It is now widely accepted that inflammation and immunity play important roles in the pathogenesis of atherosclerosis and diabetes. Over the past three decades, it has been demonstrated that cells of both the innate and adaptive immune systems contribute to progression of atherosclerosis and modulation of plaque stability. 1 Similarly, recent studies in experimental models of obesity and type II diabetes have shown that not only macrophages, but T cells and B cells, are involved in modulation of adipose depots and development of insulin resistance. 2–5 However, for all that is known regarding important cellular players in atherogenesis, a clear understanding of the mechanisms by which immune cells influence arterial plaque formation is lacking.
Macrophages are considered the predominant cell involved in foam cell formation and atherosclerotic plaque development. Numerous studies have linked macrophage activation in atherosclerosis to Toll-like receptors (TLRs) and have demonstrated that “danger signals” such as oxidized-low density lipoprotein (oxLDL), heat shock proteins, and other products of oxidative stress induce adhesion molecule and chemokine production by cells of the vessel wall as well as direct activation of macrophages in the lesion. For example, TLRs such as TLR-4, TLR-2 and TLR-6 (in concert with the scavenger receptor CD36 and TLR-4), 6–10 are activated by oxLDL and may exacerbate the inflammatory nature of the atherosclerotic plaque.
In addition to macrophages, other immune cells, including natural killer (NK) cells, T cells and a unique group of cells, NKT cells, have been identified in atherosclerotic plaques of both humans and mice and depletion of these cells by either antibodies or genetic approaches have shown that these lymphocytes play important roles in lesion formation. 1 Specifically, NK and CD4+ T helper 1 (Th1) cells, which produce interferon-gamma (IFN-γ), are known to promote atherosclerosis and plaque instability, 11,12 whereas FoxP3+ regulatory T cells that produce IL-10, are protective.13 NKT cells, like their Th1 and NK cell counterparts, are also viewed as pro-atherogenic ,14 although recent studies have challenged this view. 15
Unlike macrophages, which are activated by non-specific signals associated with inflammation or injury/infection, T cells require specific cues in the form of peptide antigens presented by antigen presenting cells such as B cells, macrophages and dendritic cells. While T cell activation promotes inflammation in the atherosclerotic lesion, the exact atherosclerosis-associated antigen recognized by T cells has not been identified. Molecules such as native LDL, oxLDL and bacteria-associated antigens (in the case of infection) have been implicated as potential “neo-antigens”. 16, 17 Identification of the exact antigen might prove impossible as it is likely that several are involved and vary from one individual to the next. Therefore, targeting specific antigens as a means of therapy will likely prove impractical.
Conventional activation of T cells involves at least two signals. The first is initial recognition of antigen in the context of the appropriate major histocompatibility complex. The second signal is the ligation of a co-stimulatory receptor, which, depending on the molecule can result in either enhanced immune responses, as is the case for CD28 and CD40L or decreased immune responses, seen with ligation of CTLA-4, a mechanism thought to turn off T cell responses once inflammation or infection is resolved. Usually, there is a third signal for differentiation and/or proliferation in the form of a cytokine mediator. Unfortunately, although immune players in atherosclerosis have been well identified, specific molecular mechanisms for immune-mediated progression of atherosclerosis remain somewhat elusive.
Because lymphocytes are found in abundance in the plaque, understanding their means of activation and relative contribution to intra-lesion inflammation is of high priority in the atherosclerotic field. In this issue of Circulation, Xia, et. al. introduce a new and important player in lymphocyte activation and atherogenesis.18 This group examined the role of a specific activating molecule, natural killer cell lectin-like receptor subfamily K (NKG2D) in atherosclerosis. NKG2D, also referred to Klrk1, is a membrane-bound receptor expressed on NK cells, NKT cells, γδ T cells and CD8+ αβ T cells, 19 which as discussed above have all been implicated in atherosclerosis. NKG2D has several ligands, including retinoic acid early transcripts 1 (Rae-1), the minor histocompatibility antigen 60 (H60) and the mouse and human UL16-binding protein-like proteins (Mult1 and ULBP, respectively). 19 Similar to ligands for TLRs, the NKG2D ligands can be considered “danger signals” as they are usually expressed at low levels under normal healthy conditions but are highly up-regulated following injury and/or infection. Because of this, Xia et. al. set out to determine whether NKG2D and its ligands are up-regulated in atherosclerotic plaques of mice and humans. Interestingly, one such NKG2D ligand, MIC, was detected at high levels in sera and isolated plaques from patients with Type-2 diabetes. Likewise, using immunohistochemistry, the authors demonstrated that atherosclerotic plaques of apoE-deficient (apoE−/−) mice express high levels of two other NKG2D ligands, Rae-1and H60, while non-atherosclerotic aortic regions do not. Analysis of specific cells using flow cytometry revealed that NKG2D ligands are up-regulated on macrophages, endothelial cells and hepatocytes of mice with experimental atherosclerosis. Although it is not clear what the stimuli are that increase expression of Rae-1 and H60 on cells of apoE−/− mice during atherogenesis, Xie et. al. nicely demonstrate that LDL, oxLDL and advanced glycation end products up-regulate Rae-1 expression on macrophages. 18 These data indicate that products associated with atherosclerosis and diabetes may be responsible for providing signals of cellular stress and damage.
To investigate the causal relationship between NKG2D and its ligands in atherosclerosis, Xie et. al. crossed NKG2D-deficient Klrk1−/− mice with apoE−/− mice and demonstrated that, compared to wild type apoE−/− mice, the Klrk−/−apoE−/− animals have a dramatic 80% reduction in aortic arch atherosclerosis. 18 The authors also studied the role of NKG2D in animals with a combination of type-1 diabetes and hyperlidiemia; both commonly associated with aggressive atherosclerosis. By treating mice with the beta cell toxin streptozotocin (STZ), Xie et. al. demonstrated that the absence of signaling via the NKG2D pathway results in an impressive decrease in atherosclerotic burden in the aortae of diabetic Klrk−/−apoE−/− animals. Similar results were observed in STZ-treated mice injected with a blocking antibody for NKG2D receptor. The mechanism for this protection appeared to be related to overt decreases in inflammation as levels of serum cytokines such as IL-6, IFN-γ and IL-12 were reduced. Surprisingly, levels of the anti-inflammatory cytokine IL-10 were likewise decreased in all three tested conditions. Whether the reduction in IL-10 is a direct result of NKG2D blockade or the indirect result of an overall reduction in inflammation was not apparent. These findings by Xie et. al, have provided us with new insight into the inflammatory process in atherosclerosis (Figure 1). Their work has identified an alternate activation pathway in which injury or oxidative stress associated with atherogenesis might exacerbate detrimental immune responses in the artery wall and the atherosclerotic lesion leading ultimately to plaque instability and rupture. In addition to showing that NKG2D participates in atherosclerosis, Xie et. al. also demonstrate that this receptor/ligand axis might also contribute to diabetes-associated inflammation which could accelerate atherosclerosis. 18 As STZ-induced diabetes leads to direct destruction of beta cells by the drug and not by autoimmune T cells, future experiments to determine whether NKG2D blockage is a viable option for reducing autoimmune-associated islet destruction or improvement of islet transplantation could prove interesting. In addition, it would be even more fascinating if inhibition of NKG2D signaling could prove beneficial in models of metabolic disease and associated exacerbated atherosclerosis.
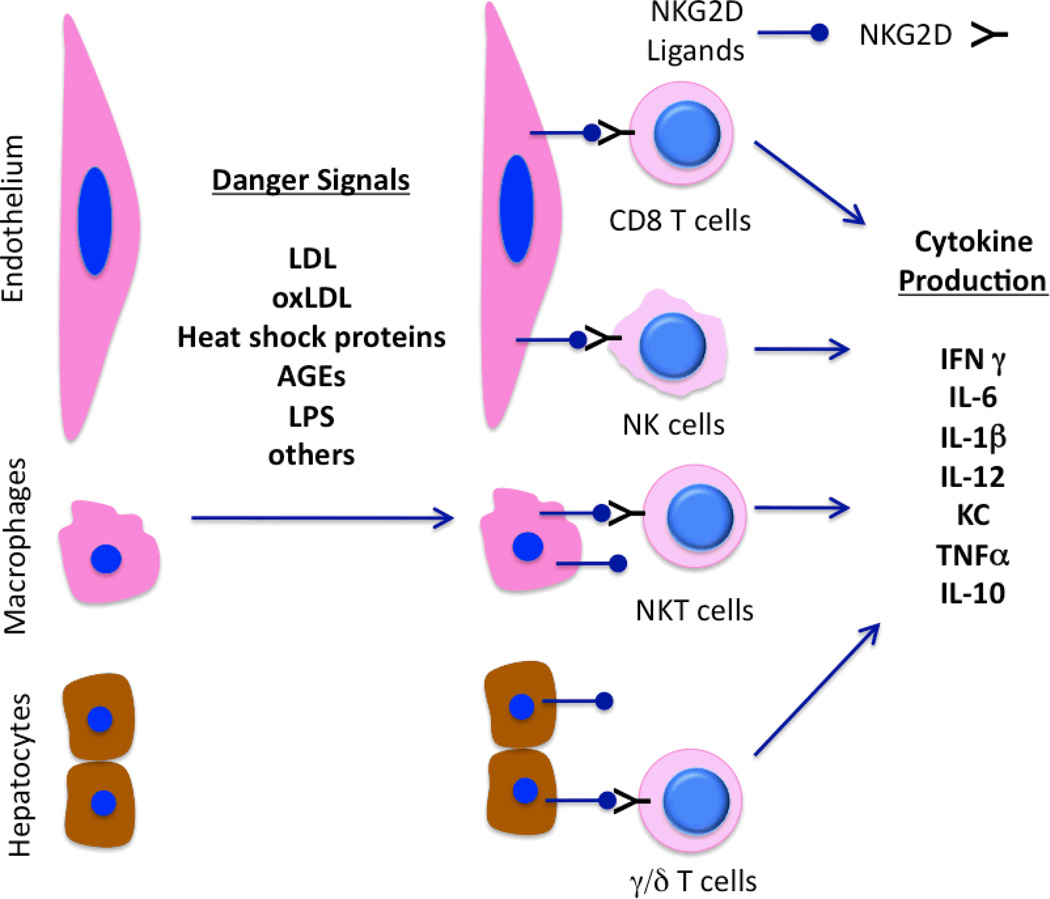
Figure 1. Schematic of NKG2D and ligands in promoting inflammation.
Danger signals such as lipids (LDL, oxLDL), heat shock proteins (HSPs), advanced glycosylation end products (AGEs) and components of infections agents such as lipopolysaccharide (LPS) can upregulate specific ligands of NKG2D on endothelial cells, macrophages and/or hepatocytes. This, in turns, leads to activation of subsets of T cells NKT cells (in concert with TCR and MHC I/II or CD1d, respectively) and NK cells with subsequent production of immuno-modulatory cytokines. It is also possible that cytokines such as IL-1β and IL-6 can be produced by endothelial cells, macrophages and/or hepatocytes in direct response to danger signals, or via interaction with activated lymphocytes.
One area left undetermined by this study is exactly which cells expressing the NKG2D receptor mediate the inflammation in concert with the ligand expressing macrophages, endothelial cells and hepatocytes. While the authors clearly show that various NKG2D ligands are up-regulated in atherosclerotic plaques of humans and apoE−/− mice, it is not as clear which NKG2D-expressing immune cells are completing the signaling axis. Because NKG2D is expressed by NK cells, NKT cells, γδ T cells and CD8+ T cells, any of these could be involved, as illustrated in Figure 1. However, of note, these cells make up a small fraction of the atherosclerotic plaque where, other than macrophages, CD4+ T cells dominate. It is possible that activated NKG2D+ CD4+ T cells in the plaque or other positive lymphocytes in the adventitia or distal tissues, such as the liver, modulate the systemic inflammatory environment and that reduced activation of these cells, where ever they may reside, improves cardiovascular outcomes.
This study emphasizes the concept that atherosclerosis is much more than a disease of lipid dysregulation. Clinically, this is perhaps most evident in patients with autoimmunity who often have severely accelerated atherosclerosis and increased in risk of myocardial infarction in the absence of elevated LDL cholesterol. 20 With the exception of statins, which work by modifying both cholesterol and decreasing inflammation, treatments that specifically target immune activation in a controlled manner have not been identified. The work presented by Xie et. al. move us one step forward to identifying therapeutic targets such as NKG2D, that more precisely modulated immunity and inflammation to ultimately decrease atherosclerosis and other chronic inflammatory diseases.
Acknowledgements
This work was supported by NIH R01HL039006 (DGH), P01HL058000 (DGH), P01HL095070 (DGH) and NIH grant R01HL088364 and R01HL089310 (to ASM).
Literature Cited
- 1.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 2.Mathis D, Shoelson SE. Immunometabolism: An emerging frontier. Nat Rev Immunol. 2011;11:81–83. doi: 10.1038/nri2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S, Mathis D. Lean, but not obese, fat is enriched for a unique population of regulatory t cells that affect metabolic parameters. Nat Med. 2009;15:930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Winer DA, Winer S, Shen L, Wadia PP, Yantha J, Paltser G, Tsui H, Wu P, Davidson MG, Alonso MN, Leong HX, Glassford A, Caimol M, Kenkel JA, Tedder TF, McLaughlin T, Miklos DB, Dosch HM, Engleman EG. B cells promote insulin resistance through modulation of t cells and production of pathogenic igg antibodies. Nat Med. 2011;17:610–617. doi: 10.1038/nm.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Winer S, Paltser G, Chan Y, Tsui H, Engleman E, Winer D, Dosch HM. Obesity predisposes to th17 bias. Eur J Immunol. 2009;39:2629–2635. doi: 10.1002/eji.200838893. [DOI] [PubMed] [Google Scholar]
- 6.Bjorkbacka H, Kunjathoor VV, Moore KJ, Koehn S, Ordija CM, Lee MA, Means T, Halmen K, Luster AD, Golenbock DT, Freeman MW. Reduced atherosclerosis in myd88-null mice links elevated serum cholesterol levels to activation of innate immunity signaling pathways. Nat Med. 2004;10:416–421. doi: 10.1038/nm1008. [DOI] [PubMed] [Google Scholar]
- 7.Gibson FC, 3rd, Hong C, Chou HH, Yumoto H, Chen J, Lien E, Wong J, Genco CA. Innate immune recognition of invasive bacteria accelerates atherosclerosis in apolipoprotein e-deficient mice. Circulation. 2004;109:2801–2806. doi: 10.1161/01.CIR.0000129769.17895.F0. [DOI] [PubMed] [Google Scholar]
- 8.Mullick AE, Tobias PS, Curtiss LK. Modulation of atherosclerosis in mice by toll-like receptor 2. Journal of Clinical Investigation. 2005;115:3149–3156. doi: 10.1172/JCI25482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, Rayner KJ, Boyer L, Zhong R, Frazier WA, Lacy-Hulbert A, El Khoury J, Golenbock DT, Moore KJ. Cd36 ligands promote sterile inflammation through assembly of a toll-like receptor 4 and 6 heterodimer. Nat Immunol. 2010;11:155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hasu M, Thabet M, Tam N, Whitman SC. Specific loss of toll-like receptor 2 on bone marrow derived cells decreases atherosclerosis in ldl receptor null mice. Can J Physiol Pharmacol. 2011;89:737–742. doi: 10.1139/y11-071. [DOI] [PubMed] [Google Scholar]
- 11.Whitman SC, Rateri DL, Szilvassy SJ, Yokoyama W, Daugherty A. Depletion of natural killer cell function decreases atherosclerosis in low-density lipoprotein receptor null mice. Arterioscler Thromb Vasc Biol. 2004;24:1049–1054. doi: 10.1161/01.ATV.0000124923.95545.2c. [DOI] [PubMed] [Google Scholar]
- 12.Whitman SC, Ravisankar P, Daugherty A. Ifn-gamma deficiency exerts gender-specific effects on atherogenesis in apolipoprotein e−/− mice. J Interferon Cytokine Res. 2002;22:661–670. doi: 10.1089/10799900260100141. [DOI] [PubMed] [Google Scholar]
- 13.Ait-Oufella H, Salomon BL, Potteaux S, Robertson AK, Gourdy P, Zoll J, Merval R, Esposito B, Cohen JL, Fisson S, Flavell RA, Hansson GK, Klatzmann D, Tedgui A, Mallat Z. Natural regulatory t cells control the development of atherosclerosis in mice. Nat Med. 2006;12:178–180. doi: 10.1038/nm1343. [DOI] [PubMed] [Google Scholar]
- 14.Tupin E, Nicoletti A, Elhage R, Rudling M, Ljunggren HG, Hansson GK, Berne GP. Cd1d-dependent activation of nkt cells aggravates atherosclerosis. J Exp Med. 2004;199:417–422. doi: 10.1084/jem.20030997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Puijvelde GH, van Wanrooij EJ, Hauer AD, de Vos P, van Berkel TJ, Kuiper J. Effect of natural killer t cell activation on the initiation of atherosclerosis. Thromb Haemost. 2009;102:223–230. doi: 10.1160/TH09-01-0020. [DOI] [PubMed] [Google Scholar]
- 16.Ketelhuth DF, Hansson GK. Cellular immunity, low-density lipoprotein and atherosclerosis: Break of tolerance in the artery wall. Thromb Haemost. 2011;106 doi: 10.1160/TH11-05-0321. [DOI] [PubMed] [Google Scholar]
- 17.Milioti N, Bermudez-Fajardo A, Penichet ML, Oviedo-Orta E. Antigen-induced immunomodulation in the pathogenesis of atherosclerosis. Clin Dev Immunol. 2008;2008:723539. doi: 10.1155/2008/723539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie M, Guerre N, Sukhova GK, Yang K, Miller C, Shi G-P, Raulet DH, Xiong N. Immune activation resulting from nkg2d/ligand interaction promotes atherosclerosis. Circulation. 2011 doi: 10.1161/CIRCULATIONAHA.111.034850. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raulet DH. Roles of the nkg2d immunoreceptor and its ligands. Nat Rev Immunol. 2003;3:781–790. doi: 10.1038/nri1199. [DOI] [PubMed] [Google Scholar]
- 20.Frostegard J. Atherosclerosis in patients with autoimmune disorders. Arterioscler Thromb Vasc Biol. 2005;25:1776–1785. doi: 10.1161/01.ATV.0000174800.78362.ec. [DOI] [PubMed] [Google Scholar]