Abstract
Background
Interlaminar epidural steroid injections (ILESIs) are commonly employed in the management of patients with symptomatic degenerative lumbar spinal canal stenosis despite little experimental evidence to guide technique optimization. One untested performance parameter is the intervertebral level at which the ILESI should be performed for maximum patient relief.
Methods
This study randomized patients with symptomatic degenerative lumbar spinal canal stenosis to receive an ILESI at the level of maximal spinal canal stenosis or at a normal/less stenotic intervertebral site 2 intervertebral levels cephalad to the level of maximal stenosis. Pain with ambulation and Roland Morris Disability Questionnaire scores were collected prior to the procedure and at 1-, 4-, and 12-week follow-ups.
Results
Fifty-seven patients were enrolled. Thirty patients (Group 1) received an ILESI at the level of maximal stenosis; 27 patients (Group 2) received an ILESI at a less stenotic level. The mean baseline preprocedural maximal pain with ambulation and disability scores for the 2 groups were not significantly different (P=0.94 and P=0.13, respectively). Patients' pain with ambulation scores were significantly lower in Group 1 compared to Group 2 at 1 and 4 weeks postinjection, but they were not significantly lower at 12 weeks (1 week, P=0.045; 4 weeks, P=0.049; 12 weeks, P=0.08). The mean Roland Morris Disability Questionnaire scores at 1, 4, and 12 weeks postinjection were significantly lower in Group 1 as compared to Group 2 (P=0.001, P=0.009, P=0.003, respectively).
Conclusion
Results suggest that patient symptom improvement is optimized when the ILESI is performed at the intervertebral level of maximal stenosis.
Keywords: Injections–epidural, low back pain, spinal stenosis
INTRODUCTION
Symptomatic degenerative lumbar spinal canal stenosis (DLSCS) is a common cause of spinal origin pain and dysfunction. Despite several studies demonstrating that surgery tends to achieve more favorable long-term outcomes,1-3 nonsurgical options are advocated for first-line therapy. Interlaminar epidural steroid injections (ILESIs) are an increasingly employed nonsurgical intervention for patients' short-term relief.3-11 However, a paucity of data exists with regard to the technical components of proper ILESI performance. The lack of standardization and validation of many ILESI technique parameters may lead to variability in DLSCS patient response. Many practitioners cite the need for more randomized controlled trials that investigate technical parameter validation.12,13
This study focuses on one important untested performance parameter: the intervertebral level at which an ILESI should be performed to achieve optimal effectiveness. A review of the English-language literature yields little guidance for selecting the most appropriate intervertebral level for ILESI performance. One source suggests that the ILESI may be performed at any level, most often at L3-L4 or L4-L5.14 Another source states that the injection can be performed at any level, preferably at or below the intervertebral level of pathology.15 Neither of these citations offers any rationale for these statements. The foundation for these unsupported claims is likely that the L4-L5 intervertebral level is the most commonly afflicted level in patients with DLSCS. Therefore, our supposition is that in current practice the intervertebral level of ILESI performance is selected on the basis of opinion, prior training, and experience, rather than on experimentally derived data.
At Ochsner Clinic Foundation, we review the cross-sectional imaging of all patients with DLSCS who are referred for ILESI to determine the most stenotic intervertebral level. We confirm that the anatomical distribution of the patient's pain is referable to or below the maximally stenotic intervertebral level. Without a clear standard, we then perform the ILESI at the level of maximal spinal canal narrowing. We use this performance strategy because the most stenotic level is likely the principal source for the patient's symptoms and delivering medication directly to this location will likely maximize therapeutic effect. Some interventionalists may disagree with this methodology, citing concern that pressure from the injectate may exacerbate the patients' symptoms. Our clinical experience does not support this concern, but this notion has not been studied experimentally.
Driven by the absence of empirical data to guide the site of injection, we investigated the hypothesis that performance of the ILESI in patients with symptomatic DLSCS at the level of maximal stenosis is more effective than injection at a nearby, less stenotic level. The study evaluated the effect on the patient's pain with ambulation (neurogenic claudication—the clinical hallmark of DLSCS) as well as functional disability. We also evaluated the immediate postprocedural pain in both groups to evaluate the potential negative effect of injecting at the level of stenosis.
METHODS
This study was conducted under the oversight of the institutional review board and was deemed Health Insurance Portability and Accountability Act compliant. From September 2006 to October 2007, the clinical histories of all patients (n=236) referred to the interventional radiology section for ILESI performance were reviewed. To be included in the study, the patient had to suffer from bilateral lower extremity pain referable to the lumbar spine, caused and/or exacerbated by ambulation. In addition, the patient was required to have undergone cross-sectional imaging (magnetic resonance imaging or computed tomography) consistent with DLSCS. The clinical history and preprocedural imaging were reviewed by the attending staff authors (JM, DK) for study eligibility. The clinical symptoms had to correlate with the intervertebral level of maximal stenosis for inclusion. Patients were included if they had additional less stenotic levels unless a dominant level was unable to be determined. Patients who had exposure to steroids in the past 3 months or who had undergone lumbar spinal surgery were excluded.
Qualifying patients were invited to participate and informed consent was obtained. Using an analog pain scale of 0 to 10 (0=no pain, 10=worst pain imaginable), the enrolled patients' maximal pain during ambulation scores and resting pain level scores were recorded at the time of the interview (5 minutes prior to the ILESI). Patients were also administered a Roland Morris Disability Questionnaire (RMDQ), a widely used health status measure for low back pain at baseline.16,17
The patients were then randomized to have their fluoroscopically guided lumbar ILESI performed either at the level of maximal stenosis or 2 intervertebral levels cephalad, corresponding to a less stenotic level. Injection was performed with a 20-gauge Tuohy needle using a loss of resistance technique. The injectate consisted of 2 mL of 40 mg/mL methylprednisolone (Pfizer), 2 mL of bupivacaine 0.25% (Hospira), and 2 mL of normal saline for a total injectate volume of 6 mL. The patient was blinded to the injection level. After the procedure, the patient was allowed a 5-minute break period. Following this break, the patient's resting pain postprocedural score using the 0-10 analog pain scale was obtained. The patient was then discharged per normal protocol.
At 1, 4, and 12 weeks postinjection, the patients were reinterviewed via phone by a person blinded to the level at which the patient's ILESI was performed. The patient's maximal pain with ambulation and the RMDQ scores were again queried. The patient's status regarding postinjection steroid exposure or surgery was also determined. The primary endpoint for this study was the 12-week postinjection interview. Patients were no longer followed if they declined continued participation, became unreachable for follow-up interviews, had additional steroid exposure, or underwent spinal surgery.
Statistical Tests
Study variables included maximal pain scores with ambulation and RMDQ scores preinjection and at 1, 4, and 12 weeks postinjection, as well as pain scores at rest several minutes prior to the injection and approximately 5 minutes postinjection. These factors were analyzed using linear regression with maximum likelihood estimation. This analysis allowed for inclusion of subjects with incomplete data and controlled for clustering due to the repeated measures on each subject. Significance was defined as P≤0.05.
RESULTS
Fifty-seven patients met inclusion criteria, agreed to participate, and were enrolled. Twenty patients were male; 37 were female. Mean patient age was 65.3 years (range, 32-88 years). Average duration of symptomatology (pain and/or disability) was 42 months. The mean degree of canal narrowing at the most stenotic level was 6.1 mm (range, 2.5-9.1 mm). The most common maximally stenotic intervertebral level was L4-L5 (n=42) followed by L3-L4 (n=11) and L5-S1 (n=4).
Thirty patients received their ILESI at the level of maximal stenosis (Group 1); the remaining 27 received their ILESI 2 intervertebral levels cephalad to the level of maximal stenosis (Group 2).
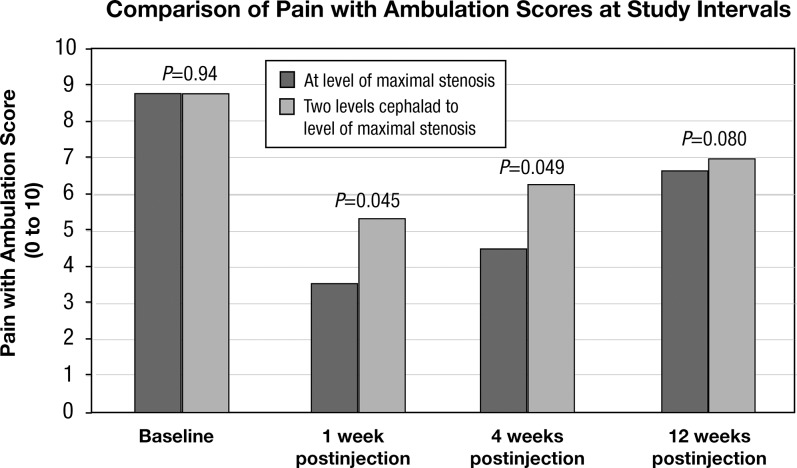
Group 1 had a mean baseline maximal pain with ambulation score of 8.8 (standard deviation [SD] 1.6, n=30) that decreased to 3.6 (SD 3.3, n=28) at 1 week postinjection and increased to 4.5 (SD 3.9, n=27) at 4 weeks and 6.7 (SD 5.3, n=15) at 12 weeks postinjection. Group 2 had a mean baseline maximal pain with ambulation score of 8.8 (SD 2.0, n=27) that decreased to 5.4 (SD 3.5, n=24) at 1 week postinjection and increased to 6.3 (SD 3.2, n=20) at 4 weeks and 7.0 (SD 2.9, n=13) at 12 weeks postinjection (Figure 1).
Figure 1.
Graph showing maximum pain with ambulation scores at baseline, 1, 4, and 12 weeks postinjection for both study groups. Comparison of the values demonstrates greater reduction in pain with ambulation scores at 1 and 4 weeks postinjection for the study group participants who received their interlaminar epidural steroid injections at the level of maximal stenosis as compared to the other group; this difference was statistically significant. The trend continued at 12 weeks but the difference was not statistically significant, possibly due to the decrease in the number of patients included at this point in the study.
The difference between the 2 groups in mean baseline maximal pain with ambulation scores (P=0.94) and disability scores (P=0.13) was not statistically significant. The mean maximal pain with ambulation scores at 1, 4, and 12 weeks postinjection in Group 1 were significantly lower than the mean baseline score (1 week, P<0.001; 4 weeks, P<0.001; 12 weeks, P<0.001). Group 2 also demonstrated significantly lower mean maximal pain with ambulation scores at 1 and 4 weeks postinjection (1 week, P<0.001; 4 weeks, P<0.001) compared to the mean baseline score. Pain scores were also lower at 12-week follow-up for Group 2, but the difference did not reach statistical significance (P=0.52).
The mean maximal pain with ambulation scores at 1 and 4 weeks postinjection were significantly lower in Group 1 compared to Group 2 (1 week, P=0.045; 4 weeks, P=0.049). The Group 1 score was also lower than the Group 2 score at 12-week follow-up, but the difference did not reach statistical significance (P=0.08).
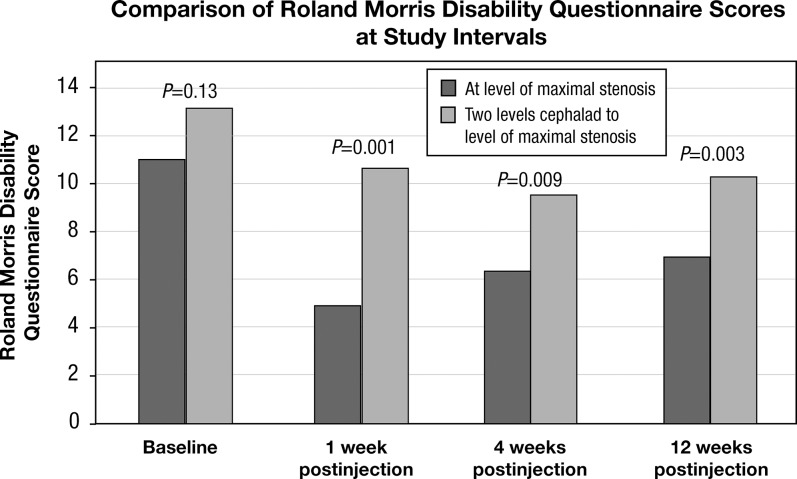
Group 1 had a mean baseline RMDQ score of 11.2 (SD 4.3, n=30) that decreased to 4.7 (SD 4.7, n=28) at 1 week postinjection and increased to 6.2 (SD 5.5, n=27) at 4 weeks and 6.7 (SD 5.3, n=15) at 12 weeks postinjection. Group 2 had a mean baseline disability score of 13.2 (SD 4.3, n=27) that decreased to 10.5 (SD 5.2, n=24) at 1 week postinjection and 9.7 (SD 5.5, n=20) at 4 weeks and increased to 10.1 (SD 5.0, n=13) at 12 weeks postinjection (Figure 2).
Figure 2.
Roland Morris Disability Questionnaire scores at baseline, 1, 4, and 12 weeks postinjection for both study groups. Scores were significantly reduced at 1, 4, and 12 weeks postinjection for the study group participants who received their interlaminar epidural steroid injections at the level of maximal stenosis (Group 1) compared to Group 2.
The mean RMDQ scores for Group 1 at 1, 4, and 12 weeks postinjection were significantly less than the mean baseline score (1 week, P<0.001; 4 weeks, P<0.001; 12 weeks, P<0.001). Group 2 patients also demonstrated significantly lower RMDQ scores at 1 and 4 weeks postinjection (1 week, P<0.001; 4 weeks, P<0.001) compared to the mean baseline score. Their 12-week score was also below baseline, but the difference did not reach statistical significance (P=0.14) due to the decreased number of patients at that point.
The mean disability scores at 1, 4, and 12 weeks postinjection were significantly lower for Group 1 as compared to Group 2 (1 week, P=0.001; 4 weeks, P=0.009; 12 weeks, P=0.003).
The mean baseline resting pain scores were similar between the 2 groups: 3.3 (SD 3.2) for Group 1 and 3.5 (SD 3.3) for Group 2. The 5-minute postinjection resting pain scores also were not significantly different: 2.0 (SD 2.6) for Group 1 and 2.4 (SD 2.4) for Group 2.
Fifteen patients injected at the site of maximal stenosis and 14 patients in the other group failed to reach the 12-week endpoint due to medical exposure to additional steroids or because they elected to undergo surgery. No procedural complications occurred. No deaths occurred.
DISCUSSION
The study confirms that ILESIs, irrespective of intervertebral level of performance, improve DLSCS-related symptomatology in the short term (1 and 4 weeks). This outcome is in concordance with other studies demonstrating the short-term benefit of ILESIs in the management of symptomatic DLSCS.6,8,10
The results indicate that Group 1 experienced greater pain relief compared to Group 2 at all follow-up intervals; this difference only reached statistical significance at 1 and 4 weeks postinjection, possibly due to the decrease in the number of patients included at the 12-week point in the study.
The reduction in the mean RMDQ score was statistically significant for Group 1 compared to Group 2 at 1, 4, and 12 weeks postinjection.
No difference was observed between the 2 groups in preinjection and postinjection pain at rest scores. In fact, both scores demonstrated no significant increase from baseline. Patients in our study did not seem to experience any significant increase in pain associated with the procedure irrespective of the level of ILESI performance.
For maximal benefit in patients with DLSCS, an ILESI should be performed at the most stenotic intervertebral level as determined by cross-sectional imaging. Operators should not be concerned that this technique will cause additional discomfort during the postprocedural period. The results of this study suggest that an ILESI performed at any level other than that of the patient's maximal stenosis may not provide maximum pain relief.
Administering the injection at the site of maximal stenosis ensures that the highest concentration of steroid and anesthetic are delivered to the area of maximal nerve irritation.18,19 Results suggest that patients will experience greater pain relief without the risk of additional discomfort.
To the best of our knowledge, a study of this nature has never been conducted. In fact, this study may be the first experimental analysis of this important ILESI technical parameter, based on our review of the literature.
This study is limited by patient subjectivity with regard to the qualitative determination of pain and disability, as well as the patient's memory with regard to the magnitude of his or her pain and disability. In addition, the use of a phone interview may have caused some patients to exaggerate the benefits of their injection to satisfy the interviewer. As stated previously, this study assumes that the maximal level of stenosis is the source of the patient's clinical symptoms. While the patient was blinded to the intervertebral level at which the ILESI was performed, the operator (JM or DK) was aware of the patient's level of maximal stenosis and to which group the patient was randomized. Finally, approximately half of each cohort failed to achieve the primary endpoint at 12 weeks postinjection.
CONCLUSION
This study validates the hypothesis that ILESIs for patients with DLSCS should be performed at the level of maximal stenosis. Operators should review patient cross-sectional imaging studies prior to ILESI performance, determine the level of maximal spinal canal stenosis, and use image guidance to perform the ILESI at the intervertebral level of maximal stenosis to ensure optimal patient benefit.
Footnotes
The authors have no financial or proprietary interest in the subject matter of this article.
This article meets the Accreditation Council for Graduate Medical Education and the American Board of Medical Specialties Maintenance of Certification competencies for Patient Care, Medical Knowledge, and Practice-Based Learning and Improvement.
REFERENCES
- 1.Kovacs FM, Urrútia G, Alarcón JD. Surgery versus conservative treatment for symptomatic lumbar spinal stenosis: a systematic review of randomized controlled trials. Spine (Phila Pa 1976) 2011 Sep 15;36(20):E1335–E1351. doi: 10.1097/BRS.0b013e31820c97b1. [DOI] [PubMed] [Google Scholar]
- 2.Weinstein JN, Tosteson TD, Lurie JD, et al. SPORT Investigators. Surgical versus nonsurgical therapy for lumbar spinal stenosis. N Engl J Med. 2008 Feb 21;358(8):794–810. doi: 10.1056/NEJMoa0707136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.North American Spine Society. Evidence-Based Clinical Guidelines for Multidisciplinary Spine Care: Diagnosis and Treatment for Degenerative Lumbar Spinal Stenosis. Burr Ridge, IL: North American Spine Society;; 2007. [Google Scholar]
- 4.Briggs VG, Li W, Kaplan MS, Eskander MS, Franklin PD. Injection treatment and back pain associated with degenerative lumbar spinal stenosis in older adults. Pain Physician. 2010 Nov-Dec;13(6):E347–E355. Erratum in: Pain Physician. 2011 Mar-Apr;14(2):217. [PubMed] [Google Scholar]
- 5.Tran de QH, Duong S, Finlayson RJ. Lumbar spinal stenosis: a brief review of the nonsurgical management. Can J Anaesth. 2010 Jul;57(7):694–703. doi: 10.1007/s12630-010-9315-3. Epub 2010 Apr 29. [DOI] [PubMed] [Google Scholar]
- 6.Koc Z, Ozcakir S, Sivrioglu K, Gurbet A, Kucukoglu S. Effectiveness of physical therapy and epidural steroid injections in lumbar spinal stenosis. Spine (Phila Pa 1976) 2009 May 1;34(10):985–989. doi: 10.1097/BRS.0b013e31819c0a6b. [DOI] [PubMed] [Google Scholar]
- 7.Parr AT, Diwan S, Abdi S. Lumbar interlaminar epidural injections in managing chronic low back and lower extremity pain: a systematic review. Pain Physician. 2009 Jan-Feb;12(1):163–188. [PubMed] [Google Scholar]
- 8.Wilson-MacDonald J, Burt G, Griffin D, Glynn C. Epidural steroid injection for nerve root compression. A randomised, controlled trial. J Bone Joint Surg Br. 2005 Mar;87(3):352–355. doi: 10.1302/0301-620x.87b3.15338. [DOI] [PubMed] [Google Scholar]
- 9.Campbell MJ, Carreon LY, Glassman SD, McGinnis MD, Elmlinger BS. Correlation of spinal canal dimensions to efficacy of epidural steroid injection in spinal stenosis. J Spinal Disord Tech. 2007 Apr;20(2):168–171. doi: 10.1097/01.bot.0000211162.43982.55. [DOI] [PubMed] [Google Scholar]
- 10.Smith CC, Booker T, Schaufele MK, Weiss P. Interlaminar versus transforaminal epidural steroid injections for the treatment of symptomatic lumbar spinal stenosis. Pain Med. 2010 Oct;11(10):1511–1515. doi: 10.1111/j.1526-4637.2010.00932.x. Epub 2010 Aug 23. [DOI] [PubMed] [Google Scholar]
- 11.Friedly J, Chan L, Deyo R. Increases in lumbosacral injections in the Medicare population: 1994 to 2001. Spine (Phila Pa 1976) 2007 Jul 15;32(16):1754–1760. doi: 10.1097/BRS.0b013e3180b9f96e. [DOI] [PubMed] [Google Scholar]
- 12.Manchikanti L, Boswell MV, Singh V, et al. ASIPP-IPM. Comprehensive evidence-based guidelines for interventional techniques in the management of chronic spinal pain. Pain Physician. 2009 Jul-Aug;12(4):699–802. [PubMed] [Google Scholar]
- 13.Chou R, Atlas SJ, Stanos SP, Rosenquist RW. Nonsurgical interventional therapies for low back pain: a review of the evidence for an American Pain Society clinical practice guideline. Spine (Phila Pa 1976) 2009 May 1;34(10):1078–1093. doi: 10.1097/BRS.0b013e3181a103b1. [DOI] [PubMed] [Google Scholar]
- 14.Botwin KP, Gruber RD. Lumbar epidural steroid injections in the patient with lumbar spinal stenosis. Phys Med Rehabil Clin N Am. 2003 Feb;14(1):121–141. doi: 10.1016/s1047-9651(02)00048-7. [DOI] [PubMed] [Google Scholar]
- 15.Kastler B. Interventional Radiology in Pain Treatment. Berlin, Germany: Springer-Verlag;; 2005. [Google Scholar]
- 16.Stratford PW, Binkley JM. Measurement properties of the RM-18. A modified version of the Roland-Morris Disability Scale. Spine (Phila Pa 1976) 1997 Oct 15;22(20):2416–2421. doi: 10.1097/00007632-199710150-00018. [DOI] [PubMed] [Google Scholar]
- 17.Roland M, Morris R. A study of the natural history of back pain. Part I: development of a reliable and sensitive measure of disability in low-back pain. Spine (Phila Pa 1976) 1983 Mar;8(2):141–144. doi: 10.1097/00007632-198303000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Flower RJ, Blackwell GJ. Anti-inflammatory steroids induce biosynthesis of a phospholipase A2 inhibitor which prevents prostaglandin generation. Nature. 1979 Mar 29;278(5703):456–459. doi: 10.1038/278456a0. [DOI] [PubMed] [Google Scholar]
- 19.Manchikanti L. Role of neuraxial steroids in interventional pain management. Pain Physician. 2002 Apr;5(2):182–199. [PubMed] [Google Scholar]