Abstract
YWHAE (14-3-3ε) protein levels are considered to be a reliable biomarker for neurodegeneration. The YWHAE protein interacts both directly and indirectly with human immunodeficiency virus (HIV) accessory proteins, leading to cell death. The purpose of this study was to examine the relationship between YWHAE polymorphisms and HIV-associated neurocognitive disorder (HAND) and the relationship between YWHAE protein levels and HAND. A cross-sectional study using random samples of HIV-seropositive (n=20) and HIV–seronegative (controls) (n=16) women from the Hispanic-Latino Longitudinal Cohort of Women was conducted. Individuals who are HIV-seropositive and heterozygous at the rs4790084/rs1204828 loci in the YWHAE gene were 3X more likely to display reduced cognitive functioning, to have received a HAND diagnosis, and to have less YHWAE protein expressed than homozygotes. Western blots from cerebral spinal fluid (CSF) indicate that the HIV-seropositive women with HAND expressed 4.5X less YWHAE compared to HIV-seropositive cognitively normal women (94% sensitivity, 84% specificity; HIV-seropositive vs. controls). Therefore, polymorphism in YWHAE may be a genetic risk factor for HAND and levels of YWHAE protein are a likely biomarker for neurocognitive status in HIV-seropositive women.
Keywords: Dementia, 14-3-3, HAND, neuropsychological, polymorphism
Introduction
Individuals infected with the human immunodeficiency virus (HIV) are at risk of developing HIV-associated neurocognitive disorders (HAND), regardless of adequate response to the use of combined antiretroviral treatment (cART) (Rackstraw 2011). In the handful of studies reported, HIV-seropositive women appear to be more likely to manifest cognitive impairments (Wojna et al. 2007; Wojna et al. 2006). As more women are infected, there is a need for understanding whether potential differences in cognition exist with respect to biomarkers (Wojna et al. 2006). In men, the 14-3-3 gene family of proteins is a proposed biomarker for HIV-associated dementia (HAD) or HIV-encephalitis (HIVE) (Gelman and Nguyen 2010; Wakabayashi et al. 2001). Thus, studies in HIV-seropositive women will contribute to further understanding of the mechanisms involved between 14-3-3’s and HAND.
The 14-3-3s are a group of highly conserved proteins with seven isoforms (σ, β, γ, ε, ζ, τ/θ, and η), and two phosphorylated isotypes (α and δ), which together comprise approximately 1% of the total brain proteins (Aitken et al. 1995). These small acidic molecules interact physically with over 300 proteins and are involved in cell cycling and apoptosis pathways (Berg et al. 2003). In humans, 14-3-3ε (known as YWHAE) is expressed throughout the brain and is the most ancient isoform conserved in every species examined (Mignon-Ravix et al. 2010). In healthy individuals, YWHAE is not detectable in cerebral spinal fluid (CSF); however, YWHAE protein has been detected in the CSF of men diagnosed with HAD (Wakabayashi et al. 2001). Similarly, in men there are higher levels of YWHAE in the postmortem brains of those with HIV infection when compared to the levels seen in HIV-seronegative individuals who do not have the virus (Gelman and Nguyen 2010). That conclusion supports the idea that YWHAE is a biomarker of neuronal degeneration. Also, the presence of YWHAE in the CSF is considered a biomarker of neurodegeneration induced by Creutzfeldt–Jakob disease (CJD), but it has not been seen to be specifically upregulated in the CSF from patients with any other neurodegenerative disorders (reviewed in (Morales et al. 2012b)). In CJD studies, gender does not appear to affect levels of YWHAE expression (Collins et al. 2006). To date, there are no published studies on the relationship between YWHAE protein levels and less severe forms of neurocognitive decline, nor are there any studies that were conducted with more than a few women.
CSF collection is an invasive procedure; therefore an alternative to identify genetic biomarkers for risk of neurocognitive decline would be recommended. Loss of YWHAE and neighbor genes in humans leads to Miller-Dieker Syndrome; a severe form of retardation (Berg et al. 2003). Mice that lack YWHAE (Ywhae−/−) display impaired memory and enhanced anxiety in addition defects in neuronal migration. However, no studies to date have examined single polymorphism changes in YWHAE in relation to any confirmed neurodegenerative disease.
Results
The HIV-seropositive women in the study were similar in respect to age, education, and other demographic/socioeconomic factors compared with the HIV-seronegative women (Morales et al. 2012a; Wojna et al. 2007; Wojna et al. 2006). In examining the frequency of YWHAE polymorphisms, we found that individuals who are heterozygotes at rs4790084 (A/G_A) were also heterozygous at the rs1204828 (T/C_T) loci with strong linkage disequilibrium (LD) (D-prime = 0.933, r-squared=0.871, LOD=9.64).
We examined whether YWHAE polymorphisms are related to any biological measures, HAND diagnosis and/or neuropsychological domains. The only biological measure that differed between genotypes was HIV RNA plasma viral load (log10 copies/ml) with the rs4790084(rs1204828) heterozygous [A/G_A(T/C_T)] which show lower levels of CSF YWHAE when compared to homozygotes [G/G(C/C)] (Table 1). We found that 9 out of the 10 HIV-seropositive individuals that are heterozygous displayed cognitive impairments as compared to only 3 out of 10 of the homozygotes (p<0.006, Table 1). According to the Mann-Whitney U-test, the overall neuropsychological performance z-score (NPZ) (p<0.02, Table 1) and the Frontal Executive function was different between polymorphism groups in HIV-seropositive women (p<0.02, Table 1). No genotype differences in neurocognitive performance were seen in HIV-seronegative subjects (Table 1). At the rs1873827 site, only 3 individuals per group displayed T/C variations, so further analysis was not deemed necessary.
Table 1.
Effects of polymorphisms in YWHAE on neurocognitive testing
| HIV-seronegative | p | HIV-seropositive | p | |||
|---|---|---|---|---|---|---|
| rs4790084(rs1204828) | rs4790084(rs1204828) | |||||
| G/G(C/C) | A/G_A(T/C_T) | G/G(C/C) | A/G_A(T/C_T) | |||
| N | 5 | 11 | 10 | 10 | ||
| Age | 37.2 ± 4.1 | 43.3 ± 2.8 | 0.14 | 42.8 ± 2.6 | 41.5 ± 4.1 | 0.58 |
| Education (years) | 13.2 ± 0.6 | 13.6 ± 0.8 | 0.56 | 13.0 ± 1.0 | 12.8 ± 0.3 | 0.74 |
| Cognitive Status | ||||||
| Normal | 70% | 10% | 0.006 | |||
| Impaired | 30% | 90% | ||||
| HIV RNA viral load | ||||||
| log10 copies/ml | ||||||
| CSF (n) | 2.0 ± 0.2 (8) | 1.7 ± 0.02 (10) | 0.28 | |||
| Plasma (n) | 2.8 ± 0.3 (10) | 1.8 ± 0.03 (10) | 0.03 | |||
| CD4 (cells/mm3) | 595 ± 101 | 781 ± 126 | 0.31 | |||
| CD4 nadir (cells/mm3) | 354 ± 81 | 562 ± 118 | 0.21 | |||
| Treatment | ||||||
| Naïve (n) | 20% (2) | 28.6% (2) | ||||
| % ART (n) | 0% (0) | 14.3% (1) | ||||
| % HAART (n) | 80% (8) | 57.1% (6) | ||||
| CPE (n) | 9.6 ± 0.8 (8) | 7.0 ± 1.4 (8) | 0.32 | |||
| YWHAE in CSF1 | 16.8± 4.0 (9) | 5.5 ± 0.9 (8) | 0.02 | |||
| pan-14-3-3 in CSF1 | 15.1 ± 3.6 (9) | 11.3 ± 2.9 (7) | 0.45 | |||
| BDI | 8.8 ± 4.5 | 7.1 ± 2.6 | 0.70 | 6.3 ± 1.4 | 12.9 ± 4.2 | 0.08 |
| I. NPZ2 | 0.3 ± 0.2 | 0.3 ± 0.1 | 0.42 | 0.4 ± 0.2 | − 0.1 ± 0.1 | 0.02 |
| II. Neuropsychological domains/subtests | ||||||
| 1. Frontal Executive2 | 0.3 ± 0.5 | 0.1 ± 0.3 | 0.07 | 0.3 ± 0.2 | −0.1 ± 0.3 | 0.02 |
| 2. Psychomotor Speed2 | 0.3 ± 0.1 | 0.2 ± 0.2 | 0.95 | 0.1 ± 0.2 | −0.3 ± 0.2 | 0.20 |
| 3. Verbal Memory2 | 0.1 ± 0.1 | 0.3 ± 0.2 | 0.87 | 0.6 ± 0.2 | 0.3 ± 0.2 | 0.64 |
| 4. Motor Speed2 | 0.4 ± 0.2 | 0.6 ± 0.2 | 0.87 | 0.7 ± 0.4 | −0.3 ± 0.3 | 0.08 |
Mean ± SEM, Mann-Whitney U-test, significant difference p<0.05 in bold
z-scores
Mean optical density of western blot expression
CSF = cerebral spinal fluid
RNA = ribonucleic acid
CPE = Central nervous system penetration index
NPZ = Overall neurological performance z-score
BDI = Beck Depression Inventory
The rs4790084 and rs1204828 polymorphisms are located in introns and are likely an effect of expression at the level of transcription or splicing. This conclusion is supported by our evidence that there are lower YWHAE levels in the homozygotes than there are in the heterozygotes (p<0.02, Table 1). Genotype differences were not seen when the western blots were reprobed with pan-14-3-3 antibody (Table 1).
Non-parametric Mann-Whitney U-tests indicated no differences between normal vs. impaired cognition with regard to any biological measure in the HIV-seropositive subjects except of higher cluster of differentiation 4 (CD4) levels in the cognitive impaired group which included all individuals ANI, MCMD, and HAD combined (p<0.03, Table 2). The highest levels of CD4 are seen in those with MCMD (1081±118, n=4) followed by ANI (849±266, n=2), and then by HAD (771±241, n=4). Limited sample numbers did not allow for further comparison between levels of HAND.
Table 2.
Profile of HIV-seropositive women by HAND diagnosis
| Neurocognitive Status | p | ||
|---|---|---|---|
| Normal | Impaired | ||
| N | 7 | 10 | |
| Age (years) | 40.3 ± 3.0 | 42.0 ± 4.2 | 0.74 |
| Education (years) | 13.1 ± 1.1 | 12.7 ± 0.5 | 0.68 |
| Cognitive Status | |||
| % ANI | 20% | ||
| % MCMD | 40% | ||
| % HAD | 40% | ||
| HIV RNA viral load | |||
| log10 copies/ml | |||
| CSF (n) | 2.1 ± 0.3 (7) | 1.7 ± 0.1 (10) | 0.38 |
| Plasma (n) | 2.7 ± 0.5 (6) | 2.0 ± 0.2 (10) | 0.18 |
| CD4 (cells/mm3) | 464 ± 43 | 831 ± 118 | 0.03 |
| CD4 nadir (cells/mm3) | 306 ± 61 | 560 ± 109 | 0.07 |
| Treatment | |||
| Naïve (n) | 28.6% (2) | 11.1% (1) | |
| % ART (n) | 0% (0) | 11.1% (1) | |
| % HAART (n) | 71.4% (5) | 77.9% (7) | |
| CPE (n) | 9.0 ± 0.9 (5) | 8.0 ± 1.2 (9) | 0.91 |
| YWHAE in CSF1 | 19.5 ± 4.6 | 5.7 ± 1.1 | 0.005 |
| pan-14-3-3 in CSF1 | 15.9 ± 4.2 | 11.5 ± 2.7 | 0.56 |
| % YWHAE heterozygotes2 | 14.3% | 70% | 0.02 |
| Beck Depression Inventory | 6.5 ± 2.4 | 13.1 ± 3.3 | 0.07 |
| I. NPZ3 | 0.5 ± 0.1 | −0.1 ± 0.1 | 0.003 |
| II. Neuropsychological Domains/subtests | |||
| 1. Frontal Executive3 | 0.7 ± 0.1 | −0.1 ± 0.1 | 0.002 |
| 2. Psychomotor Speed3 | 0.2 ± 0.2 | −0.4 ± 0.2 | 0.19 |
| 3. Verbal Memory3 | 0.4 ± 0.3 | 0.4 ± 0.2 | 0.57 |
| 4. Motor Speed3 | 0.7 ± 0.1 | −0.1 ± 0.4 | 0.10 |
Mean ± SEM, Mann-Whitney U-test, significant difference p<0.05 in bold;
CSF = cerebral spinal fluid,
RNA = ribonucleic acid;
ANI = Asymptomatic Neurocognitive Impairment;
MCMD = Mild Cognitive Motor Disorder;
HAD = HIV-associated Dementia;
NPZ = Neurological performance z-score
Mean optical density of western blot expression
z-scores,
rs4790084(rs1204828) genotype
Of 6 controls CSF samples provided by John Hopkins University one showed a detectable level of YWHAE (0.43 ± 0.10) and pan 14-3-3 (0.07 ± 0.07). We compared this to 17 out of 19 samples from the HIV-seropositive women (Table 2, Figure 1) (CSF from 1 individual not sufficient for analysis). Control and HIV-seropositive samples were run together on at least 3 to 5 replicate gels. The two CSF samples within the HIV-seropositive samples that were not positive for YWHAE or pan 14-3-3 were identified as having come from individuals who had been diagnosed as cognitively impaired. This result supports the idea that YWHAE (94% specificity, 83% sensitivity) is a potential biomarker for HIV-seropositive individuals. Our findings are consistent with a previous one showing higher rates YWHAE in men those with HAD compared to non-HIV subjects (Wakabayashi et al. 2001).
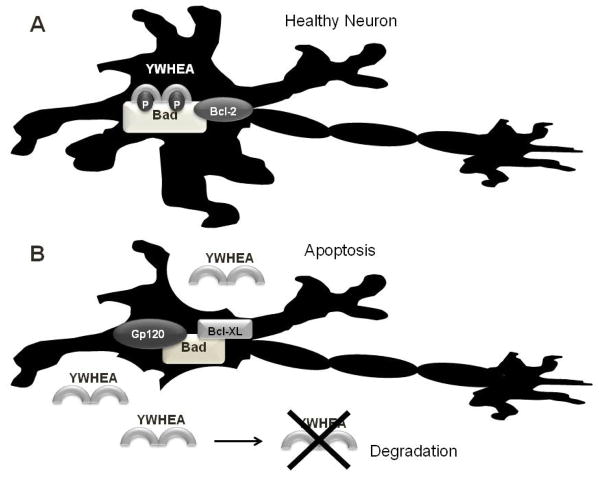
Figure 1. Proposed model of relationship between YWHAE (14-3-3ε) and HIV accessory protein gp120.
A. Binding of YWHAE (14-3-3ε) is necessary to prevent apoptosis in neurons via is association with phosphorylated B-cell lymphoma 2 (Bcl-2) antagonist of cell death (BAD) protein bound to Bcl-2, thereby suppressing apoptosis. B. Gp120-dependent dephosphorylation of BAD at Serine-112 prevents YWHAE binding allowing for its association with the Bcl-XL leading to apoptosis. Apoptosis of the neuron leads to the release of YWHAE and its eventual degradation.
Although the presence of YWHAE is confirmed in other neurological disorders, we investigated whether YWHAE might be useful as an indicator of cognitive impairments in HIV patients. Using the 17 subjects who were positive for YWHAE, a Mann-Whitney U-test of HIV-seropositive women stratified by cognitive function was conducted. The impaired cognition group displayed lower CSF YWHAE levels (Table 2), while the pan-14-3-3 levels were similar between groups (Table 2). The impaired group had lower CSF YWHAE levels and was more likely to be heterozygous at both the rs4790084 and rs1204828 loci. The cognitively impaired group had lower NPZ scores and frontal executive function. In all HIV-seropositive CSF samples (n=19), Spearman’s correlations indicated reduced frontal executive function (r=0.50, p<0.04) and psychomotor speed (r=0.59, p<0.02) z-scores which correlated to lower YWHAE protein levels (n=17). No correlations found were between CSF YWHAE/pan-14-3-3 protein levels and other biological measures or neurocognitive domains.
Discussion
The identification of strong association between polymorphic changes in YWHAE and HAND diagnosis in HIV-seropositive women, suggest that YWHAE is a potential genetic risk factor for the development of HAND. In addition, YHWAE appears to be a protein biomarker in CSF that reflects the state of cognitive function in women with HIV. The high rate of specificity and sensitivity of YHWAE protein levels in HIV subjects is consistent with previous studies in HIV-seropositive men (Gelman and Nguyen 2010; Wakabayashi et al. 2001).
We propose that individuals heterozygous at rs4790084 (rs1204828) in YWHAE are at risk for HAND as a consequence of neurodegeneration. The YWHAE polymorphism may represent a simple genetic indicator of risk for cognitive decline in HIV-seropositive individuals, thus an indicator that can be assessed easily using saliva. However, an increased sample size, the examination of men, and additional longitudinal studies are necessary to confirm these results. The genetic polymorphism we identified to be associated with HIV neurocognitive decline are not within the protein coding region they lie within introns. Therefore, we propose that polymorphic changes affect the transcription or the splicing of YWHAE. Since lower levels are detected in the heterozygotes and that YWHAE expression is necessary for healthy neuronal functioning (Berg et al. 2003). Loss of YWHAE is one possible explanation for why heterozygotes display higher levels of cognitive impairments. Another explanation is that HIV-seropositive women with chronic longstanding HAD have incurred chronic neurodegeneration leading to depleted YWHAE levels. Future post-mortem histological analysis of HIV patients would help to determine the levels of YWHAE in the brain in association to genotype. Considering that YWHAE protein is needed to prevent cell apoptosis (Figure 1) (Che et al. 2010; Iskander et al. 2004; Lipton 1992; Morales et al. 2012b; Ushijima et al. 1995), we would expected based on studies in HIV-seropositive males and CJD patients to see higher levels of YWHAE in post-mortem brains from females, compared to HIV-seronegative female brains (Gelman and Nguyen 2010; Torres et al. 2012). However, based on our results we would expect to see an inverse relationship between levels of neurocognitive impair at the time of death and the level of YWHAE protein in the HIV-seropositive brains. Our hypothesis is also consistent with a study in CJD patients where 14-3-3’s where present in the CSF of those in the early stages of the disease, but not in those with the most severe neuronal atrophy (Boesenberg-Grosse et al. 2006; Shiga et al. 2006; Torres et al. 2012).
One of the greatest challenges faced by clinicians is how to track the progression of particular diseases. Is it possible to measure a specific protein expression to determine disease progress? Our study in women supports the idea that YWHAE is a potential candidate for tracking disease progression in HIV-seropositive patients. This idea is supported by evidence that YWHAE is also present is the CSF of men with HAD (Gelman and Nguyen 2010; Wakabayashi et al. 2001). Gender comparison studies of HIV-seropositive patients have not been conducted; however, studies that have looked at gender in relation to YWHAE expression in the CSF found no differences (Cohen 1988; Collins et al. 2006; Geschwind et al. 2003). This supports the idea that YWHAE should be a universal biomarker.
Our analyses of HIV-seropositive normal vs. impaired cognition women demonstrated that the impaired group expresses less YWHAE protein; this impaired group also had lower overall NPZ scores and reduced frontal executive function. Correlations indicate that lower NPZ scores are associated with lower YWHAE protein levels. These findings suggest that YWHAE protein level reduction may indicate a decline in overall neurocognitive function. No correlations with pan-14-3-3 in any of the groups were found. Consistent with our results in healthy humans, 14-3-3’s are rarely detected immunologically; this finding was confirmed in macaque’s models (Hsich et al. 1996).
In addition, 14-3-3s (including YWHAE) have been shown to directly and indirectly interact with HIV-accessory proteins (reviewed in (Morales et al. 2012b)). The HIV-1-encoded glycoprotein 120 (gp120) which is an envelope protein, stimulates the entry of the virus into the host cell and induces neurotoxicity via the B-cell lymphoma-extra large (Bcl-XL) protein/B-cell lymphoma 2 (Bcl-2) antagonist of cell death (BAD) apoptosis pathway, which is mediated by the binding of 14-3-3s (Figure 1) (Iskander et al. 2004; Lipton 1992; Morales et al. 2012b; Ushijima et al. 1995). Cell culture studies suggest that 14-3-3ε levels are inversely associated with gp120 expression, with lower levels of 14-3-3ε observed at higher concentrations on gp120 (Kapasi et al. 2001). Our research supports this theory. The lower levels of CSF YWHAE in HIV-seropositive women were associated with more severe neurocognitive impairments. Although most HIV-infected individuals are now being treated with cART and are responding to treatment with less viral replication and improved immune status, there still may be a continuous production of virus proteins including gp120 responsible in part for the continual HIV-related neurodegeneration (Heaton et al. 2010). In the presence of gp120, we propose that neuronal cells are no longer able utilize YWHAE to prevent apoptosis, YWHAE is release when the neurons apoptosis then the protein is degraded leading to reduced protein levels in the CSF as the disease progresses (Figure 1) (Boesenberg-Grosse et al. 2006; Morales et al. 2012b; Shiga et al. 2006; Torres et al. 2012). Consistently with our theory in CJD patients, higher levels of 14-3-3s in CSF correspond to higher lactate dehydrogenase (LDH) activity which is a marker for cell death (Torres et al. 2012).
Viral protein R (Vpr) is an HIV-accessory protein that plays a role in CD4+ T-cell and macrophage viral infection by transporting the virus for integration into the host genome (Cohen et al. 1990; Kino and Pavlakis 2004; Kogan and Rappaport 2011; Vodicka et al. 1998; Zhao et al. 1994a; Zhao et al. 1994b), as well as by, cell-cycle arrest at the G2/Mitosis (M) transition (Matsuda et al. 2006). 14-3-3 proteins, including 14-3-3ε, normally regulate cell-cycle progression by binding to a phosphatase (cyclin division cycle [Cdc] 25C [Cdc25C]) leading to the dephosphorylation of B1-p34Cdc2 (Bolton et al. 2008; Gardino and Yaffe 2011; He et al. 1995). The C-terminal region of Vpr interacts with the C-terminal region of 14-3-3ε and when it binds with Cdc25C cannot remove the phosphate from B1-p34Cdc2; therefore, the cell cycle is arrested at G2/M leading to cell VPR-medicated apoptosis (Kino et al. 2005a; Kino et al. 2005b).
In the post era of cART, the nadir CD4 cell count is consistently associated with the presence of HAND (Heaton et al. 2010). In our study, we observed higher levels of CD4 and lower levels of viral HIV in the HIV impaired women. However, the CD4 cell count analyzed represents the actual CD4 cell counts does not reflect the nadir value. It is likely that HIV-seropositive women with cognitive impairment are using or responding better to cART since most of them were on cART (80% of those with cognitive impairment vs. 70% with normal cognition, Table 1). This cross-sectional study used only one sample taken within 24 hours before or after testing. The grouping of ANI, MCMD, and HAD together affects significantly observation of differences between groups the can be and should be assessed in future studies using larger cohort analysis. To fully understand the relationship between YWHAE and other biological measures, future studies should track the subjects with repeated biological testing.
Although a plasma biomarker for HAND is urgently needed, at present there are no reliable plasma markers for HAND. One of the most consistent marker associated with monocyte activation and inflammation is soluble CD14 (sCD14) (Lyons et al. 2011), which has been shown to be associated with neurocognitive status in HIV-seropositive individuals. In our study, we did not examine the potential relationship between plasma YWHAE and neurocognitive performance, which would be appropriate in future studies. There is evidence that 14-3-3σ is upregulated in plasma of patients with lung cancer (Xiao et al. 2005) and ovarian cancer (Mhawech-Fauceglia et al. 2009) possibly due to the body trying to fight the infection and prevent apoptosis. Also, determining the relationship between YWHAE polymorphism and blood plasma levels would be advantageous in the future. Taking into account the limitations of this study, our research indicates that YWHAE protein expression is highly sensitive and specific in the CSF of women infected with HIV. Additionally, that individuals with the rs4790084(rs1204828) polymorphisms in YWHAE are more likely to be present when there is a HAND diagnosis and lower levels of YWHAE. The fact that YWHAE it also interacts with HIV-accessory proteins strongly supports the notion that it is a good candidate to be a biomarker for neurodegeneration in those individuals with HIV.
Methods
Study participants
The present study includes subjects from the Hispanic-Latino Longitudinal Cohort of Women, which is part of the NeuroAIDS Specialized Neuroscience Research Program (SNRP) at the University of Puerto Rico (UPR), Medical Sciences Campus (MSC). This unique cohort of Puerto Rican, Spanish-speaking, HIV-seropositive women was characterized by viral immune profile, neurological exams, and neuropsychological performance. Neurocognitive performance was determined using the modified American Academy of Neurology HAD criteria (Luo et al. 2003; Wojna et al. 2006). All our studies have been approved by the UPR-MSC Institutional Review Board (IRB). This cross-sectional study was directed at a random sample collected in 2009 of 20 HIV-seropositive women and 16 HIV-seronegative women (controls) (Luo et al. 2003; Wojna et al. 2006). The neuropsychological assessment of the HIV-seropositive women included four different functional domains: Frontal Executive Function, Psychomotor Speed, Verbal Memory, and Motor Speed. Individual z-scores were calculated using a reference group of 35 HIV-negative women (Wojna et al. 2007). Dr. Carlos Pardo at Johns Hopkins University, Baltimore, MD (JHU), provided the HIV-seronegative (control) CSF samples from patients examined by a neurologist for non-HIV related conditions (3/6 normal participants [no neurological disorders], 2/6 Multiple Sclerosis, 1/6 with Common Variable Immune Deficiency) with appropriate consent from JHU IRB as banked samples for biological testing.
HIV cognitive impairment determination
Cognitive status was determined using the American Academy of Neurology HIV dementia criteria (AAN criteria) (American Academy of Neurology AIDS Task Force 1991; 1996), which includes criteria for asymptomatic cognitive impairment. For the purpose of this study, we compared subjects with normal cognitive function (n=8) and those with 1) impaired cognition (asymptomatic impairment (ANI), with 2) minor cognitive motor disturbance (MCMD) and 3) HIV-associated dementia (HAD), Table 1).
Polymorphisms
Quantitative polymerase chain reaction (qPCR) was performed using custom-made TaqMan probes for three single nucleotide polymorphisms (SNPs) in YWHAE, rs4790084 (60°C), rs3752826 (61°C), and rs1873827 (60°C) (Ikeda et al. 2008), using the Applied Biosystem standard protocol for saliva or blood.
Protein analysis
Western immunoblots were conducted following standard procedures (Bio-Rad Laboratories, Inc.), using 20μL aliquots of CSF, 1:1, with sample buffer run on a 12% SDS-PAGE gel. CSF samples were quantified with a Bradford assay, and corrections were made to final protein levels. The membranes were treated with a primary dilution of 1:5000 rabbit YWHAE (cat.#sc-1020) or rabbit pan-14-3-3 (cat.#sc-1657) from Santa Cruz Biotechnology, Inc., and 1:5000 of anti-rabbit HRP for the secondary antibody. The NIH/3T3 fibroblast whole cell lysate (sc-2210) was used to identify the YWHAE positive band. Both the HIV-seropositive samples and the control samples (were run together in random order on at least three replicate gels (Figure 2). Protein levels were calculated by subtracting mean gray values from the background with the same positive sample on each gel to correct for variations between gels; The Adobe Photoshop Creative Suite 3 (CS3) analysis tool was used for this process.
Figure 2.

Representative western blot of CSF samples. CSF samples were added to 12% SDS-PAGE gel, transferred to PDVF membrane and blotted for YWHAE with subsequent reblots with pan-14-3-3. #1 NIH/3T3 whole cell lysate, #2 HIV-seropositive impaired subject and #3 HIV-seropositive normal subjects.
Data analysis
All data were collected using standardized data forms provided with assessments and analysis using SPSS software, version 15. Mann-Whitney U- or Chi-Square tests and were conducted between genotypes homozygotes (rs4790084 [G/G] and rs1204828 [C/C]) vs. heterozygotes (rs4790084 [A/G, n=9, or A/A, n=1] and rs1204828 [T/C, n=9, or T/T, n=1]), within HIV status groups or between HIV cognitive status (normal vs. impaired) groups. Spearman’s Correlations were conducted between YWHAE protein levels, biological markers, and neuropsychological test z-scores. For all analyses a p-value lower than 0.05 was considered statistically significant. Sensitivity and specificity of CSF analysis was done with cross-tabulation and Chi-Square analysis using control (HIV-seronegative) samples provided from the Johns Hopkins University CSF repository.
Acknowledgments
The study was funded by National Center for Research Resources (NCRR) grant 1U54RR026139-01A1 and the National Institute on Minority Health and Health Disparities 8U54MD007587 to the University of Puerto Rico-Medical Science Campus, and National Institute of Mental Health (NIMH) Center for Novel Therapeutics of HIV-associated Cognitive Disorders Pilot Project Grant G12 RR003050 to the John Hopkins University. The study was supported partially by National Institute of Neurological Disorders and Stroke (NINDS) grants S11NS46278 and U54NS43011 (SNRP). The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of NCRR, NIMHD, NIMH, or NINDS. Thanks to Dr. Carlos Pardo at John Hopkins University for the control CSF samples. Thanks to Dr. Jacob Raber at Oregon Health & Science University for permission to use the Memory Island and for serving as an excellent mentor. Also we appreciate the contribution from Dr. Raul Mayo who conducted neuropsychological testing of cohort participants and Dr. Avindra Nath for his contribution in the development of the cohort and for his continuous collaboration with the SNRP. We acknowledge the support of Tirtsa Porrata-Doria and the Molecular Biology Core Lab, (Grant RR003050). Special thanks go to Robert Ritchie of the RCMI Publications Office (G12 RR003050). I would like to thank Madeline Collazo for her help with genotyping.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Aitken A, Jones D, Soneji Y, Howell S. 14-3-3 proteins: biological function and domain structure. Biochem Soc Trans. 1995;23:605–611. doi: 10.1042/bst0230605. [DOI] [PubMed] [Google Scholar]
- American Academy of Neurology AIDS Task Force. Nomenclature and research case definitions for neurologic manifestations of human immunodeficiency virus-type 1 (HIV-1) infection. Report of a Working Group of the American Academy of Neurology AIDS Task Force. Neurology. 1991;41:778–785. doi: 10.1212/wnl.41.6.778. [DOI] [PubMed] [Google Scholar]
- American Academy of Neurology AIDS Task Force. Clinical confirmation of the American Academy of Neurology algorithm for HIV-1-associated cognitive/motor disorder. The Dana Consortium on Therapy for HIV Dementia and Related Cognitive Disorders. Neurology. 1996;47:1247–1253. doi: 10.1212/wnl.47.5.1247. [DOI] [PubMed] [Google Scholar]
- Berg D, Holzmann C, Riess O. 14-3-3 proteins in the nervous system. Nat Rev Neurosci. 2003;4:752–762. doi: 10.1038/nrn1197. [DOI] [PubMed] [Google Scholar]
- Boesenberg-Grosse C, Schulz-Schaeffer WJ, Bodemer M, Ciesielczyk B, Meissner B, Krasnianski A, Bartl M, Heinemann U, Varges D, Eigenbrod S, Kretzschmar HA, Green A, Zerr I. Brain-derived proteins in the CSF: do they correlate with brain pathology in CJD? BMC Neurol. 2006;6:35. doi: 10.1186/1471-2377-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton DL, Barnitz RA, Sakai K, Lenardo MJ. 14-3-3 theta binding to cell cycle regulatory factors is enhanced by HIV-1 Vpr. Biol Direct. 2008;3:17. doi: 10.1186/1745-6150-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che XH, Chen H, Xu ZM, Shang C, Sun KL, Fu WN. 14-3-3epsilon contributes to tumour suppression in laryngeal carcinoma by affecting apoptosis and invasion. BMC Cancer. 2010;10:306. doi: 10.1186/1471-2407-10-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen EA, Terwilliger EF, Jalinoos Y, Proulx J, Sodroski JG, Haseltine WA. Identification of HIV-1 vpr product and function. J Acquir Immune Defic Syndr. 1990;3:11–18. [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2. Lawrence Erlbaum Associates, Inc; Hillsdale, NJ: 1988. [Google Scholar]
- Collins SJ, Sanchez-Juan P, Masters CL, Klug GM, van Duijn C, Poleggi A, Pocchiari M, Almonti S, Cuadrado-Corrales N, de Pedro-Cuesta J, Budka H, Gelpi E, Glatzel M, Tolnay M, Hewer E, Zerr I, Heinemann U, Kretszchmar HA, Jansen GH, Olsen E, Mitrova E, Alperovitch A, Brandel JP, Mackenzie J, Murray K, Will RG. Determinants of diagnostic investigation sensitivities across the clinical spectrum of sporadic Creutzfeldt-Jakob disease. Brain. 2006;129:2278–2287. doi: 10.1093/brain/awl159. [DOI] [PubMed] [Google Scholar]
- Gardino AK, Yaffe MB. 14-3-3 proteins as signaling integration points for cell cycle control and apoptosis. Semin Cell Dev Biol. 2011 doi: 10.1016/j.semcdb.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman BB, Nguyen TP. Synaptic proteins linked to HIV-1 infection and immunoproteasome induction: proteomic analysis of human synaptosomes. J Neuroimmune Pharmacol. 2010;5:92–102. doi: 10.1007/s11481-009-9168-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind MD, Martindale J, Miller D, DeArmond SJ, Uyehara-Lock J, Gaskin D, Kramer JH, Barbaro NM, Miller BL. Challenging the clinical utility of the 14-3-3 protein for the diagnosis of sporadic Creutzfeldt-Jakob disease. Arch Neurol. 2003;60:813–816. doi: 10.1001/archneur.60.6.813. [DOI] [PubMed] [Google Scholar]
- He J, Choe S, Walker R, Di Marzio P, Morgan DO, Landau NR. Human immunodeficiency virus type 1 viral protein R (Vpr) arrests cells in the G2 phase of the cell cycle by inhibiting p34cdc2 activity. J Virol. 1995;69:6705–6711. doi: 10.1128/jvi.69.11.6705-6711.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Jr, Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsich G, Kenney K, Gibbs CJ, Lee KH, Harrington MG. The 14-3-3 brain protein in cerebrospinal fluid as a marker for transmissible spongiform encephalopathies. N Engl J Med. 1996;335:924–930. doi: 10.1056/NEJM199609263351303. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Hikita T, Taya S, Uraguchi-Asaki J, Toyo-oka K, Wynshaw-Boris A, Ujike H, Inada T, Takao K, Miyakawa T, Ozaki N, Kaibuchi K, Iwata N. Identification of YWHAE, a gene encoding 14-3-3epsilon, as a possible susceptibility gene for schizophrenia. Hum Mol Genet. 2008;17:3212–3222. doi: 10.1093/hmg/ddn217. [DOI] [PubMed] [Google Scholar]
- Iskander S, Walsh KA, Hammond RR. Human CNS cultures exposed to HIV-1 gp120 reproduce dendritic injuries of HIV-1-associated dementia. J Neuroinflammation. 2004;1:7. doi: 10.1186/1742-2094-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapasi AA, Fan S, Singhal PC. Role of 14-3-3epsilon, c-Myc/Max, and Akt phosphorylation in HIV-1 gp 120-induced mesangial cell proliferation. Am J Physiol Renal Physiol. 2001;280:F333–342. doi: 10.1152/ajprenal.2001.280.2.F333. [DOI] [PubMed] [Google Scholar]
- Kino T, De Martino MU, Charmandari E, Ichijo T, Outas T, Chrousos GP. HIV-1 accessory protein Vpr inhibits the effect of insulin on the Foxo subfamily of forkhead transcription factors by interfering with their binding to 14-3-3 proteins: potential clinical implications regarding the insulin resistance of HIV-1-infected patients. Diabetes. 2005a;54:23–31. doi: 10.2337/diabetes.54.1.23. [DOI] [PubMed] [Google Scholar]
- Kino T, Gragerov A, Valentin A, Tsopanomihalou M, Ilyina-Gragerova G, Erwin-Cohen R, Chrousos GP, Pavlakis GN. Vpr protein of human immunodeficiency virus type 1 binds to 14-3-3 proteins and facilitates complex formation with Cdc25C: implications for cell cycle arrest. J Virol. 2005b;79:2780–2787. doi: 10.1128/JVI.79.5.2780-2787.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kino T, Pavlakis GN. Partner molecules of accessory protein Vpr of the human immunodeficiency virus type 1. DNA Cell Biol. 2004;23:193–205. doi: 10.1089/104454904773819789. [DOI] [PubMed] [Google Scholar]
- Kogan M, Rappaport J. HIV-1 accessory protein Vpr: relevance in the pathogenesis of HIV and potential for therapeutic intervention. Retrovirology. 2011;8:25. doi: 10.1186/1742-4690-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton SA. Requirement for macrophages in neuronal injury induced by HIV envelope protein gp120. Neuroreport. 1992;3:913–915. doi: 10.1097/00001756-199210000-00023. [DOI] [PubMed] [Google Scholar]
- Luo X, Carlson KA, Wojna V, Mayo R, Biskup TM, Stoner J, Anderson J, Gendelman HE, Melendez LM. Macrophage proteomic fingerprinting predicts HIV-1-associated cognitive impairment. Neurology. 2003;60:1931–1937. doi: 10.1212/01.wnl.0000064396.54554.26. [DOI] [PubMed] [Google Scholar]
- Lyons JL, Uno H, Ancuta P, Kamat A, Moore DJ, Singer EJ, Morgello S, Gabuzda D. Plasma sCD14 is a biomarker associated with impaired neurocognitive test performance in attention and learning domains in HIV infection. J Acquir Immune Defic Syndr. 2011;57:371–379. doi: 10.1097/QAI.0b013e3182237e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda N, Tanaka H, Yamazaki S, Suzuki J, Tanaka K, Yamada T, Masuda M. HIV-1 Vpr induces G2 cell cycle arrest in fission yeast associated with Rad24/14-3-3-dependent, Chk1/Cds1-independent Wee1 upregulation. Microbes Infect. 2006;8:2736–2744. doi: 10.1016/j.micinf.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Mhawech-Fauceglia P, Herrmann FR, Andrews C, South S, Beck A, Lele S, Odunsi K. 14-3-3sigma expression and prognostic value in patients with epithelial ovarian carcinoma: a high throughput tissue microarray analysis. Eur J Surg Oncol. 2009;35:763–767. doi: 10.1016/j.ejso.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Mignon-Ravix C, Cacciagli P, El-Waly B, Moncla A, Milh M, Girard N, Chabrol B, Philip N, Villard L. Deletion of YWHAE in a patient with periventricular heterotopias and pronounced corpus callosum hypoplasia. J Med Genet. 2010;47:132–136. doi: 10.1136/jmg.2009.069112. [DOI] [PubMed] [Google Scholar]
- Morales D, Acevedo SF, Skolasky RL, Hechavarria R, Santiago S, De La Torre T, Maldonado E, Wojna V. Translational spatial task and its relationship to HIV-associated neurocognitive disorders and apolipoprotein E in HIV-seropositive women. J Neurovirol. 2012a;18:488–502. doi: 10.1007/s13365-012-0128-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales D, Skoulakis EC, Acevedo SF. 14-3-3s are potential biomarkers for HIV-related neurodegeneration. J Neurovirol. 2012b;18:341–353. doi: 10.1007/s13365-012-0121-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rackstraw S. HIV-related neurocognitive impairment--a review. Psychol Health Med. 2011;16:548–563. doi: 10.1080/13548506.2011.579992. [DOI] [PubMed] [Google Scholar]
- Shiga Y, Wakabayashi H, Miyazawa K, Kido H, Itoyama Y. 14-3-3 protein levels and isoform patterns in the cerebrospinal fluid of Creutzfeldt-Jakob disease patients in the progressive and terminal stages. J Clin Neurosci. 2006;13:661–665. doi: 10.1016/j.jocn.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Torres M, Cartier L, Matamala JM, Hernandez N, Woehlbier U, Hetz C. Altered Prion protein expression pattern in CSF as a biomarker for Creutzfeldt-Jakob disease. PLoS One. 2012;7:e36159. doi: 10.1371/journal.pone.0036159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushijima H, Nishio O, Klocking R, Perovic S, Muller WE. Exposure to gp120 of HIV-1 induces an increased release of arachidonic acid in rat primary neuronal cell culture followed by NMDA receptor-mediated neurotoxicity. Eur J Neurosci. 1995;7:1353–1359. doi: 10.1111/j.1460-9568.1995.tb01126.x. [DOI] [PubMed] [Google Scholar]
- Vodicka MA, Koepp DM, Silver PA, Emerman M. HIV-1 Vpr interacts with the nuclear transport pathway to promote macrophage infection. Genes Dev. 1998;12:175–185. doi: 10.1101/gad.12.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi H, Yano M, Tachikawa N, Oka S, Maeda M, Kido H. Increased concentrations of 14-3-3 epsilon, gamma and zeta isoforms in cerebrospinal fluid of AIDS patients with neuronal destruction. Clin Chim Acta. 2001;312:97–105. doi: 10.1016/s0009-8981(01)00595-2. [DOI] [PubMed] [Google Scholar]
- Wojna V, Robles L, Skolasky RL, Mayo R, Selnes O, de la Torre T, Maldonado E, Nath A, Melendez LM, Lasalde-Dominicci J. Associations of cigarette smoking with viral immune and cognitive function in human immunodeficiency virus-seropositive women. J Neurovirol. 2007;13:561–568. doi: 10.1080/13550280701620747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojna V, Skolasky RL, Hechavarria R, Mayo R, Selnes O, McArthur JC, Melendez LM, Maldonado E, Zorrilla CD, Garcia H, Kraiselburd E, Nath A. Prevalence of human immunodeficiency virus-associated cognitive impairment in a group of Hispanic women at risk for neurological impairment. J Neurovirol. 2006;12:356–364. doi: 10.1080/13550280600964576. [DOI] [PubMed] [Google Scholar]
- Xiao T, Ying W, Li L, Hu Z, Ma Y, Jiao L, Ma J, Cai Y, Lin D, Guo S, Han N, Di X, Li M, Zhang D, Su K, Yuan J, Zheng H, Gao M, He J, Shi S, Li W, Xu N, Zhang H, Liu Y, Zhang K, Gao Y, Qian X, Cheng S. An approach to studying lung cancer-related proteins in human blood. Mol Cell Proteomics. 2005;4:1480–1486. doi: 10.1074/mcp.M500055-MCP200. [DOI] [PubMed] [Google Scholar]
- Zhao LJ, Mukherjee S, Narayan O. Biochemical mechanism of HIV-I Vpr function. Specific interaction with a cellular protein. J Biol Chem. 1994a;269:15577–15582. [PubMed] [Google Scholar]
- Zhao LJ, Wang L, Mukherjee S, Narayan O. Biochemical mechanism of HIV-1 Vpr function. Oligomerization mediated by the N-terminal domain. J Biol Chem. 1994b;269:32131–32137. [PubMed] [Google Scholar]