Abstract
Background
Peripheral nerve stimulation has been used to treat patients with occipital nerve–related chronic headaches who have been unsuccessful with less invasive therapeutic approaches. Patients with pacemaker-dependent cardiac conduction abnormalities require unique consideration prior to the implantation of peripheral nerve stimulators because the placement of the devices may lead to failure of the systems secondary to electromagnetic interference or crosstalk between the devices.
Case Report
An 86-year-old female who suffered from chronic right-sided cervicogenic headaches and neck pain had received only temporary relief from previous treatments. Additional comorbidities included longstanding pacemaker-dependent atrioventricular node conduction disease. Because the extent to which nerve stimulators electrically interact with pacemakers is unclear, we tunneled the leads to the lumbar region of the back and placed the generator on the contralateral side to the pacemaker to minimize the chance that the 2 devices would interfere. The patient has remained pain free for 1 year since implantation.
Conclusion
Although no current published trials evaluate the degree of interference between medical devices, case reports increasingly suggest that simultaneous implantation of a spinal cord stimulator and pacemaker is safe as long as precautions are taken and the devices are checked periodically, particularly when the devices are adjusted.
Keywords: Implantable neurostimulators, neuralgia, pacemaker–artificial, transcutaneous electric nerve stimulation
INTRODUCTION
Patients with occipital neuralgia can present with headaches that involve the hemicranium from the occiput to the supraorbital ridge.1 The dorsal ramus of C2 gives rise to branches that eventually form the greater and lesser occipital nerves. Stretching or entrapment of the occipital nerve anywhere along its anatomical path can result in chronic headaches.
Peripheral nerve stimulation has been used to treat various neuropathic pain disorders in patients who have failed less invasive therapeutic approaches.1 Nerve-stimulating electrodes placed in the subcutaneous tissue overlying the greater and lesser occipital nerves and the paracervical muscles have been used successfully to treat persistent occipital neuralgia refractory to more conservative medical treatments.2 However, patients with permanent pacemaker (PPM)-dependent cardiac conduction abnormalities require unique consideration prior to the implantation of peripheral nerve stimulation. The placement of both a PPM and a pulse generator with peripheral electrodes in the same patient may lead to failure of 1 or both of the systems secondary to electromagnetic interference or crosstalk between the 2 devices.
CASE REPORT
An 86-year-old female presented with chronic right-sided cervicogenic headaches and neck pain that limited her daily activities. She had been treated previously with medications and diagnostic nerve blocks followed by radiofrequency ablation of the cervical facet joints and greater and lesser occipital nerves. All previous treatments offered only temporary relief. She described her headaches as continuous, right-sided, sharp, stabbing pain extending from the occiput to the supraorbital ridge. In addition, she reported pain on the right side of her neck with rotation and lateral flexion. Her additional comorbidities included longstanding PPM-dependent atrioventricular node conduction disease. Her pacemaker's bipolar lead configuration was set to DDDR pacing (ALTRUA 60, Model S602, Boston Scientific) at 70 bpm. The pacemaker generator was embedded in an anterior left-sided subcutaneous pocket inferior to the clavicle.
A peripheral nerve field stimulation trial successfully covered the patient's area of pain and brought her reported pain to 0/10 on the pain scale. Subsequently, 2 bipolar octad leads with compact spacing (Restore with 1 × 8 Compact Test Lead Model 3874-45, Medtronic) were placed percutaneously in the subcutaneous tissue. One lead was placed at the level of C1-C2 overlying the distribution of distal sensory branches of the occipital nerve on the right side. The other lead was set in the subcutaneous tissue overlying the right paracervical muscles (Figures 1 and 2). Intraoperative testing of the leads was performed with representatives for the stimulator and the pacemaker present and reprogramming equipment readily available. Intraoperative testing indicated that stimulation from the leads in this configuration completely captured all areas of pain the patient had previously experienced. The leads were then tunneled to the generator that was placed in a subcutaneous pocket created on the right side, lateral to the lumbar spine and just superior to the iliac crests. This placement maximized the distance between the generator for the pacemaker and the pulse generator for the stimulating electrodes.
Figure 1.
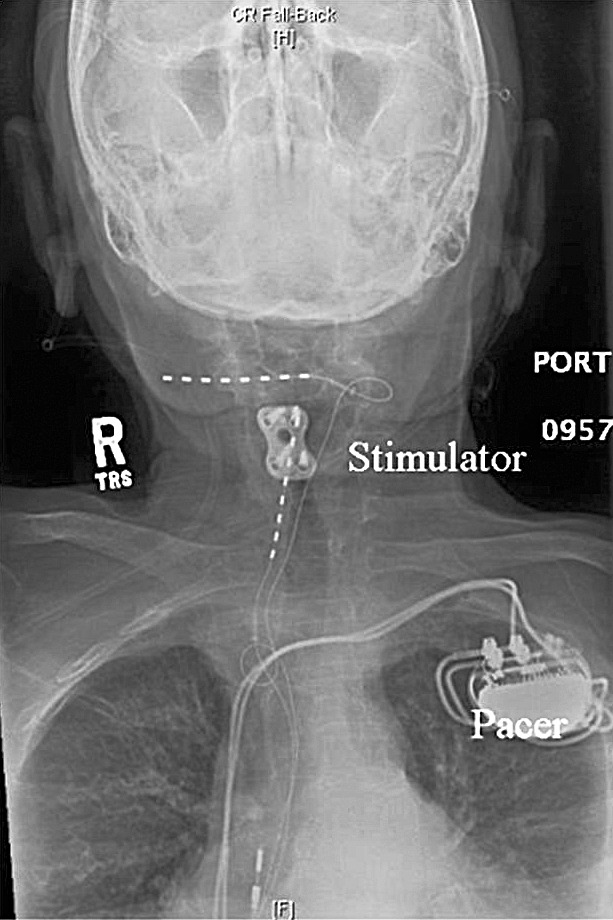
Anteroposterior view of the final electrode positions.
Figure 2.
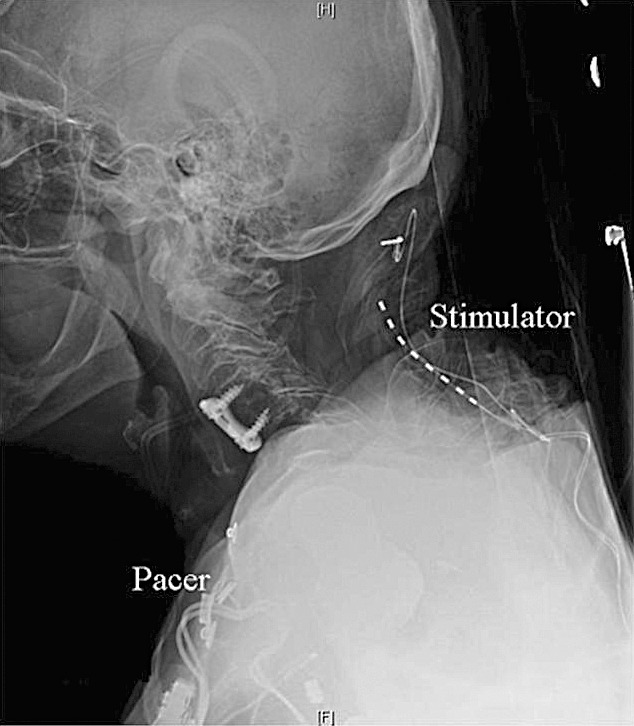
Lateral view of final electrode positions.
The stimulation setting that most optimally covered all the painful areas in the head was 1.7 V with a pulse width of 300 μs at 40 Hz. For the paracervically placed lead, the optimal configuration was 1.6 V with a pulse width of 300 μs at 40 Hz. When the pacemaker was interrogated at these settings, no evidence of interference between the 2 devices was detected. Voltage was increased from the stimulator to 6.6 V, the maximum tolerated by the patient, and no evidence of interference between the 2 devices was detected. The sensitivity of the ventricular leads was changed to the lowest setting on the model (0.25 mV), and no interference was detected (Figures 3 and 4).
Figure 3.
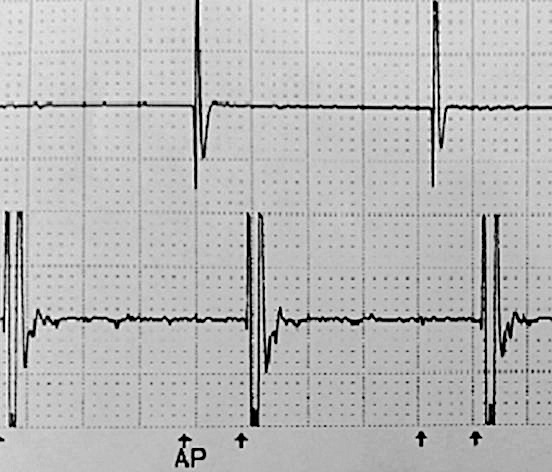
Electrocardiogram with sensitivity set to 0.25 mV and stimulation turned off.
Figure 4.
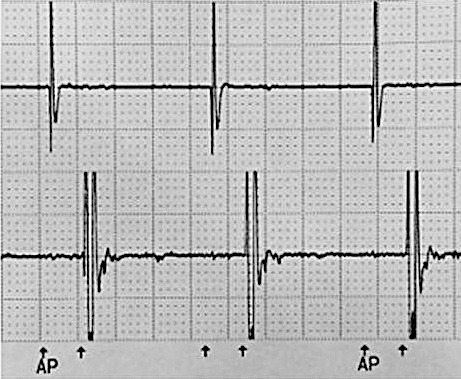
Electrocardiogram with sensitivity set to 0.25 mV and stimulation turned on.
After implantation, the patient was followed weekly for 3 weeks to ensure proper wound healing and then monthly for 3 months. The patient was subsequently seen on an as-needed basis. Our patient had become depressed and withdrawn secondary to her continuous headaches, but after placement of the stimulator she consistently reported 100% pain relief in follow-up visits up to 1 year after the implant. She has had no interactions or problems with her pacemaker.
DISCUSSION
Occipital neuralgia refractory to medical treatment has been successfully treated with peripheral nerve field stimulation.1 Concerns about electromagnetic interference between stimulators used to treat chronic pain and PPMs or implantable cardiac defibrillators (ICDs) have been reported previously.3 A literature review by Ooi et al revealed 57 unique cases, 41 of which involved PPMs and spinal cord stimulators.4 In one case, intermittent inhibition of the pacemaker occurred with increasing stimulation amplitudes from a spinal cord stimulator, and another case reported the total reset of a deep brain stimulator's settings after delivery of a therapeutic shock from an ICD but no interference of the proper functioning of the life-saving ICD. In their conclusion, the authors suggested that PPMs and neurostimulators can be used together safely, but their review did not include any reports of peripheral nerve field stimulation interactions with PPMs.
While the implantation of neuraxial spinal cord stimulators in patients with PPMs increasingly can be done safely with proper planning and testing, the extent to which peripheral nerve stimulators electrically interact with PPMs is unclear. In contrast to spinal cord stimulators that place leads in the epidural space and tend to locate the generators in subcutaneous tissues at the level of the lumbar spine, our peripheral nerve field stimulator was implanted in the same plane of tissue as our patient's PPM and the leads for the 2 devices were proximate.
In patients who do not have an implantable PPM or ICD, our normal practice is to place the pulse generator in the subcutaneous tissue overlying the trapezius muscle for electrodes placed in the region of the occipital nerve. In planning this case, however, we decided to tunnel the leads to the lumbar region of the back and place the generator on the contralateral side of the PPM to minimize the chance that the 2 devices would interfere with each other. Fortunately, no evidence of interference between the 2 devices occurred in this case, and the patient has remained pain free for 1 year since the date of implantation.
CONCLUSION
While no current published trials evaluate the degree of interference between medical devices, an increasing number of case reports suggest that simultaneous implantation is safe as long as precautions are taken and the devices are checked periodically, particularly when adjustments to either device are being made.
Footnotes
Presented in poster form at the 16th annual meeting of the North American Neuromodulation Society in Las Vegas, NV, December 6-9, 2012.
The authors have no financial or proprietary interest in the subject matter of this article.
This article meets the Accreditation Council for Graduate Medical Education and the American Board of Medical Specialties Maintenance of Certification competencies for Patient Care and Medical Knowledge.
REFERENCES
- 1.Kapural L, Sable J. Peripheral nerve stimulation for occipital neuralgia: surgical leads. Prog Neurol Surg. 2011; 24:86–95. doi: 10.1159/000323042. Epub 2011 Mar 21. [DOI] [PubMed] [Google Scholar]
- 2.Serra G, Marchioretto F. Occipital nerve stimulation for chronic migraine: a randomized trial. Pain Physician. 2012 May-Jun;15(3):245–253. [PubMed] [Google Scholar]
- 3.Molon G, Perrone C, Maines M, et al. ICD and neuromodulation devices: is peaceful coexistence possible? Pacing Clin Electrophysiol. 2011 Jun;34(6):690–693. doi: 10.1111/j.1540-8159.2011.03036.x. Epub 2011 Feb 8. [DOI] [PubMed] [Google Scholar]
- 4.Ooi YC, Falowski S, Wang D, Jallo J, Ho RT, Sharan A. Simultaneous use of neurostimulators in patients with a preexisting cardiovascular implantable electronic device. Neuromodulation. 2011 Jan;14(1):20–25. doi: 10.1111/j.1525-1403.2010.00314.x. discussion 25-26. Epub 2010 Nov 2. [DOI] [PubMed] [Google Scholar]


