Abstract
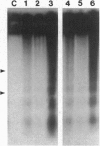
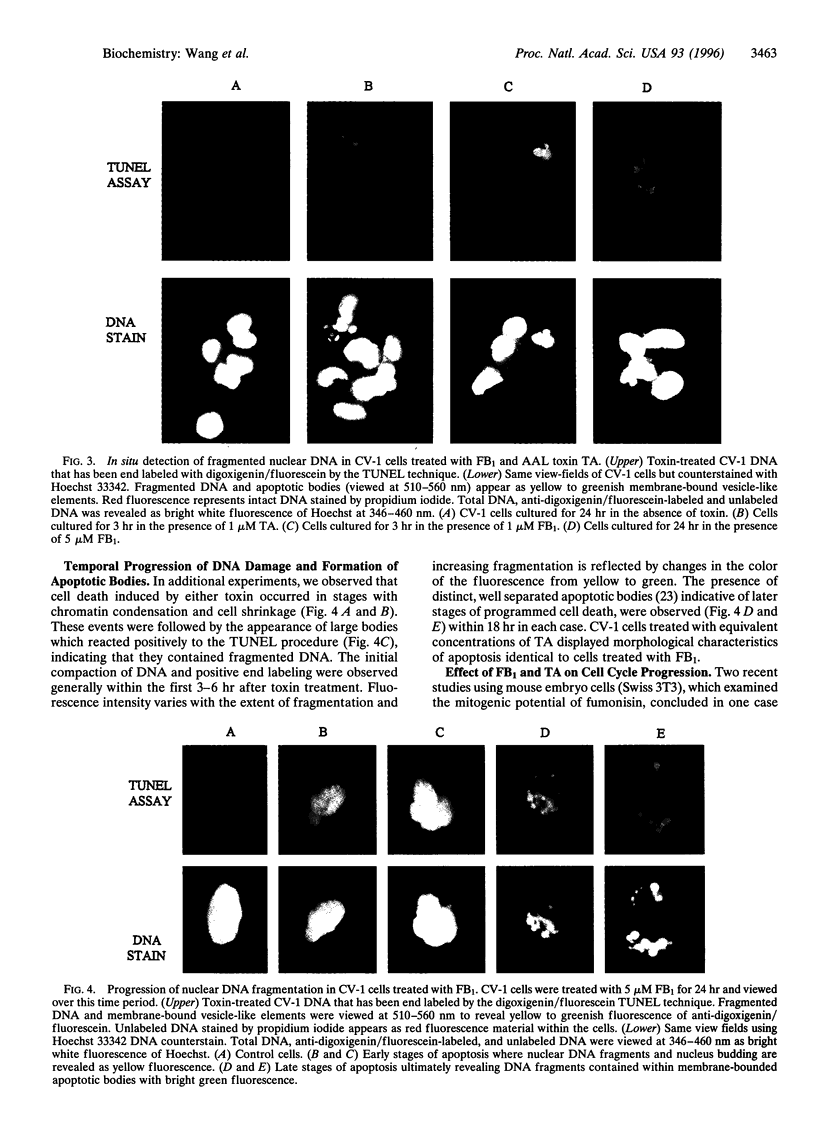
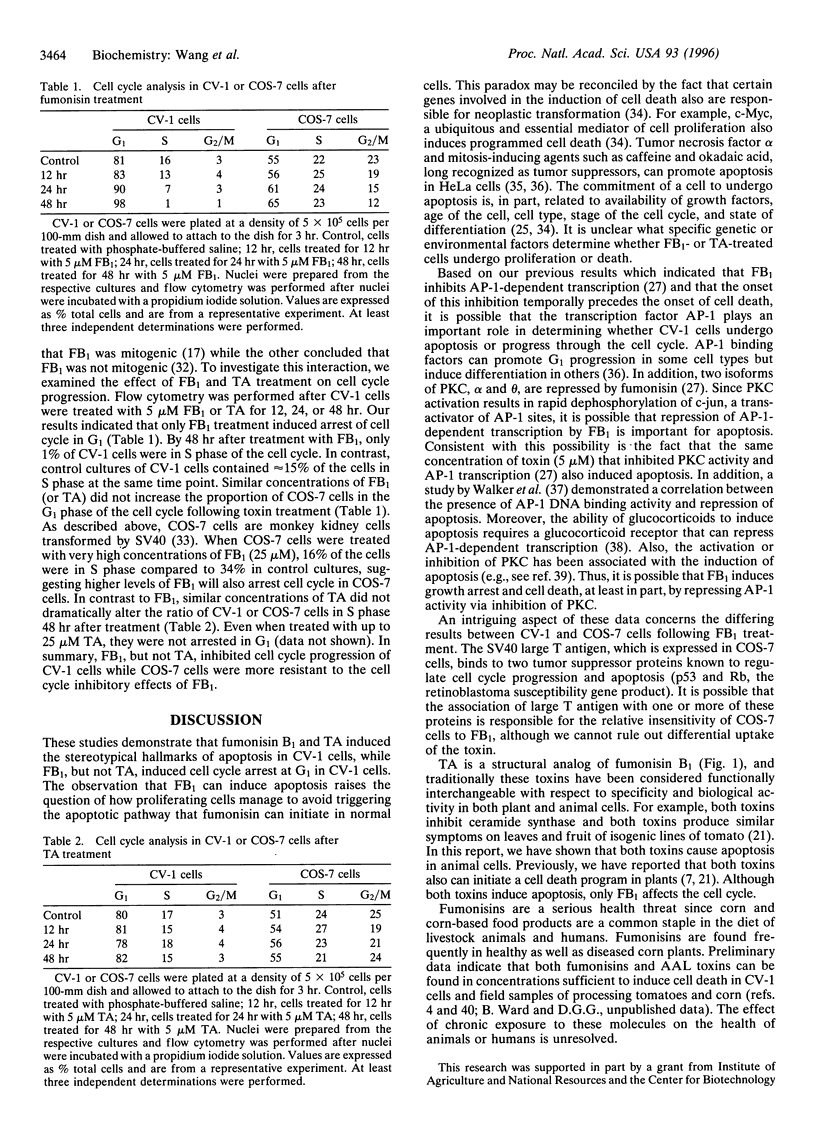
Fusarium moniliforme toxins (fumonisins) and Alternaria alternata lycopersici (AAL) toxins are members of a new class of sphinganine analog mycotoxins that occur widely in the food chain. These mycotoxins represent a serious threat to human and animal health, inducing both cell death and neoplastic events in mammals. The mechanisms by which this family of chemical congeners induce changes in cell homeostasis were investigated in African green monkey kidney cells (CV-1) by assessing the appearance of apoptosis, cell cycle regulation, and putative components of signal transduction pathways involved in apoptosis. Structurally, these mycotoxins resemble the sphingoid bases, sphingosine and sphinganine, that are reported to play critical roles in cell communication and signal transduction. The addition of fumonisin B1 or AAL toxin, TA, to CV-1 cells induced the stereotypical hallmarks of apoptosis, including the formation of DNA ladders, compaction of nuclear DNA, and the subsequent appearance of apoptotic bodies. Neither mycotoxin induced cell death, DNA ladders, or apoptotic bodies in CV-1 cells expressing simian virus 40 large T antigen (COS-7) at toxin concentrations that readily killed CV-1 cells. Fumonisin B1 induced cell cycle arrest in the G1 phase in CV-1 cells but not in COS-7 cells. AAL toxin TA did not arrest cell cycle progression in either cell line. The induction of apoptosis combined with the widespread presence of these compounds in food crops and animal feed identifies a previously unrecognized health risk to humans and livestock. These molecules also represent a new class of natural toxicants that can be used as model compounds to further characterize the molecular and biochemical pathways leading to apoptosis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Darzynkiewicz Z., Bruno S., Del Bino G., Gorczyca W., Hotz M. A., Lassota P., Traganos F. Features of apoptotic cells measured by flow cytometry. Cytometry. 1992;13(8):795–808. doi: 10.1002/cyto.990130802. [DOI] [PubMed] [Google Scholar]
- Fanning E., Knippers R. Structure and function of simian virus 40 large tumor antigen. Annu Rev Biochem. 1992;61:55–85. doi: 10.1146/annurev.bi.61.070192.000415. [DOI] [PubMed] [Google Scholar]
- Gelderblom W. C., Marasas W. F., Jaskiewicz K., Combrinck S., van Schalkwyk D. J. Cancer promoting potential of different strains of Fusarium moniliforme in a short-term cancer initiation/promotion assay. Carcinogenesis. 1988 Aug;9(8):1405–1409. doi: 10.1093/carcin/9.8.1405. [DOI] [PubMed] [Google Scholar]
- Gelderblom W. C., Semple E., Marasas W. F., Farber E. The cancer-initiating potential of the fumonisin B mycotoxins. Carcinogenesis. 1992 Mar;13(3):433–437. doi: 10.1093/carcin/13.3.433. [DOI] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Gorczyca W., Gong J., Darzynkiewicz Z. Detection of DNA strand breaks in individual apoptotic cells by the in situ terminal deoxynucleotidyl transferase and nick translation assays. Cancer Res. 1993 Apr 15;53(8):1945–1951. [PubMed] [Google Scholar]
- Harrington E. A., Fanidi A., Evan G. I. Oncogenes and cell death. Curr Opin Genet Dev. 1994 Feb;4(1):120–129. doi: 10.1016/0959-437x(94)90100-7. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H., Kastan M. B. Cell cycle control and cancer. Science. 1994 Dec 16;266(5192):1821–1828. doi: 10.1126/science.7997877. [DOI] [PubMed] [Google Scholar]
- Hauser J. M., Buehrer B. M., Bell R. M. Role of ceramide in mitogenesis induced by exogenous sphingoid bases. J Biol Chem. 1994 Mar 4;269(9):6803–6809. [PubMed] [Google Scholar]
- Helmberg A., Auphan N., Caelles C., Karin M. Glucocorticoid-induced apoptosis of human leukemic cells is caused by the repressive function of the glucocorticoid receptor. EMBO J. 1995 Feb 1;14(3):452–460. doi: 10.1002/j.1460-2075.1995.tb07021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Dickman M., Henderson G., Jones C. Repression of protein kinase C and stimulation of cyclic AMP response elements by fumonisin, a fungal encoded toxin which is a carcinogen. Cancer Res. 1995 Apr 15;55(8):1655–1659. [PubMed] [Google Scholar]
- Jarvis W. D., Kolesnick R. N., Fornari F. A., Traylor R. S., Gewirtz D. A., Grant S. Induction of apoptotic DNA damage and cell death by activation of the sphingomyelin pathway. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):73–77. doi: 10.1073/pnas.91.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marasas W. F., Jaskiewicz K., Venter F. S., Van Schalkwyk D. J. Fusarium moniliforme contamination of maize in oesophageal cancer areas in Transkei. S Afr Med J. 1988 Aug 6;74(3):110–114. [PubMed] [Google Scholar]
- Marasas W. F., Kellerman T. S., Gelderblom W. C., Coetzer J. A., Thiel P. G., van der Lugt J. J. Leukoencephalomalacia in a horse induced by fumonisin B1 isolated from Fusarium moniliforme. Onderstepoort J Vet Res. 1988 Dec;55(4):197–203. [PubMed] [Google Scholar]
- Merrill A. H., Jr, Hannun Y. A., Bell R. M. Introduction: sphingolipids and their metabolites in cell regulation. Adv Lipid Res. 1993;25:1–24. [PubMed] [Google Scholar]
- Merrill A. H., Jr, Wang E., Gilchrist D. G., Riley R. T. Fumonisins and other inhibitors of de novo sphingolipid biosynthesis. Adv Lipid Res. 1993;26:215–234. [PubMed] [Google Scholar]
- Mirocha C. J., Gilchrist D. G., Shier W. T., Abbas H. K., Wen Y., Vesonder R. F. AAL toxins, fumonisins (biology and chemistry) and host-specificity concepts. Mycopathologia. 1992 Feb;117(1-2):47–56. doi: 10.1007/BF00497278. [DOI] [PubMed] [Google Scholar]
- Nelson P. E. Taxonomy and biology of Fusarium moniliforme. Mycopathologia. 1992 Feb;117(1-2):29–36. doi: 10.1007/BF00497276. [DOI] [PubMed] [Google Scholar]
- Norred W. P. Fumonisins--mycotoxins produced by Fusarium moniliforme. J Toxicol Environ Health. 1993 Mar;38(3):309–328. doi: 10.1080/15287399309531720. [DOI] [PubMed] [Google Scholar]
- Norred W. P., Wang E., Yoo H., Riley R. T., Merrill A. H., Jr In vitro toxicology of fumonisins and the mechanistic implications. Mycopathologia. 1992 Feb;117(1-2):73–78. doi: 10.1007/BF00497281. [DOI] [PubMed] [Google Scholar]
- Ohta H., Yatomi Y., Sweeney E. A., Hakomori S., Igarashi Y. A possible role of sphingosine in induction of apoptosis by tumor necrosis factor-alpha in human neutrophils. FEBS Lett. 1994 Dec 5;355(3):267–270. doi: 10.1016/0014-5793(94)01218-0. [DOI] [PubMed] [Google Scholar]
- Piacentini M., Fesus L., Melino G. Multiple cell cycle access to the apoptotic death programme in human neuroblastoma cells. FEBS Lett. 1993 Apr 5;320(2):150–154. doi: 10.1016/0014-5793(93)80081-5. [DOI] [PubMed] [Google Scholar]
- Schroeder J. J., Crane H. M., Xia J., Liotta D. C., Merrill A. H., Jr Disruption of sphingolipid metabolism and stimulation of DNA synthesis by fumonisin B1. A molecular mechanism for carcinogenesis associated with Fusarium moniliforme. J Biol Chem. 1994 Feb 4;269(5):3475–3481. [PubMed] [Google Scholar]
- Steller H. Mechanisms and genes of cellular suicide. Science. 1995 Mar 10;267(5203):1445–1449. doi: 10.1126/science.7878463. [DOI] [PubMed] [Google Scholar]
- Sun Y., Pommier Y., Colburn N. H. Acquisition of a growth-inhibitory response to phorbol ester involves DNA damage. Cancer Res. 1992 Apr 1;52(7):1907–1915. [PubMed] [Google Scholar]
- Thiel P. G., Marasas W. F., Sydenham E. W., Shephard G. S., Gelderblom W. C. The implications of naturally occurring levels of fumonisins in corn for human and animal health. Mycopathologia. 1992 Feb;117(1-2):3–9. doi: 10.1007/BF00497272. [DOI] [PubMed] [Google Scholar]
- Walker P. R., Kwast-Welfeld J., Gourdeau H., Leblanc J., Neugebauer W., Sikorska M. Relationship between apoptosis and the cell cycle in lymphocytes: roles of protein kinase C, tyrosine phosphorylation, and AP1. Exp Cell Res. 1993 Jul;207(1):142–151. doi: 10.1006/excr.1993.1173. [DOI] [PubMed] [Google Scholar]
- Wang E., Norred W. P., Bacon C. W., Riley R. T., Merrill A. H., Jr Inhibition of sphingolipid biosynthesis by fumonisins. Implications for diseases associated with Fusarium moniliforme. J Biol Chem. 1991 Aug 5;266(22):14486–14490. [PubMed] [Google Scholar]
- Wang E., Ross P. F., Wilson T. M., Riley R. T., Merrill A. H., Jr Increases in serum sphingosine and sphinganine and decreases in complex sphingolipids in ponies given feed containing fumonisins, mycotoxins produced by Fusarium moniliforme. J Nutr. 1992 Aug;122(8):1706–1716. doi: 10.1093/jn/122.8.1706. [DOI] [PubMed] [Google Scholar]