Abstract
The inositol pyrophosphates are multifunctional signalling molecules. One of the families of enzymes that synthesize the inositol pyrophosphates are the Vip1/PPIP5Ks (PP-InsP5 kinases). The kinase domains in Vip1/PPIP5Ks have been mapped to their N-terminus. Each of these proteins also possess a phosphatase-like domain of unknown significance. In the present study, we show that this phosphatase-like domain is not catalytically active. Instead, by using SPR (surface plasmon resonance) to study protein binding to immobilized lipid vesicles, we show that this domain is specialized for binding PtdIns(3,4,5)P3 (PPIP5K1 Kd = 96 nM; PPIP5K2 Kd = 705 nM). Both PtdIns(3,4)P2 and PtdIns(4,5)P2 are significantly weaker ligands, and no significant binding of PtdIns(3,5)P2 was detected. We confirm the functional importance of this domain in inositol lipid binding by site-directed mutagenesis. We present evidence that the PtdIns(3,4,5)P3-binding domain is an unusual hybrid, in which a partial PH (pleckstrin homology) consensus sequence is spliced into the phosphatase-like domain. Agonist-dependent activation of the PtdIns 3-kinase pathway in NIH 3T3 cells drives translocation of PPIP5K1 from the cytosol to the plasma membrane. We have therefore demonstrated receptor-regulated compartmentalization of inositol pyrophosphate synthesis in mammalian cells.
Keywords: cell-signalling, compartmentalization, diphosphoinositol polyphosphates, inositol pyrophosphates
INTRODUCTION
The phospholipase C-initiated synthesis of the inositol pyrophosphates (e.g. PP-InsP5 and [PP]2-InsP4) is proposed to be a primordial signalling pathway that predates the evolution of the Ins(1,4,5)P3-activated Ca2+-mobilization cascade [1]. These inositol pyrophosphates have been proposed to regulate a variety of cellular activities (for reviews see [2–4]), including apoptosis, vesicle trafficking, cytoskeletal dynamics, exocytosis, telomere maintenance and adaptations to environmental stress. Eukaryotic cells typically contain one isomer of [PP]2-InsP4 (with diphosphates at the 1/3 and 5 positions [5]) which is synthesized from either of two isomers of PP-InsP5 (the 1/3 and 5 isomers). Two families of proteins collaborate to synthesize these molecules. Three members of the IP6K (InsP6 kinase) family [6] convert InsP6 to 5-PP-InsP5 [7] and these enzymes can also synthesize 1/3,5-[PP]2-InsP4 from 1/3-PP-InsP5 [5]. There are also two PPIP5K (PP-InsP5 kinase) enzymes [8,9] (Vip1 in yeasts [10]) that add a 1/3-diphosphate to both InsP6 and 5-PP-InsP5 [5].
Even the most abundant of the inositol pyrophosphates (5-PP-InsP5 [11]) is typically present in cells at 20-fold lower levels than those of InsP6 [3,4]. This difference in polyphosphate concentrations has raised some questions concerning proposed mechanisms of action of the inositol pyrophosphates, particularly as they are so similar in structure to InsP6 [3,4]. For example, a receptor for one particular inositol pyrophosphate would have to be remarkably specific in order to prevent competition or inhibition from InsP6, or indeed from any of the other inositol pyrophosphates; it is challenging to anticipate at a molecular level how the requisite specificity could be achieved [3]. To illustrate this point, it is notable that even the IP6K and Vip1/PPIP5K kinases recognize both InsP6 and PP-InsP5 (see above). Even when it has been shown that InsP6 neither mimics nor inhibits the binding of an inositol pyrophosphate (i.e. 1/3-PP-InsP5) to its receptor (a yeast cyclin–cyclin kinase complex [12,13]) there can sometimes be concern over biological relevance. In the latter case, the affinity for 1/3-PP-InsP5 appears too low to permit it, at ‘global’ physiologically relevant levels, to bind effectively to its target [4]. These are persistent questions in this field.
An alternative and less traditional mechanism of action of inositol pyrophosphates has come from studies in vitro that have described a novel mechanism of post-translation modification by the inositol pyrophosphates: the kinase-independent transfer to appropriate target proteins of their ‘high-energy’ diphosphate groups [14–16]. The proliferation of the number of apparent protein targets [14,16] could help explain why these pyrophosphates appear to regulate so many diverse cellular processes. However, as InsP6 strongly inhibits transphosphorylation by inositol pyrophosphates [15], this mechanism of action still faces the difficulty that InsP6 is relatively abundant [4]. There is also the issue that the entire family of inositol pyrophosphates has the ability to transphosphorylate proteins [14]. That observation raises the question of why are there multiple members of the inositol pyrophosphate family that each perform an identical function? Furthermore, in a scenario in which inositol pyrophosphates are, like all soluble signals, free to diffuse quickly through the cell, how could a single member of this family of molecules be recruited to perform one specific signalling task by a particular extracellular stimulus?
All of these difficulties in understanding the molecular basis for specificity in signalling by inositol pyrophosphates have a potential solution in the proposal that there might be spatially restricted synthesis of an inositol pyrophosphate [3]. For example, locally elevated levels of a particular inositol pyrophosphate would make InsP6 a less effective competitor, both in the context of mechanisms that involve receptor binding or transphosphorylation. Co-localization of the synthesis of one particular inositol pyrophosphate with a protein target could also facilitate specificity of signalling.
This idea that any ‘freely diffusible’ intracellular signal might be compartmentalized within a single cell was for a long time a heretical concept. Now the idea is mainstream, and the paradigm shift owes much to the characterization of spatial localization of cAMP signalling [17]. In this example, the enzymes that synthesize cAMP are spatially separated from the enzymes that hydrolyse cAMP, thereby generating an intracellular concentration gradient for this signal. By the same principle, an intracellular inositol phosphate concentration gradient is possible [18]. Certain inositol phosphate kinases possess nuclear retention signals [19,20]. It can therefore be anticipated that there might be some inositol phosphate concentration gradients across the nuclear membrane. However, the spatial organization of cytoplasmic signalling by inositol phosphates is less well understood. Arguably the most striking observation to date is the Ca2+-dependent exodus of the type A Ins(1,4,5)P3 3-kinase out of the spines of pyramidal neurons [21]. This change in the localization of Ins(1,4,5)P3 metabolism is believed to contribute to setting the temporal and spatial dynamics of neuronal Ca2+ signalling [21]. Among the other components of the cytoplasmic inositol phosphate cascade, evidence of enzyme compartmentalization is patchy and there is little information on molecular mechanisms or biological significance. For example, it is unknown how and why the Ins(1,3,4)P3 5/6-kinase is sometimes restricted to the plasma membrane [22], or why the Ins(1,3,4,5,6)P5 2-kinase is concentrated in cytoplasmic stress granules [19]. Nevertheless, in the light of recent evidence that Ins(1,3,4,5,6)P5 and InsP6 may each be distributed between two or more metabolically-distinct pools, there is growing interest in the general significance to cell signalling of compartmentalization of inositol phosphates [23].
In the present study we demonstrate that cytoplasmic compartmentalization of inositol pyrophosphate synthesis is not only possible, but it is also receptor-regulated. We show that upon activation of the appropriate cell-surface receptors to stimulate PtdIns(3,4,5)P3 synthesis, human PPIP5K1 translocates from the cytoplasm to the plasma membrane. We map a cryptic polyphosphoinositide-binding site in PPIP5Ks to an unusual hybrid domain, the N-terminal portion of a PH (pleckstrin homology) consensus sequence that is spliced into a phosphatase-like domain which itself has further evolved loss of catalytic function. This demonstration that PtdIns 3-kinase pathway can regulate compartmentalization of inositol pyrophosphate synthesis offers new opportunities to understand how signalling specificity can be controlled by cross-talk between signalling cascades. The present study is also of significance because, for the first time, we ascribe a physiologically relevant function to the so-called phosphatase-like domain of PPIP5K1, a topic that had previously generated considerable speculation [8,24].
MATERIALS AND METHODS
Protein expression and purification
PPIP5K1 kinase domain
The vector pGEX-4T-1 containing the cDNA encoding human GST (glutathione transferase)–PPIP5K1 was constructed exactly as described previously [8]. The construct was transformed into the ArcticExpress Escherichia coli strain (Stratagene) and grown in LB (Luria–Bertani) medium containing ampicillin (100 μg/ml) at 37 °C. When D600nm was 0.7, cells were transferred to 10 °C and protein was induced for 24 h with 0.1 mM IPTG (isopropyl β-D-thiogalactopyranoside).
Cells were harvested by centrifugation and the pellets were resuspended in lysis buffer [25 mM Tris/HCl (pH 8.0), 350 mM NaCl, 1 mM DTT (dithiothreitol) and EDTA-free protease inhibitor mixture tablets (Roche Applied Science)]. After sonication (medium output; 6×10 s) and centrifugation (15 000 g at 4 °C for 30 min), the supernatant was loaded on to a 5 ml glutathione–Sepharose column (GE Healthcare Life Sciences), which was pre-equilibrated with lysis buffer. The protein was purified using an ÄKTA FPLC system (Amersham Bioscience) at 4 °C. The column was washed with 10 CV (column volumes) of buffer A [25 mM Tris/HCl (pH 8.0), 350 mM NaCl and 1 mM DTT] at 1 ml/min, followed by 2 CV of buffer B [25 mM Tris/HCl (pH 8.0), 50 mM NaCl and 1 mM DTT]. The final step was a gradient elution created by mixing buffer A with buffer C [25 mM Tris/HCl (pH 8.0), 50 mM NaCl, 1 mM DTT and 30 mM glutathione] using 0–100 % buffer C; 2 ml fractions of eluted protein were collected, mixed with 50 % glycerol and stored in aliquots at −80 °C.
PBD (polyphosphoinositide binding domain) 1 and PBD2
Full-length cDNAs for human PPIP5K1 and human PPIP5K2 [9] were used as templates for subcloning their acid-phosphatase-like domains, which we redesignate in the present study as PBDs (type 1, amino acids 382–917 of PPIP5K1; type 2, amino acids 371–901 of PPIP5K2). The PBDs were given C-terminal poly(His) tags using the pET-21a vector (EMD Chemicals). For PBD1, the EcoRI 5′-primer was CGGAATTCCCCACCACATC-TGGCACTATG and the XhoI 3′-primer was CCGCTCGAG-CTCAACACCTTTCACTCC (the restriction sites are underlined). For PBD2 the EcoRI 5′-primer was CGGAATTCC-CAACTACATCTGGAACTATG and the XhoI 3′-primer was CCGCTCGAGTTCACAACCTTTGGCTCC. For PBD1R399A, the mutational primer ATTGCAATTATTGCTCATGGGGATCGT (the mutagenic codon is depicted in bold font) and its corresponding reverse complement were used during the overlap extension PCR. The second mutant (PBD1R417A) was generated in a similar way, using GTGAAACACCCAGCTTTTTTTGCTCTG and its reverse complement, along with the respective end primers mentioned above. All of the above constructs were transformed into DH5α cells for plasmid isolation and sequencing. Each of these constructs were transformed into the pGro7 chaperone-containing competent cells (Clontech), which were grown at 37 °C in LB containing 50 μg/ml ampicillin, 20 μg/ml chloramphenicol and 2 g of L-arabinose. When D600nm was 0.6, the cells were transferred to 25 °C and protein was induced for 15 h with 1 mM IPTG. The cells were next collected by centrifugation (2400 g for 10 min at 4 °C), and resuspended in ice-cold lysis buffer containing 25 mM Hepes (pH 8), 300 mM NaCl, 10 mM imidazole, protease inhibitor cocktail tablets (one tablet per 10 ml of the buffer) and 0.1 % Triton X-100. The suspension was sonicated on ice (10 × 20 s). The lysate was cleared by centrifugation, filtered (0.22 μm pore) and 25 ml was added to 1 ml of Ni-NTA (Ni2+-nitrilotriacetate)–agarose (Qiagen) for 40 min at 0–4 °C. The resin was then placed in a column, which was washed with 250 ml of 40 mM imidazole added to buffer A. This was followed by 10 ml of a second wash of buffer A plus 100 mM imidazole. Then, bound protein was eluted with 5 ml of buffer A plus 300 mM imidazole. The protein was next dialysed against buffer B and applied to a 5 ml HiTrap™ SP HP column (GE Healthcare) and eluted at 5 μl/min with buffer B plus a 0–100 %linear gradient of 2M NaCl, using an ÄKTA FPLC system.
Enzyme assays
All assays of inositol phosphate and inositol lipid metabolism were performed in lubricated microfuge tubes (PGC Scientifics). Kinase assays were performed by incubating the appropriate 3H-labelled inositol phosphate substrate at 37 °C in buffer containing 1mM EDTA, 50 mM KCl, 20 mM Hepes (pH 7.2), 8 mM MgSO4, 5 mM ATP and 10 mM NaF. All of the phosphatase assays were performed by incubating the appropriate 3H-labelled inositol phosphate substrate [or C8PtdIns(3,4,5)P3] at 37 °C in buffer containing 1 mM EDTA and 5 mM Hepes (pH 7.2), with 100 mM KCl, 2 mM MgCl2 and 0.5 mg/ml BSA.
The degree of PtdIns(3,4,5)P3 hydrolysis was recorded by assaying phosphate release [25]. For the inositol phosphate assays, these were acid quenched (0.2 volumes 2 M perchloric acid and 1 mg/ml InsP6), neutralized and analysed by Partisphere SAX HPLC as described previously [5]. Usually, the 3H in individual 1 ml fractions was determined after mixing with 4 ml of MonoFlow 4 scintillant (National Diagnostics), although occasionally a Flo-1 in-line radioactivity detector (Radiomatic Instruments) was used. Recovery of 3H in all assays was >85 % and did not vary with the degree of metabolism, indicating that there was no systematic loss of any particular product.
The hydrolysis of p-nitrophenyl phosphate was studied in the phosphatase assay buffer (see above), except that EDTA was omitted. At the end of each incubation period, the reaction was stopped by the addition of 1 M NaOH and the absorbance of the resulting solution at 405 nm was recorded with a SpectraMax 340 microplate spectrophotometer (Molecular Devices).
PEG (polyethylene glycol) precipitation
This assay is based on methods that were used in a previous study [26]. PBD2 (32 μg) was incubated at 4 °C for 20 min in 50 μl of buffer containing 25 mM Hepes (pH 7.2), 5 mg/ml bovine γ-globulin, 100 mM KCl, 1 mM EDTA, 1 mM DTT, [3H]InsP6 (approx. 15000 d.p.m.) and increasing amounts of non-radioactive InsP6 (or C8-PtdIns(3,4,5)P3; Cabiochem. Then 35 μl of ice-cold 30 % (w/v) PEG was added, with vortexing, followed by 10 min at 0–4 °C. Samples were subsequently centrifuged at 10 000 g (10 min at 4 °C). The supernatants were removed (and saved for counting free [3H]InsP6) and the resulting pellet was carefully washed with 1 ml of the incubation buffer (but without ligand or bovine γ-globulin). The pellet and protein-bound [3H]InsP6 was resuspended in 1 ml of 1 % SDS and 8 ml of Monoflow scintillation fluid.
SPR (surface plasmon resonance)
The Biacore sensor chip Pioneer L1 was used for immobilizing large unilamellar vesicles [77:20:3 by vol. PtdCho (phosphatidylcholine)/PtdE (phosphatidylethanolamine)/test phosphoinositide] [27], as described previously [28]. Then, 90 μl of the vesicles were injected over the chip surface at a flow rate of 5 μl/min until the response reached 5000 RU (response units). The protein under study was injected at a flow rate of 5 μl/min in buffer containing 100 mM KCl, 6 mM MgCl2, 5 mM Na2ATP and 25 mM Hepes (pH 7.2). The RU in the resulting sensorgram were automatically corrected for any response arising from protein interacting with control vesicles from which the test phosphoinositide was omitted. The values of the saturating RU (Req) were plotted against the protein concentrations (C) and the equilibrium binding affinity (Kd) was subsequently determined by performing a non-linear least-squares analysis of the binding isotherm using the equation , where Rmax is the maximal Req value. The S.E.M. of individual Req determinations did not exceed 1 % of the mean values.
Confocal immunofluorescence
NIH 3T3 cells were seeded on to 35 mm glass bottom micro-well dishes and transfected 24 h later with cDNA coding FLAG-PPIP5K1 (cloned into the pDEST515 plasmid) using Lipofectamine™ 2000 (Invitrogen) according to the manufacturer’s instructions. On the following day, cells were serum starved for 3 h and treated as indicated with 50 ng/ml PDGF (platelet-derived growth factor; Sigma). For immunostaining, cells were rinsed twice with PBS, fixed with 3 %paraformaldehyde in PBS containing 4 % sucrose for 15 min at room temperature (23 °C), permeabilized with 0.2 % Triton X-100 for 5 min and washed with PBS. After blocking with 10 % (v/v) BSA/PBS for 30 min at 37 °C, cells were incubated with the primary antibody (mouse monoclonal anti-FLAG, Abcam #ab18230) for 2 h at 37 °C and washed with PBS, followed by 1 h incubation at 37 °C with the secondary antibody (Alexa Fluor® 488 goat anti-mouse; Invitrogen #A-11001) and washed with PBS before incubation with DAPI (4′,6-diamidino-2-phenylindole; Invitrogen, #D1306) for 10 min at 37 °C. After washing the cells twice with PBS, confocal images were taken on a Zeiss LSM 510 META using a Plan-Apochromat ×63/1.4 oil objective. The cellular distribution of the FLAG-PPIP5K1 was recorded by line intensity profiles [29] using LSM Image Examiner software. Data were exported into SigmaPlot and the ratios of pixel intensity (plasma membrane/cytoplasm) were then calculated as described previously [30].
Other materials
[3H]InsP6 was purchased from PerkinElmer and was purified by HPLC using a 4 mm×250 mm IonPac AS7 column (Dionex) eluted with a linear gradient of 0–1 M nitric acid. Fractions containing pure [3H]InsP6 were saved and lyophilized. 5-PP-[3H]InsP5, 1/3-PP-[3H]InsP5 and 1/3,5-[PP]2-[3H]InsP4 were prepared as previously described [5,9,31]. All of the restriction enzymes were purchased from New England Biolabs. All of the phospholipids (1-stearoyl-2-arachidonoyl) were purchased from Avanti Polar Lipids. Non-radioactive InsP6 was purchased from Calbiochem. CHAPS, octyl glucoside, LY294002 and L-arabinose were purchased from Sigma–Aldrich. The GRP-1 (general receptor for phosphoinositides-1) PH domain construct was provided by Dr David Lambright (University of Massachusetts Medical School, Worcester, MA, U.S.A.) [32]; it was expressed and purified as previously described [33]. The protease inhibitor cocktail tablets were bought from Roche Applied Science and the Pioneer L1 Biacore sensor chip was purchased from GE Healthcare Life Sciences. Recombinant hMIPP (human multiple inositol polyphosphate phosphatase) was expressed and purified as previously described [34].
RESULTS
The catalytic activity of the kinase domain of PPIP5K1
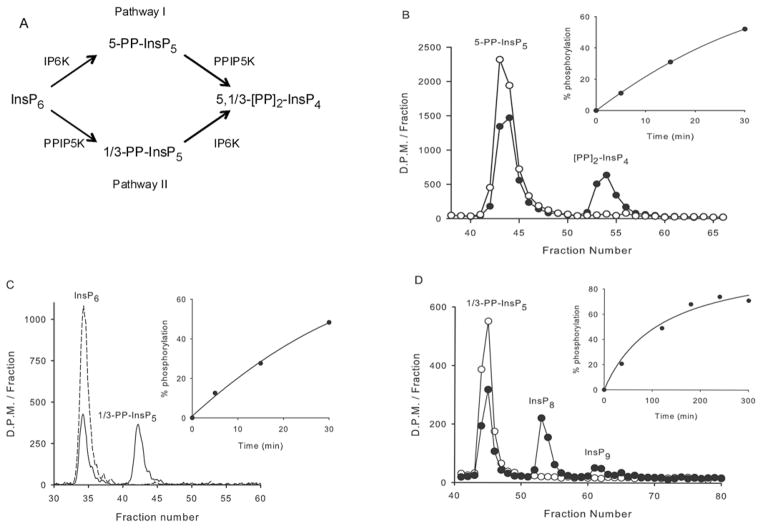
Mammalian PPIP5Ks, and the yeast homologue Vip1, have previously been shown to phosphorylate both InsP6 and 5-PP-InsP5 [5,8–10] and thereby participate in two pathways of [PP]2-InsP4 synthesis (Figure 1A), here named ‘I’ and ‘II’, as in an earlier study [35]. The kinase activity is self-contained in the N-terminal third of the Vip1/PPIP5Ks (Figure 1 and [8]). Fridy et al. [8], previously determined that the catalytic specificity of this kinase domain for 5-PP-InsP5 is 2.5-fold higher than the corresponding value for InsP6. A much greater (80-fold) catalytic preference for 5-PP-InsP5 was observed in our experiments (Table 1). This catalytic preference of the kinase for 5-PP-InsP5 can be predicted to be less dramatic in vivo, where the cellular levels of InsP6 substantially exceed those of 5-PP-InsP5 (Table 1). However, if there were to be any compartmentalization of PPIP5K within a single cell, there could be a localized metabolism of either of these alternate substrates.
Figure 1. The contributions of PPIP5Ks to [PP]2 -InsP4 synthesis.
(A) The two pathways of [PP]2 -InsP4 synthesis in mammalian cells. The structures of the diphosphoinositol polyphosphates are taken from information in [5,7,11]. The uncertainty concerning the precise placement of the 1/3-diphosphate group reflects an analytical impediment created by the axis of symmetry in the inositol ring. Thus 1-PP-InsP5 and 3-PP-InsP5 are an enantiomeric pair, and a stereoselective technique to distinguish between them has not yet been developed. This figure does not include the uncharacterized InsP8 and InsP9 observed in the present study, because of questions concerning their biological significance in mammalian cells (see the Results section). (B) A total of 0.2 ng of the PPIP5K1 kinase domain was incubated for either 0 min (open circles) or 15 min (filled circles) in 30 μl of kinase assay buffer, with approx. 5000 d.p.m. of 5-PP-[3H]InsP5. (C) In 30 μl of kinase assay buffer,17 ng of the kinase domain was incubated with approx. 0.035 μCi [3H]InsP6 for either 0 min (broken line) or for 20 min (solid line). (D) In 150 μl of kinase assay buffer, 500 ng of the kinase domain was incubated with approx. 1500 d.p.m. [3H]InsP8 for either 0 min (open circles) or 4 h (filled circles). All assays were acid quenched, neutralized and analysed by Partisphere SAX HPLC as described in the Materials and methods section, and radioactivity was assessed in either 1 ml fractions (B and D) or by using an in-line counter (C). The insets show the time course for each reaction. Recoveries of 3H in all experiments were >85 % (see the Materials and methods section).
Table 1. Kinetic constants for PPIP5K1 catalytic domain.
The kcat/Km ratios (specificity constants) were determined from the enzyme concentration and first-order rate constants for the metabolic reactions described in Figure 1, except for Ins(1,3,4,5,6)P5 phosphorylation. The data for intracellular concentrations were taken from various sources. Several publications have put the cellular concentration of PP-InsP5 in the 1–5 μM range (see [4] for a summary); a mid-range value of 2 μM is given in this Table. The values for the concentrations of Ins(1,3,4,5,6)P5 and InsP6 [48] are at the high end of a range of estimates [49]. MDD–HPLC analysis indicates that 1/3-PP-InsP5 must be <10 % of the cellular concentration of 5-PP-InsP5 [5,11].
| Substrate | Concentration in vivo (M) | kcat/Km (104×M−1 s−1) | [S]×kcat/Km (s −1) |
|---|---|---|---|
| 5-PP-InsP5 | 2 × 10 −6 | 384 ± 10 | 7.7 |
| InsP6 | 40 × 10 −6 | 4.8 ± 0.3 | 1.9 |
| Ins(1,3,4,5,6)P5 | 90 × 10 −6 | 0.3 ± 0.01 | 0.021 |
| 1/3-PP-InsP5 | 0.2 × 10 −6 | 0.11 ± 0.04 | 0.0002 |
We noted (Figure 1D) that the kinase domain can further phosphorylate the 1/3-PP-InsP5 to a compound that we have designated as an InsP8, since it co-elutes with [PP]2-InsP4 (which also has eight phosphates). Some further phosphorylation to InsP9 was also observed (Figure 1D). Vip1 can also convert 1/3-PP-InsP5 into InsP8 and InsP9; such molecules, which were speculated to contain triphosphate groups, have been suggested to significantly expand the family of diphosphoinositol polyphosphates [36]. It was further indicated [36] that such molecules are acid-labile and might degrade during acid-based cell lysis, or even during their HPLC separation; if so, this could have led us to significantly underestimate the efficiency of 1/3-PP-InsP5 phosphorylation. To check this possibility, we HPLC-purified 3HInsP8 and then took this material through another round of our acid-quench, neutralization and HPLC procedures. We recovered >85 % of the InsP8 and we did not detect any dephosphorylation of this compound. We are therefore confident that we have accurately determined that the catalytic efficiency of 1/3-PP-InsP5 phosphorylation by PPIP5K1 is very low (Table 1) and of doubtful biological significance. Ins(1,3,4,5,6)P5 was another relatively weak substrate (Table 1); the 2-phosphate group presumably makes a significant contribution to substrate specificity. We conclude that the physiological substrates for phosphorylation by PPIP5K1 are InsP6 and 5-PP-InsP5.
The phosphatase-like domains of PPIP5K1 and PPIP5K2 do not express phosphatase activity
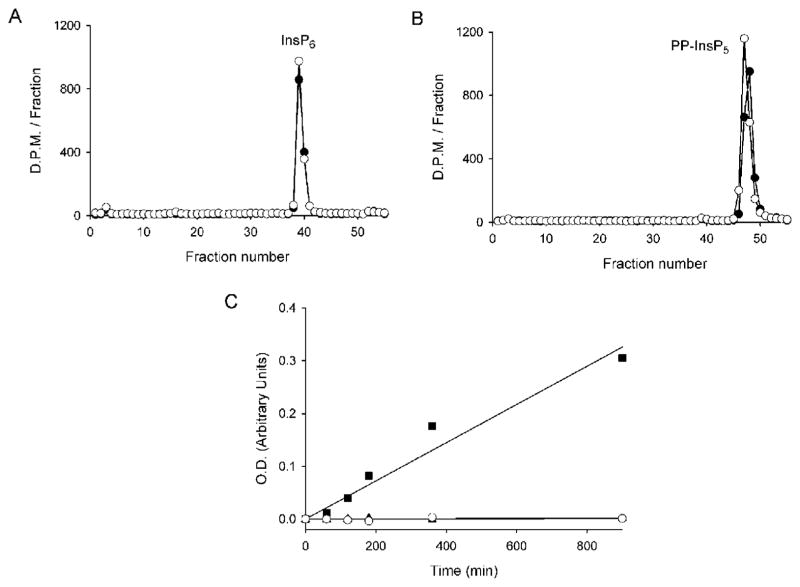
Previous molecular modelling studies [10] have identified the central third portion of Vip1/PPIP5Ks as a putative acid-phosphatase domain (see Figure 5). In particular, these domains contain the RHGXRXP catalytic motif which, in phytases [37] and hMIPP [38], is specialized for the hydrolysis of InsP6 and other inositol phosphates. There has therefore been considerable speculation concerning what might be the function of this domain in Vip/PPIP5Ks [8,24]. We have previously shown [9] that in vitro full-length recombinant human PPIP5K1 does not de-phosphorylate either Ins(1,3,4,5,6)P5, InsP6, 5-PP-InsP5 or [PP]2-InsP4. In the present study, we examined whether the phosphatase-like domains from each protein would possess catalytic activity. This reductionist approach is more sensitive because we were able to express in E. coli larger quantities of the domains at higher purity than we could achieve with full-length proteins. The phosphatase-like domain of PPIP5K1 was incubated for up to 15 h at 37 °C with InsP6 (Figure 2A), or 5-PP-InsP5 (Figure 2B), or either 1/3-PP-InsP5, [PP]2-InsP4 or the putative InsP8 triphosphate (results not shown; all assays used trace amounts of 3H-labelled material and in additional assays with InsP6 we used 1 μM amounts); none of these polyphosphates were hydrolysed. Identical results were obtained with the phosphatase-like domain of PPIP5K2 (results not shown). We additionally incubated the phosphatase-like domains of either PPIP5K1 or PPIP5K2 with p-nitrophenyl phosphate, a generic substrate for all acid phosphatases; again, there was no activity (Figure 2C). In contrast, when added at a similar concentration to that of the PPIP5Ks, hMIPP [38] hydrolysed p-nitrophenyl phosphate at a rate of approx. 10 nM/mg of protein per min (Figure 2C). We conclude that these phosphatase-like domains are catalytically inactive.
Figure 5. Evidence from multiple sequence alignments that PBD1 and PBD2 are unusual hybrid PtdIns(3,4,5)P3-binding domains.
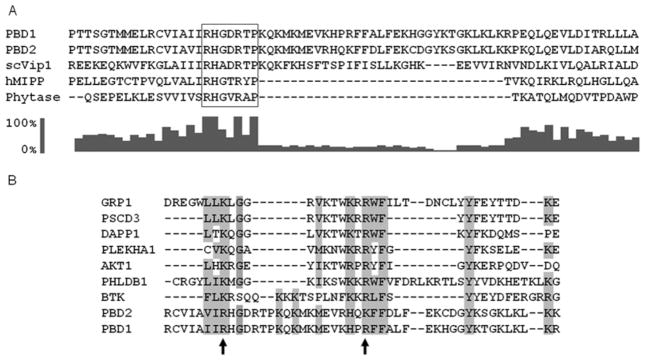
(A) Previous molecular modelling studies [10] have identified residues 530–1047 as a phosphatase-like region of Vip1 from Saccharomyces cerevisiae (scVip); the N-terminus of this domain was aligned (using ClustalX) with corresponding residues in the PBDs of PPIP5K1 (residues 382–453), PPIP5K2 (residues 371–442), hMIPP and the E. col. phytase. The RHGXRXP acid-phosphatase motif in the PBDs is boxed. The bar graph (generated with ClustalX 2.0.10) shows the conservation score (on a scale of 0 to 100 %) for each residue in the alignment. (B) A separate ClustalX sequence alignment of PBD1, PBD2 and several PtdIns(3,4,5)P3 -specific PH domains (GRP-1, PHLDB1 and PSCD3 are from mice; DAPP1, PLEKHA1, AKT1 and BTK are human sequences). The arrows mark two conserved basic residues in PBD1 (Arg399 and Arg417) that were selected for mutagenesis studies. Other conserved amino acid residues that are identical or similar in nature have also been highlighted. GenBank® accession numbers: scVip1, NP_013514; hMIPP, AAH32504.1; Phytase, 9954897; PBD2, NP_056031.2; PBD1, AAH57395.1; GRP-1, AAB60876.1; PSCD3, EDL19040.1; DAPP1, AAH12924.1; PLEKHA1, AAH01136.1; AKT1, AAL55732.1; PHLDB1, AAH69853.1; BTK, NP_000052.1.
Figure 2. The phosphatase-like domains of PPIP5Ks do not have phosphatase activity.
PBD1 (6 μg) was incubated with approx. 1500–2000 d.p.m. of either [3H]InsP6 (A) or 5-PP-[3H]InsP5 (B) in 120 μl of phosphatase assay buffer for 15 h at 37 °C (closed circles). Controls (open circles) did not contain enzyme. (C) 50 mM p-nitrophenyl phosphate replaced the inositol phosphates, and the assays contained 90 μg of either PBD1 (open circles) or PBD2 (closed triangles; these symbols are largely hidden by the open circles). Reactions were quenched with 1M NaOH and the A450 indicates the accumulation of p-nitrophenol. The closed squares represents the hydrolysis of p-nitrophenylphosphate by 90 ug of hMIPP. O.D., optical density (absorbance).
The phosphatase-like domain of PPIP5K1 binds InsP6 and PtdIns(3,4,5)P3
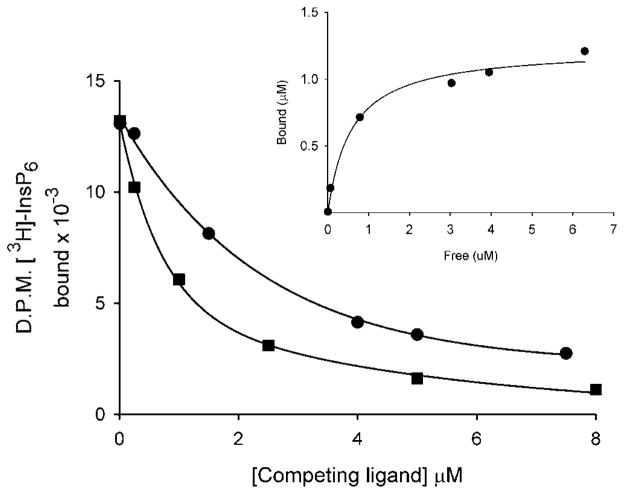
In addition to the substrate binding domain that is constructed around the RHGXRXP motif, acid phosphatases utilize a remote and catalytically essential histidine to hydrolyze substrate. This histidine normally occurs as a His-Asp dipeptide [37] (or His-Ala-Glu tripeptide in the specific case of hMIPP [38]). The absence in the phosphatase-like domains of such a histidine in the appropriate context [8] could help explain our demonstration that this domain lacks phosphatase activity. So what could be the functional significance of the tight conservation of the RHGXRXP motif? To answer that question, we investigated whether the phosphatase-like domain is important in binding of ligand, rather than its metabolism. We first examined whether the phosphatase-like domain of PPIP5K2 would bind InsP6. After equilibration of InsP6 with the domain, we separated bound and free ligand by precipitating protein with PEG [39]. In these experiments, InsP6 was found to bind to the protein (Kd = 0.6 μM; Figure 3).
Figure 3. Use of a PEG precipitation assay to identify binding of InsP6 and PtdIns(3,4,5)P3 to PBD2.
PBD2 (32 μg) was incubated with 3HInsP6 and increasing amounts of either non-radioactive InsP6 (circles) or C8 -PtdIns(3,4,5)P3 (squares) as described in the Materials and methods section. Bound and free ligand was separated by a PEG precipitation technique (see Materials and methods section). The Kd for InsP6 (0.57 μM) was calculated from the saturation binding plot (inset). The value of Bmax was 0.6 mol/mol of protein.
The binding of InsP6 to certain proteins does not always involve a specific recognition of the three-dimensional pattern of phosphate groups around the inositol ring. Instead, because of its especially high concentration of electronegative charge around the inositol ring, InsP6 can interact with some proteins through non-specific delocalized electrostatic interactions, particularly to proteins with an electrostatically polarized region such as a PH domain [40]. However, the most biologically relevant ligands for PH domains are usually the inositol lipids [40]. These considerations led us to directly compare InsP6-and PtdIns(3,4,5)P3-binding to the phosphatase-like domain of PPIP5K2. In PEG-precipitation assays, a C8 soluble analogue of PtdIns(3,4,5)P3 was 2- to 3-fold more potent than InsP6 itself at displacing 3HInsP6 from the domain (Figure 3). That is, these assays indicate that PtdIns(3,4,5)P3 is the preferred ligand. This is also likely to be case in vivo after activation of the PtdIns 3-kinase pathway, which can elevate PtdIns(3,4,5)P3 levels to approx. 200 μM [41], well above InsP6 levels (Table 1). These considerations, and the further experiments described below, have led us to re-designate this region of the PPIP5K proteins as a PBD.
The PtdIns(3,4,5)P3-binding affinities of the PBDs of PPIP5K1 and PPIP5K2
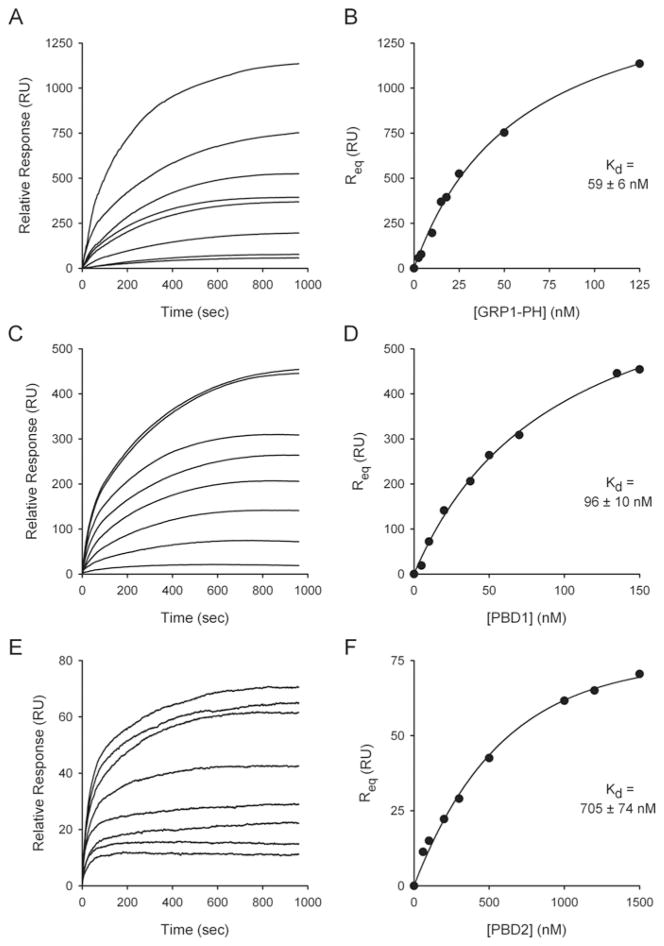
To more rigorously define phosphoinositide recognition by the PBD domains of PPIP5K1 and PPIP5K2, we measured equilibrium binding by SPR [27,28]. To maximize biological relevance, these experiments were performed in buffer containing 100 mM KCl, 1 mM free Mg2+ and 5 mM MgATP. We utilized membrane mimetics, that is we immobilized phospholipid vesicles made from PtdCho and PtdE into which we incorporated 3 %(v/v) PtdIns(3,4,5)P3 [27]. As a control, we first examined the binding of the GRP-1 PH domain to these vesicles. Sensorgrams were obtained at several different protein concentrations (Figure 4A), from which equilibrium binding affinity was determined (Figure 4B). The Kd value that we obtained for the GRP-1-PH (59 nM) is within the range of values published by others using the same technique (28–170 nM; [33,42]).
Figure 4. SPR-based equilibrium-binding analysis of the affinity for PtdIns(3,4,5)P3 for the GRP1 PH domain, PBD1 and PBD2.
Test protein was injected at 5 μl/min over immobilized PtdCho/PtdE/PtdIns(3,4,5)P3 vesicles and the resulting changes in the RU are plotted as a function of time to yield the SPR binding sensorgrams. The resulting Req values are plotted as a function of the protein concentration, from which a Kd value was determined, as described in the Materials and methods section. (A and B) Data obtained from the GRP1 PH domain (2.5, 4, 10, 15, 18, 25, 50 and 125 nM). (C and D) Data obtained from PBD1 (5, 10, 20, 37.5, 50, 70, 135 and 150 nM). (E and F) Data obtained from PBD2 (60, 100, 200, 300, 500, 1000, 1200 and 1500 nM). S.E.M. of individual Req values are smaller than the symbols denoting the means.
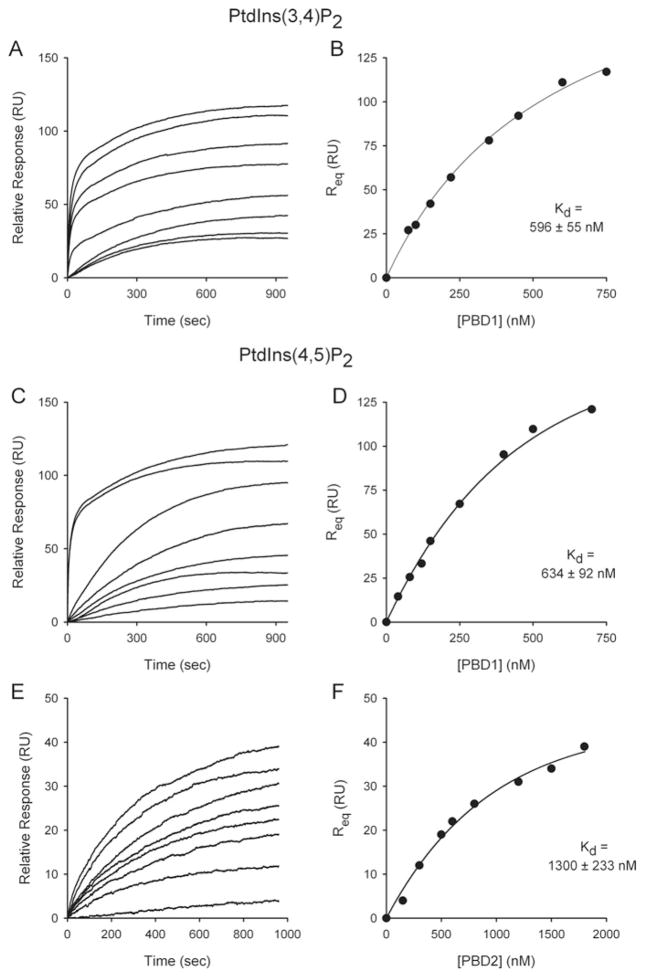
We next determined the affinity with which PtdIns(3,4,5)P3 binds to the newly renamed PBD domains of the PPIP5Ks (Figure 4). PBD1 bound PtdIns(3,4,5)P3 with high affinity (Kd = 96 nM; Figures 4C and 4D). This affinity is within the range of values that has previously been determined for a subset of PH domains, including GRP-1 (see above), that are specialized for binding to PtdIns(3,4,5)P3 [27,33]. We also found that PtdIns(3,4,5)P3 is a ligand for PBD2 (Figures 4E and 4F), but with considerably lower affinity (Kd = 705 nM; Figure 4F) than that for PBD1.
The data described above demonstrate that the PBDs are catalytically inactive against inositol phosphates and even p-nitrophenyl phosphate, a generic acid-phosphatase substrate. Nevertheless, in view of the high affinity of these domains for PtdIns(3,4,5)P3 (see above) we also incubated PBD1 (35 μg/ml) for 24 h at 37 °C with 1 mM of the C8 soluble analogue of PtdIns(3,4,5)P3. Using a sensitive phosphate release assay [25], we were unable to detect any hydrolysis of the lipid (results not shown).
The nature of the PBDs: novel, hybrid domains
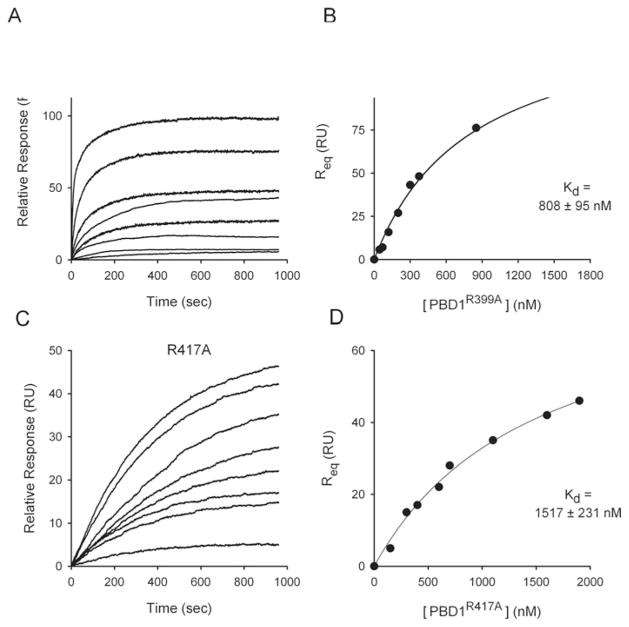
The amino acid sequences of the PBD domains of PPIP5K1 and PPIP5K2 were aligned with two related InsP6-metabolizing acid phosphatases, namely the hMIPP [38] and an E. coli phytase. This alignment identifies an approx. 30 amino acid residue insert, beginning immediately C-terminal of the RHGXRXP motif in the PBDs, which does not correspond to the genuine acid phosphatase sequences (Figure 5A). This insert in the PDBs, in separate alignments (Figure 5B), was found to be similar in several respects to the N-terminal portion of PtdIns(3,4,5)P3-specific PH domains [43]: for example (Figure 5B), the second β-strand of such PH domains has an R/KXRXF/L motif [43] that is well conserved in PBD1 (HXRXF), although slightly less conserved in PBD2 (HXKXF). Additionally, a tyrosine residue that is frequently present in the third β-strand of the PH domain [43] was aligned with a tyrosine in PBD1 and PBD2 (Figure 5B). Some additional similarities between the PBD insert and the PtdIns(3,4,5)P3-binding subset of PH domains are highlighted in Figure 5(B). Furthermore, in PPIP5Ks, just before the N-terminus of the putative PH domain insert, the first arginine in the RHGXRXP phosphatase motif aligns with a conserved lysine in the first β-strand of these PH domains (Figure 5B). That is, an unusual hybrid motif within PBDs aligns with a partial PH domain consensus. We experimentally tested the significance of these alignments by mutagenesis of two residues in PBD1 (corresponding to Arg399 and Arg417 in full-length PPIP5K1, marked by arrows in Figure 5B). These electropositive amino acids are good candidates for binding to the negatively charged phosphates in the headgroup of PtdIns(3,4,5)P3. The Kd values for the binding of PtdIns(3,4,5)P3 by PBD1R399A and PBD1R417A mutants were 8-fold and 16-fold lower than the Kd values for the wild-type PBD1, confirming the functionality of these two arginine residues (Figure 6). Arg399 in PBD1 is conservatively replaced with a lysine in PBD2 (Figure 5), which might contribute to the latter’s lower affinity for PtdIns(3,4,5)P3 (Figure 4). Note that Vip1, the yeast homologue of the PPIP5Ks, cannot bind PtdIns(3,4,5)P3 in vivo, as this lipid is not synthesized by that organism. Could Vip1 bind PtdIns(4,5)P2 instead? We have not directly answered that question, but we note that Vip1 lacks a residue equivalent to Arg417 in PBD1 (Figure 5). Mutagenesis of this particular arginine in PBD1 eliminated all detectable binding of PtdIns(4,5)P2 (results not shown). Our data therefore predict that Vip1 is unlikely to bind to inositol lipids in vivo.
Figure 6. SPR-based equilibrium-binding analysis of the affinity for PtdIns(3,4,5)P3 of two PBD1 mutants.
Two arginine residues in PBD1 (that correspond with Arg399 and Arg417 in PPIP5K1) were mutated to alanine and the constructs R399A (at 45, 70, 120, 200, 300, 375, 850 and 1700 nM) (A and B) and R417A (at 150, 300, 400, 600, 700, 1100, 1600 and 1900 nM) (C and D) were injected at 5 μl/min over immobilized PtdCho/PtdE/PtdIns(3,4,5)P3 vesicles and the resulting changes in the RU are plotted as a function of time to yield the SPR-binding sensorgrams (A and C), from which the Kd values were determined (B and D). The buffer contained 100 mM KCl, 6 mM MgCl2, 5 mM Na2 ATP and 25 mM Hepes (pH 7.2).
The specificity of lipid binding to the PBDs of PPIP5K1 and PPIP5K2
PBD1 was found to bind PtdIns(4,5)P2 and PtdIns(3,4)P2 with approx. 6-fold lower affinity than does PtdIns(3,4,5)P3 (Figures 7A–7D). These data indicate that both the 3- and 5-phosphates of PtdIns(3,4,5)P3 contribute to ligand affinity. However, there was no significant binding of PtdIns(3,5)P2 to PBD1 (results not shown). This observation that polyphosphoinositide binding requires vicinal phosphate groups is known to be a characteristic of the PH domain [44]. Ptd3P, Ptd4P and Ptd5P did not bind to PBD1 (results not shown). PBD2 bound PtdIns(4,5)P2 relatively weakly (Figures 7E and 7F) and did not bind PtdIns(3,5)P2, Ptd3P, Ptd4P or Ptd5P (results not shown). Clearly, PBD1 has a considerably greater affinity for inositol lipids than does PBD2 (Figures 4 and 7).
Figure 7. SPR-based equilibrium-binding analysis of the affinity for PtdIns(4,5)P2 and PtdIns(4,5)P2 for PBD1 and PBD2.
Either PBD1 (A–D) or PBD2 (E and F) were injected at 5 μl/min over immobilized PtdCho/PtdE/PtdIns(3,4)P2 vesicles (A and B) or PtdCho/PtdE/PtdIns(4,5)P2 vesicles (C–F) and the resulting changes in the RU are plotted as a function of time to yield the SPR binding sensorgrams, from which the Kd values were determined. Peptide concentrations ranged from 75 to 1900 nM.
In intact cells, PPIP5K1 translocates to plasma membranes after activation of the PtdIns 3-kinase signalling pathway
The experiments described above demonstrate that PPIP5K1 contains a domain (PBD1) that binds PtdIns(3,4,5)P3 and, with a 6-fold lower affinity, PtdIns(4,5)P2. We therefore next examined the intracellular distribution of PPIP5K1 in NIH 3T3 cells. To maximize the physiological relevance of these experiments, we transfected the cells with full-length human PPIP5K1 (FLAG-tagged), rather than just the protein’s PBD domain. The intracellular distribution of the protein was monitored by confocal immunofluorescence (Figure 8).
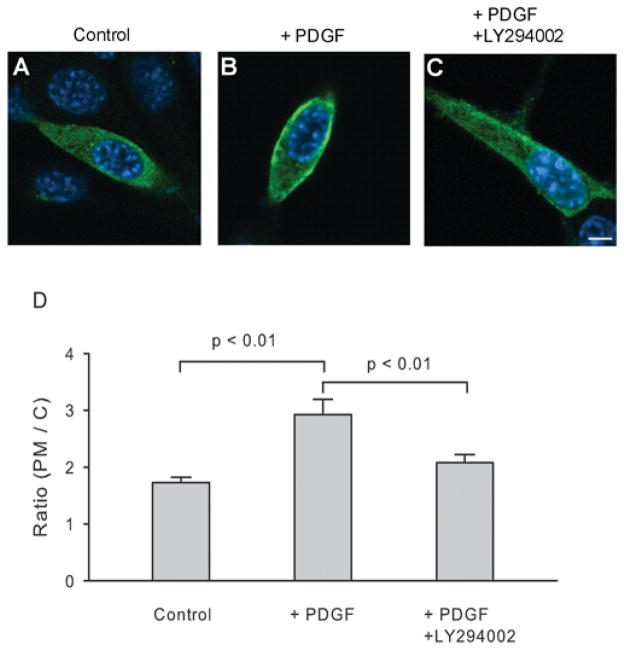
Figure 8. PDGF promotes translocation of PPIP5K to the plasma membrane in NIH 3T3 cells.
At 1 day after transfecting NIH 3T3 cells with cDNA encoding FLAG-tagged PPIP5K1, the intracellular location of the enzyme was determined by confocal immunofluorescence microscopy. (A) Control. (B) Treatment with 50 ng/ml PDGF for 5 min. (C) 15 min pre-treatment with 100 μM LY294002 [50] before 50 ng/ml PDGF was added for 5 min. The scale bar represents 5 μm. (D) The concentration of PPIP5K at the plasma membrane (PM) is shown as a ratio to the concentration of kinase in the cytosol (C) [30]. Results are the means ± S.E.M. from 31–34 cells.
Representative examples of single cells are shown in Figure 8. The data were quantified by recording line intensity profiles [29] from which the ratios of pixel intensity (plasma membrane/cytoplasm) were calculated ([30]; Figures 8A and 8D). We found that PPIP5K1 was distributed throughout the cytoplasm, although a slightly elevated level of PPIP5K1 was found at the plasma membrane (Figures 8A and 8D). This may reflect some binding of PPIP5K1 to PtdIns(4,5)P2, but, given that we studied localization of the full-length protein, there may be additional protein–protein interactions.
PtdIns(3,4,5)P3 is a plasmalemmal second messenger, the levels of which increase dramatically after receptor-dependent activation of the PtdIns 3-kinase pathway [41]. Upon 5 min of treatment of NIH 3T3 cells with PDGF, a PtdIns 3-kinase activator, there was a significant increase in the proportion of PPIP5K1 that was present at the plasma membrane (Figure 8). This translocation of PPIP5K1 was largely prevented when the PtdIns 3-kinase pathway was inhibited by pre-treatment of cells with LY294002 (Figures 8C and 8D). Thus the PtdIns 3-kinase pathway enhances compartmentalization of inositol pyrophosphate synthesis in vivo.
DISCUSSION
The purification and cloning of the PPIP5K family [8,9] (Vip1 in yeasts [10]) solved a long-standing problem in the inositol pyrophosphate field: the identification of the enzymes that synthesize [PP]2-InsP4 from PP-InsP5. However, this development brought with it an unexpected conundrum. In addition to a kinase domain, the Vip1/PPIP5K proteins were found to contain a region with similarities in amino acid sequence to certain acid phosphatases (such as phytase and MIPP) that are specialized for the dephosphorylation of inositol phosphates. The presence of competing phosphatase and kinase activities within a single protein is rare, but of considerable significance when it occurs [45]. However, one of the achievements of our present study is to demonstrate that this domain is not a functional phosphatase, but instead it is shown to be specialized for binding of inositol lipids. Our experiments also indicate that the lipid-binding site is formed from a novel hybrid sequence constructed from partial PH and acid-phosphatase domains. We further show that the activation of the PtdIns 3-kinase pathway is associated with PPIP5K1 translocation to the plasma membrane. This new observation of a mechanism for receptor-dependent compartmentalization of inositol pyrophosphate synthesis represents another important finding in the present study.
Multiple sequence alignments provide evidence that the PtdIns(3,4,5)P3-binding region of PPIP5Ks, the PBDs, includes a partial PH domain (Figure 5). This conclusion is supported by our characterization of the specificity of inositol lipid binding to these domains: PtdIns(4,5)P2 and PtdIns(3,4)P2, which both have vicinal phosphates, were shown to bind to the PBD1 albeit with weaker affinity than did PtdIns(3,4,5)P3. In contrast, neither PtdIns(3,5)P2 nor any of the PtdInsPs exhibited any significant binding to PBDs (see the Results section). Thus, just like other PH domains that preferentially bind PtdIns(3,4,5)P3, PBDs demonstrates an absolute requirement for two vicinal phosphate groups [44]. Moreover, Arg417 in the PBD of PPIP5K1 was shown by mutagenesis to make a critical contribution to ligand binding (Figure 6). This arginine residue aligns with a conserved arginine in the second β-strand of certain PH domains (Figure 5), which also participates in ligand binding [43]. It is also intriguing that a conserved and functionally important lysine residue in the first β-strand of the PH domain [43] was aligned with the first arginine residue in the RHGXRXP phosphatase motif within the PBDs (Figure 5). Our mutagenic experiments revealed that this residue, Arg399, also makes a significant contribution to ligand binding in PPIP5K1 (Figure 6). Thus we conclude that the PBD may be considered a novel hybrid domain.
It is possible that the presence of this PH domain-like insert may contribute to the phosphatase-like sequence being catalytically inactive, but in addition previous alignments [8] have shown that PPIP5Ks lack a remote histidine that is catalytically essential in genuine acid phosphatases such as phytases and MIPP [37,38]. In any case, we believe that the new findings in the present study, together with our earlier work [9], should end speculation [8,24] that PPIP5Ks might harbour phosphatase activity. To reiterate all of our findings on this topic: in earlier work [9], the levels of inositol phosphates in HEK (human embryonic kidney) cells were unchanged following overexpression of a kinase-dead mutant of full-length PPIP5K1. That is an experimental paradigm that would be expected to unmask any significant phosphatase activity. Furthermore, the full-length recombinant PPIP5K1 does not dephosphorylate either Ins(1,3,4,5,6)P5, InsP6, 5-PP-InsP5 or [PP]2-InsP4 [9]. In the present study, the latter polyphosphates were again demonstrated not to be substrates, but now our conclusion is more rigorous because we incubated the PBDs from both PPIP5K1 and PPIP5K2 at higher concentrations and for longer times than in previous experiments with full-length PPIP5K1. Finally, we have extended the list of molecules that are not substrates to also include 1/3-PP-InsP5, InsP8 (a putative triphosphate, see [36]), PtdIns(3,4,5)P3 and even p-nitrophenyl phosphate, a generic acid-phosphatase substrate.
One of the most important mechanisms for achieving signalling specificity by a particular pathway is through regulated spatial organization of its key components [46]. Our present study shows that this is a property of the inositol pyrophosphate signalling cascade. Inositol lipid-dependent compartmentalization of PPIP5K1 in animal cells is significant because it provides a novel means of regulating inositol phosphate signalling pathways. Thus it seems likely there can be PtdIns 3-kinase-dependent localized elevations in the concentrations of the products of 1/3-PP-InsP5 and [PP]2-InsP4 (Figure 1 and Table 1). This indication that under certain circumstances inositol pyrophosphate synthesis can be compartmentalized adds new features to our understanding of the mechanisms of action of inositol pyrophosphates. As outlined in the Introduction section, irrespective of whether these pyrophosphates act by transphosphorylating proteins or by binding to specific receptors, compartmentalization of inositol pyrophosphate synthesis can enhance their biological activities. Exocytosis is at least one plasma membrane located event that is regulated by inositol pyrophosphates [47]. Nevertheless, the importance of compartmentalization of PPIP5K may well go beyond local elevations in inositol pyrophosphate concentrations. Perhaps translocation of PPIP5K1 to the plasma membrane modifies its kinase activity. Additionally, PPIP5K might engage in functionally significant, but non-catalytic, protein–protein interactions. The exceptionally high-affinity binding of PPIP5K to PtdIns(3,4,5)P3 (Figure 4) could also prohibit or displace the binding to this lipid of other proteins that might have lower affinity for PtdIns(3,4,5)P3; such ligand competition could also have regulatory significance. One or more of these options are likely to be productive directions for future research into the biological significance of the PPIP5K family.
Acknowledgments
We thank Agnes Janoshazi and Jeff Tucker for assistance with the confocal microscopy and for data evaluation.
FUNDING
This work was supported by the Intramural Research Program of the National Institutes of Health/National Institute of Environmental Health Sciences.
Abbreviations used
- CV
column volumes
- DTT
dithiothreitol
- GRP-1
general receptor for phosphoinositides-1
- IP6K
InsP6 kinase
- hMIPP
human multiple inositol polyphosphate phosphatase
- IPTG
isopropyl β-D-thiogalactopyranoside
- LB
Luria–Bertani
- PBD
polyphosphoinositide binding domain
- PDGF
platelet-derived growth factor
- PEG
polyethylene glycol
- PH
pleckstrin homology
- PPIP5K
PP-InsP5 kinase
- PtdCho
phosphatidylcholine
- PtdE
phosphatidylethanolamine
- RU
response units
- SPR
surface plasmon resonance
Footnotes
AUTHOR CONTRIBUTION
All three of the authors designed and performed experiments, and contributed to writing the manuscript.
References
- 1.Seeds AM, York JD. Inositol polyphosphate kinases: regulators of nuclear function. Biochem Soc Symp. 2007;74:183–197. doi: 10.1042/BSS0740183. [DOI] [PubMed] [Google Scholar]
- 2.Barker CJ, Illies C, Gaboardi GC, Berggren PO. Inositol pyrophosphates: structure, enzymology and function. Cell Mol Life Sci. 2009;66:3851–3871. doi: 10.1007/s00018-009-0115-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burton A, Hu X, Saiardi A. Are inositol pyrophosphates signalling molecules? J Cell Physiol. 2009;220:8–15. doi: 10.1002/jcp.21763. [DOI] [PubMed] [Google Scholar]
- 4.Shears SB. Diphosphoinositol polyphosphates: metabolic messengers? Mol Pharmacol. 2009;76:236–252. doi: 10.1124/mol.109.055897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin H, Fridy PC, Ribeiro AA, Choi JH, Barma DK, Vogel G, Falck JR, Shears SB, York JD, Mayr GW. Structural analysis and detection of biological inositol pyrophosphates reveals that the VIP/PPIP5K family are 1/3-kinases. J Biol Chem. 2009;284:1863–1872. doi: 10.1074/jbc.M805686200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saiardi A, Nagata E, Luo HR, Snowman AM, Snyder SH. Identification and characterization of a novel inositol hexakisphosphate kinase. J Biol Chem. 2001;276:39179–39185. doi: 10.1074/jbc.M106842200. [DOI] [PubMed] [Google Scholar]
- 7.Draskovic P, Saiardi A, Bhandari R, Burton A, Ilc G, Kovacevic M, Snyder SH, Podobnik M. Inositol hexakisphosphate kinase products contain diphosphate and triphosphate groups. Chem Biol. 2008;15:274–286. doi: 10.1016/j.chembiol.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 8.Fridy PC, Otto JC, Dollins DE, York JD. Cloning and characterization of two human VIP1-like inositol hexakisphosphate and diphosphoinositol pentakisphosphate kinases. J Biol Chem. 2007;282:30754–30762. doi: 10.1074/jbc.M704656200. [DOI] [PubMed] [Google Scholar]
- 9.Choi JH, Williams J, Cho J, Falck JR, Shears SB. Purification, sequencing, and molecular identification of a mammalian PP-InsP5 kinase that is activated when cells are exposed to hyperosmotic stress. J Biol Chem. 2007;282:30763–30775. doi: 10.1074/jbc.M704655200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mulugu S, Bai W, Fridy PC, Bastidas RJ, Otto JC, Dollins DE, Haystead TA, Ribeiro AA, York JD. A conserved family of enzymes that phosphorylate inositol hexakisphosphate. Science. 2007;316:106–109. doi: 10.1126/science.1139099. [DOI] [PubMed] [Google Scholar]
- 11.Albert C, Safrany ST, Bembenek ME, Reddy KM, Reddy KK, Falck JR, Bröker M, Shears SB, Mayr GW. Biological variability in the structures of diphosphoinositol polyphosphates in Dictyostelium discoideum and mammalian cells. Biochem J. 1997;327:553–560. doi: 10.1042/bj3270553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee YS, Huang K, Quiocho FA, O’Shea EK. Molecular basis of cyclin-CDK–CKI regulation by reversible binding of an inositol pyrophosphate. Nat Chem Biol. 2008;4:25–32. doi: 10.1038/nchembio.2007.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee YS, Mulugu S, York JD, O’Shea EK. Regulation of a cyclin-CDK–CDK inhibitor complex by inositol pyrophosphates. Science. 2007;316:109–112. doi: 10.1126/science.1139080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhandari R, Saiardi A, Ahmadibeni Y, Snowman AM, Resnick AC, Kristiansen TZ, Molina H, Pandey A, Werner JK, Jr , Juluri KR, et al. Protein pyrophosphorylation by inositol pyrophosphates is a post-translational event. Proc Natl Acad Sci USA. 2007;104:15305–15310. doi: 10.1073/pnas.0707338104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saiardi A, Bhandari A, Resnick R, Cain A, Snowman AM, Snyder SH. Inositol pyrophosphate: physiologic phosphorylation of proteins. Science. 2004;306:2101–2105. doi: 10.1126/science.1103344. [DOI] [PubMed] [Google Scholar]
- 16.Azevedo C, Burton A, Ruiz-Mateos E, Marsh M, Saiardi A. Inositol pyrophosphate mediated pyrophosphorylation of AP3B1 regulates HIV-1 Gag release. Proc Natl Acad Sci USA. 2009;106:21161–21166. doi: 10.1073/pnas.0909176106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Houslay MD. Underpinning compartmentalised cAMP signalling through targeted cAMP breakdown. Trends Biochem Sci. 2010;35:91–100. doi: 10.1016/j.tibs.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 18.Nezu A, Tanimura A, Morita T, Tojyo Y. Visualization of Ins(1,4,5)P3 dynamics in living cells: two distinct pathways for Ins(1,4,5)P3 generation following mechanical stimulation of HSY-EA1 cells. J Cell Sci. 2010;123:2292–2298. doi: 10.1242/jcs.064410. [DOI] [PubMed] [Google Scholar]
- 19.Brehm MA, Schenk TM, Zhou X, Fanick W, Lin H, Windhorst S, Nalaskowski MM, Kobras M, Shears SB, Mayr GW. Intracellular localization of human inositol (1,3,4,5,6)-P5 2-kinase. Biochem J. 2007;408:335–345. doi: 10.1042/BJ20070382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nalaskowski MM, Deschermeier C, Fanick W, Mayr GW. The human homologue of yeast ArgRIII protein is an inositol phosphate multikinase with predominantly nuclear localization. Biochem J. 2002;366:549–556. doi: 10.1042/BJ20020327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schell MJ, Irvine RF. Calcium-triggered exit of F-actin and IP3 3-kinase A from dendritic spines is rapid and reversible. Eur J Neurosci. 2006;24:2491–2503. doi: 10.1111/j.1460-9568.2006.05125.x. [DOI] [PubMed] [Google Scholar]
- 22.Yang L, Reece J, Gabriel SE, Shears SB. Apical localization of ITPK1 enhances its ability to be a modifier gene product in a murine tracheal cell model of cystic fibrosis. J Cell Sci. 2006;119:1320–1328. doi: 10.1242/jcs.02836. [DOI] [PubMed] [Google Scholar]
- 23.Otto JC, Kelly P, Chiou ST, York JD. Alterations in an inositol phosphate code through synergistic activation of a G protein and inositol phosphate kinases. Proc Natl Acad Sci USA. 2007;104:15653–15658. doi: 10.1073/pnas.0705729104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pohlmann J, Fleig U. Asp1, a conserved 1/3 inositol polyphosphate kinase, regulates the dimorphic switch in S. pombe. Mol Cell Biol. 2010;30:4535–4547. doi: 10.1128/MCB.00472-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoenig M, Lee RJ, Ferguson DC. A microtiter plate assay for inorganic phosphate. J Biochem Biophys Methods. 1989;19:249–252. doi: 10.1016/0165-022x(89)90031-6. [DOI] [PubMed] [Google Scholar]
- 26.Ye W, Ali N, Bembenek ME, Shears SB, Lafer EM. Inhibition of clathrin assembly by high-affinity binding of specific inositol polyphosphates to the synapse-specific clathrin assembly protein AP-3. J Biol Chem. 1995;270:1564–1568. [PubMed] [Google Scholar]
- 27.Narayan K, Lemmon MA. Determining selectivity of phosphoinositide-binding domains. Methods. 2006;39:122–133. doi: 10.1016/j.ymeth.2006.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gokhale NA, Abraham A, Digman MA, Gratton E, Cho W. Phosphoinositide specificity of and mechanism of lipid domain formation by annexin A2-p11 heterotetramer. J Biol Chem. 2005;280:42831–42840. doi: 10.1074/jbc.M508129200. [DOI] [PubMed] [Google Scholar]
- 29.Balla T, Varnai P. Visualizing cellular phosphoinositide pools with GFP-fused protein-modules. Sci STKE. 2002;2002:L3. doi: 10.1126/stke.2002.125.pl3. [DOI] [PubMed] [Google Scholar]
- 30.Marechal Y, Pesesse X, Jia Y, Pouillon V, Perez-Morga D, Daniel J, Izui S, Cullen PJ, Leo O, Luo HR, et al. Inositol 1,3,4,5-tetrakisphosphate controls proapoptotic Bim gene expression and survival in B cells. Proc Natl Acad Sci USA. 2007;104:13978–13983. doi: 10.1073/pnas.0704312104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caffrey JJ, Safrany ST, Yang X, Shears SB. Discovery of molecular and catalytic diversity among human diphosphoinositol polyphosphate phosphohydrolases: an expanding NUDT family. J Biol Chem. 2000;275:12730–12736. doi: 10.1074/jbc.275.17.12730. [DOI] [PubMed] [Google Scholar]
- 32.Lietzke SE, Bose S, Chawla A, Czech MP, Lambright DG. Structural basis of 3-phosphoinositide recognition by pleckstrin homology domains. Mol Cell. 2000;6:385–394. doi: 10.1016/s1097-2765(00)00038-1. [DOI] [PubMed] [Google Scholar]
- 33.Manna D, Albanese A, Park WS, Cho W. Mechanistic basis of differential cellular responses of phosphatidylinositol 3,4-bisphosphate- and phosphatidylinositol 3,4,5-trisphosphate-binding pleckstrin homology domains. J Biol Chem. 2007;282:32093–32105. doi: 10.1074/jbc.M703517200. [DOI] [PubMed] [Google Scholar]
- 34.Cho J, King JS, Qian X, Harwood AJ, Shears SB. Dephosphorylation of 2,3-bisphosphoglycerate by MIPP expands the regulatory capacity of the Rapoport–Luebering glycolytic shunt. Proc Natl Acad Sci USA. 2008;105:5998–6003. doi: 10.1073/pnas.0710980105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Padmanabhan U, Dollins DE, Fridy PC, York JD, Downes CP. Characterization of a selective inhibitor of inositol hexakisphosphate kinases: use in defining biological roles and metabolic relationships of inositol pyrophosphates. J Biol Chem. 2009;284:10571–10582. doi: 10.1074/jbc.M900752200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Losito O, Szijgyarto Z, Resnick AC, Saiardi A. Inositol pyrophosphates and their unique metabolic complexity: analysis by gel electrophoresis. PloS ONE. 2009;4:e5580. doi: 10.1371/journal.pone.0005580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rao DE, Rao KV, Reddy TP, Reddy VD. Molecular characterization, physicochemical properties, known and potential applications of phytases: an overview. Crit Rev Biotechnol. 2009;29:182–198. doi: 10.1080/07388550902919571. [DOI] [PubMed] [Google Scholar]
- 38.Caffrey JJ, Hidaka K, Matsuda M, Hirata M, Shears SB. The human and rat forms of multiple inositol polyphosphate phosphatase: functional homology with a histidine acid phosphatase up-regulated during endochondral ossification. FEBS Lett. 1999;442:99–104. doi: 10.1016/s0014-5793(98)01636-6. [DOI] [PubMed] [Google Scholar]
- 39.Theibert AB, Estevez VA, Ferris C, Danoff SK, Barrow RK, Prestwich GD, Snyder SH. Inositol 1,3,4,5–tetrakisphosphate and inositol hexakisphosphate receptor proteins: isolation and characterization from brain. Proc Nat Acad Sci USA. 1991;88:3165–3169. doi: 10.1073/pnas.88.8.3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lemmon MA, Ferguson KM, Abrams CS. Pleckstrin homology domains and the cytoskeleton. FEBS Lett. 2002;513:71–76. doi: 10.1016/s0014-5793(01)03243-4. [DOI] [PubMed] [Google Scholar]
- 41.Stephens LR, Jackson TR, Hawkins PT. Agonist-stimulated synthesis of phosphatidylinositol(3,4,5)-trisphosphate: a new intracellular signalling system? Biochim Biophys Acta. 1993;1179:27–75. doi: 10.1016/0167-4889(93)90072-w. [DOI] [PubMed] [Google Scholar]
- 42.He J, Haney RM, Vora M, Verkhusha VV, Stahelin RV, Kutateladze TG. Molecular mechanism of membrane targeting by the GRP-1 PH domain. J Lipid Res. 2008;49:1807–1815. doi: 10.1194/jlr.M800150-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park WS, Heo WD, Whalen JH, O’Rourke NA, Bryan HM, Meyer T, Teruel MN. Comprehensive identification of PIP3-regulated PH domains from C. elegans to H. sapiens by model prediction and live imaging. Mol Cell. 2008;30:381–392. doi: 10.1016/j.molcel.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lemmon MA. Pleckstrin Homology (PH) domains and phosphoinositides. Biochem Soc Symp. 2007;74:81–93. doi: 10.1042/BSS0740081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rider MH, Bertrand L, Vertommen D, Michels PA, Rousseau GG, Hue L. 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase: head-to-head with a bifunctional enzyme that controls glycolysis. Biochem J. 2004;381:561–579. doi: 10.1042/BJ20040752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dumont JE, Dremier S, Pirson I, Maenhaut C. Cross signaling, cell specificity, and physiology. Am J Phys Cell Phys. 2002;283:C2–C28. doi: 10.1152/ajpcell.00581.2001. [DOI] [PubMed] [Google Scholar]
- 47.Illies C, Gromada J, Fiume R, Leibiger B, Yu J, Juhl K, Yang SN, Barma DK, Falck JR, Saiardi A, et al. Inositol pyrophosphates determine exocytic capacity. Science. 2007;318:1299–1302. doi: 10.1126/science.1146824. [DOI] [PubMed] [Google Scholar]
- 48.Barker CJ, Wright J, Hughes PJ, Kirk CJ, Michell RH. Complex changes in cellular inositol phosphate complement accompany transit through the cell cycle. Biochem J. 2004;380:465–473. doi: 10.1042/BJ20031872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Torres J, Domínguez S, Cerdá FM, Obal G, Mederos A, Irvine RF, Díaz A, Kremer C. Solution behaviour of myoinositol hexakisphosphate in the presence of multivalent cationsPrediction of a neutral pentamagnesium species under cytosolic/nuclear conditions. J Inorg Biochem. 2005;99:828–840. doi: 10.1016/j.jinorgbio.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 50.Weiner OD, Neilsen PO, Prestwich GD, Kirschner MW, Cantley LC, Bourne HR. A PtdInsP(3)- and Rho GTPase-mediated positive feedback loop regulates neutrophil polarity. Nat Cell Biol. 2002;4:509–513. doi: 10.1038/ncb811. [DOI] [PMC free article] [PubMed] [Google Scholar]