Abstract
Our aim in the current study was to determine the necessity of satellite cells for long-term muscle growth and maintenance. We utilized a transgenic Pax7-DTA mouse model, allowing for the conditional depletion of > 90% of satellite cells with tamoxifen treatment. Synergist ablation surgery, where removal of synergist muscles places functional overload on the plantaris, was used to stimulate robust hypertrophy. Following 8 wk of overload, satellite cell-depleted muscle demonstrated an accumulation of extracellular matrix (ECM) and fibroblast expansion that resulted in reduced specific force of the plantaris. Although the early growth response was normal, an attenuation of hypertrophy measured by both muscle wet weight and fiber cross-sectional area occurred in satellite cell-depleted muscle. Isolated primary myogenic progenitor cells (MPCs) negatively regulated fibroblast ECM mRNA expression in vitro, suggesting a novel role for activated satellite cells/MPCs in muscle adaptation. These results provide evidence that satellite cells regulate the muscle environment during growth.—Fry, C. S., Lee, J. D., Jackson, J. R., Kirby, T. J., Stasko, S. A., Liu, H., Dupont-Versteegden, E. E., McCarthy, J. J., Peterson, C. A. Regulation of the muscle fiber microenvironment by activated satellite cells during hypertrophy.
Keywords: Pax7, fibroblast, myogenic progenitor cells, Tcf4, extracellular matrix
Skeletal muscle resident stem cells, satellite cells, lie between the basal lamina and the muscle fiber plasma membrane; they remain quiescent under resting conditions but become activated in response to injury and mechanical load (1). In addition to anatomical location, satellite cells are identified by expression of several genes, including paired box 7 (Pax7). We utilized the discrete expression of Pax7 in satellite cells to develop the Pax7-DTA mouse, whereby the use of Cre-lox technology allows for the specific and conditional ablation of satellite cells following tamoxifen-induced expression of diphtheria toxin (2). We recently demonstrated that satellite cells are dispensable for robust muscle hypertrophy (2), contradicting historical views that satellite cells were required for growth (3–5). Through the use of synergist ablation (SA) surgery, where the removal of the gastrocnemius and soleus muscles facilitates functional overload of the plantaris, we showed that in the short term, satellite cell-depleted muscle hypertrophies to the same extent as plantaris muscles in mice with their full complement of satellite cells (2).
Contrary to muscle hypertrophy, the necessity of satellite cells during the regenerative process following muscle injury has been well documented (6–8), as has the extensive remodeling of the muscle extracellular matrix (ECM) following acute muscle injury (8–11). ECM in muscle is synthesized largely by fibroblasts found in the interstitial space between fibers (12–14). Recent work by Murphy et al. (8) demonstrated an interaction between satellite cells and muscle fibroblasts in the regeneration of muscle following barium chloride injection. The necessity of both satellite cells and fibroblasts for complete regeneration highlighted the importance of both cell types and the interplay between them. Less appreciated is the remodeling of the ECM that occurs during muscle hypertrophy; the role of satellite cells in that process, if any, has not been explored.
That satellite cell-depleted muscle did not display attenuated hypertrophy following overload (2) challenges the myonuclear domain hypothesis (15); that an expanding fiber volume requires the fusion/addition of a new myonucleus to maintain a constant nuclear to cytoplasmic ratio. However, whether the increased mass can be maintained for an extended period, and whether further muscle growth can occur in the absence of myonuclear accretion, remain unanswered questions. The findings from this study indicate that satellite cells are not required to maintain a significantly increased muscle mass for an extended period of time; however, growth plateaus in the absence of satellite cells, associated with excessive ECM accumulation. Results suggest that satellite cells are required for healthy remodeling of the ECM during muscle adaptation through regulation of fibroblast activity.
MATERIALS AND METHODS
Mice
All animal procedures were conducted in accordance with institutional guidelines for the care and use of laboratory animals as approved by the Institutional Animal Care and Use Committee of the University of Kentucky. Mice were housed in a temperature- and humidity-controlled room and maintained on a 14:10-h light-dark cycle with food and water ad libitum. The Pax7CreER/+;Rosa26DTA/+ strain, designated Pax7-DTA, was generated by crossing Pax7CreER/CreER and Rosa26DTA/DTA strains (2). The Pax7-DTA mouse allows for tamoxifen-induced Cre recombination, which drives the expression of the diphtheria toxin A chain, killing satellite cells expressing the Pax7 gene.
Conditional ablation of satellite cells
Adult (4 mo of age), male Pax7-DTA mice were administered by intraperitoneal injection either vehicle (15% ethanol in sunflower seed oil) or tamoxifen (2 mg/d) for 5 consecutive days. Following a 2-wk washout period, vehicle- and tamoxifen-treated mice were randomly divided into sham-surgery or SA (SA-1, -2, -4, -8 wk) groups. A visual representation of the study design with tamoxifen treatment, surgery, and harvest can be seen in Fig. 1A. Mice from the parental strain, Pax7CreER/CreER (Pax7-CreER), were also randomized to vehicle or tamoxifen treatment, followed by a 2-wk washout, and then subjected to SA surgery and 8 wk of overload (SA-8) to determine the effects of tamoxifen, independent of satellite cell depletion.
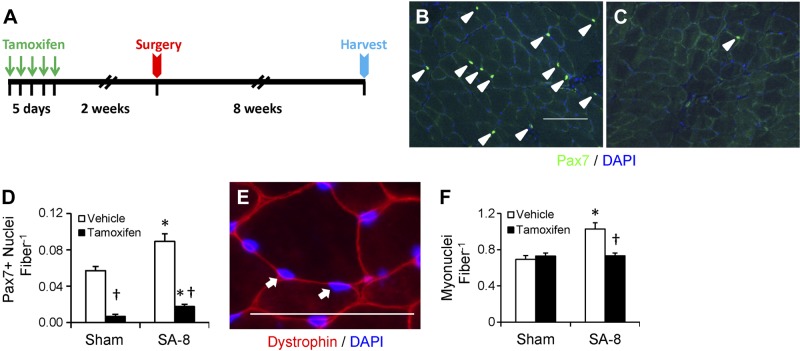
Figure 1.
Conditional depletion of satellite cells results in attenuation of myonuclear accretion 8 wk following SA surgery (SA-8). A) Study design diagram demonstrating time of tamoxifen treatment, SA surgery, and harvest of tissue. B) Pax7 immunohistochemistry on plantaris muscle cross-section from vehicle-treated Pax7-DTA mice to identify satellite cell nuclei (green), counterstained with DAPI (blue). Pax7+/DAPI+ nuclei (arrowheads) were counted. Scale bar = 100 μm. C) Pax7 immunohistochemistry on plantaris muscle cross section from tamoxifen-treated Pax7-DTA mice. D) Quantification of Pax7+/DAPI+ nuclei, presented as mean ± se Pax7+ nuclei per fiber; n = 10–12 mice/group. E) Dystrophin (red) immunohistochemistry, costained with DAPI (blue). DAPI+ nuclei residing within the dystrophin border were classified as myonuclei (arrows). Scale bar = 50 μm. F) Quantification of myonuclei, presented as mean ± se myonuclei per fiber; n = 6. *P < 0.05 for surgery between condition-matched groups; †P < 0.05 for tamoxifen between condition-matched groups.
SA surgery
Surgical removal of synergist muscles (gastrocnemius and soleus) places a mechanical overload on the remaining plantaris muscle, as described in detail previously (2, 16). Briefly, a longitudinal incision on the dorsal aspect of the lower hind limb was made, exposing the tendon of the gastrocnemius muscle for the excision of the gastrocnemius and soleus muscles. Following 1, 2, 4, or 8 (n=4–12) wk of overload, mice were anesthetized, the plantaris muscle was excised, and the animal was euthanized by cervical dislocation. Each plantaris muscle was weighed; processed accordingly for histochemistry, RNA isolation, single fiber function, or whole-muscle function; and stored at −80°C until analysis.
Immunohistochemistry and Western blot reagents
Antibodies and reagents used were as follows: anti-transcription factor 4 (Tcf4; cat. no. 2569), anti-phospho-SMAD3 (Ser423/425; 9520), and anti-SMAD2/3 (8685) from Cell Signaling Technology (Beverly, MA, USA); anti-Pax7, anti-myosin heavy chain I (BA.D5), anti-myosin heavy chain IIa (SC.71), and anti-myosin heavy chain IIb (BF.F3) from Developmental Studies Hybridoma Bank (Iowa City, IA, USA); anti-dystrophin (VP D505); mouse IgG blocking reagent (MKB-2213), Vectashield mounting medium (H-1000), streptavidin-FITC (SA-5001), and streptavidin-Texas Red (SA-5006) from Vector Laboratories (Burlingame, CA, USA); Texas Red-conjugated goat anti-mouse (610-109-121) from Rockland Immunochemicals (Gilbertsville, PA, USA); biotinylated anti-mouse IgG (115-065-205) from Jackson ImmunoResearch (West Grove, PA, USA); Tyramide Signal Amplification (TSA) Kit (T20935), 4′,6-diamidino-2-phenylindole (DAPI; D1306), Alexa Fluor 594 goat anti-rabbit IgG (H+L; A11037), wheat germ agglutinin, TX Red-X conjugate (W21405), goat anti-mouse IgG2b Alexa Fluor 647-conjugated 2°Ab (1:250; A21242), goat anti-mouse IgG1 Alexa Fluor 488 conjugated 2°Ab (1:500; A21121), and goat anti-mouse IgM biotin conjugated 2°Ab (1:150; 626840) from Invitrogen (Grand Island, NY, USA); anti-mouse pan-macrophage marker (F4/80; 14–4810-82) from eBiosciences (San Diego, CA, USA); and anti-laminin (ab14055) and goat anti-rat biotin (ab7096) from Abcam (Cambridge, MA, USA).
Immunohistochemistry
Plantaris muscle was pinned to a cork block at resting length, covered with a thin layer of Tissue Tek optimal cutting temperature (OCT) compound (Sakura Finetek, Torrance, CA, USA), and then quickly frozen in liquid nitrogen-cooled isopentane and stored at −80°C until sectioning. Frozen muscles were sectioned (7 μm), air-dried, and stored at −20°C. For Pax7 and Tcf4 detection, sections were fixed in 4% paraformaldehyde (PFA), followed by epitope retrieval using sodium citrate (10 mM, pH 6.5) at 92°C for 20 min. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide in phosphate-buffered saline (PBS) for 7 min, followed by an additional blocking step with Mouse-on-Mouse Blocking Reagent (Vector Laboratories). Incubation with Pax7 or Tcf4 antibodies was followed by incubation with the biotin-conjugated secondary antibody and streptavidin-horseradish peroxidase (HRP) included within the TSA kit. TSA-Alexa Fluor 488 or 594 was used to visualize antibody-binding. DAPI (10 nM) was added briefly, and then slides were washed and mounted with Vectashield fluorescent mounting medium. The dystrophin antibody was applied to fresh frozen sections followed by Texas Red- or AMCA-conjugated goat anti-mouse secondary antibody. Antibodies specific to myosin heavy chains type I, IIa, and IIb were applied following dystrophin for fiber type specific cross-sectional area (CSA) determination. Sections were postfixed in 4% PFA and then stained with DAPI. For detection of phospho-SMAD3, sections were fixed in 4% PFA, followed by incubation with phospho-SMAD3 antibody and a goat anti-rabbit Alexa Fluor 488 secondary antibody. For detection of F4/80, sections were fixed in ice-cold acetone, followed by incubation with F4/80 antibody and a goat anti-rat biotin secondary antibody and streptavidin-HRP included within the TSA kit.
Histochemistry
Plantaris sections were stained with hematoxylin and eosin to assess basic muscle morphology and picro-Sirius red to visualize collagen following standard protocols. Slides were mounted with cytoseal XYL (Thermo Fisher Scientific, Wilmington, DE, USA) before imaging at ×20. For detection of N-acetyl-d-glucosamine, sections were fixed with 4% PFA and then incubated with Texas red direct conjugate wheat germ agglutinin.
Immunohistochemistry/histochemistry quantification
Images were captured at ×20 at room temperature with a Zeiss upright microscope (AxioImager M1; Carl Zeiss, Oberkochen, Germany), and analysis was carried out using the AxioVision Rel 4.8 software (Zeiss). To determine fiber type-specific CSA, myosin heavy chain-stained ×20 images were used. To capture the entire plantaris muscle cross section required analysis of 15–20 images (∼700 fibers for sham-surgery muscle and ∼1000 fibers for SA-8). Dystrophin-stained tissue was also used to quantify myonuclear number. DAPI-stained nuclei that clearly resided within the dystrophin border of the muscle fiber were scored as myonuclei using the interactive measurement software. Satellite cell and fibroblast abundance was assessed using Pax7 and Tcf4 staining, respectively (×20), and only those loci that were scored as Pax7+ or Tcf4+ by the software and were DAPI+ were counted. Wheat germ agglutinin staining was quantified to measure fibrotic areas of the muscle using the thresholding feature of the AxioVision Rel software, and the area occupied by wheat germ agglutinin was expressed relative to muscle fiber number. phospho-SMAD3+ nuclei were quantified using the thresholding feature of the AxioVision Rel software, and phopsho-SMAD3+ nuclei were expressed relative to total DAPI+ nuclei.
Microarray analysis
Total RNA was isolated from the plantaris muscles of tamoxifen-treated (n=3) and vehicle-treated SA-1 mice (n=2) using TRIzol (Invitrogen) according to the manufacturer's directions. RNA samples were treated with Turbo DNase (Ambion, Austin, TX, USA) to remove genomic DNA contamination. The total RNA concentration and purity were assessed by measuring the optical density (230, 260, and 280 nm) with the Nanodrop 1000 Spectrophotometer (ThermoFisher Scientific). RNA integrity was assessed using the Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA, USA). Microarray hybridization and processing (Affymetrix, Santa Clara, CA, USA) were performed at the University of Kentucky Microarray Core Facility according to the manufacturer's protocol. Each chip (Mouse Gene 430 2.0; Affymetrix) used 1 μg of total RNA derived from a pooled sample of the right and left plantaris muscles from each animal. Partek Genomics Suite (Partek, Inc., St. Louis, MO, USA) was used to identify differentially expressed genes that were significantly different between the 2 groups. Functional annotation was performed using the Database for Annotation, Visualization, and Integrated Discovery (DAVID; National Institute of Allergy and Infectious Diseases, Bethesda, MD, USA; http://david.abcc.ncifcrf.gov/home.jsp). Pathway analysis utilized Kyoto Encyclopedia of Genes and Genomes (KEGG; Kyoto, Japan; http://www.genome.jp/kegg/). Data from the microarray analysis have been uploaded to the U.S. National Center for Biotechnology Information (NCBI; Bethesda, MD, USA) Gene Expression Omnibus (GEO) repository (accession no. GSE52597; http://www.ncbi.nlm.nih.gov/geo/).
Single-fiber functional analysis
Plantaris muscle fibers from vehicle- and tamoxifen-treated SA-8 mice (n=19–29 fibers) were chemically permeabilized with Triton X-100 and attached to a force transducer (403; resonant frequency 600 Hz; Aurora Scientific, Aurora, ON, Canada) and a motor (312B; step-time 0.6 ms; Aurora Scientific). Steady-state force and tension recovery kinetics were measured as a function of the activating Ca2+ concentration as described previously (17).
Whole-muscle functional analysis
Plantaris muscles from vehicle- and tamoxifen-treated SA-8 mice (n=8) were placed in Krebs-Ringer solution (in mM: 137 NaCl, 5 KCl, 1 MgSO4, 1 NaH2PO4, 24 NaHCO3, and 2 CaCl2) equilibrated with 95% O2-5% CO2 (pH ∼7.4) and mounted for study ex vivo. The distal tendon was tied to a glass rod, and the proximal tendon was attached to a force transducer (BG Series 100g; Kulite, Leonia, NJ, USA) using 4-0 silk sutures. The force transducer was mounted on a micrometer, which was used to adjust muscle length. The muscle was placed between platinum plate electrodes in a water-jacketed organ bath containing buffer continuously gassed with 95% O2-5% CO2. The plantaris was positioned at the length that elicited the highest twitch force (optimal length, Lo). Contractile characteristics were determined by electrical stimulation using supramaximal voltage (Grass S48; Grass Instruments, Quincy, MA, USA) and pulse and train durations of 0.3 and 300 ms, respectively. To define force-frequency characteristics, force was measured in response to stimulus frequencies of 1, 15, 30, 50, 80, 100, and 120 every 2 min, interspersed by maximal, 120-Hz stimulations. All measurements were performed in solutions containing d-tubocurarine (0.025 mM). For all measurements of muscle force, the output of the force transducer was monitored using a digital oscilloscope (546601B; Hewlett Packard, Palo Alto, CA, USA). After the end of the protocol, muscle length was measured using a hand-held electronic caliper (CD-6 CS; Mitutoyo America, Aurora, IL, USA). The plantaris was then removed from the organ bath, trimmed of connective tissue, blotted dry, and weighed. Muscle weight and Lo were used to estimate CSA (18–19). Absolute forces are expressed as kilonewtons per square meter.
Isolation and culture of primary myogenic progenitor cells (MPCs) and muscle fibroblasts
Detailed procedures have been published previously (20). Briefly, MPCs were purified from the gastrocnemius, soleus, plantaris, and tibialis anterior of 12 male adult (4-mo-old) C57B/L6 mice according to the preplate protocol (21). Muscles were thoroughly minced in PBS containing dispase II (2.4 U/ml; Roche Applied Science, Indianapolis, IN, USA) and collagenase D (1 mg/ml; Sigma-Aldrich, St. Louis, MO, USA), incubated at 37°C for 60 min, and then passed through a 40 μm filter (BD Biosciences, San Jose, CA, USA). The filtrates were spun at 1300 g for 10 min to pellet the cells. Cells were maintained in culture as described previously (22) and preplating was initiated to enrich for MPCs. Briefly, cells were plated on uncoated 60 mm tissue culture plastic and incubated for 30 min at 37°C in growth medium [GM+; composed of Dulbecco's modified Eagle's medium (DMEM), 20% fetal bovine serum, and 5 ng/ml basic fibroblast growth factor (bFGF)] to allow adherent cells (fibroblasts) to attach. The nonadherent cells (MPCs) were collected and transferred to a 60-mm collagen-coated dish (passage 0). GM+ was changed every other day between preplating. MPCs were used at a purity >90% as determined by MyoD staining. Fibroblasts were maintained on uncoated 60-mm tissue culture plastic plates in GM− (DMEM and 20% fetal bovine serum), and GM− was changed every other day. Fibroblast ECM mRNA expression was assessed in the presence and absence of bFGF and found not to be different (data not shown).
Fibroblast-fibroblast and fibroblast-MPC cocultures
Fibroblasts were cocultured with MPCs or, as a control, cocultured with fibroblasts (n=5 isolates studied in duplicate). For cocultures, fibroblasts and MPCs were plated at 2 × 105 cells on Transwell inserts accompanying the companion 6-well plate. Six-well uncoated plates were plated with fibroblasts at 8 × 105 cells. The fibroblasts and MPCs on inserts were allowed to settle and adhere for 12 h before the coculture was started. All coculture experiments were performed for 24 h in GM−. Following the 24 h coculture, the cells from the inserts and plate wells were collected separately for RNA analysis or with radioimmunoprecipitation assay (RIPA) buffer with protease and phosphatase inhibitors for protein analysis.
Conditioned medium (CM)
Medium from fibroblasts and MPCs was collected following a 24 h conditioning period, and cells and large debris were spun down at 1300 g for 10 min. Six-well uncoated plates were plated with fibroblasts at 8 × 105 cells, and MPC-CM or fibroblast-CM (control) was placed on fibroblasts for 24 h (n=3–5 isolates studied in duplicate). Following the 24 h culture with CM, the fibroblasts were collected for RNA analysis.
Fibroblast proliferation determination
Fibroblast proliferation during coculture with MPCs or fibroblasts was assessed by measuring 5-bromo-2′-deoxyuridine (BrdU) incorporation according to the manufacturer's instructions. Briefly, fibroblasts were plated on 6-well plates at an approximate confluency of 40–50% with inserts containing MPCs or fibroblasts placed into the wells (n=3 isolates studied in duplicate). Cells were incubated with BrdU labeling reagent (Invitrogen) diluted 1:100 in DMEM + 20% fetal bovine serum for 3 h. After the 3 h incubation with BrdU labeling reagent, cells were washed and replaced with DMEM plus 20% fetal bovine serum for an additional 21 h. Following the 24 h total coculture period, fibroblasts were fixed in 70% ethanol for 30 min at 4°C and then blocked with 3% H2O2 in 100% methanol for 10 min, followed by 30 min of incubation in denaturing solution and 10 min in blocking buffer (BrdU staining kit, Invitrogen). Biotinylated mouse anti-BrdU (Invitrogen) was added for 1 h after which cells were rinsed in PBS and incubated in streptavidin-peroxidase for 10 min. Plates were then treated with a 1:200 dilution of fluorescein TSA buffer for 30 min (Invitrogen). To stain all nuclei, plates were treated with DAPI (Invitrogen) at 10 nM and incubated for 20 min. Cells were counted using a fluorescent inverted microscope (Zeiss Axio Observer) at a magnification of 10×, and the ratio of BrdU+ cells to total cell number was calculated.
Total RNA isolation and real-time RT-PCR
Detailed procedures have been published previously (20). Briefly, total RNA from fibroblasts and MPCs was obtained with the RNAqueous kit (Ambion) per the manufacturer's instructions. The quantity and quality of the isolated RNA were determined by NanoDrop 2000 (Thermo Fisher Scientific). Total RNA (1 μg) was reverse transcribed, and the resultant cDNA was amplified with a 1× SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA) or 1× TaqMan Master Mix plus 0.3 μM of gene-specific upstream and downstream primers during 40 cycles on an Applied Biosystems 7900 Fast Realtime Cycler. A standard curve was generated using pooled cDNA from the experimental samples included in each set of experiments, and Ct values were within the linear portion of the amplification range. The expression of the specific genes of interest was reported as relative arbitrary units, as the value obtained for the specific gene was normalized to the value obtained for GAPDH. In every case, the Ct values were taken when the efficiency of the reaction was between 90 and 110%. In every run, product purity was confirmed by examining the melt curve that showed a single peak. All the real-time experiment runs that we present were repeated more than once. The primers used were as follows: GAPDH, 5′-TCAGGACCCATCTATGATAAAACCTA-3′ (forward) and 5′-TCAGGTCAGAGAAGAACACCATTTC-3′ (reverse). Primers for collagen 1α2, collagen 3α1, collagen 6α2, and fibronectin were predesigned TaqMan primers. All primers used are designed to span intron/exon borders.
Western blot analysis
Cells were harvested using ice-cold RIPA buffer with 10 μl/ml protease inhibitor cocktail for mammalian tissues (P8340, Sigma-Aldrich), and protein concentration was determined by the BCA (Thermo Fisher Scientific) method. Protein (30 μg) was electrophoresed in 4–15% SDS-polyacrylamide gels (Bio-Rad, Hercules, CA, USA) and transferred onto nitrocellulose membranes at 150 V for 1 h at 4°C. Membranes were blocked for 1 h with Odyssey Blocking Blocker (LI-COR, Lincoln, NE, USA) and immunoblotted with phospho-SMAD3 (Ser423/425) and SMAD2/3 antibodies (Cell Signaling) overnight at 4°C with gentle rocking. After being washed, the blots were incubated for 20 min at room temperature with goat anti-rabbit IgG (Alexa 680; LI-COR). Bands were visualized using the Odyssey infrared imaging system (LI-COR). Band intensity for a protein of interest was quantified using Odyssey Application Software 3.0.21 (LI-COR), and results are expressed as arbitrary densitometric value of phospho-SMAD3 normalized to total SMAD3.
Statistical analysis
A 2-factor ANOVA (vehicle/tamoxifen×sham/SA-8) was performed to determine whether a significant interaction existed between factors for each dependent variable under consideration. If a significant interaction was detected, Tukey's post hoc comparisons were performed to identify the source of significance with P ≤ 0.05. A Student's t test was performed to determine whether differences existed in gene expression between fibroblasts cocultured with MPCs or fibroblasts and between fibroblasts cultured in MPC- or fibroblast-CM with P ≤ 0.05.
RESULTS
Effective satellite cell depletion and lack of recovery following 8 wk of overload
Following tamoxifen treatment of Pax7-DTA mice, satellite cell content in the plantaris muscle, determined by Pax7 immunohistochemistry (Fig. 1B, C), was effectively (∼90%) depleted (P<0.05; Fig. 1D). Following 8 wk of overload (SA-8), a significant increase in satellite cell content occurred in both vehicle- and tamoxifen-treated muscle (P<0.05); however, in the latter, satellite cells remained >80% depleted compared with plantaris from vehicle-treated mice, demonstrating a lack of recovery of the satellite cell population following a robust growth stimulus. Recent findings demonstrating heterogeneity in Pax7 expression, with high expressors associated with quiescence and a more stem-cell-like phenotype (23), suggest that these cells are preferentially depleted in our study, precluding the recovery of the muscle satellite cell niche. Tamoxifen treatment of Pax7-CreER mice did not yield a decrease in satellite cell content (Supplemental Fig. S1A), demonstrating the specificity of satellite cell depletion with tamoxifen treatment of the Pax7-DTA strain.
Attenuation of myonuclear accretion and fiber regeneration
With the expansion of cytoplasmic volume that occurs during muscle hypertrophy, there is a characteristic increase in the number of myonuclei through the fusion of satellite cells into mature fibers. To determine whether myonuclear abundance increased in response to 8 wk of overload, the total number of myonuclei for the entire muscle CSA was quantified; myonuclei were identified as DAPI+ nuclei residing within the muscle fiber, delineated by dystrophin immunoreactivity (Fig. 1E). The number of myonuclei per fiber increased 48% in the vehicle-treated SA-8 group (P<0.05) but was unchanged in the tamoxifen-treated SA-8 group (Fig. 1F), consistent with our previous findings following 2 wk of overload in Pax7-DTA mice (2). We also quantified centrally located myonuclei, a hallmark of newly regenerated fibers in hematoxylin and eosin stained sections (Supplemental Fig. S1D). Centrally nucleated fibers were very rare in muscle from sham-surgery mice. Approximately 9% of the fibers in the vehicle-treated SA-8 group had centrally located nuclei, whereas only 2% of the fibers in the satellite cell-depleted SA-8 muscle were centrally nucleated (Supplemental Fig. S1E). Previous reports demonstrate an association of muscle spindle fibers with Pax7/Pax3+ satellite cells (24). In the current study, neither satellite cell depletion nor overload affected muscle spindle fiber content, shown and quantified in Supplemental Fig. S1F, G.
Attenuated muscle hypertrophy following 8 wk of overload in satellite cell-depleted muscle
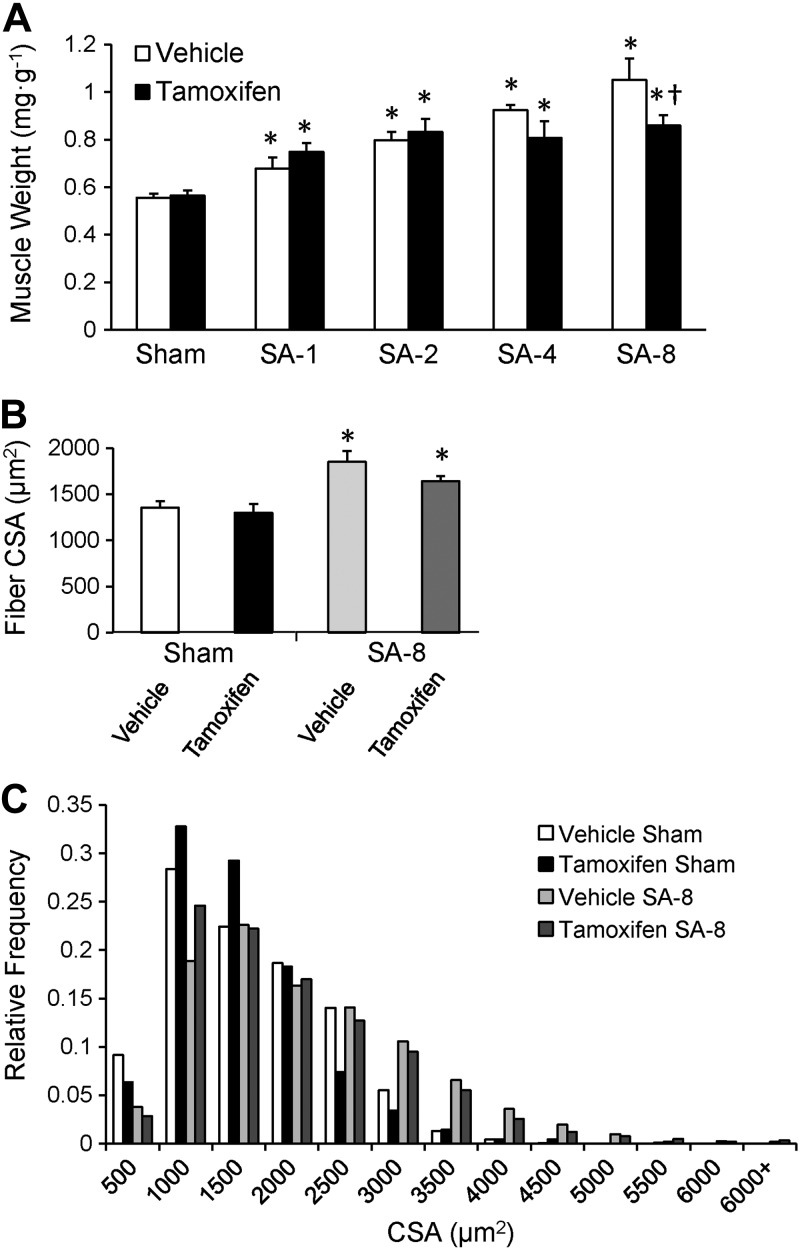
The time course of plantaris muscle hypertrophy in response to overload is shown in Fig. 2A. Vehicle- and tamoxifen-treated mice showed comparable plantaris growth in response to 1 and 2 wk of overload. Vehicle-treated mice continued to grow following 4 and 8 wk of overload, resulting in an ∼2-fold increase in muscle wet weight 8 wk following SA surgery, comparable to growth following 8 wk of overload of the plantaris in tamoxifen-treated parental Pax7-CreER mice (Supplemental Fig. S1B). By contrast, satellite cell-depleted muscle displayed a blunted hypertrophic response 8 wk after surgery (P<0.05; Fig. 2A). Muscle growth plateaued following 2 wk of overload; mass increased by 65% at 2 wk that was maintained through 8 wk of overload. Consistent with the increase in muscle weight, the increase in mean fiber CSA tended to be different between vehicle- and tamoxifen-treated SA-8 mice (P=0.1; Fig. 2B), increasing 36 compared with 26%, respectively. Distribution of fiber CSA demonstrated a rightward shift in fiber size following 8 wk of overload, with satellite cell-depleted SA-8 mice showing a decrease in the frequency of large fibers with CSA > 2500 μm2 (Fig. 2C). Immunohistochemical analyses using isoform-specific myosin heavy chain antibodies (Fig. 3A) showed that CSA increased in all fiber types after 8 wk of overload (Fig. 3B); however, there tended to be a reduced increase in CSA with satellite cell depletion in fast fiber types (IIa, IIx, and IIb) compared with vehicle-treated animals, consistent with average muscle fiber CSA changes. Following 8 wk of overload, hypertrophy of the plantaris in tamoxifen-treated Pax7-CreER mice was not different from growth in vehicle-treated Pax7-DTA (Supplemental Fig. S1B). The plantaris muscle showed the expected increase in oxidative fiber type distribution following 8 wk of overload (25–26), with decreased fiber type IIb and IIx frequency and increased fiber type I and IIa frequency (Fig. 3C). This fiber type shift occurred independently of satellite cell content.
Figure 2.
Conditional depletion of satellite cells results in attenuation of muscle hypertrophy 8 wk following SA surgery (SA-8). A) Time course of plantaris growth. Muscle wet weight normalized to body weight (mg/g) 1, 2, 4, and 8 wk following surgery, presented as means ± se; n = 4–12. B) Quantification of plantaris muscle fiber CSA following dystrophin immunohistochemistry (see Fig. 1E), presented as mean ± se CSA (μm2); n = 6. C) Histogram of binned distribution of fiber CSA following dystrophin immunohistochemistry. *P < 0.05 for surgery between condition-matched groups; †P < 0.05 for tamoxifen between condition-matched groups.
Figure 3.
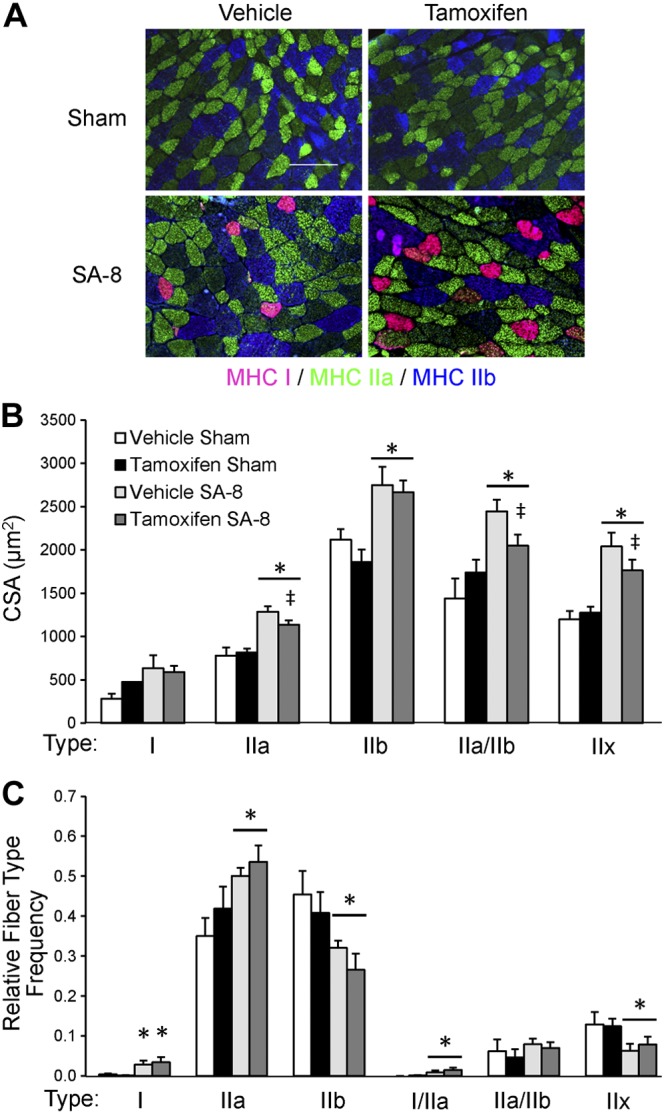
Plantaris fiber type shift 8 wk following SA surgery (SA-8) is independent of satellite cell content. A) Immunohistochemical analysis of plantaris muscle cross sections for myosin heavy chain (MHC) type I (pink), type IIa (green) and type IIb (blue). Unstained fibers are MHC type IIx. Scale bar = 100 μm. B) Quantification of MHC-specific fiber CSA, presented as mean ± se CSA (μm2); n = 6. C) Quantification of the relative frequency of the different fiber types, presented as mean ± se relative frequency; n = 6. *P < 0.05 for surgery between condition-matched groups; ‡P = 0.1 for tamoxifen between condition-matched groups (trend toward significant effect).
Decreased whole-muscle function, but not single-fiber function, in satellite cell-depleted muscle
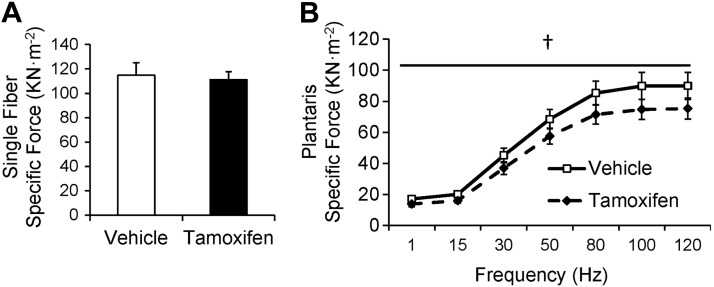
To investigate whether the differences observed in myonuclear content affected single-fiber function, 48 fibers (19 vehicle, 29 tamoxifen) from plantaris muscles of SA-8 animals were prepared for functional analyses. The ktr in maximally activating solution (maximal rate of cross-bridge cycling) was unaffected by satellite cell depletion, as was pCa50 (the concentration of Ca2+ necessary to produce one-half maximal tension expressed as pCa50 units; Supplemental Fig. S2A). Similarly, single fiber specific force (force per unit area) at maximally activating Ca2+ concentration was not affected by satellite cell depletion (Fig. 4A).
Figure 4.
Decreased whole-muscle function, but not single-fiber function, in satellite cell-depleted muscle 8 wk following SA surgery (SA-8). A) Specific force (kN/m2) in permeabilized single fibers isolated from the plantaris of vehicle-treated and satellite cell-depleted animals, presented as mean ± se specific force; n = 19–29 fibers/group. B) Specific force in the plantaris of vehicle-treated and satellite cell-depleted animals, presented as mean ± se specific force; n = 8 mice/group. †P < 0.05 for tamoxifen between condition-matched groups.
Contrary to single-fiber specific force, the specific force-frequency relationship of the entire plantaris was depressed by satellite cell depletion (Fig. 4B). Satellite cell-depleted plantaris muscle developed 20% less specific force than vehicle-treated muscle at stimulus frequencies of 1 to 120 Hz (P<0.05). This protocol involved repetitive measurements of maximal tetanic force. These values did not decline over time (overall changes: vehicle SA-8 −2.3±3.7%, tamoxifen SA-8 1.3±2.0%), demonstrating the stability of the plantaris muscle over the course of measurement.
Increased accumulation of ECM in satellite cell-depleted muscle
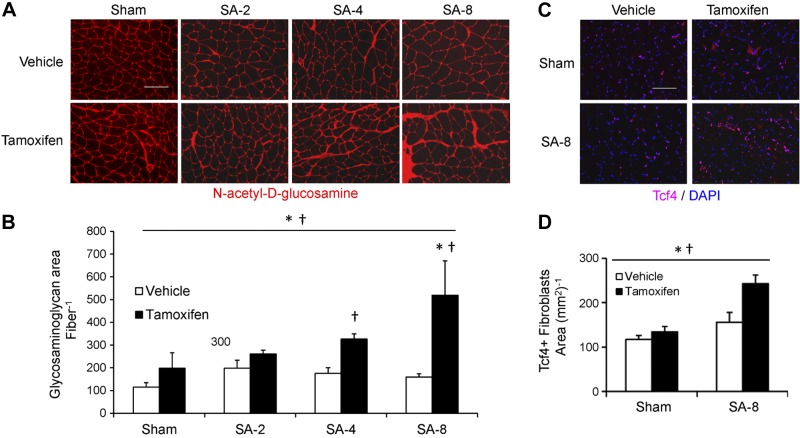
To begin to understand mechanisms underlying the blunted long-term growth response and impaired force generation in overloaded satellite cell-depleted muscle, microarray analyses were performed. RNA from vehicle SA-1 and tamoxifen SA-1 muscles were analyzed with Affymetrix mouse 430 2.0 chips. Functional annotation showed significant (P<0.0001) up-regulation of genes classified as “extracellular structure organization and biogenesis” (GO:0043062) in tamoxifen-treated overloaded muscle. KEGG pathway analysis demonstrated enrichment for ECM-receptor genes following satellite cell depletion; representative gene expression differences are shown in Supplemental Fig. S2B. To determine whether the changes in gene expression observed in response to 1 wk of overload resulted in altered ECM accumulation, histochemistry with picro-Sirius red staining was used, which demonstrated increased collagen accumulation, confirming the microarray results (Supplemental Fig. S2C). To quantify the accumulation of the ECM fluorescently, wheat germ agglutinin histochemistry was performed (Fig. 5A). The area occupied by muscle ECM was increased in response to satellite cell depletion and overload (Fig. 5A, B). In response to 4 and 8 wk of overload (SA-4, SA-8), there was increased ECM accumulation in satellite cell-depleted muscle (P<0.05). Area occupied by muscle ECM was increased when expressed per fiber and as a percentage of the total muscle CSA (vehicle SA-8: 7.5±0.9%, tamoxifen SA-8: 12.0±1.9%; P<0.05). Following 8 wk of overload, there was no increase in the accumulation of ECM in the plantaris of vehicle-treated Pax7-DTA mice (Fig. 5A, B) or in tamoxifen-treated Pax7-CreER mice (Supplemental Fig. S1C).
Figure 5.
Depletion of satellite cells is associated with increased ECM deposition and fibroblast content 8 wk following SA surgery (SA-8). A) Plantaris muscle cross sections incubated with fluorescently labeled wheat germ agglutinin quantifies N-acetyl-d-glucosamine (red) a major component of the ECM. Scale bar = 100 μm. B) Quantification of area occupied by wheat germ agglutinin staining, presented as mean ± se area of wheat germ agglutinin per fiber; n = 4–6. C) Tcf4 immunohistochemistry (red) on plantaris muscle cross sections counterstained with DAPI (blue). Scale bar = 100 μm. D) Quantification of Tcf4+/DAPI+ fibroblasts, presented as mean ± se Tcf4+ fibroblasts per area of muscle (mm2); n = 6. *P < 0.05 for surgery between condition-matched groups; †P < 0.05 for tamoxifen between condition-matched groups.
Increased muscle fibroblast content in satellite cell-depleted muscle
In addition to increased ECM accumulation, hematoxylin and eosin and DAPI staining suggested cellular infiltration in the ECM in satellite cell-depleted SA-8 plantaris muscle, but immunohistochemistry with the F/480 antibody that recognizes macrophages showed no difference between tamoxifen- and vehicle-treated SA-8 muscles (Supplemental Fig. S3A). Because the cells appeared to be embedded in the ECM, Tcf4 immunohistochemistry was performed to identify muscle fibroblasts (Fig. 5C). Following 8 wk of overload, the number of muscle fibroblasts was higher in satellite cell-depleted muscle (P<0.05; Fig. 5D), consistent with the increase in muscle ECM deposition. Tcf4+ fibroblasts were located in the extracellular space surrounding muscle fibers and in areas of collagen accumulation, but not in the satellite cell niche, beneath the laminin-containing basal lamina (Supplemental Fig. S3B).
Decreased transforming growth factor-β (TGF-β)-SMAD3 signaling in overloaded muscle is unaffected by satellite cell depletion
Numerous ECM genes are known to be targets of TGF-β-SMAD, including several collagen isoforms (27). To assess the activity of the TGF-β-SMAD3 signaling pathway in the plantaris, we used phospho-SMAD3 immunohistochemistry and DAPI staining to identify nuclei that were positive for phospho-SMAD3 (Supplemental Fig. S4A, B). Following 8 wk of overload, there was a decrease in the proportion of phospho-SMAD3+ nuclei (P<0.05) that was unaffected by satellite cell depletion (Supplemental Fig. S4C).
MPC secretory products alter fibroblast ECM gene expression in vitro, independent of TGF-β-SMAD signaling
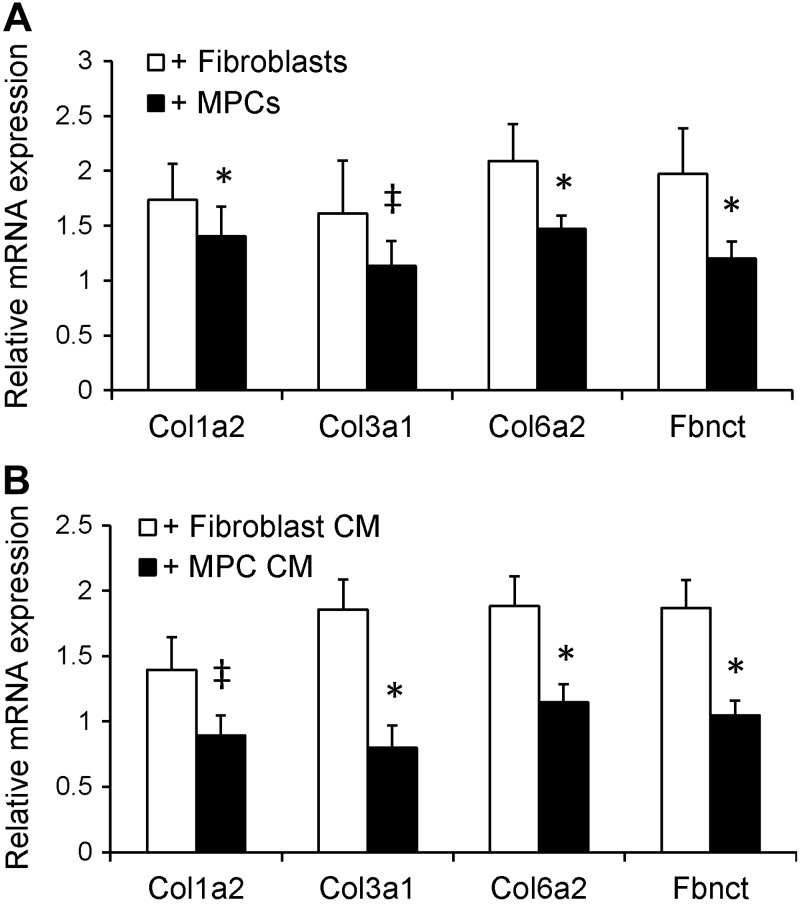
An early response to muscle overload is satellite cell activation and proliferation, so that satellite cell daughters, MPCs, increase by >300% at 2 wk (2) and remain elevated through ≥8 wk (see Fig. 1C). With the increase we observed in ECM accumulation in satellite cell-depleted SA-8 mice (see Fig. 5B), we wanted to assess whether MPCs regulate fibroblast ECM expression. To determine whether MPCs directly affect fibroblast function, MPCs and fibroblasts were isolated from adult muscle and studied in coculture in vitro. Fibroblasts that were cocultured with MPCs had lower mRNA expression of collagen 1α2 and 6α2 and fibronectin (P<0.05) and a trend for lower expression of collagen 3α1 (P=0.1) compared with fibroblasts that were cocultured with fibroblasts (Fig. 6A). Fibroblasts were also cultured in media that had been conditioned by MPCs or fibroblasts. Fibroblasts that were cultured in MPC-CM had lower mRNA expression of collagen 3α1 and 6α2 and fibronectin (P<0.05) and a trend for lower expression of collagen 1α2 (P=0.1) compared with fibroblasts that were cultured in fibroblast-CM (Fig. 6B). The reduced ECM gene expression observed in fibroblasts in response to MPC coculture or CM does not appear to be mediated by the TGF-β signaling pathway, as Western blot analysis of protein isolated from fibroblasts showed no difference in phospho-SMAD3 content in fibroblasts cocultured with MPCs compared with fibroblasts (Supplemental Fig. S4C).
Figure 6.
MPCs and MPC-CM decrease ECM gene expression in mouse primary muscle fibroblasts. A) Relative mRNA expression of various ECM components in fibroblasts that were cocultured with MPCs or fibroblasts; n = 5 isolates/group. B) Relative mRNA expression of various ECM components in fibroblasts that were cocultured in MPC- or fibroblast-CM; n = 3–5 isolates. *P < 0.05 for coculture with MPCs/MPC-CM: ‡P = 0.1 for coculture with MPCs/MPC-CM (trend toward significant effect).
Proliferation of fibroblasts was also assessed in coculture with the use of BrdU labeling (Supplemental Fig. S4D). Following coculture of fibroblasts with MPCs or fibroblasts, no difference existed in the overall number of fibroblasts or the proportion of fibroblasts that were labeled with BrdU (fibroblasts cocultured with MPCs: 62.6±0.0%; fibroblasts cocultured with fibroblasts: 62.7±0.0%; Supplemental Fig. S4E).
DISCUSSION
The most significant finding from this study is the novel role of activated satellite cells in the regulation of skeletal muscle ECM during hypertrophy. With depletion of satellite cells, accumulation of ECM occurred during hypertrophy, as did expansion of the number of Tcf4+ fibroblasts. Release of a MPC secretory product influenced fibroblast ECM expression in vitro, providing further support for a regulatory role of activated satellite cells and MPCs in fibroblast-mediated ECM remodeling.
Muscle fibroblasts are largely thought to be responsible for the excessive production of ECM components that can lead to muscle fibrosis (13, 28–29). The necessity of fibroblasts for complete muscle regeneration, proper myogenesis, and interaction with satellite cells has been demonstrated recently (8, 30). Data from the current study support the hypothesis that in the absence of satellite cell activation and MPC accumulation, the number of fibroblasts increases, correlating with an increase in ECM accumulation (8). In satellite cell-depleted plantaris muscle overloaded for 8 wk, a significant increase (81%) in Tcf4+ fibroblasts occurred compared with vehicle-treated overloaded muscle. In sham-surgery animals, depletion of satellite cells did not influence muscle fibroblast number or ECM accrual, emphasizing the importance of satellite cell activation to the process. Thus, activated satellite cells and MPCs regulate fibroblast proliferation and remodeling of the ECM to enable fiber growth. The requirement of satellite cells during typical muscle maintenance may be minimal, and it is only when the muscle is robustly challenged to adapt that the necessity of satellite cells to regulate fibroblast ECM production becomes apparent.
To further address satellite cell regulation of fibroblast ECM production, we utilized an in vitro coculture system where MPC-containing inserts were placed inside fibroblast-containing wells allowing diffusion of media between cell types but preventing direct contact. Culture of fibroblasts with MPCs or with MPC-CM resulted in decreased (25–130%) mRNA expression of various collagen isoforms and fibronectin, principle components of the muscle ECM, compared with fibroblasts cultured with fibroblasts or in fibroblast-CM. These data provide further evidence in support of the hypothesis that activated satellite cells/MPCs regulate muscle fibroblast ECM production. It has been previously demonstrated that fibroblasts can promote MPC survival and facilitate differentiation into myotubes (30–31), but in the current study we demonstrate a novel influence of MPCs on fibroblast ECM gene expression.
Recent work has also identified alternate populations of cells capable of contributing to fibrosis in muscle. These fibrogenic/adipogenic precursors (FAPs) are identified by expression of platelet-derived growth factor receptor α (PDGFRα) and stem cell antigen-1 (Sca-1) (32–34). FAPs appear to overlap with muscle fibroblasts as evidenced by dual expression of both Tcf4 and PDGFRα (8). In the current study, coculture with MPCs did not influence fibroblast proliferation over a 24 h period. We hypothesize that activated satellite cells/MPCs inhibit recruitment of FAPs into the fibroblast lineage, as opposed to directly influencing fibroblast proliferation. As a result, during adaptation in the absence of activated satellite cell/MPC expansion, the Tcf4+ fibroblast population expands. Thus, in addition to the muscle microenvironment influencing satellite cell function (35), activated satellite cells/MPCs influence the muscle microenvironment during adaptation, apparently limiting fibroblast expansion and regulating fibroblast ECM gene expression. While we cannot rule out the possibility that other cell populations contribute to ECM deposition, the dramatic expansion of the Tcf4+ fibroblast pool following overload in satellite cell-depleted muscle provides strong evidence for their role in the overproduction of ECM observed.
The increased accumulation of ECM in the plantaris of satellite cell-depleted muscle following 8 wk of overload may have hindered the hypertrophic response. We demonstrate no attenuation of hypertrophy following 2 wk of overload but further growth plateaus at 4 and 8 wk in satellite cell-depleted muscle. Following 4 and 8 wk of overload, we observed increased ECM accumulation, only in satellite cell-depleted muscle. We hypothesize that this excessive ECM limits continued hypertrophic growth. Abnormal ECM deposition and fibrosis of muscle tissue are known to impede regeneration after acute injury (36–37), as well as during chronic diseases like muscular dystrophy (38–39). During overload and resistance training, the ECM is strengthened via net collagen synthesis (40); however, the influence of excess ECM accumulation on muscle hypertrophy is relatively unexplored. In the current study, 8 wk of mechanical overload of satellite cell-depleted muscle led to a significant (161%) increase in ECM accumulation relative to vehicle-treated mice of the same strain, or tamoxifen-treated Pax7-CreER mice. The depletion of satellite cells also led to a 20% reduction in specific force of overloaded plantaris muscle, but not isolated single plantaris fibers, likely through the negative contribution of excess ECM present in the muscle. We showed previously that force production of single fibers overloaded for 2 wk is unaffected by depletion of satellite cells (2), which we extend to 8 wk in this study. We propose that the accumulation of fibrotic, noncontractile tissue in satellite cell-depleted muscle at this later 8 wk time point contributes to the loss of force production of the plantaris as a whole. The ECM is involved in the lateral transmission of force (41), and perturbations in the glycoprotein complex of the ECM can result in impaired force transmission (42). Thus, excess ECM accumulation may physically hinder both hypertrophy and force production. Components of the muscle ECM are known to regulate satellite cell self renewal and muscle regeneration following injury (43); the ECM is an integral part of the signaling mechanism involved in myogenesis via MPC adhesion and proliferation (12, 44). The ECM is also essential in providing scaffolding for complete muscle regeneration, that is dysregulated in the absence of satellite cells (8). A similar outcome may arise during muscle hypertrophy, where the removal of satellite cells yields a net accumulation of muscle ECM, which negatively affects the hypertrophic potential and contractile function of the muscle.
Activity of the TGF-β signaling pathway has been linked to fibroblast proliferation (45–46), overproduction of ECM, and fibrosis in the lung and liver (47–48), as well as in dystrophic muscle (49). We hypothesized that up-regulation of the TGF-β signaling pathway may explain the observed increase in ECM production and fibroblast expansion. We did not detect differences in the relative proportion of phospho-SMAD3+ nuclei in satellite cell-depleted muscle; in fact, we detected a decrease in phospho-SMAD3+ nuclei with overload irrespective to satellite cell content. We also failed to detect a difference in phospho-SMAD3 protein in fibroblasts cultured with MPCs or fibroblasts. From these data, it does not appear that modulation of the TGF-β signaling pathway is responsible for the observed increased fibroblast content and ECM accumulation. However, our in vitro experimental results suggest that a secreted MPC factor modulates fibroblast ECM gene expression. Future experiments will be directed toward the identification and characterization of this factor.
Through the use of γ-irradiation, several studies concluded a necessity of satellite cells in the hypertrophic response of skeletal muscle to a growth stimulus (4–5, 50–51). The development of the Pax7-DTA strain represents a significant improvement in the ability to specifically target satellite cells in adult skeletal muscle and assess their role during hypertrophy (2). While previous data (2), as well as results in the current study, demonstrate equivalent hypertrophy in satellite cell-depleted muscle through the first several weeks of functional overload, a longer period of overload (8 wk) yielded a blunted hypertrophic response. In addition to attenuated hypertrophy in the satellite cell-depleted SA-8 mice, characteristic myonuclear accretion that occurs during hypertrophy was also prevented. Previous work in our laboratory illustrated a larger myonuclear domain in satellite cell-depleted muscle that underwent overload for 2 wk (2), suggesting that each myonucleus was capable of governing a larger cytosolic volume. The plasticity of skeletal muscle can accommodate increases and decreases in the size of the myonuclear domain (50, 52). However, the attenuated hypertrophy observed in the current study could be due to an inability of the myonuclear domain to expand beyond a certain point. A ceiling may exist beyond which the domain can no longer increase without addition of a new myonucleus, as has been proposed for human muscle (53–55). Although we hypothesize that the increased accumulation of ECM negatively affects muscle hypertrophy, it is also possible that restricted fiber growth may promote accumulation of ECM. The present study does not determine cause and effect but does demonstrate a clear association between blunted growth and ECM accumulation. We hypothesize that in the absence of satellite cell-dependent myonuclear accretion, both increased ECM accumulation and a myonuclear domain ceiling may limit muscle fiber adaptation.
Supplementary Material
Acknowledgments
The authors acknowledge Ben Lawson and Ken Campbell (Center of Muscle Biology Single Fiber Function core services, University of Kentucky).
Research was supported by the Jeane B. Kempner Postdoctoral Scholar Award (to C.S.F.); Ellison Medical Foundation/American Federation of Aging Research Fellow EPD 12102 (to J.D.L.); and U.S. National Institutes of Health (NIH) grants AG-34453 (to C.A.P.) and AR-60701 (to C.A.P. and J.J.M.). S.A.S. was supported by NIH grant T32-HL-086341. The project described was also partially supported by the National Center for Advancing Translational Sciences, NIH grant UL1-TR-000117.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- bFGF
- basic fibroblast growth factor
- BrdU
- 5-bromo-2′-deoxyuridine
- CM
- conditioned medium
- CSA
- cross-sectional area
- DAPI
- 4′,6-diamidino-2-phenylindole
- DMEM
- Dulbecco's modified Eagle's medium
- ECM
- extracellular matrix
- F4/80
- mouse pan-macrophage marker
- FAP
- fibrogenic/adipogenic precursor
- GM
- growth medium
- HRP
- horseradish peroxidase
- Lo
- optimal length
- MPCs
- myogenic progenitor cells
- Pax7
- paired box 7
- PBS
- phosphate-buffered saline
- PDGFRα
- platelet-derived growth factor receptor α
- PFA
- paraformaldehyde
- RIPA
- radioimmunoprecipitation assay
- SA
- synergist ablation
- Tcf4
- transcription factor 4
- TGF-β
- transforming growth factor-β
- TSA
- tyramide signal amplification
REFERENCES
- 1. Zammit P. S., Partridge T. A., Yablonka-Reuveni Z. (2006) The skeletal muscle satellite cell: the stem cell that came in from the cold. J. Histochem. Cytochem. 54, 1177–1191 [DOI] [PubMed] [Google Scholar]
- 2. McCarthy J. J., Mula J., Miyazaki M., Erfani R., Garrison K., Farooqui A. B., Srikuea R., Lawson B. A., Grimes B., Keller C., Van Zant G., Campbell K. S., Esser K. A., Dupont-Versteegden E. E., Peterson C. A. (2011) Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development 138, 3657–3666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schiaffino S., Bormioli S. P., Aloisi M. (1976) Fate of newly formed satellite cells during compensatory muscle hypertrophy. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 21, 113–118 [DOI] [PubMed] [Google Scholar]
- 4. Rosenblatt J. D., Parry D. J. (1992) Gamma-irradiation prevents compensatory hypertrophy of overloaded mouse extensor digitorum longus muscle. J. Appl. Physiol. 73, 2538–2543 [DOI] [PubMed] [Google Scholar]
- 5. Rosenblatt J. D., Yong D., Parry D. J. (1994) Satellite cell-activity is required for hypertrophy of overloaded adult-rat muscle. Muscle Nerve 17, 608–613 [DOI] [PubMed] [Google Scholar]
- 6. Sambasivan R., Yao R., Kissenpfennig A., Van Wittenberghe L., Paldi A., Gayraud-Morel B., Guenou H., Malissen B., Tajbakhsh S., Galy A. (2011) Pax7-expressing satellite cells are indispensable for adult skeletal muscle regeneration. Development 138, 3647–3656 [DOI] [PubMed] [Google Scholar]
- 7. Lepper C., Partridge T. A., Fan C.-M. (2011) An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development 138, 3639–3646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Murphy M. M., Lawson J. A., Mathew S. J., Hutcheson D. A., Kardon G. (2011) Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development 138, 3625–3637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goetsch S. C., Hawke T. J., Gallardo T. D., Richardson J. A., Garry D. J. (2003) Transcriptional profiling and regulation of the extracellular matrix during muscle regeneration. Physiol. Genomics 14, 261–271 [DOI] [PubMed] [Google Scholar]
- 10. Caldwell C. J., Mattey D. L., Weller R. O. (1990) Role of basement-membrane in the regeneration of skeletal-muscle. Neuropathol. Appl. Neurobiol. 16, 225–238 [DOI] [PubMed] [Google Scholar]
- 11. Kaariainen M., Jarvinen T., Jarvinen M., Rantanen J., Kalimo H. (2000) Relation between myofibers and connective tissue during muscle injury repair. Scand. J. Med. Sci. Sports 10, 332–337 [DOI] [PubMed] [Google Scholar]
- 12. Kuhl U., Timpl R., Vondermark K. (1982) Synthesis of type IV collagen and laminin in cultures of skeletal-muscle cells and their assembly on the surface of myotubes. Dev. Biol. 93, 344–354 [DOI] [PubMed] [Google Scholar]
- 13. Sasse J., Vondermark H., Kuhl U., Dessau W., Vondermark K. (1981) Origin of collagen type-I, type-III and type-V in cultures of avian skeletal-muscle. Dev. Biol. 83, 79–89 [DOI] [PubMed] [Google Scholar]
- 14. Lipton B. H. (1977) Collagen-synthesis by normal and bromodeoxyuridine-modulated cells in myogenic culture. Dev. Biol. 61, 153–165 [DOI] [PubMed] [Google Scholar]
- 15. Cheek D. B., Powell G. K., Scott R. E. (1965) Growth of muscle mass and skeletal collagen in rat. I. Normal growth. II. Effect of ablation of pituitary thyroid or testes. Bull. Johns Hopkins Hosp. 116, 378–395 [PubMed] [Google Scholar]
- 16. Gordon S. E., Davis B. S., Carlson C. J., Booth F. W. (2001) ANG II is required for optimal overload-induced skeletal muscle hypertrophy. Am. J. Physiol. Endocrinol Metab. 280, E150–E159 [DOI] [PubMed] [Google Scholar]
- 17. Campbell K. S. (2006) Tension recovery in permeabilized rat soleus muscle fibers after rapid shortening and restretch. Biophys. J. 90, 1288–1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Close R. I. (1972) Dynamic properties of mammalian skeletal muscles. Physiol. Rev. 52, 129–197 [DOI] [PubMed] [Google Scholar]
- 19. Ferreira L. F., Moylan J. S., Stasko S., Smith J. D., Campbell K. S., Reid M. B. (2012) Sphingomyelinase depresses force and calcium sensitivity of the contractile apparatus in mouse diaphragm muscle fibers. J. Appl. Physiol. 112, 1538–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hidestrand M., Richards-Malcolm S., Gurley C. M., Nolen G., Grimes B., Waterstrat A., Van Zant G., Peterson C. A. (2008) Sca-1-expressing nonmyogenic cells contribute to fibrosis in aged skeletal muscle. J. Gerontol. A Biol. Sci. Med. Sci. 63, 566–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rando T. A., Blau H. M. (1994) Prinary mouse myoblast purification, characterization, and transplantation for cell-mediated gene-therapy. J. Cell Biol. 125, 1275–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Taylor-Jones J. M., McGehee R. E., Rando T. A., Lecka-Czernik B., Lipschitz D. A., Peterson C. A. (2002) Activation of an adipogenic program in adult myoblasts with age. Mech. Ageing Dev. 123, 649–661 [DOI] [PubMed] [Google Scholar]
- 23. Rocheteau P., Gayraud-Morel B., Siegl-Cachedenier I., Blasco M. A., Tajbakhsh S. (2012) A subpopulation of adult skeletal muscle stem cells retains all template DNA strands after cell division. Cell 148, 112–125 [DOI] [PubMed] [Google Scholar]
- 24. Kirkpatrick L. J., Yablonka-Reuveni Z., Rosser B. W. C. (2010) Retention of Pax3 expression in satellite cells of muscle spindles. J. Histochem. Cytochem. 58, 317–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Noble E. G., Dabrowski B. L., Ianuzzo C. D. (1983) Myosin transformation in hypertrophied rat muscle. Pflügers Archiv. Eur. J. Physiol. 396, 260–262 [DOI] [PubMed] [Google Scholar]
- 26. Roy R. R., Baldwin K. M., Martin T. P., Chimarusti S. P., Edgerton V. R. (1985) Biochemical and physiological-changes in overloaded rat fast-twitch and slow-twitch ankle extensors. J. Appl. Physiol. 59, 639–646 [DOI] [PubMed] [Google Scholar]
- 27. Verrecchia F., Chu M. L., Mauviel A. (2001) Identification of novel TGF-beta/Smad gene targets in dermal fibroblasts using a combined cDNA microarray/promoter transactivation approach. J. Biol. Chem. 276 [DOI] [PubMed] [Google Scholar]
- 28. Zou Y., Zhang R. Z., Sabatelli P., Chu M. L., Bonnemann C. G. (2008) Muscle interstitial fibroblasts are the main source of collagen VI synthesis in skeletal muscle: Implications for congenital muscular dystrophy types Ullrich and Bethlem. J. Neuropathol. Expl. Neurol. 67, 144–154 [DOI] [PubMed] [Google Scholar]
- 29. Sanderson R. D., Fitch J. M., Linsenmayer T. R., Mayne R. (1986) Fibroblasts promote the formation of a continuous basal lamina during myogenesis invitro. J. Cell Biol. 102, 740–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mathew S. J., Hansen J. M., Merrell A. J., Murphy M. M., Lawson J. A., Hutcheson D. A., Hansen M. S., Angus-Hill M., Kardon G. (2011) Connective tissue fibroblasts and Tcf4 regulate myogenesis. Development 138, 371–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang Y., Li H., Lian Z., Li N. (2010) Myofibroblasts protect myoblasts from intrinsic apoptosis associated with differentiation via beta 1 integrin-PI3K/Akt pathway. Dev. Growth Differ. 52, 725–733 [DOI] [PubMed] [Google Scholar]
- 32. Uezumi A., Fukada S.-i., Yamamoto N., Takeda S. I., Tsuchida K. (2010) Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat. Cell Biol. 12, 143–152 [DOI] [PubMed] [Google Scholar]
- 33. Uezumi A., Ito T., Morikawa D., Shimizu N., Yoneda T., Segawa M., Yamaguchi M., Ogawa R., Matev M. M., Miyagoe-Suzuki Y., Takeda S. I., Tsujikawa K., Tsuchida K., Yamamoto H., Fukada S. (2011) Fibrosis and adipogenesis originate from a common mesenchymal progenitor in skeletal muscle. J. Cell Sci. 124, 3654–3664 [DOI] [PubMed] [Google Scholar]
- 34. Joe A. W. B., Yi L., Natarajan A., Le Grand F., So L., Wang J., Rudnicki M. A., Rossi F. M. (2010) Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat. Cell Biol. 12, 153–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cosgrove B. D., Sacco A., Gilbert P. M., Blau H. M. (2009) A home away from home: Challenges and opportunities in engineering in vitro muscle satellite cell niches. Differentiation 78, 185–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sato K., Li Y., Foster W., Fukushima K., Badlani N., Adachi N., Usas A., Fu F. H., Huard J. (2003) Improvement of muscle healing through enhancement of muscle regeneration and prevention of fibrosis. Muscle Nerve 28, 365–372 [DOI] [PubMed] [Google Scholar]
- 37. Li Y., Foster W., Deasy B. M., Chan Y. S., Prisk V., Tang Y., Cummins J., Huard J. (2004) Transforming growth factor-beta 1 induces the differentiation of myogenic cells into fibrotic cells in injured skeletal muscle - A key event in muscle fibrogenesis. Am. J. Pathol. 164, 1007–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zanotti S., Saredi S., Ruggieri A., Fabbri M., Blasevich F., Romaggi S., Morandi L., Mora M. (2007) Altered extracellular matrix transcript expression and protein modulation in primary Duchenne muscular dystrophy myotubes. Matrix Biol. 26, 615–624 [DOI] [PubMed] [Google Scholar]
- 39. Porter J. D., Khanna S., Kaminski H. J., Rao J. S., Merriam A. P., Richmonds C. R., Leahy P., Li J. J., Guo W., Andrade F. H. (2002) A chronic inflammatory response dominates the skeletal muscle molecular signature in dystrophin-deficient mdx mice. Hum. Mol. Genet. 11, 263–272 [DOI] [PubMed] [Google Scholar]
- 40. Kjaer M. (2004) Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol. Rev. 84, 649–698 [DOI] [PubMed] [Google Scholar]
- 41. Zhang C., Gao Y. (2012) Finite element analysis of mechanics of lateral transmission of force in single muscle fiber. J. Biomech. 45, 2001–2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ramaswamy K. S., Palmer M. L., van der Meulen J. H., Renoux A., Kostrominova T. Y., Michele D. E., Faulkner J. A. (2011) Lateral transmission of force is impaired in skeletal muscles of dystrophic mice and very old rats. J. Physiol. 589, 1195–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Urciuolo A., Quarta M., Morbidoni V., Gattazzo F., Molon S., Grumati P., Montemurro F., Tedesco F.S., Blaauw B., Cossu G., Vozzi G., Rando T. A., Bonaldo P. (2013) Collagen VI regulates satellite cell self-renewal and muscle regeneration. Nat. Commun. 4, 1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Foster R. F., Thompson J. M., Kaufman S. J. (1987) A laminin substrate promotes myogenesis in rat skeletal-muscle cultures–analysis of replcation and development using antidesmin and anti-BrdU monoclonal-antibodies. Dev. Biol. 122, 11–20 [DOI] [PubMed] [Google Scholar]
- 45. Kay E. P., Lee M. S., Seong G. J., Lee Y. G. (1998) TGF-betas stimulate cell proliferation via an autocrine production of FGF-2 in corneal stromal fibroblasts. Curr. Eye Res. 17, 286–293 [DOI] [PubMed] [Google Scholar]
- 46. Strutz F., Zeisberg M., Renziehausen A., Raschke B., Becker V., van Kooten C., Muller G. A. (2001) TGF-beta 1 induces proliferation in human renal fibroblasts via induction of basic fibroblast growth factor (FGF-2). Kidney Int. 59, 579–592 [DOI] [PubMed] [Google Scholar]
- 47. Broekelmann T. J., Limper A. H., Colby T. V., McDonald J. A. (1991) Transforming growth factor-beta-1 is present at sites of extracellular-matrix gene expression in human pulmonary fibrosis. Proc. Natl. Acad. Sci. U. S. A. 88, 6642–6646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Castilla A., Prieto J., Fausto N. (1991) Transforming growth factor-beta-1 and factor-alpha in chronic liver-disease–effects of interferon alpha therapy. N. Engl. J. Med. 324, 933–940 [DOI] [PubMed] [Google Scholar]
- 49. Bernasconi P., Torchiana E., Confalonieri P., Brugnoni R., Barresi R., Mora M., Cornelio F., Morandi L., Mantegazza R. (1995) Expression of transforming growth-factor-beta-1 in dystrophic patient muscles correlates with fibrosis - pathogenetic role of a fibrogenic cytokine. J. Clin. Investig. 96, 1137–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Adams G. R., Caiozzo V. J., Haddad F., Baldwin K. M. (2002) Cellular and molecular responses to increased skeletal muscle loading after irradiation. Am. J. Physiol. Cell Physiol. 283, C1182–C1195 [DOI] [PubMed] [Google Scholar]
- 51. Phelan J. N., Gonyea W. J. (1997) Effect of radiation on satellite cell activity and protein expression in overloaded mammalian skeletal muscle. Anat. Rec. 247, 179–188 [DOI] [PubMed] [Google Scholar]
- 52. Bruusgaard J. C., Johansen I. B., Egner I. M., Rana Z. A., Gundersen K. (2010) Myonuclei acquired by overload exercise precede hypertrophy and are not lost on detraining. Proc. Natl. Acad. Sci. U. S. A. 107, 15111–15116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Petrella J. K., Kim J. S., Cross J. M., Kosek D. J., Bamman M. M. (2006) Efficacy of myonuclear addition may explain differential myofiber growth among resistance-trained young and older men and women. Am. J. Physiol. Endocrinol. Metab. 291, E937–E946 [DOI] [PubMed] [Google Scholar]
- 54. Verdijk L. B., Gleeson B. G., Jonkers R. A. M., Meijer K., Savelberg H., Dendale P., van Loon L. J. C. (2009) Skeletal muscle hypertrophy following resistance training is accompanied by a fiber type-specific increase in satellite cell content in elderly men. J. Gerontol. A Biol. Sci. Med. Sci. 64, 332–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Petrella J. K., Kim J.-S., Mayhew D. L., Cross J. M., Bamman M. M. (2008) Potent myofiber hypertrophy during resistance training in humans is associated with satellite cell-mediated myonuclear addition: a cluster analysis. J. Appl. Physiol. 104, 1736–1742 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.