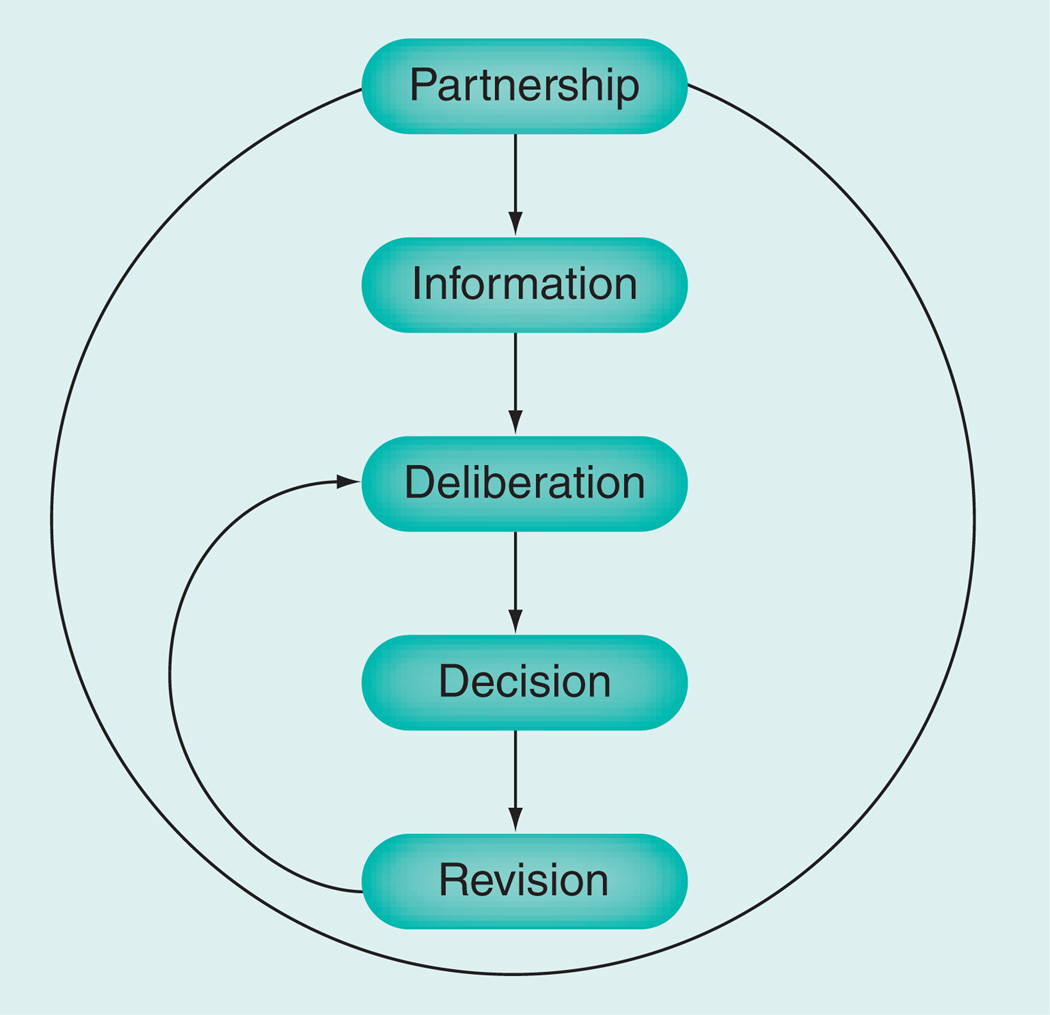
In 2006, Montori, Charles and Gafni modified the Charles et al. framework [8] to make it more applicable to the care of patients with chronic conditions [16]. This modification stressed the need for an ongoing partnership between clinicians and patients that is essential in the context of chronic conditions, but may not be a necessary or possible requisite in the acute setting. The other components of the framework remained nominally similar with the recognition that in chronic care decision-making there is the opportunity to revise decisions. This longitudinal deliberation cycle is illustrated in Figure 1 and requires that SDM is modeled as a partnership between the patient and the clinician [16]. The opportunity for these revisions results from the long-term effects of disease and treatment such that only small losses may accrue when the chosen paths turn out to be undesirable (e.g., when a patient develops an uncommon side effect) and a different approach is then chosen without loss in the patient–clinician partnership.
Figure 1.
Model of shared decision-making in chronic conditions.
There are 52 randomized trials of SDM interventions tested in the context of 16 chronic conditions and reported in 57 publications [17–79]. Most of these trials refer to the implementation of patient decision aids (tools that communicate the evidence about the relative merits of the available options in order to facilitate patient participation in decision-making) and not necessarily measure whether SDM took place. These trials also evaluated patient education, audiotaping of consultations, patient training or coaching programs, and physician training programs. In most cases, comparators were either usual care or the provision of generic patient education materials with no study comparing between SDM interventions. Furthermore, the narrow range of populations (overselected by strict eligibility criteria or by the rigor of trial participation) and settings (often single academic centers) reduces the confident application of this evidence outside of those contexts. All of these limitations preclude the generation of firm conclusions about the effectiveness of SDM interventions and, as such, may be holding back the acceptance and implementation of SDM interventions in the care of people with chronic conditions. In other words, CER about SDM delivery models is both lacking and needed.
The task of conducting CER about SDM requires some additional work. First, considerable variation exists in the processes of operationalization and implementation of SDM interventions. This is in part due to differences in the developers’ perspective of what constitutes SDM. As Durand et al. have found, this variation, however, remains hidden as most decision aid developers fail to disclose the definition or framework or theoretical rationale for their approach [80]. The resulting tools differ in their structure and function, but also in the environment in which they are assumed to work. Some tools are designed as patient education materials for use prior to the consultation to promote informed patient choice. Other tools are designed to promote an informed interaction between the patient and the clinician during the encounter [81]. The consequence of this is that it becomes difficult to assign value to interventions when it is unclear where the credit for effectiveness should lie. As a result, those appraising the SDM CER evidence must operate under the assumption that the mode or method of SDM makes no difference on outcomes. This is probably an invalid assumption.
Up until recently, there has been no standardized approach for the development of SDM tools. However, in 2006, the International Patient Decision Aids Standardization (IPDAS) Collaboration created a checklist to evaluate the quality of decision aids [82] and further guidance was released in 2012 [102]. It is difficult to know to what these standards apply, given the diversity of tools and approaches that might fall in the category of patient decision aids. If one were to conceptualize decision aids as in an early stage of development, standards might represent a form of premature closure on the features that are needed and sufficient for their success in producing SDM. The impact of these standards on the quality of decision aids and on the likelihood of SDM remains unclear. In this discussion, we run the risk of conflating the use of tools or interventions to promote SDM with the attitude, approach or actions of SDM in practice, a relationship that is at best incomplete.
A second challenge to the development of comparative effectiveness about SDM relates to the lack of a standardized measurement set for SDM. Many authors have attempted to develop measures to gauge the quality of SDM [83,84]. These measures have proven largely to be discordant [85,86]. This lack of concordance reflects variations in the conceptualization of SDM used to develop the measures and variations in the particular construct that is being measured (e.g., clinician performance, patient experience, deliberation process and decision as outcome) [87]. Limitations in SDM measurement undermine our confidence in the estimates of SDM efficacy. While there are a few measures that are commonly used (e.g., the Control Preferences scale [88] and the Decisional Conflict scale [89]), their common usage does not imply that these measures have both adequate measurement properties (e.g., all relevant forms of validity and responsiveness to change) and congruence with the conceptualization of SDM used to develop the intervention being tested. The same lack of measurement clarity affects outcomes not directly related to SDM but felt to be consequential to its presence or absence. These include medication adherence, patient satisfaction, decisional conflict, patient knowledge, clinical encounter time and a variety of condition-specific outcomes.
SDM–CER synergy in chronic conditions
In the introduction to this paper, we outlined the important relationships that need to be fostered and promoted to help advance the agendas of both SDM and CER and encourage meaningful improvements for diverse patient populations. Funding agencies and institutes such as the Institute of Medicine, NIH, Agency for Healthcare Research and Quality, Patient-Centered Outcomes Research Institute, Centers for Medicare and Medicaid Services, and others are committed to the ideals of both fields and seem primed to support work that can fully embrace their potential. Specifically, CER should provide the evidence backbone for SDM; CER should be applied to compare alternative implementations of SDM in practice; and SDM should be used to translate CER into practice.
Several recent conferences [90] and grants (e.g., Agency for Healthcare Research and Quality’s Innovative Adaptation and Dissemination of CER), have highlighted the potential synergy between SDM and CER. Most recently, in June of 2013, SDM stakeholders from around the globe gathered in Lima (Peru) for the 7th International Shared Decision-Making Conference [103]. A symposium at the conference was devoted to the role SDM can play in CER translation and promising work was demonstrated in the context of a variety of chronic conditions:
-
▪
An ongoing study (TRICEP, started in 2011) is assessing the primary care use of a decision aid (Diabetes Medication Choice Issue Cards) [62] to translate CER about diabetes medications for patients with Type 2 diabetes. Results from a survey of primary care physicians of sites involved with this study [91] demonstrated that most primary care physicians were not familiar with CER, but were much more familiar with SDM and felt it might be a good way to translate evidence;
-
▪
An SDM–CER partnership seemed to demonstrate particular promise in the context of diverse or vulnerable populations where it may serve as a way to reduce disparity, normalize practice variations and improve outcomes. Work from a study in the Carolinas Healthcare system showed significant reductions in emergency room visits, hospitalizations and oral steroid use in a relatively vulnerable patient population that received an SDM toolkit [92];
-
▪
In the context of rheumatoid arthritis, a low literacy decision aid adapted from an Agency for Healthcare Research and Quality CER-based tool and translated into three languages at the University of California (CA, USA) was feasibly used in outpatient clinics leading to improvements in knowledge and decisional conflict [93];
-
▪
Finally, in a practical randomized trial, another set of issue cards, this time focused on antidepressants, based on CER showed improvement in decisional conflict in rural and urban primary care sites [94]. These examples show how SDM might correct practice patterns, health disparities and CER translation failures by subjecting treatment and management decisions to the deliberative conversations between informed patients and clinicians.
These practical examples illustrate how CER, even when the research warrants low confidence in the results, can provide estimates of the likelihood of relevant outcomes across alternatives to populate SDM interventions and support the provider’s guidance. Most of the evidence supporting decision-making and included in decision aids, is comprised of indirect comparisons between interventions, as most fields lack trials testing direct comparisons between active interventions, with most assessing the efficacy of interventions against placebo [95]. Improvements in CER might, therefore, improve the evidence base supporting clinical decision-making, an urgency acknowledged in the founding mission of Patient-Centered Outcomes Research Institute.
The existing literature suggests that there is great policy interest in SDM for chronic conditions [12,13]. Indeed, 110 randomized trials included in the latest update of the Cochrane Collaboration Systematic Review of decision aids (a type of SDM intervention) for screening and treatment [96] clearly support the efficacy of these tools. However, questions remain about how SDM interventions are implemented, deployed and routinized in clinics, and about the comparative effectiveness of these SDM implementations. These questions require careful study.
CER results could be translated into practice through two general strategies: practice- or population-based policies, or patient-centered approaches. Practice guidelines, formulary design and quality-of-care frameworks for accountability, public reporting and reward are examples of the former. The practice of SDM, with its requirement of considering information about the available options and outcomes, offers a patient-centered approach to the translation of CER. The relative effectiveness or complementarity of these approaches deserves full exploration [97].
Conclusion
SDM should be applied to the translation of CER about management options into practice in a patient-centered manner. CER results should provide the evidence backbone of SDM interventions. CER methods should be applied to evaluate SDM implementations. Without CER about alternative management strategies, using SDM to find the best option would lack validity. Without SDM, CER translation might not improve patient-centered care and outcomes. Without CER about SDM, SDM interventions might never get implemented into practice. This synergy, when embraced, can advance the agendas of both fields and improve the health of patients – particularly those with chronic conditions – in a way that is evidence-based, patient-centered and practically relevant.
Future perspective
We have identified the following unanswered (or partially unanswered) questions as particularly relevant to advancing the field of SDM research:
-
▪
What are the characteristics of interventions that must be present to define an intervention as promoting SDM?
-
▪
What are the characteristics of measurement approaches that make them required components for the evaluation of interventions to promote SDM and to compare the effectiveness of SDM interventions?
-
▪
What are the characteristics of CER, for example, selection of patients, interventions, comparisons, outcomes and designs, that make it amenable to patient-centered translation via SDM?
-
▪
In what ways can work to advance CER and SDM be synergized to improve health quality and patient care?
-
▪
Is SDM superior or complementary to policy approaches to the translation of CER into practice?
As these questions receive attention and effort is made to answer them, it ought to be our minimal goal to ensure that CER results find their way, when timely, into the clinical decision-making process. SDM interventions may provide a useful conduit for this, and we should identify optimal implementation strategies for them, since lack of routine use of SDM tools may annul their value. Finally, high quality CER should provide the evidence base of SDM tools [102]. Coming full circle, the synergy of CER and SDM will realize the value of both the comparative effectiveness enterprise and of the collaborative engagement of clinicians and patients with chronic conditions working together to make clinical decisions.
Executive summary.
Background
-
▪
Shared decision-making (SDM) is the work patients and clinicians do together to consider alternative management options and their desirable and undesirable characteristics as informed by the best available evidence from comparative effectiveness research (CER). SDM translates CER in a patient-centered fashion. CER provides the evidence backbone for SDM interventions. CER can be applied to compare the effectiveness of different ways of implementing SDM interventions.
-
▪
The use of SDM interventions in patients with chronic conditions is not well documented in randomized trials of SDM interventions. The extant literature, while inconsistent, suggests that SDM interventions, particularly decision aids, are efficacious. Sparse data about effectiveness may be contributing to the limited use of SDM in the care of patients with chronic conditions.
SDM–CER synergy in chronic conditions
-
▪
We have identified a conceptual and practical synergy between SDM and CER. Improvements in CER (e.g., direct comparisons) will improve the content of SDM. Improvements in the CER of SDM (e.g., conceptual and methodological consistency and larger studies) will improve the evidence base about the relative effectiveness of SDM implementations. Improvements in SDM will, among other effects, help translate CER to improve patient outcomes.
Conclusion
-
▪
A synergy between SDM and CER may be able to advance the agendas of both fields and improve the health of patients – particularly those with chronic conditions – in a way that is evidence-based, patient-centered and practically relevant.
Acknowledgments
This publication was supported by CCaTS Grant Number TL1 TR000137 from the National Center for Advancing Translational Science (NCATS).
Footnotes
Publisher's Disclaimer: Disclaimer
The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Institue of Medicine. Initial National Priorities for Comparative Effectiveness Research. Washington, DC, USA: National Academies Press; 2009. [Google Scholar]
- 2. Legare F, Witteman HO. Shared decision making: examining key elements and barriers to adoption into routine clinical practice. Health Aff. (Millwood) 2013;32(2):276–284. doi: 10.1377/hlthaff.2012.1078. ▪ Provides an analysis of key elements of shared decision-making (SDM) and examines the barriers to its implementation into practice.
- 3.Bodenheimer T, Chen E, Bennett HD. Confronting the growing burden of chronic disease: can the U.S. health care workforce do the job? Health Aff. (Millwood) 2009;28(1):64–74. doi: 10.1377/hlthaff.28.1.64. [DOI] [PubMed] [Google Scholar]
- 4. Making Health Care Decisions: A Report on the Ethical and Legal Implications of Informed Consent in the Patient–Practitioner Relationship. Washington, DC, USA: US Government Printing Office; 1982. President’s Commission for the Study of Ethical Problems in Medicine and Behavioral Research. ▪ Report by the Presidential Commission that called for SDM and stated that it was the “appropriate ideal for patient–professional relationships”.
- 5. Szasz TS, Hollender MH. A contribution to the philosophy of medicine; the basic models of the doctor-patient relationship. AMA Arch. Intern. Med. 1956;97(5):585–592. doi: 10.1001/archinte.1956.00250230079008. ▪ Early report on the different modes of patient–physician interaction. The authors suggest that the model of mutual particpation (a philosophical precursor to SDM) would be most appropriate in the treatment of chronic conditions.
- 6.Lalonde M. A New Perspective on the Health of Canadians: A Working Document. Ottawa, ON, Canada: Government of Canada; 1974. [Google Scholar]
- 7.Hoving C, Visser A, Mullen PD, van den Borne B. A history of patient education by health professionals in Europe and North America: from authority to shared decision making education. Patient Educ. Couns. 2010;78(3):275–281. doi: 10.1016/j.pec.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 8. Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango) Soc. Sci. Med. 1997;44(5):681–692. doi: 10.1016/s0277-9536(96)00221-3. ▪▪ This is the most cited definition of SDM and laid the ground work for many future discussions about SDM.
- 9. Makoul G, Clayman ML. An integrative model of shared decision making in medical encounters. Patient Educ. Couns. 2006;60(3):301–312. doi: 10.1016/j.pec.2005.06.010. ▪ Provides an overview of conceptual definitions of SDM.
- 10.Institute of Medicine. Crossing the Quality Chasm: a New Health System for the 21st Century. Washington, DC, USA: The National Academies Press; 2001. [PubMed] [Google Scholar]
- 11.Legare F, Stacey D, Pouliot S, et al. Interprofessionalism and shared decision-making in primary care: a stepwise approach towards a new model. J. Interprof. Care. 2011;25(1):18–25. doi: 10.3109/13561820.2010.490502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frosch DL, Moulton BW, Wexler RM, Holmes-Rovner M, Volk RJ, Levin CA. Shared decision making in the United States: policy and implementation activity on multiple fronts. Z. Evid. Fortbild. Qual. Gesundhwes. 2011;105(4):305–312. doi: 10.1016/j.zefq.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Harter M, van der Weijden T, Elwyn G. Policy and practice developments in the implementation of shared decision making: an international perspective. Z. Evid. Fortbild. Qual. Gesundhwes. 2011;105(4):229–233. doi: 10.1016/j.zefq.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 14.Salzburg statement on shared decision making. BMJ. 2011;342:d1745. doi: 10.1136/bmj.d1745. [DOI] [PubMed] [Google Scholar]
- 15.US Department of Health and Human Services. Multiple Chronic Conditions – A Strategic Framework: Optimum Health and Quality of Life for Individuals with Multiple Chronic Conditions. Washington, DC, USA: US Department of Health and Human Services; 2010. [Google Scholar]
- 16. Montori VM, Gafni A, Charles C. A shared treatment decision-making approach between patients with chronic conditions and their clinicians: the case of diabetes. Health Expect. 2006;9(1):25–36. doi: 10.1111/j.1369-7625.2006.00359.x. ▪ Frames SDM in a chronic condition context.
- 17. Stacey D, Bennett CL, Barry MJ, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst. Rev. 2011;10:CD001431. doi: 10.1002/14651858.CD001431.pub3. ▪▪ Presents a systematic review of decision aids.
- 18.Stacey D, Kryworuchko J, Bennett C, Murray MA, Mullan S, Legare F. Decision coaching to prepare patients for making health decisions: a systematic review of decision coaching in trials of patient decision AIDS. Med. Decis. Making. 2012;32(3):E22–E33. doi: 10.1177/0272989X12443311. [DOI] [PubMed] [Google Scholar]
- 19.Legare F, Turcotte S, Stacey D, Ratte S, Kryworuchko J, Graham ID. Patients’ perceptions of sharing in decisions: a systematic review of interventions to enhance shared decision making in routine clinical practice. Patient. 2012;5(1):1–19. doi: 10.2165/11592180-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 20.Jimbo M, Rana GK, Hawley S, et al. What is lacking in current decision aids on cancer screening? CA Cancer J. Clin. 2013;63(3):193–214. doi: 10.3322/caac.21180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spiegle G, Al-Sukhni E, Schmocker S, et al. Patient decision aids for cancer treatment: are there any alternatives? Cancer. 2013;119(1):189–200. doi: 10.1002/cncr.27641. [DOI] [PubMed] [Google Scholar]
- 22.Carpenter JS, Studts JL, Byrne MM. A systematic review of menopausal symptom management decision aid trials. Maturitas. 2011;69(1):11–21. doi: 10.1016/j.maturitas.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 23.Arterburn DE, Westbrook EO, Bogart TA, Sepucha KR, Bock SN, Weppner WG. Randomized trial of a video-based patient decision aid for bariatric surgery. Obesity. 2011;19(8):1669–1675. doi: 10.1038/oby.2011.65. [DOI] [PubMed] [Google Scholar]
- 24.Auvinen A, Hakama M, Ala-Opas M, et al. A randomized trial of choice of treatment in prostate cancer: the effect of intervention on the treatment chosen. BJU Int. 2004;93(1):52–56. doi: 10.1111/j.1464-410x.2004.04554.x. discussion 56. [DOI] [PubMed] [Google Scholar]
- 25.Barry MJ, Cherkin DC, YuChiao C, Fowler FJ, Skates S. A randomized trial of a multimedia shared decision-making program for men facing a treatment decision for benign prostatic hyperplasia. Dis. Manag. Clin. Outcomes. 1997;1(1):5–14. [Google Scholar]
- 26.Berry DL, Halpenny B, Hong F, et al. The personal patient profile-prostate decision support for men with localized prostate cancer: a multi-center randomized trial. Urol. Oncol. 2013;31(7):1012–1021. doi: 10.1016/j.urolonc.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bieber C, Müller KG, Blumenstiel K, et al. A shared decision-making communication training program for physicians treating fibromyalgia patients: effects of a randomized controlled trial. J. Psychosomat. Res. 2008;64(1):13–20. doi: 10.1016/j.jpsychores.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 28.Bieber C, Muller KG, Blumenstiel K, et al. Long-term effects of a shared decision-making intervention on physician–patient interaction and outcome in fibromyalgia. A qualitative and quantitative 1 year follow-up of a randomized controlled trial. Patient Educ. Couns. 2006;63(3):357–366. doi: 10.1016/j.pec.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 29.Bosco JL, Halpenny B, Berry D. Personal preferences and discordant prostate cancer treatment choice in an intervention trial of men newly diagnosed with localized prostate cancer. Health Qual. Life Outcomes. 2012;10(1):123. doi: 10.1186/1477-7525-10-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown R, Butow PN, Boyer MJ, Tattersall MH. Promoting patient participation in the cancer consultation: evaluation of a prompt sheet and coaching in question-asking. Br. J Cancer. 1999;80(1–2):242–248. doi: 10.1038/sj.bjc.6690346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown RF, Butow PN, Dunn SM, Tattersall MH. Promoting patient participation and shortening cancer consultations: a randomised trial. Br. J. Cancer. 2001;85(9):1273–1279. doi: 10.1054/bjoc.2001.2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown RF, Butow PN, Sharrock MA, et al. Education and role modelling for clinical decisions with female cancer patients. Health Expect. 2004;7(4):303–316. doi: 10.1111/j.1369-7625.2004.00294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Butow P, Devine R, Boyer M, Pendlebury S, Jackson M, Tattersall MH. Cancer consultation preparation package: changing patients but not physicians is not enough. J. Clin. Oncol. 2004;22(21):4401–4409. doi: 10.1200/JCO.2004.66.155. [DOI] [PubMed] [Google Scholar]
- 34.Davison BJ, Degner LF. Empowerment of men newly diagnosed with prostate cancer. Cancer Nursing. 1997;20(3):187–196. doi: 10.1097/00002820-199706000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Davison BJ, Goldenberg SL, Wiens KP, Gleave ME. Comparing a generic and individualized information decision support intervention for men newly diagnosed with localized prostate cancer. Cancer Nursing. 2007;30(5):E7–E15. doi: 10.1097/01.NCC.0000290819.22195.d6. [DOI] [PubMed] [Google Scholar]
- 36.De Lorenzo F, Ballatori E, Di Costanzo F, Giacalone A, Ruggeri B, Tirelli U. Improving information to Italian cancer patients: results of a randomized study. Ann. Oncol. 2004;15(5):721–725. doi: 10.1093/annonc/mdh190. [DOI] [PubMed] [Google Scholar]
- 37.Deinzer A, Veelken R, Kohnen R, Schmieder RE. Is a shared decision-making approach effective in improving hypertension management? J. Clin. Hypertens (Greenwich) 2009;11(5):266–270. doi: 10.1111/j.1751-7176.2009.00112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Emmett CL, Montgomery AA, Peters TJ, Fahey T. Three-year follow-up of a factorial randomised controlled trial of two decision aids for newly diagnosed hypertensive patients. Br. J. Gen. Pract. 2005;55(516):551–553. [PMC free article] [PubMed] [Google Scholar]
- 39.Ford S, Fallowfield L, Hall A, Lewis S. The influence of audiotapes on patient participation in the cancer consultation. Eur. J. Cancer. 1995;31A(13–14):2264–2269. doi: 10.1016/0959-8049(95)00336-3. [DOI] [PubMed] [Google Scholar]
- 40.Goel V, Sawka CA, Thiel EC, Gort EH, O’Connor AM. Randomized trial of a patient decision aid for choice of surgical treatment for breast cancer. Med. Decis. Making. 2001;21(1):1–6. doi: 10.1177/0272989X0102100101. [DOI] [PubMed] [Google Scholar]
- 41.Hack TF, Pickles T, Bultz BD, Ruether JD, Degner LF. Impact of providing audiotapes of primary treatment consultations to men with prostate cancer: a multi-site, randomized, controlled trial. Psychooncology. 2007;16(6):543–552. doi: 10.1002/pon.1094. [DOI] [PubMed] [Google Scholar]
- 42.Hack TF, Pickles T, Bultz BD, et al. Impact of providing audiotapes of primary adjuvant treatment consultations to women with breast cancer: a multisite, randomized, controlled trial. J. Clin. Oncol. 2003;21(22):4138–4144. doi: 10.1200/JCO.2003.12.155. [DOI] [PubMed] [Google Scholar]
- 43.Hacking B, Wallace L, Scott S, Kosmala-Anderson J, Belkora J, McNeill A. Testing the feasibility, acceptability and effectiveness of a ‘decision navigation’ intervention for early stage prostate cancer patients in Scotland – a randomised controlled trial. Psychooncology. 2013;22(5):1017–1024. doi: 10.1002/pon.3093. [DOI] [PubMed] [Google Scholar]
- 44.Hamann J, Cohen R, Leucht S, Busch R, Kissling W. Shared decision making and long-term outcome in schizophrenia treatment. J. Clin. Psychiatry. 2007;68(7):992–997. doi: 10.4088/jcp.v68n0703. [DOI] [PubMed] [Google Scholar]
- 45.Hamann J, Langer B, Winkler V, et al. Shared decision making for in-patients with schizophrenia. Acta Psychiatr. Scand. 2006;114(4):265–273. doi: 10.1111/j.1600-0447.2006.00798.x. [DOI] [PubMed] [Google Scholar]
- 46.Hamann J, Mendel R, Meier A, et al. ‘How to speak to your psychiatrist’: shared decision-making training for inpatients with schizophrenia. Psychiatr. Serv. 2011;62(10):1218–1221. doi: 10.1176/ps.62.10.pss6210_1218. [DOI] [PubMed] [Google Scholar]
- 47.Hochlehnert A, Richter A, Bludau HB, et al. A computer-based information-tool for chronic pain patients. Computerized information to support the process of shared decision-making. Patient Educ. Couns. 2006;61(1):92–98. doi: 10.1016/j.pec.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 48.Jibaja-Weiss ML, Volk RJ, Granchi TS, et al. Entertainment education for breast cancer surgery decisions: a randomized trial among patients with low health literacy. Patient Educ. Couns. 2011;84(1):41–48. doi: 10.1016/j.pec.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 49.Kasper J, Kopke S, Muhlhauser I, Nubling M, Heesen C. Informed shared decision making about immunotherapy for patients with multiple sclerosis (ISDIMS): a randomized controlled trial. Eur. J. Neurol. 2008;15(12):1345–1352. doi: 10.1111/j.1468-1331.2008.02313.x. [DOI] [PubMed] [Google Scholar]
- 50.Kopke S, Kasper J, Muhlhauser I, Nubling M, Heesen C. Patient education program to enhance decision autonomy in multiple sclerosis relapse management: a randomized-controlled trial. Mult. Scler. 2009;15(1):96–104. doi: 10.1177/1352458508095921. [DOI] [PubMed] [Google Scholar]
- 51.Lalonde L, O’Connor AM, Duguay P, Brassard J, Drake E, Grover SA. Evaluation of a decision aid and a personal risk profile in community pharmacy for patients considering options to improve cardiovascular health: the OPTIONS pilot study. Int. J. Pharm. Pract. 2006;14(1):51–62. [Google Scholar]
- 52.Leighl NB, Shepherd HL, Butow PN, et al. Supporting treatment decision making in advanced cancer: a randomized trial of a decision aid for patients with advanced colorectal cancer considering chemotherapy. J. Clin. Oncol. 2011;29(15):2077–2084. doi: 10.1200/JCO.2010.32.0754. [DOI] [PubMed] [Google Scholar]
- 53.Loh A, Simon D, Wills CE, Kriston L, Niebling W, Harter M. The effects of a shared decision-making intervention in primary care of depression: a cluster-randomized controlled trial. Patient Educ. Couns. 2007;67(3):324–332. doi: 10.1016/j.pec.2007.03.023. [DOI] [PubMed] [Google Scholar]
- 54.Manns BJ, Taub K, Vanderstraeten C, et al. The impact of education on chronic kidney disease patients’ plans to initiate dialysis with self-care dialysis: a randomized trial. Kidney Int. 2005;68(4):1777–1783. doi: 10.1111/j.1523-1755.2005.00594.x. [DOI] [PubMed] [Google Scholar]
- 55.Man-Son-Hing M, Laupacis A, O’Connor AM, et al. A patient decision aid regarding antithrombotic therapy for stroke prevention in atrial fibrillation: a randomized controlled trial. JAMA. 1999;282(8):737–743. doi: 10.1001/jama.282.8.737. [DOI] [PubMed] [Google Scholar]
- 56.Mathers N, Ng CJ, Campbell MJ, Colwell B, Brown I, Bradley A. Clinical effectiveness of a patient decision aid to improve decision quality and glycaemic control in people with diabetes making treatment choices: a cluster randomised controlled trial (PANDAs) in general practice. BMJ Open. 2012;2(6):e001469. doi: 10.1136/bmjopen-2012-001469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McAlister FA, Man-Son-Hing M, Straus SE, et al. Impact of a patient decision aid on care among patients with nonvalvular atrial fibrillation: a cluster randomized trial. CMAJ. 2005;173(5):496–501. doi: 10.1503/cmaj.050091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mishel MH, Germino BB, Lin L, et al. Managing uncertainty about treatment decision making in early stage prostate cancer: a randomized clinical trial. Patient Educ. Couns. 2009;77(3):349–359. doi: 10.1016/j.pec.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 59.Montgomery AA, Fahey T, Peters TJ. A factorial randomised controlled trial of decision analysis and an information video plus leaflet for newly diagnosed hypertensive patients. Br. J. Gen. Pract. 2003;53(491):446–453. [PMC free article] [PubMed] [Google Scholar]
- 60.Montori VM, Shah ND, Pencille LJ, et al. Use of a decision aid to improve treatment decisions in osteoporosis: the osteoporosis choice randomized trial. Am. J. Med. 2011;124(6):549–556. doi: 10.1016/j.amjmed.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 61.Morgan MW, Deber RB, Llewellyn-Thomas HA, et al. Randomized, controlled trial of an interactive videodisc decision aid for patients with ischemic heart disease. J. Gen. Intern. Med. 2000;15(10):685–693. doi: 10.1046/j.1525-1497.2000.91139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mullan RJ, Montori VM, Shah ND, et al. The diabetes mellitus medication choice decision aid: a randomized trial. Arch. Intern. Med. 2009;169(17):1560–1568. doi: 10.1001/archinternmed.2009.293. [DOI] [PubMed] [Google Scholar]
- 63.Murray E, Davis H, Tai SS, Coulter A, Gray A, Haines A. Randomised controlled trial of an interactive multimedia decision aid on benign prostatic hypertrophy in primary care. BMJ. 2001;323(7311):493–496. doi: 10.1136/bmj.323.7311.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Oakley S, Walley T. A pilot study assessing the effectiveness of a decision aid on patient adherence with oral bisphosphonate medication. Pharm. J. 2006;276(7399) [Google Scholar]
- 65.Peele PB, Siminoff LA, Xu Y, Ravdin PM. Decreased use of adjuvant breast cancer therapy in a randomized controlled trial of a decision aid with individualized risk information. Med. Decis. Making. 2005;25(3):301–307. doi: 10.1177/0272989X05276851. [DOI] [PubMed] [Google Scholar]
- 66.Sawka AM, Straus S, Rotstein L, et al. Randomized controlled trial of a computerized decision aid on adjuvant radioactive iodine treatment for patients with early-stage papillary thyroid cancer. J. Clin. Oncol. 2012;30(23):2906–2911. doi: 10.1200/JCO.2011.41.2734. [DOI] [PubMed] [Google Scholar]
- 67.Sepucha KR, Belkora JK, Tripathy D, Esserman LJ. Building bridges between physicians and patients: results of a pilot study examining new tools for collaborative decision making in breast cancer. J. Clin. Oncol. 2000;18(6):1230–1238. doi: 10.1200/JCO.2000.18.6.1230. [DOI] [PubMed] [Google Scholar]
- 68.Siminoff LA, Gordon NH, Silverman P, Budd T, Ravdin PM. A decision aid to assist in adjuvant therapy choices for breast cancer. Psychooncology. 2006;15(11):1001–1013. doi: 10.1002/pon.1040. [DOI] [PubMed] [Google Scholar]
- 69.Simon D, Kriston L, von Wolff A, et al. Effectiveness of a web-based, individually tailored decision aid for depression or acute low back pain: a randomized controlled trial. Patient Educ. Couns. 2012;87(3):360–368. doi: 10.1016/j.pec.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 70.Street RL, Jr, Voigt B, Geyer C, Jr, Manning T, Swanson GP. Increasing patient involvement in choosing treatment for early breast cancer. Cancer. 1995;76(11):2275–2285. doi: 10.1002/1097-0142(19951201)76:11<2275::aid-cncr2820761115>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 71.Thomson RG, Eccles MP, Steen IN, et al. A patient decision aid to support shared decision-making on anti-thrombotic treatment of patients with atrial fibrillation: randomised controlled trial. Qual. Saf. Health Care. 2007;16(3):216–223. doi: 10.1136/qshc.2006.018481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.van Tol-Geerdink JJ, Willem Leer J, Weijerman PC, et al. Choice between prostatectomy and radiotherapy when men are eligible for both: a randomized controlled trial of usual care vs decision aid. BJU Int. 2013;111(4):564–573. doi: 10.1111/j.1464-410X.2012.11402.x. [DOI] [PubMed] [Google Scholar]
- 73.Vandemheen KL, O’Connor A, Bell SC, et al. Randomized trial of a decision aid for patients with cystic fibrosis considering lung transplantation. Am. J. Respir. Crit. Care Med. 2009;180(8):761–768. doi: 10.1164/rccm.200903-0421OC. [DOI] [PubMed] [Google Scholar]
- 74.Veroff DR, Sullivan LA, Shoptaw EJ, et al. Improving self-care for heart failure for seniors: the impact of video and written education and decision aids. Popul. Health Manag. 2012;15(1):37–45. doi: 10.1089/pop.2011.0019. [DOI] [PubMed] [Google Scholar]
- 75.Vodermaier A, Caspari C, Koehm J, Kahlert S, Ditsch N, Untch M. Contextual factors in shared decision making: a randomised controlled trial in women with a strong suspicion of breast cancer. Br. J. Cancer. 2009;100(4):590–597. doi: 10.1038/sj.bjc.6604916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vodermaier A, Caspari C, Wang L, Koehm J, Ditsch N, Untch M. How and for whom are decision aids effective? Long-term psychological outcome of a randomized controlled trial in women with newly diagnosed breast cancer. Health Psychol. 2011;30(1):12–19. doi: 10.1037/a0021648. [DOI] [PubMed] [Google Scholar]
- 77.Whelan T, Levine M, Willan A, et al. Effect of a decision aid on knowledge and treatment decision making for breast cancer surgery: a randomized trial. JAMA. 2004;292(4):435–441. doi: 10.1001/jama.292.4.435. [DOI] [PubMed] [Google Scholar]
- 78.Whelan T, Sawka C, Levine M, et al. Helping patients make informed choices: a randomized trial of a decision aid for adjuvant chemotherapy in lymph node-negative breast cancer. J. Natl Cancer Inst. 2003;95(8):581–587. doi: 10.1093/jnci/95.8.581. [DOI] [PubMed] [Google Scholar]
- 79.Wilson SR, Strub P, Buist AS, et al. Shared treatment decision making improves adherence and outcomes in poorly controlled asthma. Am. J. Respir. Crit. Care Med. 2010;181(6):566–577. doi: 10.1164/rccm.200906-0907OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Durand MA, Stiel M, Boivin J, Elwyn G. Where is the theory? Evaluating the theoretical frameworks described in decision support technologies. Patient Educ. Couns. 2008;71(1):125–135. doi: 10.1016/j.pec.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 81.Elwyn G, Frosch D, Volandes AE, Edwards A, Montori VM. Investing in deliberation: a definition and classification of decision support interventions for people facing difficult health decisions. Med. Decis. Making. 2010;30(6):701–711. doi: 10.1177/0272989X10386231. [DOI] [PubMed] [Google Scholar]
- 82.Elwyn G, O’Connor A, Stacey D, et al. Developing a quality criteria framework for patient decision aids: online international Delphi consensus process. BMJ. 2006;333(7565):417. doi: 10.1136/bmj.38926.629329.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Elwyn G, Edwards A, Mowle S, et al. Measuring the involvement of patients in shared decision-making: a systematic review of instruments. Patient Educ. Couns. 2001;43(1):5–22. doi: 10.1016/s0738-3991(00)00149-x. [DOI] [PubMed] [Google Scholar]
- 84.Scholl I, Koelewijn-van Loon M, Sepucha K, et al. Measurement of shared decision making – a review of instruments. Z. Evid. Fortbild. Qual. Gesundhwes. 2011;105(4):313–324. doi: 10.1016/j.zefq.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 85.Weiss MC, Peters TJ. Measuring shared decision making in the consultation: a comparison of the OPTION and informed decision making instruments. Patient Educ. Couns. 2008;70(1):79–86. doi: 10.1016/j.pec.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 86.Kasper J, Heesen C, Kopke S, Fulcher G, Geiger F. Patients’ and observers’ perceptions of involvement differ. Validation study on inter-relating measures for shared decision making. PLoS ONE. 2011;6(10):e26255. doi: 10.1371/journal.pone.0026255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kasper J, Hoffmann F, Heesen C, Kopke S, Geiger F. MAPPIN’SDM – the multifocal approach to sharing in shared decision making. PLoS ONE. 2012;7(4):e34849. doi: 10.1371/journal.pone.0034849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Degner LF, Sloan JA, Venkatesh P. The control preferences scale. Can. J. Nurs. Res. 1997;29(3):21–43. [PubMed] [Google Scholar]
- 89.O’Connor AM. Validation of a decisional conflict scale. Med. Decis. Making. 1995;15(1):25–30. doi: 10.1177/0272989X9501500105. [DOI] [PubMed] [Google Scholar]
- 90.Politi MC, Clayman ML, Fagerlin A, Studts JL, Montori V. Insights from a conference on implementing comparative effectiveness research through shared decision-making. J. Comp. Eff. Res. 2013;2(1):23–32. doi: 10.2217/cer.12.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shah ND. Primary care providers perceptions about the role of shared decision making for translating comparative effectiveness research into practice. Presented at: 7th International Shared Decision Making Conference; 16–19 June 2013; Lima, Peru. [Google Scholar]
- 92.Tapp H. Comparative Effectiveness of Shared Decision Making and the Chronic Care Model for Asthma Management in a Clinic Population. Presented at: 7th International Shared Decision Making Conference; 16–19 June 2013; Lima, Peru. [Google Scholar]
- 93.Barton J. Reduced Decisional Conflict with Use of a Low Literacy Decision Aid Tool for Vulnerable Populations with Rheumatoid Arthritis. Presented at: 7th International Shared Decision Making Conference; 16–19 June 2013; Lima, Peru. [Google Scholar]
- 94.Leblanc A. Translation into Practice of Comparative Effectiveness of Depression Medications: The Depression Medication Choice Decision Aid Trial. Presented at: 7th International Shared Decision Making Conference; 16–19 June 2013; Lima, Peru. [Google Scholar]
- 95.Salanti G, Kavvoura FK, Ioannidis JP. Exploring the geometry of treatment networks. Ann. Intern. Med. 2008;148(7):544–553. doi: 10.7326/0003-4819-148-7-200804010-00011. [DOI] [PubMed] [Google Scholar]
- 96.Stacey D, Courtemanche C, Barry M, et al. Cochrane review of patient decision aids for treatment or screening decisions: update in 2012 reveals 24 new trials for 110 total. Presented at: 7th International Shared Decision Making Conference; 16–19 June 2013; Lima, Peru. [Google Scholar]
- 97.Shah ND, Mullan RJ, Breslin M, Yawn BP, Ting HH, Montori VM. Translating comparative effectiveness into practice: the case of diabetes medications. Med. Care. 2010;48(6 Suppl.):S153–S158. doi: 10.1097/MLR.0b013e3181d5956c. [DOI] [PubMed] [Google Scholar]
Websites
- 101.CDC. Chronic Disease and Health Promotion. 2012 www.cdc.gov/chronicdisease/overview/index.htm.
- 102.IPDAS Collaboration. The 2012 IPDAS Background Document. 2012 http://ipdas.ohri.ca/resources.html.
- 103.7th International Shared Decision-Making Conference; www.isdm2013.org. [Google Scholar]