Abstract
Objective:
To determine whether strict blood pressure (BP) control is the best medical management for patients with symptomatic carotid artery occlusion and hemodynamic cerebral ischemia.
Methods:
In this prospective observational cohort study, we analyzed data from 91 participants in the nonsurgical group of the Carotid Occlusion Surgery Study (COSS) who had recent symptomatic internal carotid artery occlusion and hemodynamic cerebral ischemia manifested by ipsilateral increased oxygen extraction fraction. The target BP goal in COSS was ≤130/85 mm Hg. We compared the occurrence of ipsilateral ischemic stroke during follow-up in the 41 participants with mean BP ≤130/85 mm Hg to the remaining 50 with higher BP.
Results:
Of 16 total ipsilateral ischemic strokes that occurred during follow-up, 3 occurred in the 41 participants with mean follow-up BP of ≤130/85 mm Hg, compared to 13 in the remaining 50 participants with mean follow-up BP >130/85 mm Hg (hazard ratio 3.742, 95% confidence interval 1.065–13.152, log-rank p = 0.027).
Conclusion:
BPs ≤130/85 mm Hg were associated with lower subsequent stroke risk in these patients.
Classification of evidence:
This study provides Class III evidence that control of hypertension ≤130/85 mm Hg is associated with a reduced risk of subsequent ipsilateral ischemic stroke in patients with recently symptomatic carotid occlusion and hemodynamic cerebral ischemia (increased oxygen extraction fraction).
Patients with symptomatic carotid artery occlusion and hemodynamic cerebral ischemia manifested by increased oxygen extraction fraction (OEF) are at 20%–30% risk for ipsilateral stroke within 2 years.1–3 Disagreement exists whether strict blood pressure (BP) control is the best medical management for these patients or whether higher BPs are needed to preserve cerebral perfusion and prevent subsequent stroke.3,4 To address this issue, we analyzed data from the nonsurgical group of the Carotid Occlusion Surgery Study (COSS).2
METHODS
The COSS was a parallel-group, prospective, 1:1 randomized, open-label, blinded-adjudication treatment trial conducted from 2002 to 2010 to test the hypothesis that extracranial-intracranial arterial bypass, when combined with best medical therapy, could reduce by 40% the subsequent occurrence of ipsilateral ischemic stroke at 2 years in patients with recent symptomatic internal carotid artery (ICA) occlusion and ipsilateral increased OEF measured by PET. COSS was carried out at 49 clinical centers and 18 PET centers in the United States and Canada. The majority were academic medical centers. The trial was terminated early for futility. Details of the trial design and results have been reported.2,5
The first follow-up visit was 30–35 days after randomization. Subsequent follow-up visits were at 3-month intervals after randomization until 24 months or the end of the trial. The nonsurgical group remained on the antithrombotic treatment preferred by their physicians. Each follow-up examination included monitoring of the efficacy of risk factor modification: BP, low-density lipoprotein cholesterol, triglycerides, hemoglobin A1C, and smoking. The target goal for BP was ≤130/85 mm Hg. If the BP was greater than this, the local COSS investigator was instructed to make recommendations to the primary physician for intervention based on the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure (JNC 7).6
Of the 98 nonsurgical participants, 91 are included in the analysis: 3 had no postrandomization BPs recorded and 4 had ipsilateral ischemic strokes occurring before the first BP recording at the 30–35 days follow-up visit including 1 crossover with a postoperative ipsilateral ischemic stroke. For the 2 other crossovers, we censored data at the time of surgery. Follow-up for the primary endpoint of ipsilateral ischemic stroke until occurrence, 2 years, or the end of the trial was complete in these 91 patients. Ipsilateral ischemic stroke was defined as the clinical diagnosis of a focal neurologic deficit due to cerebral ischemia clinically localizable within the territory of the symptomatic occluded ICA that lasted for more than 24 hours.
We divided participants into 2 groups: 41 with mean BP during follow-up who met the COSS target of ≤130/85 mm Hg and 50 whose BPs were higher than this. Since we were interested in the association between BP and stroke prevention, we only used the BP recorded before the stroke occurred for those 16 who experienced an endpoint ipsilateral ischemic stroke. For all others, we used all recorded BP measurements. Before exclusion of the poststroke BPs, there was no statistically significant difference in the total number of BP recordings during follow-up between the 16 patients who experienced a stroke during follow-up (5.4 ± 2.8) and the 75 who did not (5.4 ± 2.7, p = 0. 0.97). Once we excluded the poststroke BPs for this analysis, those who experienced a stroke had fewer BP recordings (2.1 ± 1.2, p < 0.001).
We compared baseline characteristics using generalized Fisher exact tests for categorical variables and Wilcoxon rank-sum tests for continuous variables. Since there was an imbalance at baseline between the 2 groups in time interval from qualifying event to randomization (see Results), a Cox model was used to determine if this time interval was a predictor of the primary endpoint of ipsilateral ischemic stroke.
The primary research question addressed by this study is whether there was an association between lower BPs and the occurrence of ipsilateral ischemic stroke in medically treated patients with recently symptomatic ICA occlusion and hemodynamic cerebral ischemia as manifested by an ipsilateral hemispheric increase in OEF. A Cox regression model with BP group as the predictor and time to first stroke as the outcome was used to estimate the hazard ratio for the 2 groups for the primary analysis. For this primary analysis, the hazard ratio was considered statistically significant if the 95% confidence intervals (CIs) did not include 1.
Usage of antihypertensive drugs was compared between the 2 groups using the method of generalized estimating equations with BP group as the predictor and that accounted for the multiplicity of visits on participants. Since the use of antihypertensive drugs was different between the 2 groups (see Results), we performed subgroup analyses of always treated (taking at least 1 antihypertensive drug at every follow-up visit) participants to address the secondary question of whether treatment of hypertension to ≤130/85 mm Hg was associated with a difference in subsequent stroke.
To examine for the possibility of a J-curve relationship between follow-up BP and stroke occurrence, we determined the rate of subsequent stroke when participants were categorized into 5 groups based on their mean systolic BP during follow-up according to the scheme used by Ovbiagele et al.,7 who found such a relationship in an analysis of the PROFESS trial: <120 mm Hg, 120–<130 mm Hg, 130–<140 mm Hg, 140–< 150 mm Hg, and ≥150 mm Hg.
Standard protocol approvals, registrations, and patient consents.
The original COSS study was done with full institutional review board approval of all the participating centers. Written informed consent was obtained from all participants. COSS is registered as NCT00029146 with ClinicalTrials.gov.
This study provides Class III evidence that control of hypertension ≤130/85 mm Hg is associated with a reduced risk of subsequent ipsilateral ischemic stroke in patients with recently symptomatic carotid occlusion and hemodynamic cerebral ischemia (increased OEF).
RESULTS
Forty-one participants had mean BP during follow-up of ≤130/85 mm Hg and 50 participants had mean BPs greater than this. The groups were well-matched for baseline characteristics and medical management during follow-up except for the time from entry event to randomization (tables 1 and 2). Time from entry event to randomization was not a significant predictor of subsequent stroke in these 91 patients (p = 0.3286).
Table 1.
Baseline characteristics
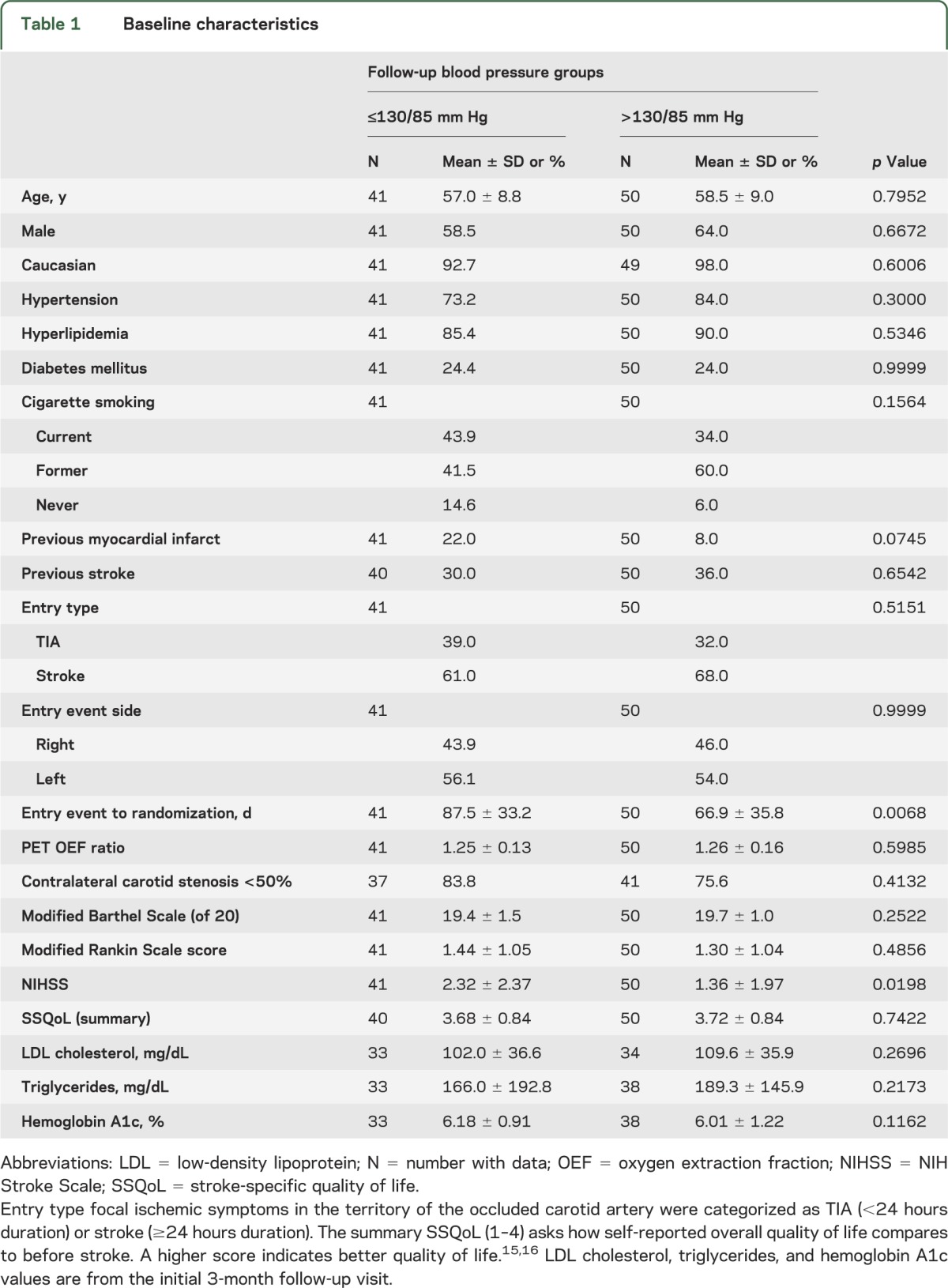
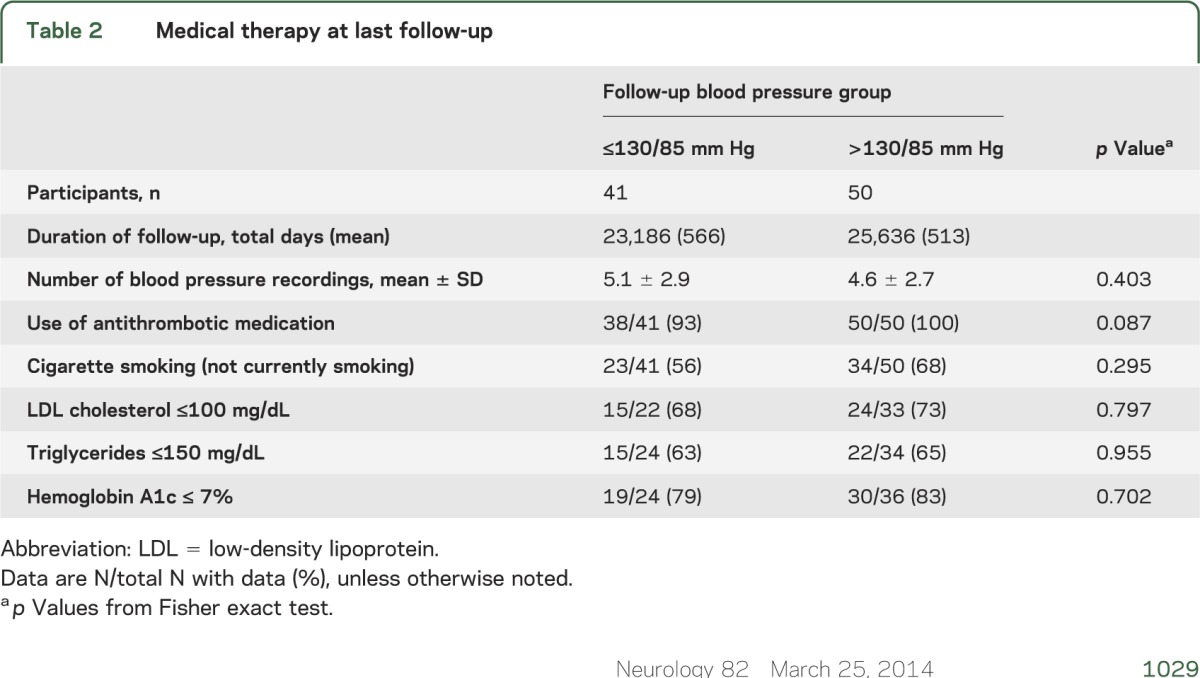
Table 2.
Medical therapy at last follow-up
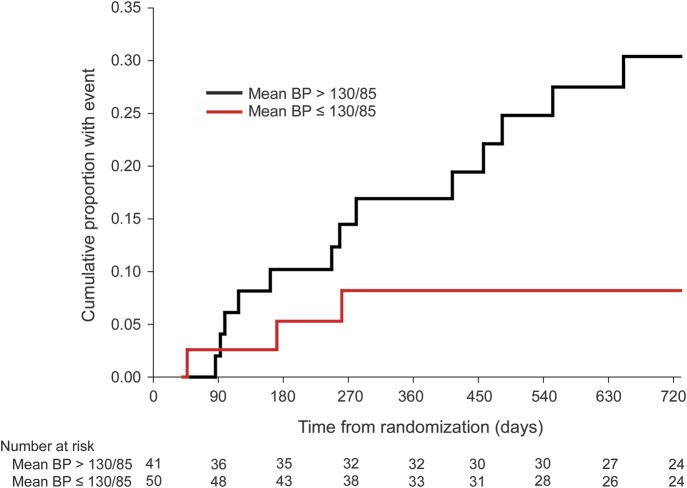
Of 16 total postrandomization ipsilateral ischemic strokes that occurred within 2 years of randomization, 3 occurred in the 41 participants with mean follow-up BP of ≤130/85 mm Hg, compared to 13 in the remaining 50 participants with mean follow-up BPs >130/85 mm Hg (hazard ratio 3.742, 95% CI 1.065–13.152, log-rank p = 0.027) (figures 1 and 2). Estimated 2-year ipsilateral ischemic stroke rates were taken from the Kaplan-Meier curves. For those whose mean BP during follow-up was ≤130/85 mm Hg, the 2-year estimated rate was 0.082 ± 0.046 (SE). For those with mean BP >130/85 mm Hg, the estimated rate was 0.304 ± 0.072 (SE).
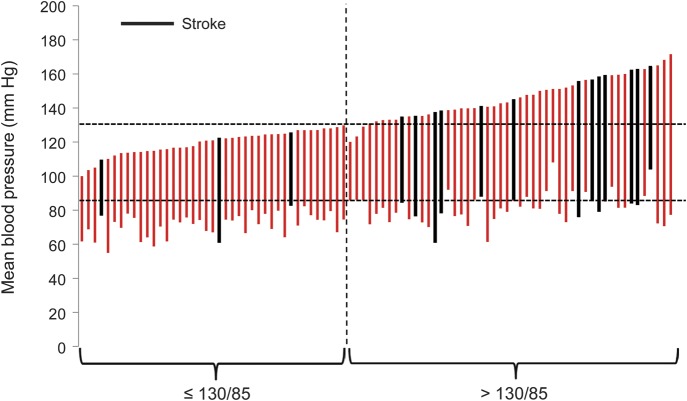
Figure 1. Individual mean systolic and diastolic blood pressures for the 91 participants in the 2 Carotid Occlusion Surgery Study groups.
Vertical bars depict the mean systolic (top of bar) and diastolic (bottom of bar) blood pressure of each participant in the study. Those who experienced an ipsilateral ischemic stroke within 2 years of randomization are shown in black. Three participants had mean systolic pressures below 130 mm Hg but diastolic pressures greater than 85 mm Hg. They are just to the right of the dotted vertical line.
Figure 2. Kaplan-Meier cumulative curves for occurrence of ipsilateral ischemic stroke.
The number of participants who remained event-free and available for follow-up evaluation at each 90-day interval is shown for each group at the bottom of the graph. BP = blood pressure.
The percent of follow-up visits at which the participants reported the use of antihypertensive drugs was different between the 2 groups: 224/353 (63%) for the ≤130/85 mm Hg group and 356/438 (81%) for the >130/85 mm Hg group (p = 0.0225). In the subgroup of always treated participants (taking at least 1 antihypertensive drug at every follow-up visit), there were 2 strokes in 23 patients in the ≤130/85 mm Hg group and 10 strokes in 32 participants in the >130/85 mm Hg group (hazard ratio 3.781, 95% CI 0.827–17.297, log-rank p = 0.065). The hazard ratio of 3.781 for this subgroup analysis was essentially identical to that of 3.742 for the primary analysis, indicating no heterogeneity of the association.
Figure 3 shows the categorization into 5 groups based on mean systolic BP during follow-up according to the scheme used by Ovbiagele et al. for the PROFESS trial analysis.
Figure 3. Rate of recurrent stroke within 2 years in the 5 blood pressure groups as defined in the PROFESS study.

Kaplan-Meier estimates with vertical bars denoting standard error of the estimates.
DISCUSSION
Prospective observational studies during middle and old age show that usual BP is strongly and directly related to stroke risk down to at least 115/75 mm Hg without any evidence of a threshold effect or J-curve.8 Treatment of high BP reduces the risk of recurrent stroke, although the optimal level is unknown and a subject of controversy.6,7,9–12 In the PROGRESS study, the lowest risk of stroke recurrence was among the lowest quartile of achieved systolic BP (<120 mm Hg).12 In contrast, in the PROFESS study, mean systolic BP <120 mm Hg was associated with a slight, but statistically significant, increase in stroke risk.7 Using the same categorization as the PROFESS analysis, we did not find evidence of a J-curve, but this conclusion must be tempered by the wide standard errors for the observed rates due to the small numbers in each group (figure 3).
Patients with severe cerebral arterial occlusive disease may represent a different situation because the hemodynamic effects of the obstruction may require a higher perfusion pressure to maintain cerebral blood flow downstream, thus leading to a higher stroke risk with lower BPs. In the Warfarin-Aspirin Symptomatic Intracranial Disease trial, lower BP during follow-up was associated with lower stroke risk. There was no evidence of an increased stroke risk in those with the lowest BPs (<119 mm Hg systolic or ≤79 mm Hg diastolic).13 In an analysis of data from the European Carotid Surgery Trial, the North American Symptomatic Carotid Endarterectomy Trial, and the United Kingdom Transient Ischaemic Attack Aspirin Trial, the association of lower BP during follow-up with lower stroke risk was not affected by the presence of a unilateral carotid stenosis or asymptomatic carotid occlusion. However, with bilateral carotid stenosis ≥70%, there was an increased risk of stroke with BPs below 150 mm Hg systolic.14 Since only 11 nonsurgical participants in COSS had contralateral ≥70% stenosis, we cannot comment on the effect of lower BP in this subgroup. Similarly, we cannot comment on the effect of lower BP on the risk of myocardial infarction. COSS did not record nonfatal myocardial infarction after the first 30 days. None occurred in the nonsurgical group within this period and no fatal myocardial infarctions occurred afterwards.
The patients in our study represent the most extreme case of cerebral hemodynamic compromise, with demonstration by PET of reduced cerebral blood flow relative to oxygen metabolic requirements (increased OEF) in the hemisphere distal to the symptomatic carotid occlusion. Nevertheless, lower BPs were associated with reduced, not increased, stroke risk. This was not simply due to the lower BP group not being hypertensive to begin with as the hazard ratio was the same for the subgroup always treated with antihypertensive drugs as for the entire cohort.
While we cannot definitively conclude from these data that control of BP to these levels will benefit such patients since this was not a randomized trial of different targets for BP control, this study provides Class III evidence that control of hypertension ≤130/85 mm Hg is associated with a reduced risk of subsequent ipsilateral ischemic stroke in patients with recently symptomatic carotid occlusion and hemodynamic cerebral ischemia (increased OEF).
Supplementary Material
ACKNOWLEDGMENT
The authors thank all the people who worked on COSS and all the patients who volunteered to participate.
GLOSSARY
- BP
blood pressure
- CI
confidence interval
- COSS
Carotid Occlusion Surgery Study
- ICA
internal carotid artery
- JNC 7
Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure
- OEF
oxygen extraction fraction
Footnotes
Editorial, page 1018
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Drs. Powers and Clarke had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Drs. Powers, Clarke, Grubb, Videen, Adams, and Derdeyn. Acquisition of data: Drs. Powers, Clarke, Grubb, Videen, Adams, and Derdeyn. Analysis and interpretation of data: Drs. Powers, Clarke, Grubb, Videen, Adams, and Derdeyn. Drafting of the manuscript: Drs. Powers and Clarke. Statistical analysis: Dr. Clarke. Obtained funding: Drs. Powers and Clarke. Administrative, technical, or material support: Drs. Powers, Clarke, Grubb, Videen, Adams, and Derdeyn. Study supervision: Drs. Powers and Clarke.
STUDY FUNDING
Supported by USPHS grants NS39526, NS42157, and NS41895 from the National Institutes of Neurological Disorders and Stroke.
DISCLOSURE
W. Powers reports receiving salary and other support from USPHS grants that funded this research. W. Clarke reports receiving salary and other support from USPHS grants that funded this research. R. Grubb, Jr., reports receiving salary and other support from USPHS grants that funded this research. Dr. Grubb reports grant support from the Barnes-Jewish Hospital Foundation. T. Videen reports receiving salary and other support from USPHS grants that funded this research. H. Adams reports receiving salary and other support from USPHS grants that funded this research. Dr. Adams reports receiving personal compensation as a consultant for Merck, Medtronics, and Pierre Fabre. C. Derdeyn reports receiving salary and other support from USPHS grants that funded this research. Dr. Derdeyn reports receiving personal compensation as a board member with W.L. Gore et al. and as a consultant for Microvention, Inc. Dr. Derdeyn also reports owning stock/stock options in Pulse Therapeutics and nFocus. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Grubb RL, Jr, Derdeyn CP, Fritsch SM, et al. Importance of hemodynamic factors in the prognosis of symptomatic carotid occlusion. JAMA 1998;280:1055–1060 [DOI] [PubMed] [Google Scholar]
- 2.Powers WJ, Clarke WR, Grubb RL, Jr, Videen TO, Adams HP, Jr, Derdeyn CP. Extracranial-intracranial bypass surgery for stroke prevention in hemodynamic cerebral ischemia: the carotid occlusion surgery study randomized trial. JAMA 2011;306:1983–1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamauchi H, Higashi T, Kagawa S, et al. Is misery perfusion still a predictor of stroke in symptomatic major cerebral artery disease? Brain 2012;135:2515–2526 [DOI] [PubMed] [Google Scholar]
- 4.Powers WJ. Management of patients with atherosclerotic carotid occlusion. Curr Treat Options Neurol 2011;13:608–615 [DOI] [PubMed] [Google Scholar]
- 5.Grubb RL, Jr, Powers WJ, Clarke WR, Videen TO, Adams HP, Jr, Derdeyn CP. Surgical results of the carotid occlusion surgery study. J Neurosurg 2013;118:25–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA 2003;289:2560–2572 [DOI] [PubMed] [Google Scholar]
- 7.Ovbiagele B, Diener HC, Yusuf S, et al. Level of systolic blood pressure within the normal range and risk of recurrent stroke. JAMA 2011;306:2137–2144 [DOI] [PubMed] [Google Scholar]
- 8.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002;360:1903–1913 [DOI] [PubMed] [Google Scholar]
- 9.Lakhan SE, Sapko MT. Blood pressure lowering treatment for preventing stroke recurrence: a systematic review and meta-analysis. Int Arch Med 2009;2:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rashid P, Leonardi-Bee J, Bath P. Blood pressure reduction and secondary prevention of stroke and other vascular events: a systematic review. Stroke 2003;34:2741–2748 [DOI] [PubMed] [Google Scholar]
- 11.Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011;42:227–276 [DOI] [PubMed] [Google Scholar]
- 12.Arima H, Chalmers J, Woodward M, et al. Lower target blood pressures are safe and effective for the prevention of recurrent stroke: the PROGRESS trial. J Hypertens 2006;24:1201–1208 [DOI] [PubMed] [Google Scholar]
- 13.Turan TN, Cotsonis G, Lynn MJ, Chaturvedi S, Chimowitz M. Relationship between blood pressure and stroke recurrence in patients with intracranial arterial stenosis. Circulation 2007;115:2969–2975 [DOI] [PubMed] [Google Scholar]
- 14.Rothwell PM, Howard SC, Spence JD. Relationship between blood pressure and stroke risk in patients with symptomatic carotid occlusive disease. Stroke 2003;34:2583–2590 [DOI] [PubMed] [Google Scholar]
- 15.Williams LS, Weinberger M, Harris LE, Biller J. Measuring quality of life in a way that is meaningful to stroke patients. Neurology 1999;53:1839–1843 [DOI] [PubMed] [Google Scholar]
- 16.Williams LS, Weinberger M, Harris LE, Clark DO, Biller J. Development of a stroke-specific quality of life scale. Stroke 1999;30:1362–1369 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.