Abstract
Osteocytes have been hypothesized to be the major mechanosensors in bone. How in situ osteocytes respond to mechanical stimuli is still unclear because of technical difficulties. In vitro studies have shown that osteocytes exhibited unique calcium (Ca2+) oscillations to fluid shear. However, whether this mechanotransduction phenomenon holds for in situ osteocytes embedded within a mineralized bone matrix under dynamic loading remains unknown. Using a novel synchronized loading/imaging technique, we successfully visualized in real time and quantified Ca2+ responses in osteocytes and bone surface cells in situ under controlled dynamic loading on intact mouse tibia. The resultant fluid-induced shear stress on the osteocyte in the lacunocanalicular system (LCS) was also quantified. Osteocytes, but not surface cells, displayed repetitive Ca2+ spikes in response to dynamic loading, with spike frequency and magnitude dependent on load magnitude, tissue strain, and shear stress in the LCS. The Ca2+ oscillations were significantly reduced by endoplasmic reticulum (ER) depletion and P2 purinergic receptor (P2R)/phospholipase C (PLC) inhibition. This study provides direct evidence that osteocytes respond to in situ mechanical loading by Ca2+ oscillations, which are dependent on the P2R/PLC/inositol trisphosphate/ER pathway. This study develops a novel approach in skeletal mechanobiology and also advances our fundamental knowledge of bone mechanotransduction.—Jing, D., Baik, A. D., Lu, X. L., Zhou, B., Lai, X., Wang, L., Luo, E., Guo, X. E. In situ intracellular calcium oscillations in osteocytes in intact mouse long bones under dynamic mechanical loading.
Keywords: lacunocanalicular system, mechanotransduction, fluorescence recovery after photobleaching, shear stress, endoplasmic reticulum, purinergic receptor
Osteocytes are mature bone cells that reside in a fluid-filled mineralized bone matrix and are interconnected through numerous intercellular processes to form an extensive network in the lacunocanalicular system (LCS; 1). Osteocytes can extend their dendritic processes to communicate with osteoblasts and possibly osteoclasts on the bone surface and regulate the matrix remodeling activities of these cells through factors such as receptor activator of nuclear factor-κB ligand (RANKL) and sclerostin (2, 3). The osteocyte network has been hypothesized to be the central mechanosensor that orchestrates bone modeling and remodeling by detecting mechanical stimuli applied to the skeleton (4) through interstitial fluid flow (5). Indeed, perturbations in osteocyte networks have been shown to drastically alter bone remodeling and/or modeling in a mechanical disuse model (6). Therefore, understanding osteocyte mechanotransduction has significant clinical implications in diseases involving dysfunctional bone remodeling, such as osteoporosis. To date, however, how in situ osteocytes detect and transduce mechanical stimuli is still poorly understood.
Many in vitro experiments have demonstrated the capability of osteocytes to respond to various forms of mechanical stimuli, such as fluid flow-induced shear stress and hydrostatic pressure (7–10). However, other types of bone cells, such as osteoblasts and osteoclasts, have also been shown to respond to these physical stimuli (11, 12) and propagate intracellular signaling molecules among neighboring cells (13–15). Thus, a delineated role of osteocytes in bone mechanotransduction remains unclear. Intracellular calcium (Ca2+) is a pivotal and ubiquitous second messenger regulating many downstream cellular activities, and it is also observed to be one of the earliest mechanotransduction events in bone cells (16, 17). Abundant in vitro studies on Ca2+ signaling in osteoblasts and osteocytes subjected to mechanical stimuli have identified two distinct transduction pathways: fast Ca2+ wave propagation via ATP/P2 purinergic receptors (P2Rs), and relatively slow Ca2+ waves via intercellular gap junctions (GJs), such as connexin 43 (13–20). Our group recently discovered that 2-dimensional (2D) in vitro micropatterned osteocyte networks were much more sensitive than osteoblasts to fluid flow stimulation in terms of Ca2+ oscillations (18). Consistent with previous findings (13–15), the ATP-related signaling pathway dominates these uniquely repetitive Ca2+ oscillations. Furthermore, Ca2+ oscillations of micropatterned individual osteocytes were spatiotemporally correlated within a cell network, demonstrating that intercellular communication between neighboring cells is also a fundamental aspect of osteocyte mechanotransduction (4, 19). Thus, osteocyte networks, at least in the 2D in vitro system, demonstrate the capability to detect daily physical mechanical activities and to function as the major mechanical sensors in bone. However, it is unknown whether these intriguing mechanotransduction phenomena hold for in situ osteocyte networks embedded in their native mineralized bone matrix with the natural LCS microenvironment under physiological mechanical loading.
There have been several previous studies using calvarial bone for examining in situ Ca2+ signaling of bone cells (21–23). Ishihara et al. (23) found that both surface osteoblasts and osteocytes in situ exhibited autonomous Ca2+ responses, with the osteoblasts appearing more active. Their study also suggested that inhibition of GJ communication significantly altered autonomous waves in osteocytes but not osteoblasts. In addition, fluid flow on the calvarial surface induced Ca2+ oscillations in both osteoblasts and osteocytes, where GJs were once again more critical in osteocytes. Adachi et al. (21) demonstrated that calvarial bone matrix deformation induced by microneedles resulted in a rapid and prolonged increase of Ca2+ concentration in an osteocyte. Although these studies have demonstrated the importance of Ca2+ signaling in bone in situ, the role of mechanical loading in osteocyte mechanotransduction is not clear in a bone not yet adapted for dynamic mechanical events. In this study, we established a novel ex vivo mechanical loading model using mouse tibia for studying real-time Ca2+ signaling in osteocytes. It is worthwhile noting that this axial tibial loading model has been used extensively in in vivo studies of bone mechanobiology (24–28). Our new synchronized loading/imaging model has the capabilities of applying controlled dynamic loading on intact, healthy long bones, measuring and estimating the shear stress on osteocytes in the LCS by a combination of fluorescence recovery after photobleaching (FRAP) imaging and transport modeling, and, most notably, recording the Ca2+ signaling of in situ osteocyte networks in the LCS when the bone is subjected to physiologically relevant dynamic loading. For the first time, we observed Ca2+ oscillations in osteocytes in the form of repetitive Ca2+ spikes in intact long bone under dynamic loading. In addition, we correlated the magnitudes of applied mechanical loads, tissue strains, LCS fluid flow velocities, and shear stresses with the spatiotemporal parameters of Ca2+ responses in osteocyte networks. We further investigated the underlying biochemical mechanisms responsible for osteocyte Ca2+ signaling by examining the roles of 7 Ca2+ signaling-related pathways.
MATERIALS AND METHODS
Bone sample preparation and Ca2+ dye loading
This study was approved by the Institutional Animal Care and Use Committee at Columbia University. Skeletally mature 3-mo C57BL/6J female mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). After euthanasia by CO2 inhalation, bilateral intact tibiae with periosteum were immediately dissected from the carcasses, with muscles being gently removed under sterile conditions. Samples were incubated in α-minimal essential medium (α-MEM) containing 5% fetal bovine serum (FBS), 5% calf serum, and 1% penicillin/streptomycin for 2–6 h before imaging (29). For fluorescent Ca2+ imaging, tibiae were incubated with 15 μM Fluo-8 AM (ABD Bioquest, Sunnyvale, CA, USA) dissolved in dimethyl sulfoxide (DMSO) with pluronic acid F-127 (20% in DMSO; Life Technologies, Carlsbad, CA, USA) in α-MEM containing 5% charcoal-stripped FBS (CSFBS) for 30 min at 37°C, followed by washing and deesterification for 15 min, before confocal imaging.
Cell viability assessment
The viability of bone cells within the cultured tibiae was assessed by live/dead staining (Live/Dead Viability/Cytotoxicity Kit; Life Technologies) at 2, 12, 24, and 48 h after animal euthanasia (3 tibiae were evaluated at each time point). Tibiae were incubated with 2 μM calcein AM and 4 μM ethidium homodimer-1 dissolved in phosphate-buffered saline (PBS) for 45 min at 37°C. Bone cells inside the tibia were then visualized using a FluoView FV1000 confocal microscope (Olympus, Tokyo, Japan) with 488/516- and 586/617-nm laser excitation/emission, respectively. Osteocyte viability and function were further confirmed by observing the release of osteocyte Ca2+ spikes in response to the exogenous ATP stimulation. Tibiae (n=9) were incubated with 15 μM Fluo-8 AM for 30 min at 37°C, followed by deesterification for 15 min, and then were imaged using the confocal microscope. ATP solution (Sigma-Aldrich, St. Louis, MO, USA) at 1 mM in α-MEM containing 5% CSFBS was added to the sample. In addition, ionomycin, a potent and selective Ca2+ ionophore agent (Life Technologies) at 10 or 20 μM dissolved in DMSO and in α-MEM containing 5% CSFBS, was also added to the samples as positive controls. Only viable cells are expected to show intracellular Ca2+ responses (30).
Mechanical loading system
A custom-designed system was built for in situ osteocyte Ca2+ and FRAP imaging of murine tibiae (Fig. 1A). Axial loading of the tibiae was applied using a piezoelectric linear actuator (M-227.10; Physik Instrumente, Karlsruhe, Germany). Loading force was measured by a 5-lb load cell (model 31; Honeywell, Columbus, OH, USA). A linear bearing was mounted along the loading axis to constrain the lateral movement of the sample holder during mechanical loading. Actual deformation applied on the tibia was measured using a capacitive displacement transducer (CapaNCDT620; Micro-Epsilon, Raleigh, NC, USA). The force and displacement data were captured using a 16-bit data acquisition (DAQ) card (NI USB-6210) and LabView software (National Instruments, Austin, TX, USA).
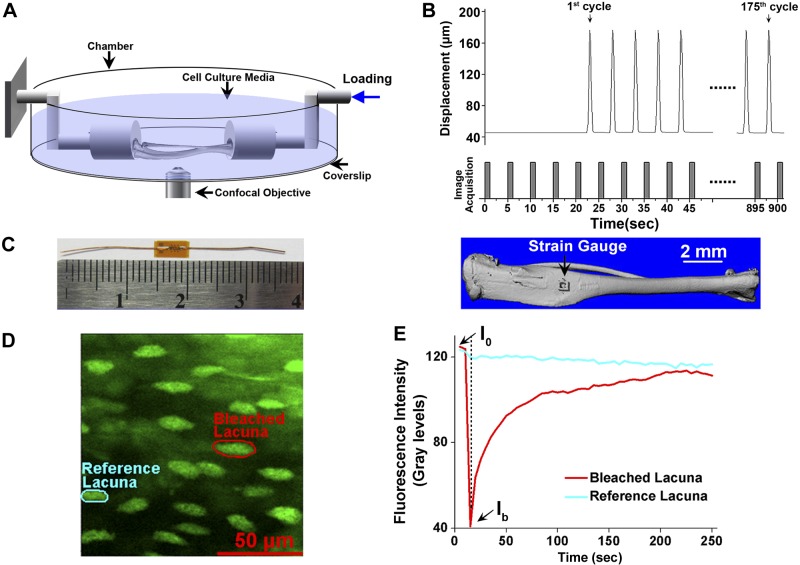
Figure 1.
Ex vivo mouse long bone mechanical loading and Ca2+ and FRAP imaging setups and protocols. A) Schematic representation of the mechanical loading system used for in situ Ca2+ imaging and FRAP experiments. Axial, cyclic compressive loading was applied on the distal end of the tibia via a piezoelectric linear actuator to generate compression and bending. B) Displacement of the linear actuator produced peak load magnitudes of 4, 6, or 8 N and was synchronized with confocal image acquisition to minimize movement artifacts. Compressive cyclic loads were applied for 175 cycles over 15 min. C) Strain gauges were placed on the anteromedial surface where the Ca2+ imaging and FRAP experiments were performed to quantify the load-induced strains. D) Representative fluorescent image showing the selected photobleached and reference lacunae for the FRAP experiment. E) Time course of the fluorescence recovery in the photobleached lacuna. Lacunae were photobleached to ∼30−50% of original intensity.
Synchronized tibial mechanical loading and confocal imaging
Tibiae loaded with a Ca2+ indicator were mounted in the loading apparatus, and the anteromedial surface that experienced significant tensile strains under compressive axial loading on the whole bone was imaged using the confocal microscope with either ×10 or ×40 objectives and 488-nm laser excitation. Because Fluo-8 AM readily penetrated into live cells, the cells lining the bone surface and osteocytes in the matrix could be easily identified by adjusting the focus depth. Surface cells were typically located at 10 μm below a bright periosteum staining, and osteocytes were identified 30–40 μm below the periosteum. The periosteum on the bone surface was able to act as a barrier against the external fluid movement and thus minimize the potential shear stress generated by the slight medium movement on surface cells. Confocal time-lapse images were synchronized with a rest-inserted mechanical loading protocol to eliminate in-plane and out-of-focus motion artifacts as reported in our previous study (Fig. 1B and ref. 31). A preload of 2 N followed by 175 cycles of 6-, 8-, or 10-N peak compressive load was applied on tibiae (thus resulting in 4-, 6-, and 8-N loading magnitudes). A dwell time of 4 s was applied between each cycle. Confocal images were recorded at 1.1 s/frame during the dwell time after each loading cycle for a total of 180 frames (512-×512-pixel images) including 5 frames for baseline recording before loading. The total imaging and loading period was 900 s. Motion artifacts were further removed using the StackReg image registration algorithm in ImageJ (U.S. National Institutes of Health; Bethesda, MD, USA; ref. 32). Cell bodies were manually traced using MetaMorph 7.0 (Molecular Devices, Downingtown, PA, USA), and the average intensity of the cell body was extracted as a function of time. The average pixel intensity of each individual cell was normalized by its corresponding baseline, and a spike was defined as a transient increase to 1.5 times the baseline intensity of each cell (22, 23). The percentage of responsive cells (number of responsive cells divided by the total cells in the field of view), average number of Ca2+ spikes (excluding nonresponsive cells), magnitude of Ca2+ spikes, and time to the first spike were quantified (18, 33).
Strain measurement
To quantify load-induced strain on our region of interest (the anteromedial surface), a separate set of nine 3-mo female mouse tibiae were used for mechanical testing. The tensile strain was measured using commercial strain gauges with 0.38-mm grid length and 0.51-mm grid width (EA-06-015DJ-120; Vishay Measurements Inc., Wendell, NC, USA). The strain gauge was glued on the tibia anteromedial surface using cyanoacrylate adhesive (M-Bond 200 adhesive; Vishay Measurements), which was consistent with the Ca2+ or FRAP imaging region (Fig. 1C). During mechanical testing, the tibiae were immersed in PBS. A 0.5-N preload was applied to secure the sample, and the linear actuator moved at 10 μm/s to compress the sample until the load reached 11 N. The strain gauge output was connected to a signal conditioning amplifier (Vishay 2100; Vishay Measurements), and strain values were simultaneously recorded by the National Instruments DAQ board.
FRAP-based measurements of LCS fluid flow and shear stress
A separate set of tibiae (n=14) were incubated in 0.2 mg/ml sodium fluorescein (376 Da) in α-MEM for 1.5 h at 37°C before imaging. Paired FRAP was performed on randomly selected lacunae ∼40 μm below the periosteum of our region of interest (Fig. 1D) when bone was mechanically loaded or statically held (31). In brief, the selected lacuna was photobleached using a high-intensity 488-nm laser for ∼3 s, reducing its fluorescence intensity to 30–50% of the original level, followed by application of 40 loading cycles with a 4-s imaging period inserted between the adjacent cycles. From the raw image data sets, temporal fluorescence profiles that showed a typical exponential recovery in the photobleached lacuna and a slight autofading because of repeated imaging in far away reference lacuna were obtained (Fig. 1E). After correction of the autofading, the transport rate k of the tracer (defined as the reciprocal of the characteristic time constant of the exponential recovery) and the transport enhancement k/k0 (defined as the ratio of tracer transport rate under loading condition k over that under static condition k0) were quantified. By simulating the diffusion-convection of tracers in an anatomically accurate LCS model, the canalicular fluid velocity that matched the measured transport enhancement for each loading condition was obtained (31). The corresponding shear stress was calculated from the velocity profile inside the annular canalicular fluid channel using the Brinkman equation (34) and scaled to the lacunar space surrounding the cell body (for details, see Supplemental Material).
Ca2+ signaling pathway studies
All 7 of the following biochemical pathways (detailed below) have been shown in our previous studies to be critical for Ca2+ responses in bone cells (18, 20, 33). Five mice (10 tibiae) were used for each specific pathway group. Left and right tibiae of each mouse were paired and separated into control and inhibitor-treated groups. A cyclic load of 6-N magnitude was applied for all groups. Tibiae were preincubated with the pathway inhibitor for 1 h at 37°C before Ca2+ dye incubation, and the inhibitor was present in the medium throughout the imaging experiment. The inhibitors for the 7 pathways are specified as follows: 150 μM pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic acid (PPADS), a nonselective P2R blocker; 1 mM neomycin, a phospholipase C (PLC) inhibitor; 50 μM GdCl3, an inhibitor of stretch-activated Ca2+ channels (SACCs); 20 μM NNC 55–0396, a selective inhibitor of T-type voltage-gated Ca2+ channels (VGCCs); 75 μM 18α-glycyrrhetinic acid (18α-GA; 0.1% DMSO), a reversible intercellular GJ blocker; 1 μM thapsigargin (TG; 0.1% DMSO), an inhibitor of the Ca2+-ATPase pump of the endoplasmic reticulum (ER); and 10 μM amlodipine (0.1% DMSO), an L-type VGCC antagonist. Because 18α-GA, TG, and amlodipine were dissolved in DMSO, the contralateral tibiae were incubated in 0.1% DMSO as a vehicle control group. All antagonists were obtained from Sigma-Aldrich. The concentrations of all inhibitors were optimized for bone cells and other type of cells by previous investigations (18, 20, 33, 35–40).
Data analysis
All data are presented as means ± sd. One-way analysis of variance (ANOVA) with Bonferroni's post hoc analysis was performed to compare the Ca2+ spike number, magnitude of spike intensity, time to reach the first spike and time between the first and second spikes between surface cells and osteocytes across the mechanical loading magnitudes. To determine the correlation between a spatiotemporal parameter and the load level, a linear regression analysis was performed. One-way ANOVA with Bonferroni's post hoc analysis was used to determine statistical differences between the Ca2+ spike spatiotemporal parameters of 7 pathway groups. PPADS-, neomycin-, GdCl3-, and NNC 55–0396-treated groups were compared with the untreated groups (the contralateral tibiae). 18α-GA-, TG-, and amlodipine-treated groups were compared with the vehicle control (DMSO-treated contralateral tibiae). The significance level was set at 0.05.
RESULTS
Validation of the ex vivo Ca2+ imaging model
Bone surface cells and osteocytes were imaged at a focal plane ∼10 and ∼40 μm below the periosteal surface, respectively (Fig. 2A, C), under a confocal microscope. Differential interference contrast (DIC) images showed the mineral-facing bone matrix with many cavity-like lacunae in the osteocyte layer but not in the bone surface cell layer (Fig. 2B, D). To validate the present ex vivo mouse tibia culture technique, live/dead staining was used to evaluate the cell viability at different time points of tissue culture (2, 12, 24, and 48 h) after tibia dissection (Fig. 2E–H). Our results demonstrate that almost all bone cells were labeled with calcein AM (green color) rather than ethidium homodimer-1 (red color), indicating that the bone cells maintained their viability for ≥12 h after animal euthanasia. The viability and function of these osteocytes cultured within 12 h after animal euthanasia were further confirmed by the release of robust Ca2+ spikes in response to exogenous ATP stimulation (Supplemental Movie S1). In addition, ionomycin, at either 10 or 20 μM, triggered robust and prolonged in situ osteocyte Ca2+ increases in intact mouse tibiae (data not shown).
Figure 2.
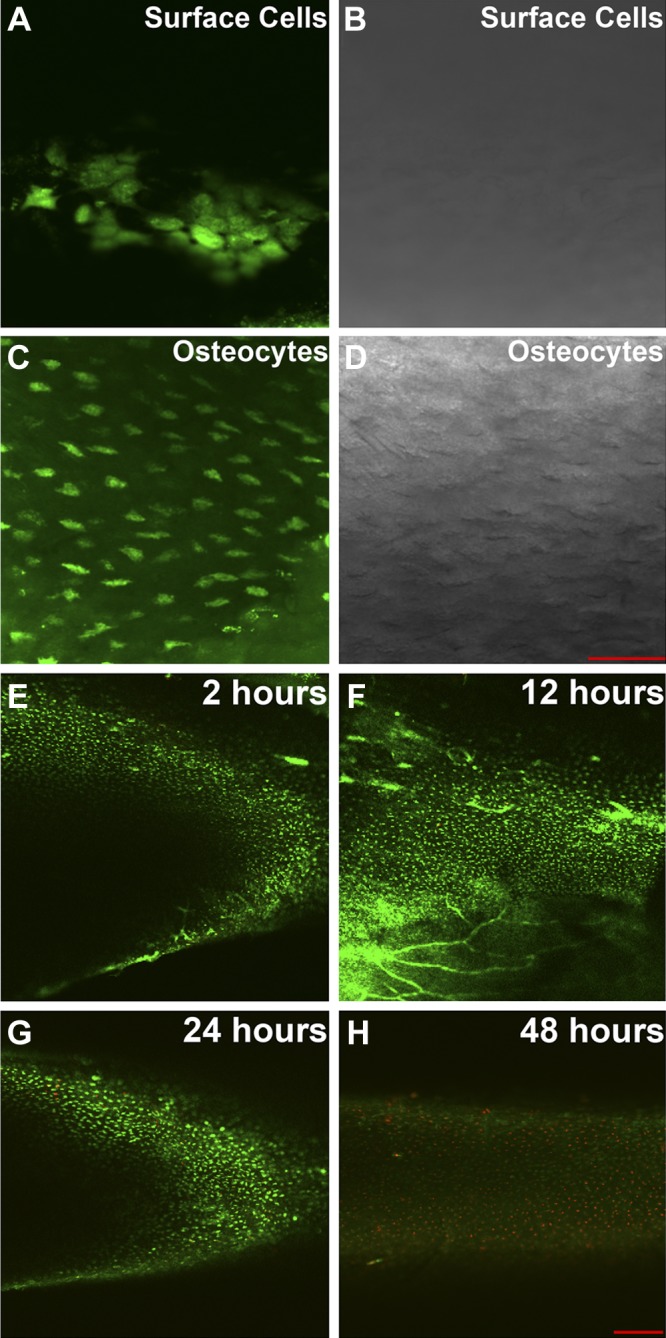
Confocal images of surface cells and osteocytes stained with Fluo-8 AM Ca2+ indicator and validation of the ex vivo intact tibia culture model by live/dead staining in intact mouse tibiae. A, B) Fluorescent (A) and DIC (B) images of surface cells ∼10 μm below periosteal surface. C, D) Fluorescent (C) and DIC (D) images of embedded osteocytes ∼40 μm below periosteal surface. Lacunae surrounding osteocytes were visible in the DIC image. Scale bar = 50 μm. E–H) Representative live/dead staining images for cultured intact mouse tibia samples at 2 (E), 12 (F), 24 (G), and 48 h (H) after animal euthanasia and sample dissection. Live cells were labeled with calcein AM (green dots in the image) and dead cells were labeled with ethidium homodimer-1 (red dots in the image). Scale bar = 200 μm.
Quantification of mechanical loading-induced tissue strain, LCS fluid flow, and shear stress in the mouse tibia
Axial compressive loading was applied on the distal end of the tibia to generate tensile strain on the anteromedial bone surface. The resultant strains of 500–2500 με were measured using strain gauges at 1- to 11-N axial loads (n=9 tibiae; Fig. 3A, B). Significantly linear positive correlation existed between the strain and force values over the tested loading range [strain (με) = 193.186 × force (N); Fig. 3B]. The average peak tensile strain in the 4-, 6-, and 8-N loading groups was 773, 1159, and 1546 με, respectively. These strain values applied on the mouse tibia fell in the range of the anabolic strain in human long bone in vivo (41).
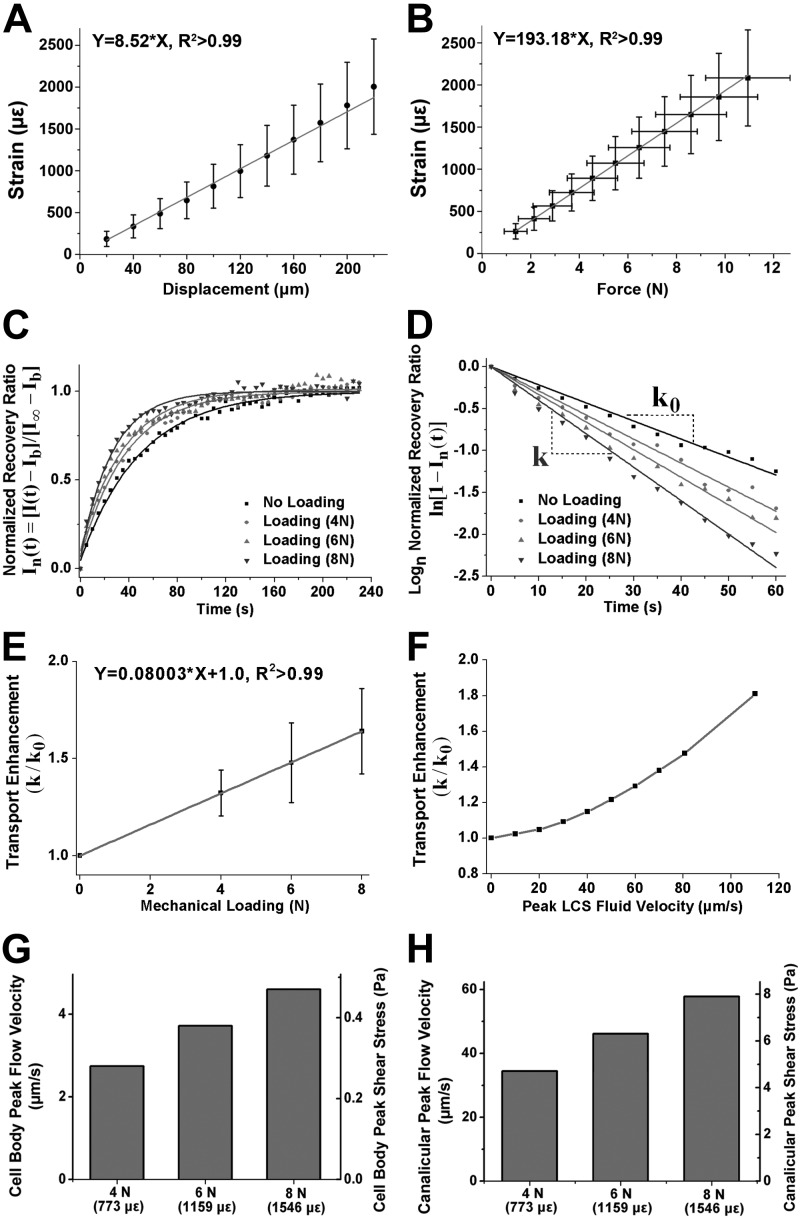
Figure 3.
Measurement of load-induced tensile strain on the mouse tibial surface and FRAP-based quantification of load-induced fluid flow velocity and shear stress in the LCS. A, B) Strain-displacement (A) and strain-force relationship (B; n=9) measured by the strain gauge under compressive axial loading in the Ca2+ or FRAP imaging region. Strain-force data demonstrated a linear relationship over a 1- to 11-N loading range [Strain (με) = 193.186 × force (N), R2 > 0.99]. C) Representative plots of the normalized fluorescence recovery ratio of a lacuna sequentially subjected to photobleaching under 0-N static loading and 4-, 6-, and 8-N cyclic mechanical loading. D) Log-transformed plot of the recovery ratio with the slope representing the characteristic transport rate, k. E) Linear regression for the average transport enhancement (k/k0) within 20 tested lacunae at varying loading conditions (4-, 6-, or 8-N peak load magnitude). F) Overall power relationship between fluid velocity and transport enhancement (k/k0) using the 3-compartment LCS transport model. G, H) Peak LCS fluid flow velocity and shear stress in the cell body (G) and canaliculi (H) calculated from a 3-compartment LCS model at the loading magnitudes tested.
FRAP-based quantification of load-induced fluid flow velocity and shear stress in the LCS is shown in Fig. 3C–H. Mechanical loading increased the fluorescence recovery (Fig. 3C) and enhanced the characteristic transport rate k (i.e., the slope of the fitting line; Fig. 3D). The solute transport enhancement (relative to diffusion, k/k0) displayed a positive linear correlation with the load magnitude (Fig. 3E). Using a 3-compartment LCS transport model combined with the measured LCS anatomic parameters listed in Supplemental Table S1 (31, 42), we established a power relationship between load-induced transport enhancement and fluid velocity in the bone LCS of the tibiae studied (Fig. 3F). The calculated peak fluid flow velocity in the bone LCS (detailed in the Supplemental Material) was 34.4 μm/s at 4-N, 46.1 μm/s at 6-N, and 57.8 μm/s at 8-N mechanical loading, which corresponded to 4.7-, 6.3-, and 7.9-Pa fluid shear stress on the osteocyte processes in the canaliculi, respectively (Fig. 3G). The peak fluid flow around the cell body was calculated as 2.7 μm/s at 4-N, 3.7 μm/s at 6-N, and 4.6 μm/s at 8-N loading levels, which corresponded to 0.28-, 0.38-, and 0.47-Pa fluid shear stress, respectively (Fig. 3H). These results demonstrate that the fluid flow within the LCS was positively correlated with loading magnitude under the loading conditions tested.
In situ osteocytes displayed fewer autonomous Ca2+ responses than surface cells
Time-lapse fluorescent images of surface cells and osteocytes were captured under static (0-N load magnitude) conditions for 15 min to record the autonomous Ca2+ responses in situ (Fig. 4A, B and Supplemental Movie S2). The total numbers of bone samples and analyzed cells in each experimental group are shown in Table 1. As shown in Fig. 4C, 13.0% of bone surface cells, but only 1.3% of osteocytes, displayed autonomous Ca2+ responses. Responsive surface cells and osteocytes had an average of 1.4 ± 0.8 and 1.3 ± 0.5 spikes during the 15-min imaging period, respectively (Fig. 4D). Our findings reveal that osteocytes have relatively quiescent autonomous Ca2+ activities in mouse long bone.
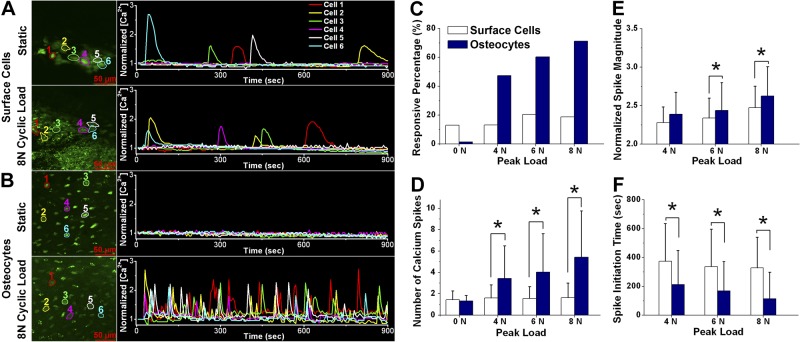
Figure 4.
Autonomous and mechanical loading-induced Ca2+ responses in bone surface cells and osteocytes of intact murine tibiae. A, B) Representative imaging fields (left panels) and Ca2+ traces (right panels) of bone surface cells (A) or osteocytes (B) under either static or cyclic mechanical loading with an 8-N peak load magnitude. C) Percentage of responsive cells (had ≥1 Ca2+ spike) of total counted cells in the field of view. D) Average number of Ca2+ spikes (excluding nonresponsive cells) over the 15-min experimental period. E, F) Peak magnitude normalized with the baseline value (E) and the time between initiation of mechanical loading and the first observed Ca2+ spike (F). Data are means ± sd. *P < 0.05.
Table 1.
Numbers of bone samples and cells (responsive cells together with nonresponsive cells) in different experimental groups for comparison studies of Ca2+ signaling between osteocytes and surface cells
| Cell type | Mechanical loading (N) |
|||
|---|---|---|---|---|
| 0 | 4 | 6 | 8 | |
| Osteocytes | ||||
| Samples | 4 | 4 | 4 | 4 |
| Cells | 479 | 448 | 439 | 449 |
| Surface cells | ||||
| Samples | 6 | 6 | 6 | 6 |
| Cells | 292 | 297 | 292 | 294 |
In situ osteocytes displayed unique Ca2+ oscillations under mechanical loading
Representative mechanical loading-induced Ca2+ responses in bone surface cells and osteocytes are shown in Fig. 4A, B and Supplemental Movie S2. Bone surface cells and osteocytes displayed dramatically different characteristics in Ca2+ signaling under mechanical loading. A small percentage of surface cells (13.1% at 4 N, 20.5% at 6 N, and 18.7% at 8 N) released 1 or 2 Ca2+ spikes, with slightly higher values under 6- and 8-N conditions. In contrast, osteocytes responded to mechanical loading with Ca2+ signaling in the form of multiple Ca2+ spikes. The percentage of responsive cells was significantly higher than that of bone surface cells over the entire range of loading magnitudes (Fig. 4C). The responsive percentage of osteocytes was linearly proportional to the magnitude of the compressive load applied on the tibiae (47.3% at 4 N, 60.4% at 6 N, and 71.3% at 8 N), whereas bone surface cells showed no consistent trend under increased load. Furthermore, a positive correlation was observed between the number of Ca2+ spikes and loading magnitude in osteocytes but not in surface cells (Fig. 4D). Osteocytes displayed 3.4 ± 3.0 spikes at 4 N, 4.0 ± 3.6 spikes at 6 N, and 5.4 ± 4.3 spikes at 8 N, which were significantly higher than those in the surface cells at all loading levels (P<0.05). Osteocytes also had Ca2+ spikes with a significantly higher magnitude (P<0.05) and took less time to initiate the first Ca2+ spike than surface cells, and both parameters for osteocytes were dependent on the loading magnitude (Fig. 4E, F). In summary, these results demonstrate that embedded osteocytes display unique and robust Ca2+ oscillations that are dependent on the mechanical loading magnitude applied to the long bone.
ER Ca2+ store and ATP-related signaling pathways contribute to mechanical loading-induced Ca2+ oscillations in osteocytes
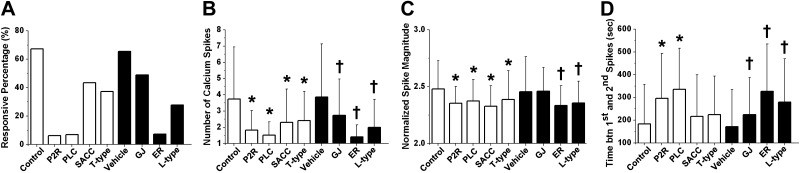
The numbers of tested tibiae and analyzed cells are shown in Table 2. Inhibitions of P2R and PLC and depletion of the ER Ca2+ store significantly reduced the percentage of responsive osteocytes (Fig. 5A). The responsive percentage was reduced from 67.3% in control conditions to 6.3% in the P2R-inhibited group and to 7.0% in the PLC-inhibited group and from 65.5% under vehicle control conditions to 7.4% in the ER Ca2+ store-inhibited group. Thus, these 3 pathways played a critical role in the initiation of Ca2+ responses under loading. Disruption of these pathways also reduced the number of Ca2+ spikes observed over the 15-min loading period (P<0.05; Fig. 5B). The average number of Ca2+ spikes was 1.8 ± 1.2 in the P2R-inhibited group, 1.5 ± 0.8 in the PLC-inhibited group, and 1.4 ± 0.7 in the ER Ca2+ store-depleted group, all showing a greater than 50% decrease compared with those in the corresponding control groups. Significant inhibition of Ca2+ responses was also observed after the SACCs, T-type VGCCs, GJs, and L-type VGCCs were inhibited. All pathway inhibitions, except GJ inhibition, displayed modest yet significant decreases in Ca2+ spike magnitude (P<0.05; Fig. 5C) and resulted in increases in the time interval between the first and second spikes (Fig. 5D). These results demonstrate that both extracellular and intracellular Ca2+ sources, including Ca2+-induced Ca2+ release mechanisms mediated through inositol trisphosphate (IP3), are critical for the initiation and oscillations of Ca2+ spikes in osteocytes when bone is subjected to cyclic loading.
Table 2.
Numbers of bone samples and cells (responsive cells together with nonresponsive cells) in different experimental groups for Ca2+ signaling studies
| Treatment | Inhibition group |
||||||
|---|---|---|---|---|---|---|---|
| PPADS | Neomycin | GdCl3 | NNC 5-0396 | 18α-GA | TG | Amlodipine | |
| Inhibition | |||||||
| Samples | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Cells | 749 | 761 | 498 | 659 | 618 | 706 | 697 |
| Untreated/vehicle | |||||||
| Samples | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Cells | 682 | 675 | 616 | 644 | 647 | 807 | 648 |
Figure 5.
Investigation of the roles of various Ca2+ signaling pathways in 6-N mechanical loading-induced Ca2+ oscillations. Extracellular ATP P2X/P2Y receptors (P2R), PLC, SACCs, T-type VGCCs (T-type), L-type VGCCs (L-type), GJs, and ER Ca2+ store (ER) have all been implicated in osteocyte Ca2+ signaling. These pathways were inhibited using small molecule inhibitors. A DMSO vehicle control was used for statistical comparisons of GJ, ER, and L-type groups. Percentage of responsive cells (A), average number of Ca2+ spikes (excluding nonresponsive cells; B), normalized spike magnitude (C), and time between first and second spikes (D) over the 15-min experimental period. *P < 0.05 vs. corresponding control group; †P < 0.05 vs. vehicle control group.
DISCUSSION
Osteocytes have long been conjectured to be the major mechanosensors in bone because of their unique positioning in the bone matrix, enabling them to directly experience mechanical loads and respond biochemically to the changes in the mechanical microenvironment through their expansive LCS network connections (43). However, how osteocytes respond and transduce the mechanical signals in situ in the LCS is still poorly understood. Several previous studies have investigated osteocyte mechanotransduction in calvarial bone fragments using microneedle displacement (21) or fluid flow over the fragment surface (22). However, to date, critical questions regarding the anatomic site and the usage of physiologically relevant mechanical loading for Ca2+ signaling of osteocytes in situ remain unanswered. Understanding the characteristics of Ca2+ signaling of bone cells in more clinically relevant load-bearing sites, e.g., tibia and femur, might be more helpful for deciphering the mechanisms of skeletal mechanotransduction. Indeed, osteocytes in long bones display numerous morphological and network differences compared with those in calvarial osteocytes, which possibly involve distinct mechanotransduction and mechanoadaptation mechanisms (44, 45). Furthermore, reproducing physiologically relevant mechanical loading on in situ osteocytes, e.g., fluid flow-induced shear stresses within the LCS resulting from mineralized matrix deformations, is impossible using currently available in vitro or calvarial ex vivo models. The mineralized bone matrix and dynamic nature of physiological loading present significant technical challenges for observing and quantifying Ca2+ signaling in osteocytes in intact bones under mechanical loading. In this study, we developed a synchronized loading/imaging approach for investigating in situ osteocyte Ca2+ mechanotransduction in long bone, which represents an important advance in organ-level cell signaling studies within dynamically deformed tissues.
In the present study, we observed in situ Ca2+ signaling with unique multiple Ca2+ spikes in osteocytes responding to dynamic mechanical loading in long bones. In contrast, bone surface cells showed negligible Ca2+ responses in response to mechanical loading. Our findings demonstrate, for the first time, that the osteocyte in the LCS, previously regarded as an inert space occupier, has the unique capability to sense and respond to mechanical loading in bone in the form of robust Ca2+ oscillations. Furthermore, we discovered a dose-dependent relationship between the applied loading magnitude on mouse long bone and the spatiotemporal parameters of osteocyte Ca2+ spikes. Many previous studies have shown that the anabolic and catabolic responses in bone were dependent on the loading profiles, including the magnitude, frequency, and pattern (46–48). Our study shows that osteocytes not only are sensitive to mechanical loading but also can modulate their immediate biochemical responses according to the loading magnitude, which may presumably regulate downstream bone modeling and/or remodeling activities. These findings provide further evidence to support the hypothesis that osteocytes might act as an orchestrator of the bone modeling and remodeling processes.
The exact nature of the physical signals that trigger Ca2+ signaling in osteocytes remains to be determined. When bone is subjected to mechanical loading, multiple physical signals are induced around the osteocytes, which include direct substrate and cell deformation, hydrostatic pressure, interstitial fluid flow in the LCS, and dynamic electrical fields (43, 49, 50). The LCS fluid flow-induced shear stress on the cell membrane of the osteocyte has been posited as a major candidate to induce osteocyte mechanotransduction (29, 34). We have previously shown that shear stresses as low as 0.5 Pa on the cell body were able to induce >95% of in vitro osteocytes to respond with Ca2+ spikes (18). The present study demonstrates that physiological loading induced significant LSC fluid flow and shear stress, with the fluid shear positively correlated with the load levels. Moreover, we showed a linear positive correlation between osteocyte Ca2+ oscillations and LCS fluid flow. Thus, taken together with our previous in vitro investigations, the present study demonstrates that, within the existence of many potential physical signals, the LCS fluid flow alone has sufficient capacity to induce the observed robust osteocyte Ca2+ oscillations. Furthermore, our modeling calculations predicted much higher shear stresses on the cell dendrites than on the cell body. It has been shown that osteocyte dendritic processes displayed a strain amplification mechanism and possess higher mechanosensitivity than the cell body (21, 51, 52). Therefore, osteocytes in situ may initiate Ca2+ oscillations from cell processes and result in the subsequent Ca2+ signaling in the cell body (52, 53). Together, our findings suggest that load-induced fluid flow may be an important and efficient means of signal transduction in the LCS.
In the present study, reduced osteocyte Ca2+ responses were observed when P2R, PLC, or the ER Ca2+ store was blocked. We conclude that the ATP-related P2R-PLC-IP3-ER pathway plays a major role in load-induced osteocyte Ca2+ oscillations (54). Our previous in vitro studies also demonstrated that extracellular ATP diffusion played a paracrine and/or autocrine role in mediating both the intercellular Ca2+ wave propagation and intracellular Ca2+ oscillations in bone cells (18, 20). Extracellular ATP can be released by osteocytes by mechanically induced Ca2+ influx (55) and readily transported to neighboring osteocytes through loading-induced convection and diffusion through the LCS (29, 31). Numerous in vivo studies have shown the significance of both P2X and P2Y receptors in maintaining bone quality and bone mechanosensitivity (56, 57). In the present study, we showed similarly prominent reductive effects on osteocyte Ca2+ spikes when P2R, PLC, and the ER Ca2+ store were blocked. Furthermore, our findings revealed moderate roles of GJs, VGCCs, and SACCs in modulating mechanically induced osteocyte Ca2+ signaling in long bone. Findings from Ishihara et al. (23) revealed that osteocytes specifically modulated autonomous or bone surface fluid-induced Ca2+ signaling via GJs in calvarial bones. However, dramatically different developmental origins, osteocyte shapes, and LCS structures between long bones and calvarial bones might result in distinct Ca2+ responding mechanisms (44, 45). Moreover, osteocytes in the surface fluid shearing model might receive minimal direct mechanical stimulation, whereas osteocytes in our current model were subjected to direct LCS fluid shear stress. Therefore, this finding suggests that intercellular communication via GJs may be a critical pathway between bone surface cells and osteocytes in the bone matrix. It is also of interest that calvariae are not considered to be a significant weight-bearing site, whereas tibiae are primarily load-bearing bones. The observed difference between tibial and calvarial responses under mechanical loading may underlie different mechanosensing mechanisms and/or various degrees of mechanosensitivity. Genetic knockout studies have demonstrated a complex role of GJs in bone mechanotransduction, whereas enhanced bone formation in response to mechanical loading was observed in connexin 43-deficient mice (58, 59). The current ex vivo model may not provide complete answers to these complex phenomena, but it may offer an interesting real-time quantification technique in those knockout mice for future studies. Notably, Thi et al. (60) suggested that pannexin might be more important in ATP release than connexin 43 hemichannels. This is consistent with our current finding that GJ inhibition is not as dramatic as direct inhibition of the ATP pathway including either P2R or PLC. Taken together, the results of our study reveal that extracellular ATP is critical for initiating the Ca2+ activities of in situ osteocytes in long bone, and Ca2+ oscillations in osteocytes rely highly on the IP3-induced Ca2+ release from the ER. It should be noted that the exact mechanism underlying the distinct Ca2+ responses between surface bone cells and osteocytes is not known, although it is consistent with our previous in vitro findings (18, 19). We speculate that the keys lie in the ATP-related P2R-PLC-IP3-ER signaling pathway and ER refilling dynamics between surface cells and osteocytes.
Osteocytes have been implicated in physiological processes as disparate as bone homeostasis and kidney phosphate metabolism (1). Our discovery of mechanically induced osteocyte Ca2+ responses with unique multiple oscillations in long bone provide direct evidence that the osteocyte acts as a major mechanosensor in bone. The positive correlation between Ca2+ oscillations and LCS fluid shear supports, for the first time, the essential role of load-induced LCS fluid flow in osteocyte mechanotransduction and also suggests that the osteocyte network is capable of detecting and differentiating various loads. We also demonstrated that the ATP-related P2R-PLC-IP3-ER signaling pathway is a critical mechanism in osteocytic Ca2+ oscillations. Understanding the role of Ca2+ oscillations and associated intercellular communications within osteocyte networks may provide a clearer mechanism of how bone tissue can integrate habitual mechanical loads into varying bone modeling and remodeling activities seen in physiological conditions and pathological states such as osteoporosis and disuse bone loss.
Supplementary Material
Acknowledgments
The authors thank Keith Yeager, Bin Wang, and Wen Li for their technical help in the experiments; Genevieve Brown and Andrea Morrell for their help in drafting the manuscript; and Andrea Morrell for her help in performing the ionomycin experiments.
This work was supported by the U.S. National Institutes of Health (grants R21 AR052417, R01 AR052461, and RC1 AR058453 to X.E.G. and R01 AR054385 to L.W.).
D.J. thanks the China Scholarship Council for its partial financial support. The authors declare no conflicts of interest.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- 2D
- 2-dimensional
- 18α-GA
- 18α-glycyrrhetinic acid
- α-MEM
- α-minimal essential medium
- ANOVA
- analysis of variance
- CSFBS
- charcoal stripped fetal bovine serum
- DIC
- differential interference contrast
- DMSO
- dimethyl sulfoxide
- ER
- endoplasmic reticulum
- FBS
- fetal bovine serum
- FRAP
- fluorescence recovery after photobleaching
- GJ
- gap junction
- IP3
- inositol trisphosphate
- LCS
- lacunocanalicular system
- PBS
- phosphate-buffered saline
- PLC
- phospholipase C
- PPADS
- pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic acid
- P2R
- P2 purinergic receptor
- RANKL
- receptor activator of nuclear factor-κB ligand
- SACC
- stretch-activated Ca2+ channel
- TG
- thapsigargin
- VGCC
- voltage-gated Ca2+ channel
REFERENCES
- 1. Bonewald L. F. (2011) The amazing osteocyte. J. Bone Miner. Res. 26, 229–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nakashima T., Hayashi M., Fukunaga T., Kurata K., Oh-Hora M., Feng J. Q., Bonewald L. F., Kodama T., Wutz A., Wagner E. F., Penninger J. M., Takayanagi H. (2011) Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat. Med. 17, 1231–1234 [DOI] [PubMed] [Google Scholar]
- 3. Moriishi T., Fukuyama R., Ito M., Miyazaki T., Maeno T., Kawai Y., Komori H., Komori T. (2012) Osteocyte network; a negative regulatory system for bone mass augmented by the induction of Rankl in osteoblasts and Sost in osteocytes at unloading. PLoS One 7, e40143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Turner C. H., Robling A. G., Duncan R. L., Burr D. B. (2002) Do bone cells behave like a neuronal network? Calcif. Tissue Int. 70, 435–442 [DOI] [PubMed] [Google Scholar]
- 5. Fritton S. P., Weinbaum S. (2009) Fluid and solute transport in bone: flow-induced mechanotransduction. Annu. Rev. Fluid Mech. 41, 347–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tatsumi S., Ishii K., Amizuka N., Li M., Kobayashi T., Kohno K., Ito M., Takeshita S., Ikeda K. (2007) Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell Metab. 5, 464–475 [DOI] [PubMed] [Google Scholar]
- 7. Klein-Nulend J., van der Plas A., Semeins C. M., Ajubi N. E., Frangos J. A., Nijweide P. J., Burger E. H. (1995) Sensitivity of osteocytes to biomechanical stress in vitro. FASEB J. 9, 441–445 [DOI] [PubMed] [Google Scholar]
- 8. Ajubi N. E., Klein-Nulend J., Alblas M. J., Burger E. H., Nijweide P. J. (1999) Signal transduction pathways involved in fluid flow-induced PGE2 production by cultured osteocytes. Am. J. Physiol. Endocrinol. Metab. 276, E171–E178 [DOI] [PubMed] [Google Scholar]
- 9. Kamel M. A., Picconi J. L., Lara-Castillo N., Johnson M. L. (2010) Activation of β-catenin signaling in MLO-Y4 osteocytic cells versus 2T3 osteoblastic cells by fluid flow shear stress and PGE2: implications for the study of mechanosensation in bone. Bone 47, 872–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Klein-Nulend J., Semeins C. M., Ajubi N. E., Nijweide P. J., Burger E. H. (1995) Pulsating fluid flow increases nitric oxide (NO) synthesis by osteocytes but not periosteal fibroblasts–correlation with prostaglandin upregulation. Biochem. Biophys. Res. Commun. 217, 640–648 [DOI] [PubMed] [Google Scholar]
- 11. Li P., Hu M., Sun S., Zhang Y., Gao Y., Long M., Huo B., Zhang D. (2012) Fluid flow-induced calcium response in early or late differentiated osteoclasts. Ann. Biomed. Eng. 40, 1874–1883 [DOI] [PubMed] [Google Scholar]
- 12. Jacobs C. R., Yellowley C. E., Davis B. R., Zhou Z., Cimbala J. M., Donahue H. J. (1998) Differential effect of steady versus oscillating flow on bone cells. J. Biomech. 31, 969–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jorgensen N. R., Henriksen Z., Brot C., Eriksen E. F., Sorensen O. H., Civitelli R., Steinberg T. H. (2000) Human osteoblastic cells propagate intercellular calcium signals by two different mechanisms. J. Bone Miner. Res. 15, 1024–1032 [DOI] [PubMed] [Google Scholar]
- 14. Jorgensen N. R., Henriksen Z., Sorensen O. H., Eriksen E. F., Civitelli R., Steinberg T. H. (2002) Intercellular calcium signaling occurs between human osteoblasts and osteoclasts and requires activation of osteoclast P2X7 receptors. J. Biol. Chem. 277, 7574–7580 [DOI] [PubMed] [Google Scholar]
- 15. Henriksen Z., Hiken J. F., Steinberg T. H., Jorgensen N. R. (2006) The predominant mechanism of intercellular calcium wave propagation changes during long-term culture of human osteoblast-like cells. Cell Calcium 39, 435–444 [DOI] [PubMed] [Google Scholar]
- 16. Chen N. X., Ryder K. D., Pavalko F. M., Turner C. H., Burr D. B., Qiu J., Duncan R. L. (2000) Ca2+ regulates fluid shear-induced cytoskeletal reorganization and gene expression in osteoblasts. Am. J. Physiol. Cell Physiol. 278, C989–C997 [DOI] [PubMed] [Google Scholar]
- 17. You J., Reilly G. C., Zhen X., Yellowley C. E., Chen Q., Donahue H. J., Jacobs C. R. (2001) Osteopontin gene regulation by oscillatory fluid flow via intracellular calcium mobilization and activation of mitogen-activated protein kinase in MC3T3-E1 osteoblasts. J. Biol. Chem. 276, 13365–13371 [DOI] [PubMed] [Google Scholar]
- 18. Lu X. L., Huo B., Chiang V., Guo X. E. (2012) Osteocytic network is more responsive in calcium signaling than osteoblastic network under fluid flow. J. Bone Miner. Res. 27, 563–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jing D., Lu X. L., Luo E., Sajda P., Leong P. L., Guo X. E. (2013) Spatiotemporal properties of intracellular calcium signaling in osteocytic and osteoblastic cell networks under fluid flow. Bone 53, 531–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huo B., Lu X. L., Costa K. D., Xu Q., Guo X. E. (2010) An ATP-dependent mechanism mediates intercellular calcium signaling in bone cell network under single cell nanoindentation. Cell Calcium 47, 234–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Adachi T., Aonuma Y., Ito S., Tanaka M., Hojo M., Takano-Yamamoto T., Kamioka H. (2009) Osteocyte calcium signaling response to bone matrix deformation. J. Biomech. 42, 2507–2512 [DOI] [PubMed] [Google Scholar]
- 22. Ishihara Y., Sugawara Y., Kamioka H., Kawanabe N., Hayano S., Balam T. A., Naruse K., Yamashiro T. (2013) Ex vivo real-time observation of Ca2+ signaling in living bone in response to shear stress applied on the bone surface. Bone 53, 204–215 [DOI] [PubMed] [Google Scholar]
- 23. Ishihara Y., Sugawara Y., Kamioka H., Kawanabe N., Kurosaka H., Naruse K., Yamashiro T. (2012) In situ imaging of the autonomous intracellular Ca2+ oscillations of osteoblasts and osteocytes in bone. Bone 50, 842–852 [DOI] [PubMed] [Google Scholar]
- 24. De Souza R. L., Matsuura M., Eckstein F., Rawlinson S. C., Lanyon L. E., Pitsillides A. A. (2005) Non-invasive axial loading of mouse tibiae increases cortical bone formation and modifies trabecular organization: a new model to study cortical and cancellous compartments in a single loaded element. Bone 37, 810–818 [DOI] [PubMed] [Google Scholar]
- 25. Fritton J. C., Myers E. R., Wright T. M., van der Meulen M. C. (2005) Loading induces site-specific increases in mineral content assessed by microcomputed tomography of the mouse tibia. Bone 36, 1030–1038 [DOI] [PubMed] [Google Scholar]
- 26. Brodt M. D., Silva M. J. (2010) Aged mice have enhanced endocortical response and normal periosteal response compared with young-adult mice following 1 week of axial tibial compression. J. Bone Miner. Res. 25, 2006–2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Silva M. J., Brodt M. D., Lynch M. A., Stephens A. L., Wood D. J., Civitelli R. (2012) Tibial loading increases osteogenic gene expression and cortical bone volume in mature and middle-aged mice. PLoS One 7, e34980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lynch M. E., Main R. P., Xu Q., Walsh D. J., Schaffler M. B., Wright T. M., van der Meulen M. C. (2010) Cancellous bone adaptation to tibial compression is not sex dependent in growing mice. J. Appl. Physiol. 109, 685–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang L., Wang Y., Han Y., Henderson S. C., Majeska R. J., Weinbaum S., Schaffler M. B. (2005) In situ measurement of solute transport in the bone lacunar-canalicular system. Proc. Natl. Acad. Sci. U. S. A. 102, 11911–11916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hutchins B. I., Kalil K. (2008) Differential outgrowth of axons and their branches is regulated by localized calcium transients. J. Neurosci. 28, 143–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Price C., Zhou X., Li W., Wang L. (2011) Real-time measurement of solute transport within the lacunar-canalicular system of mechanically loaded bone: direct evidence for load-induced fluid flow. J. Bone Miner. Res. 26, 277–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Thevenaz P., Ruttimann U. E., Unser M. (1998) A pyramid approach to subpixel registration based on intensity. IEEE Trans. Image Process. 7, 27–41 [DOI] [PubMed] [Google Scholar]
- 33. Lu X. L., Huo B., Park M., Guo X. E. (2012) Calcium response in osteocytic networks under steady and oscillatory fluid flow. Bone 51, 466–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Weinbaum S., Cowin S. C., Zeng Y. (1994) A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. J. Biomech. 27, 339–360 [DOI] [PubMed] [Google Scholar]
- 35. Sipma H., Van der Zee L., Den Hertog A., Nelemans A. (1996) Neomycin inhibits histamine and thapsigargin mediated Ca2+ entry in DDT1 MF-2 cells independent of phospholipase C activation. Eur. J. Pharmacol. 305, 207–212 [DOI] [PubMed] [Google Scholar]
- 36. Heinemann A., Shahbazian A., Bartho L., Holzer P. (1999) Different receptors mediating the inhibitory action of exogenous ATP and endogenously released purines on guinea-pig intestinal peristalsis. Br. J. Pharmacol. 128, 313–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hung C. T., Allen F. D., Mansfield K. D., Shapiro I. M. (1997) Extracellular ATP modulates [Ca2+]i in retinoic acid-treated embryonic chondrocytes. Am. J. Physiol. 272, C1611–C1617 [DOI] [PubMed] [Google Scholar]
- 38. Guo Y., Martinez-Williams C., Gilbert K. A., Rannels D. E. (1999) Inhibition of gap junction communication in alveolar epithelial cells by 18α-glycyrrhetinic acid. Am. J. Physiol. 276, L1018–L1026 [DOI] [PubMed] [Google Scholar]
- 39. Huang L., Keyser B. M., Tagmose T. M., Hansen J. B., Taylor J. T., Zhuang H., Zhang M., Ragsdale D. S., Li M. (2004) NNC 55–0396 [(1S,2S)-2-(2-(N-[(3-benzimidazol-2-yl)propyl]-N-methylamino)ethyl)-6-fluoro-1,2, 3,4-tetrahydro-1-isopropyl-2-naphtyl cyclopropanecarboxylate dihydrochloride]: a new selective inhibitor of T-type calcium channels. J. Pharmacol. Exp. Ther. 309, 193–199 [DOI] [PubMed] [Google Scholar]
- 40. Yoshida J., Ishibashi T., Nishio M. (2003) Antiproliferative effect of Ca2+ channel blockers on human epidermoid carcinoma A431 cells. Eur. J. Pharmacol. 472, 23–31 [DOI] [PubMed] [Google Scholar]
- 41. Burr D. B., Milgrom C., Fyhrie D., Forwood M., Nyska M., Finestone A., Hoshaw S., Saiag E., Simkin A. (1996) In vivo measurement of human tibial strains during vigorous activity. Bone 18, 405–410 [DOI] [PubMed] [Google Scholar]
- 42. Wang B., Zhou X., Price C., Li W., Pan J., Wang L. (2013) Quantifying load-induced solute transport and solute-matrix interactions within the osteocyte lacunar-canalicular system. J. Bone Miner. Res. 28, 1075–1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bonewald L. F., Johnson M. L. (2008) Osteocytes, mechanosensing and Wnt signaling. Bone 42, 606–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vatsa A., Breuls R. G., Semeins C. M., Salmon P. L., Smit T. H., Klein-Nulend J. (2008) Osteocyte morphology in fibula and calvaria—is there a role for mechanosensing? Bone 43, 452–458 [DOI] [PubMed] [Google Scholar]
- 45. Himeno-Ando A., Izumi Y., Yamaguchi A., Iimura T. (2012) Structural differences in the osteocyte network between the calvaria and long bone revealed by three-dimensional fluorescence morphometry, possibly reflecting distinct mechano-adaptations and sensitivities. Biochem. Biophys. Res. Commun. 417, 765–770 [DOI] [PubMed] [Google Scholar]
- 46. Judex S., Gupta S., Rubin C. (2009) Regulation of mechanical signals in bone. Orthod. Craniofac. Res. 12, 94–104 [DOI] [PubMed] [Google Scholar]
- 47. Robling A. G., Turner C. H. (2009) Mechanical signaling for bone modeling and remodeling. Crit. Rev. Eukaryot. Gene Expr. 19, 319–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Turner C. H., Robling A. G. (2005) Mechanisms by which exercise improves bone strength. J. Bone Miner. Metab. 23(Suppl.), 16− 22 [DOI] [PubMed] [Google Scholar]
- 49. Rochefort G. Y., Pallu S., Benhamou C. L. (2010) Osteocyte: the unrecognized side of bone tissue. Osteoporos. Int. 21, 1457–1469 [DOI] [PubMed] [Google Scholar]
- 50. Jacobs C. R., Temiyasathit S., Castillo A. B. (2010) Osteocyte mechanobiology and pericellular mechanics. Annu. Rev. Biomed. Eng. 12, 369–400 [DOI] [PubMed] [Google Scholar]
- 51. Burra S., Nicolella D. P., Francis W. L., Freitas C. J., Mueschke N. J., Poole K., Jiang J. X. (2010) Dendritic processes of osteocytes are mechanotransducers that induce the opening of hemichannels. Proc. Natl. Acad. Sci. U. S. A. 107, 13648–13653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Han Y., Cowin S. C., Schaffler M. B., Weinbaum S. (2004) Mechanotransduction and strain amplification in osteocyte cell processes. Proc. Natl. Acad. Sci. U. S. A. 101, 16689–16694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. You L., Cowin S. C., Schaffler M. B., Weinbaum S. (2001) A model for strain amplification in the actin cytoskeleton of osteocytes due to fluid drag on pericellular matrix. J. Biomech. 34, 1375–1386 [DOI] [PubMed] [Google Scholar]
- 54. Berridge M. J., Lipp P., Bootman M. D. (2000) The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 1, 11–21 [DOI] [PubMed] [Google Scholar]
- 55. Genetos D. C., Kephart C. J., Zhang Y., Yellowley C. E., Donahue H. J. (2007) Oscillating fluid flow activation of gap junction hemichannels induces ATP release from MLO-Y4 osteocytes. J. Cell. Physiol. 212, 207–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Li J., Liu D., Ke H. Z., Duncan R. L., Turner C. H. (2005) The P2X7 nucleotide receptor mediates skeletal mechanotransduction. J. Biol. Chem. 280, 42952–42959 [DOI] [PubMed] [Google Scholar]
- 57. Wang N., Robaye B., Agrawal A., Skerry T. M., Boeynaems J. M., Gartland A. (2012) Reduced bone turnover in mice lacking the P2Y13 receptor of ADP. Mol. Endocrinol. 26, 142–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhang Y., Paul E. M., Sathyendra V., Davison A., Sharkey N., Bronson S., Srinivasan S., Gross T. S., Donahue H. J. (2011) Enhanced osteoclastic resorption and responsiveness to mechanical load in gap junction deficient bone. PLoS One 6, e23516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Grimston S. K., Watkins M. P., Brodt M. D., Silva M. J., Civitelli R. (2012) Enhanced periosteal and endocortical responses to axial tibial compression loading in conditional connexin43 deficient mice. PLoS One 7, e44222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Thi M. M., Islam S., Suadicani S. O., Spray D. C. (2012) Connexin43 and pannexin1 channels in osteoblasts: who is the “hemichannel”? J. Membr. Biol. 245, 401–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.