Abstract
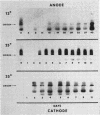
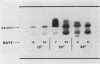
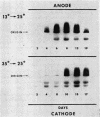
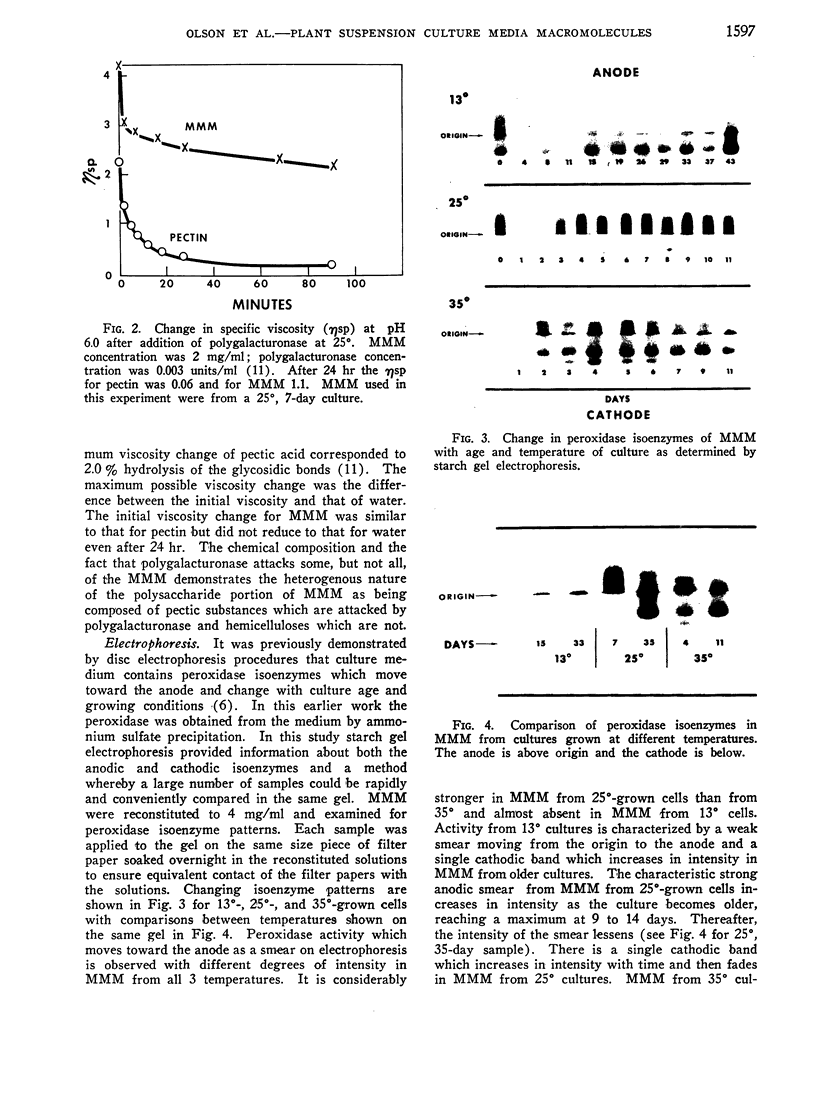
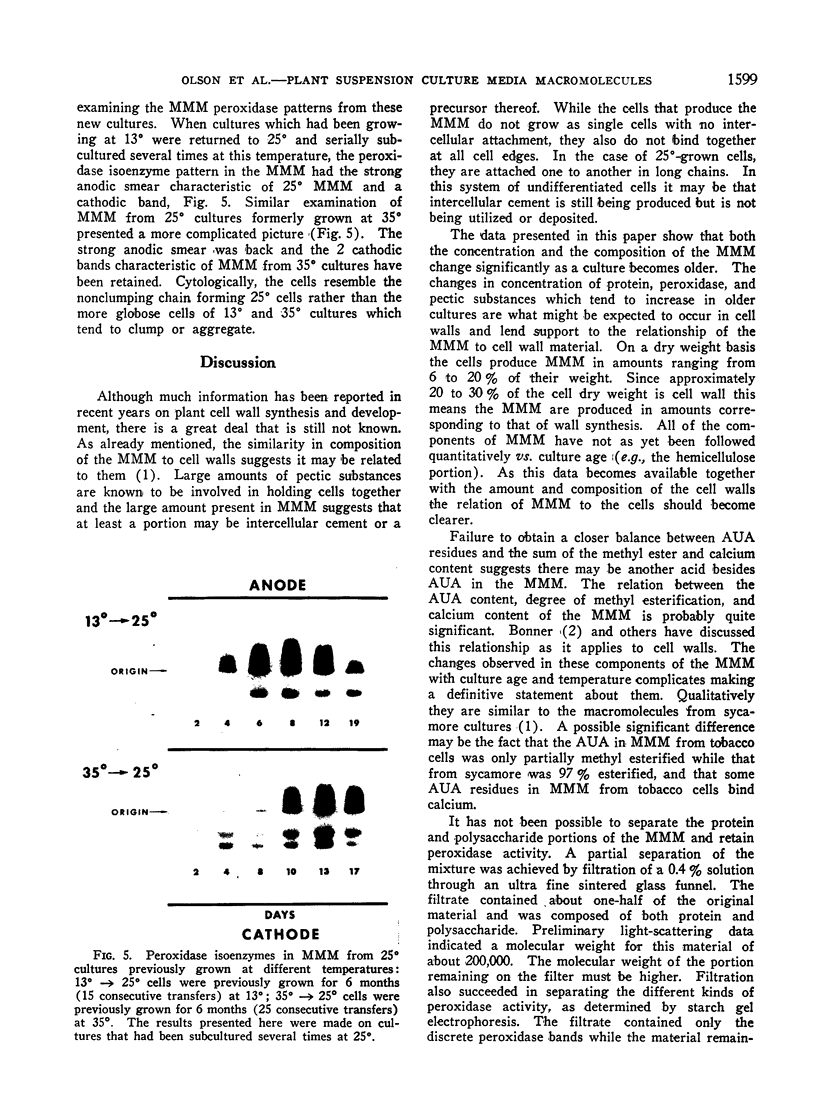
Macromolecules secreted into the media by a nondifferentiating suspension culture of tobacco cells were found to be composed of protein and polysaccharide, and to account for the viscosity of the media. The concentration, composition, and viscosity of these macromolecules changed significantly with the age of the culture and growth temperature. The concentration changed from 0.02 mg/ml in newly inoculated cultures to over 1 mg/ml in older cultures. The macromolecules contained from 6 to 18% protein and 3 to 4 μmoles hydroxy-proline/mg nitrogen, more than 20 times the level found in whole cells. The macromolecules contained 5 to 25% pectic substances whose carboxyl groups were either methyl esterified or combined with calcium. Arabinose, xylose, glucose, galactose, and mannose were identified in acid hydrolysates of the macromolecules. Peroxidase activity of the macromolecules increased as cultures became older. Peroxidase isoenzyme patterns changed with culture age and growth temperature. The relation of the macromolecules to cell walls and intercellular substances is discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker G. E., Hui P. A., Albersheim P. Synthesis of Extracellular Polysaccharide by Suspensions of Acer Pseudoplatanus Cells. Plant Physiol. 1964 Nov;39(6):913–920. doi: 10.1104/pp.39.6.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland R. Hydroxyproline Formation and Its Relation to Auxin-induced Cell Elongation in the Avena Coleoptile. Plant Physiol. 1968 Oct;43(10):1625–1630. doi: 10.1104/pp.43.10.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jong D. W., Jansen E. F., Olson A. C. Oxidoreductive and hydrolytic enzyme patterns in plant suspension culture cells. Local and time relationships. Exp Cell Res. 1967 Aug;47(1):139–156. doi: 10.1016/0014-4827(67)90218-2. [DOI] [PubMed] [Google Scholar]
- De Jong D. W., Olson A. C., Hawker K. M., Jansen E. F. Effect of cultivation temperature on peroxidase isozymes of plant cells grown in suspension. Plant Physiol. 1968 May;43(5):841–844. doi: 10.1104/pp.43.5.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J. J. Peroxidases from the extreme dwarf tomato plant. Identification, isolation, and partial purification. Plant Physiol. 1968 Jul;43(7):1037–1041. doi: 10.1104/pp.43.7.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filner P. Semi-conservative replication of DNA in a higher plant cell. Exp Cell Res. 1965 Aug;39(1):33–39. doi: 10.1016/0014-4827(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Jansen E. F., Baglan N. C. Recovery of 14C-labeled sugar and alcohol derivatives in gas chromatography. J Chromatogr. 1968 Nov 5;38(1):18–23. doi: 10.1016/0021-9673(68)85003-4. [DOI] [PubMed] [Google Scholar]
- KOLLAR S. J., JARAI M. Biochemical chlorination in Streptomvces aureofaciens. Nature. 1960 Nov 19;188:665–665. doi: 10.1038/188665a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lamport D. T. The isolation and partial characterization of hydroxyproline-rich glycopeptides obtained by enzymic degradation of primary cell walls. Biochemistry. 1969 Mar;8(3):1155–1163. doi: 10.1021/bi00831a049. [DOI] [PubMed] [Google Scholar]
- Olson A. C. Proteins and Plant Cell Walls. Proline to Hydroxyproline in Tobacco Suspension Cultures. Plant Physiol. 1964 Jul;39(4):543–550. doi: 10.1104/pp.39.4.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson A. C., White L. M., Noma A. T. Behavior of uronic acids and acid-treated uronic acids on an automatic amino acid analyzer. J Chromatogr. 1969 Sep 9;43(3):399–403. doi: 10.1016/s0021-9673(00)99221-5. [DOI] [PubMed] [Google Scholar]
- STRAUS J., CAMPBELL W. A. Release of enzymes by plant tissue cultures. Life Sci. 1963 Jan;1:50–62. doi: 10.1016/0024-3205(63)90037-7. [DOI] [PubMed] [Google Scholar]
- Shannon L. M., Kay E., Lew J. Y. Peroxidase isozymes from horseradish roots. I. Isolation and physical properties. J Biol Chem. 1966 May 10;241(9):2166–2172. [PubMed] [Google Scholar]