Abstract
N-Benzyl substitution of 5-HT2A receptor agonists of the phenethylamine structural class of psychedelics (such as 4-bromo-2,5-dimethoxyphenethylamine, often referred to as 2C-B) confer a significant increase in binding affinity as well as functional activity of the receptor. We have prepared a series of 48 compounds with structural variations in both the phenethylamine and N-benzyl part of the molecule to determine the effects on receptor binding affinity and functional activity at 5-HT2A and 5-HT2C receptors. The compounds generally had high affinity for the 5-HT2A receptor with 8b having the highest affinity at 0.29 nM but with several other compounds also exhibiting subnanomolar binding affinities. The functional activity of the compounds was distributed over a wider range with 1b being the most potent at 0.074 nM. Most of the compounds exhibited low to moderate selectivity (1- to 40-fold) for the 5-HT2A receptor in the binding assays, although one compound 6b showed an impressive 100-fold selectivity for the 5-HT2A receptor. In the functional assay, selectivity was generally higher with 1b being more than 400-fold selective for the 5-HT2A receptor.
Keywords: Serotonin, 5-HT2A receptor agonist, N-benzyl phenethylamines, selectivity
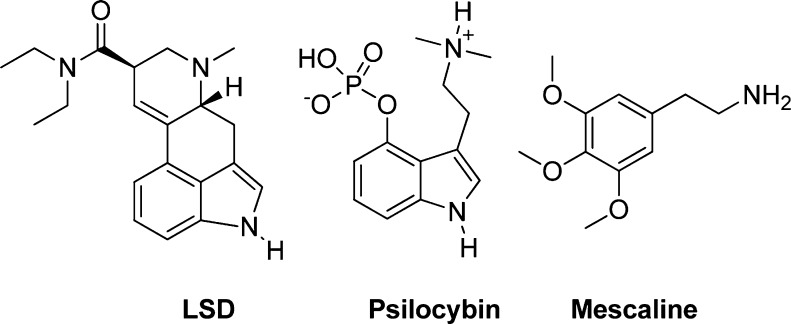
Serotonin (5-HT) receptors are widely distributed in both the CNS and the peripheral nervous system and are involved in the regulation of a plethora of physiological responses such as cognition, memory processing, mood, circadian behavior, and appetite.1 The 5-HT2A receptor appears to play a key role in a number of disease states such as addiction,2 schizophrenia,3 obsessive compulsive disorder,4,5 depression,6,7 pain,8 inflammation,9 migraine,10 and cluster headaches,11 and in the manifestation of mystical/religious-type experiences as well as in altered states of consciousness.3,12−15 Agonist activation of 5-HT2A receptors in the cortex is believed to be responsible for the remarkable psychopharmacological effects exerted by hallucinogens such as lysergic acid diethylamide (LSD) and psilocybin; see Figure 1.16 Neutral antagonists such as 2-bromolysergic acid diethylamide (BOL-148) have been investigated as a prophylactic treatment of cluster headaches, and inverse agonists such as risperidone and clozapine are well-known atypical antipsychotics.17,18
Figure 1.
Representative members of the three structural groups of 5-HT2A agonists. LSD (an ergoline), psilocybin (a tryptamine), and mescaline (a phenethylamine).
5-HT2A agonists have traditionally been divided into three structural groups: Ergolines, tryptamines, and phenethylamines.19 The ergoline group is named after the ergot fungus which was the initial source of ergot alkaloids, but ergolines are also known to occur naturally in several other species of plant and fungi.20 The ergolines comprise a varied group of compounds with a rich pharmacology and bind to many types of monoamine receptors both serotonergic and nonserotonergic. Not all ergolines are 5-HT2A agonists and several are used clinically in the treatment of migraine,21 Parkinson’s disease and in obstetrics.22,23
Tryptamines are slightly more selective compounds but usually have strong affinity for several 5-HT receptors. Serotonin itself is an agonist at all 5-HT receptors, whereas the classical hallucinogens psilocybin and N,N-dimethyltryptamine (DMT) activate 5-HT1A receptors as well as the 5-HT2 subtypes.
Phenethylamines are generally selective for the 5-HT2 receptor subtypes but lack selectivity between the individual subtypes.24 The optimal substitution pattern and molecular configuration for simple phenethylamines have been refined and improved during the past 40 years and have resulted in some very potent agonists.25,26
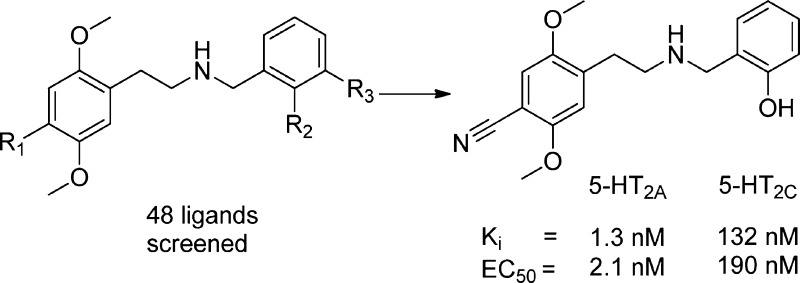
Initially, N-substituted phenethylamines were thought to be inferior compared to the parent phenethylamines because early studies involving N-alkylation with simple substituents (e.g., methyl, ethyl, propyl) produced compounds with significantly diminished activity.27,28 Therefore, it was surprising when it was discovered that N-benzyl and specifically N-(2-methoxy)benzyl substitution dramatically improved both binding affinity and functional activity and in vivo 5-HT2A activation of simple phenethylamines such as 2C-I (4-iodo-2,5-dimethoxyphenethylamine).29,30 This discovery was later expanded upon to include conformationally restricted N-benzylphenethylamines which resulted in the first truly selective 5-HT2A agonist.31
As part of our efforts to develop an agonist PET-tracer for the 5-HT2A receptor, we have examined a number of different N-benzylphenethylamines for their ability to bind to and activate 5-HT2A receptors in the search for a 5-HT2A selective agonist.32 Agonists are of special interest in PET studies because agonist PET-ligands only label receptors in the active, high-affinity state which provides more functionally relevant imaging. Furthermore, agonist PET-ligands are potentially more sensitive to changes in synaptic serotonin levels due to the higher affinity of 5-HT for agonist versus antagonist labeled receptors. Several ligands from the study have been investigated as PET-ligands, and 5a (under the alias Cimbi-36) is currently being evaluated in humans.33 Herein we wish to report the full details on the medicinal chemistry development of these ligands. We wanted to study the impact of the 4-substituent on N-benzylphenethylamines with a small set of N-benzyl substituents and determine the effects on both binding affinity and functional activity at the 5-HT2A receptor as well as the 5-HT2C receptor. Extensive structure–activity relationship (SAR) studies have previously been performed on the 4-position of psychedelic phenethylamines and amphetamines.27,34 These studies revealed that 4-substituents containing hydrogen bond donors such as −COOH, −OH, and −NH2 decrease affinity by several orders of magnitude while nonpolar substituents such as halogens and alkyl groups increased affinity. Thus, there seems to be a clear correlation between the lipophilic nature of the substituent in the 4-position and binding affinity; however, only halogens and short alkyl chains (1–4 carbons) are agonists while longer alkyl chains, aryl and benzyl groups are antagonists at the 5-HT2A receptor.
Herein we report our efforts to investigate the SAR of this compound family via a systematic variation of substituents on the phenethylamine and benzyl rings. Twelve phenethylamines were paired with 4 benzaldehydes to give a set of 48 N-benzyl phenethylamines that subsequently were evaluated on the 5-HT2A and 5-HT2C receptors in binding and functional assays.
Chemistry
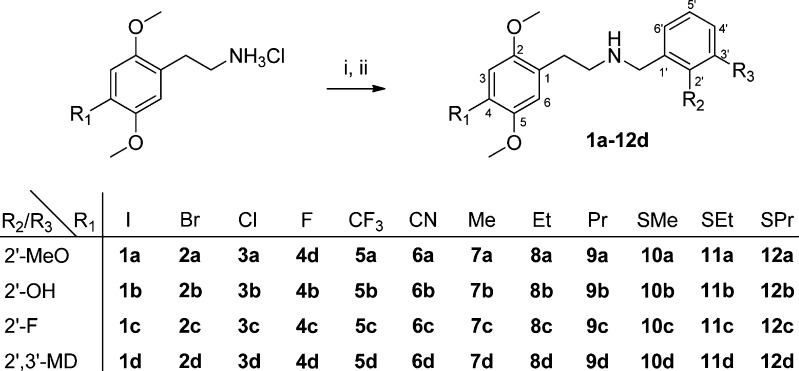
The targeted 48 compounds were all synthesized by indirect reductive amination of the respective phenethylamines and benzaldehydes as shown in Scheme 1. The N-benzylphenethylamines were precipitated as their hydrochloride salts in 46–94% isolated yields. The syntheses of the parent 12 phenethylamines have been described previously; see the Supporting Information for details.
Scheme 1. Synthesis of N-Benzyl Phenethylamines.
Reaction conditions: (i) aldehyde, EtOH, rt; (ii) NaBH4, EtOH, rt.
In Vitro Pharmacology
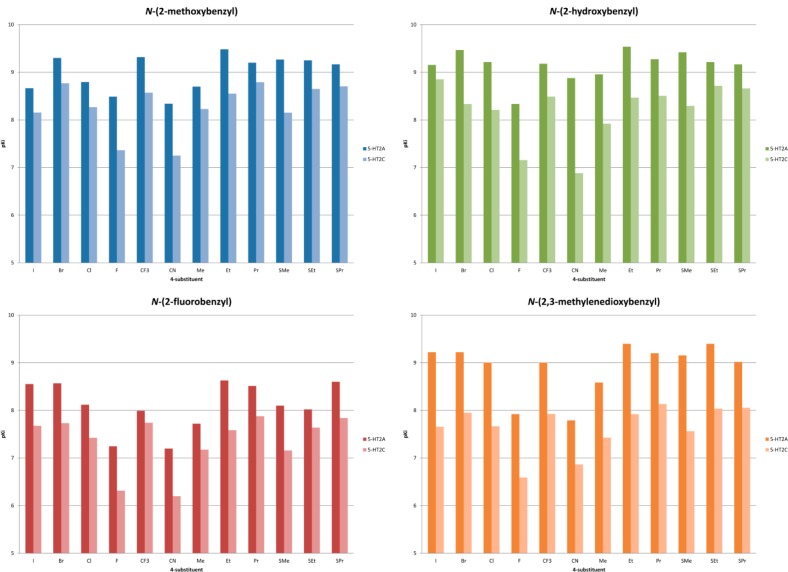
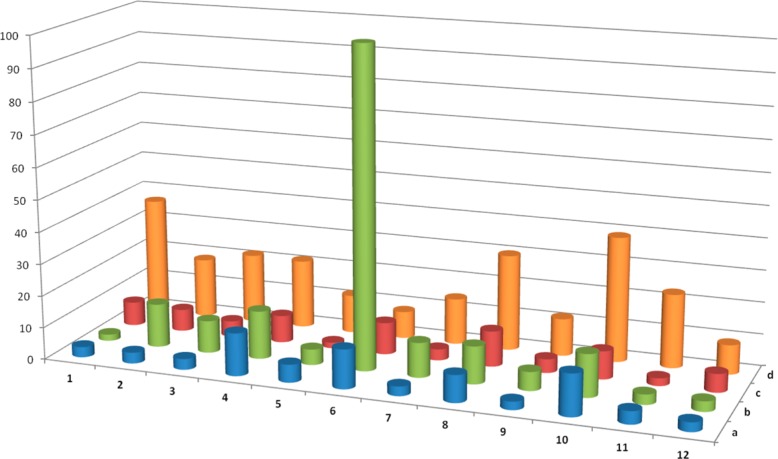
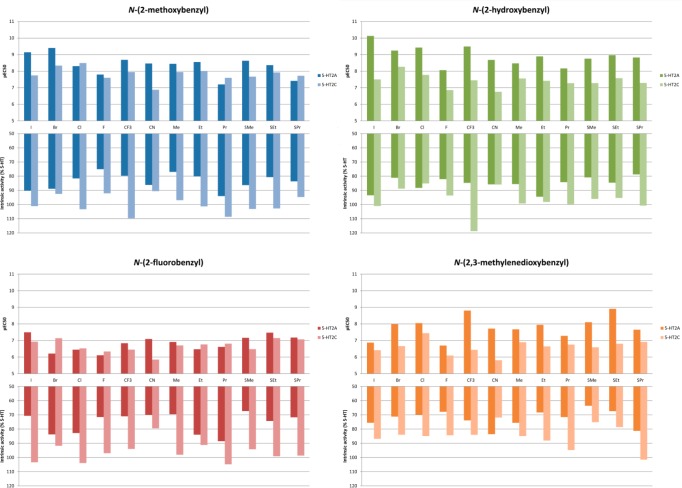
All compounds were assessed in a radioligand competition binding assay for affinity at human 5-HT2A receptors and rat 5-HT2C receptors using displacement of antagonist radioligands [3H]Ketanserin and [3H]Mesulergine via the NIMH Psychoactive Drug Screening Program (PDSP). The results are summarized in Figure 2. In Figure 3, the 5-HT2A/2C selectivities based on the data in Figure 2 are presented.
Figure 2.
Binding affinities (pKi) of N-benzylphenethylamines at the 5-HT2A and 5-HT2C receptor. See the Supporting Information for tables with all data.
Figure 3.
Graph showing the 5-HT2A/5-HT2C selectivities based on binding affinities.
Functional assays were performed on all compounds to determine the ability of the ligands to activate downstream cellular signaling pathways. The compounds were examined for their efficacy at stimulating phospholipase C mediated production of inositol phosphates (IP1–3) at both human 5-HT2A and 5-HT2C receptors. The results are summarized in Figure 4; see the Supporting Information for full details.
Figure 4.
Functional characterization of N-benzylphenethylamines at human 5-HT2A and 5-HT2C receptors. Top half shows pEC50, whereas bottom half represents intrinsic activity.
Results and Discussion
The results from the binding affinity measurements showed that the majority of the compounds bind to both 5-HT2A and 5-HT2C receptors in the low nanomolar range with several compounds having subnanomolar affinities (pKi above 9) at the 5-HT2A receptor. Previously, a smaller subset of compounds were subjected to a broader screen which showed the N-benzyl phenethylamines are highly selective for the 5-HT2 receptor subtypes over a wide selection of other neuroreceptors.32 Ligands substituted with F, CN, or Me at the 4-position of the phenethylamine core (4a–d, 6a–d, and 7a–d) have slightly lower affinities which is in accordance with affinity data published previously on simple/primary phenethylamines and amphetamines.35 In general, the ligands with a N-(2-fluorobenzyl) substituent (1c–12c) have lower affinities than the other N-benzyl substituents which could be due to a diminished hydrogen bond acceptor capability.
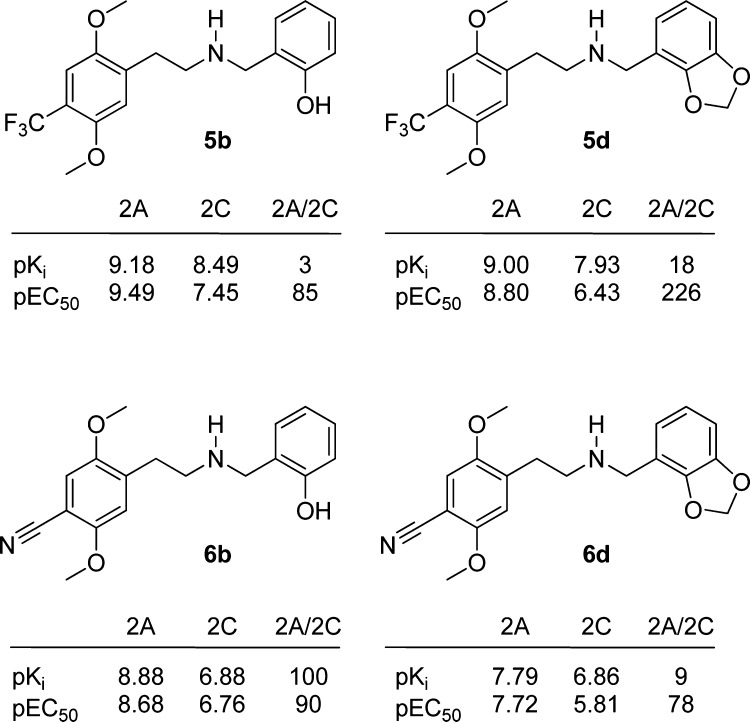
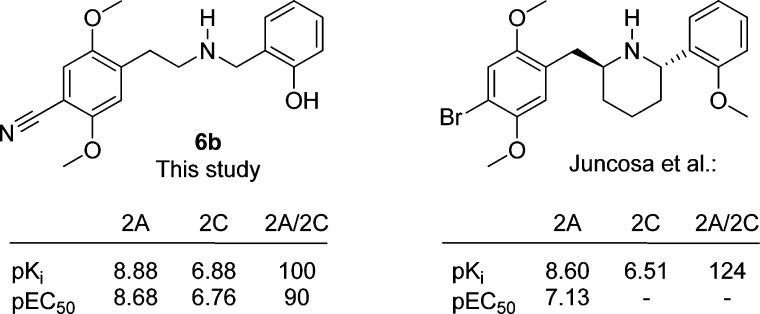
While all compounds were generally 5-HT2A-selective in the binding assays, the selectivity varied with the nature of both the 4-substituents and the N-benzyl substituents. 2,3-Methylenedioxy substitution on the N-benzyl part results in a general increase in the 5-HT2A-selective binding with most 4-substituents; see Figure 3. The influence of the other N-benzyl substituents on 5-HT2A/2C-selectivity was too erratic to show any general trends. The same applies to the 4-substituents which do not display any general trends. In Figure 5, 5b, 5d, 6b, and 6d are shown for comparison. Compound 5b is a very potent agonist with 85-fold selectivity in the functional assays, but low selectivity (3-fold) in the binding assay. In 5d, the methylenedioxy moiety in the N-benzyl group increases the selectivity to a factor of 226 and 18, respectively, while maintaining potency. Exchanging the CF3-group for a CN-group in these two compounds gives two very different results: 6b retains the high affinity for 5-HT2A, while affinity for 5-HT2C diminishes, giving a 100-fold selective compound, whereas in 6d the same CF3 to CN substitution leads to an erosion of both affinity and selectivity for 5-HT2A. In Figure 6, the concentration–response curves for 6b and 6d on 5-HT2A and 5-HT2C are compared with DOI. As can be seen in Figure 3, the combination of the 4-CN and N-(2-hydroxybenzyl) substituents in 6b gives the most selective compound in the series with respect to binding affinities. In simple amphetamines with the same substitution pattern, the 4-CN substitution results in a moderate (20-fold) selectivity toward 5-HT2A, and it appears that this property is augmented by the 2′-hydroxybenzyl substituent but not by the other N-benzyl groups examined in this study. Attempts to rationalize the structure–activity relationships via docking of the ligand set in previously reported 5-HT2A/2C homology-models were unsuccessful.35
Figure 5.
Structure and in vitro pharmacological profile of 5b, 5d, 6b, and 6d.
Figure 6.
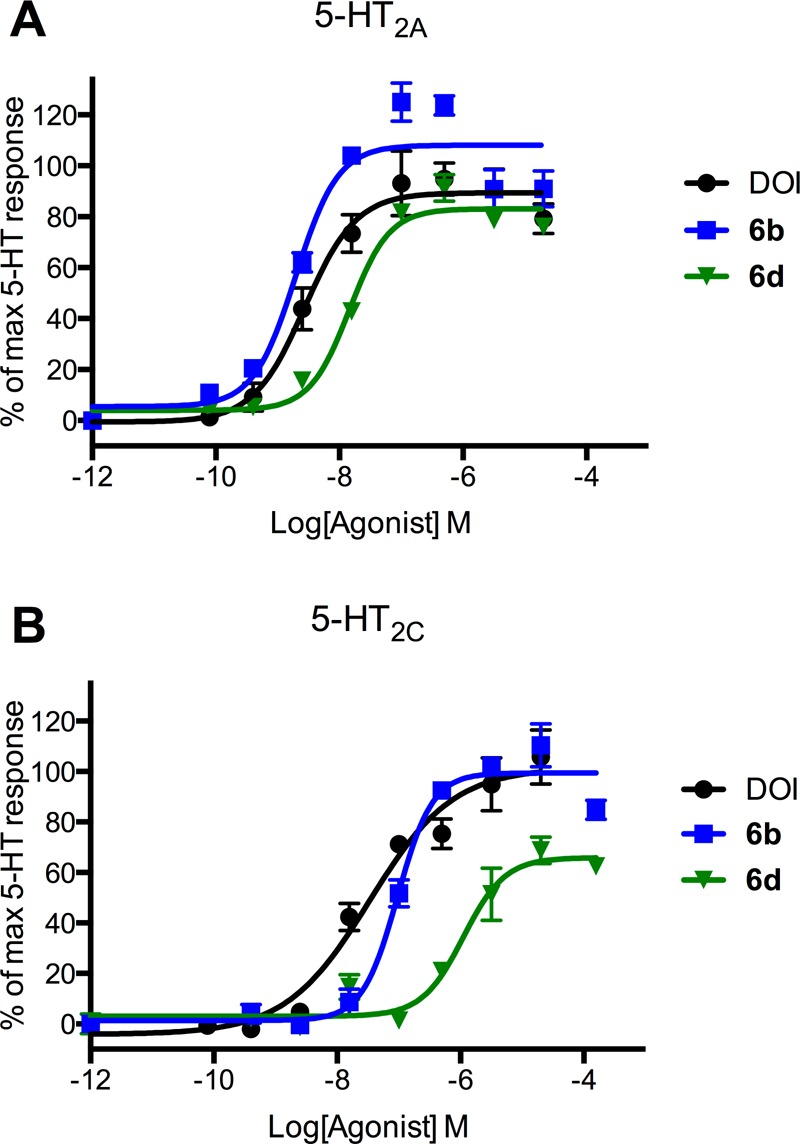
Concentration–response curves of the agonists DOI, 6b and 6d at 5-HT2A (A) and 5-HT2C (B) receptors, respectively. Concentration–response curves were generated from stimulation of inositol phosphate formation in tsA cells transiently expressing either 5-HT2A (A) or 5-HT2C (B) receptors. The formation of inositol phosphate was determined as described in Methods and calculated as percent response compared to a full 5-HT response. Data shown are mean ± SD of a single representative experiment performed in triplicate. Two additional experiments gave similar results.
In the functional assay, the N-(2-hydroxybenzyl) substituted compounds generally showed the highest activity at the 5-HT2A receptor with moderate to good selectivity. With very few exceptions, the intrinsic activity was above 70% for all compounds on both 5-HT2A and 5-HT2C. N-(2-Methoxybenzyl) compounds (1–12a) were less active and also less selective 5-HT2A agonists. From the N-(2-hydroxybenzyl) compounds, 1b emerged as the most functionally potent of all ligands tested with an EC50 of 0.074 nM with more than 400-fold selectivity for the 5-HT2A receptor. The N-(2,3-methylenedioxybenzyl) substituted compounds (1d–12d) were generally less potent. The N-(2-fluorobenzyl) compounds (1c–12c) were inferior in terms of affinity, efficacy, and selectivity compared to the other three series, mirroring the results from the binding assays, but comparable to the parent phenethylamines.
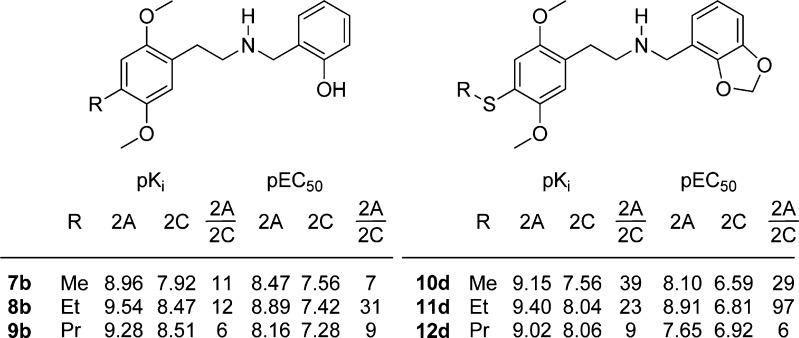
In simple phenethylamines, the functional activity drops as the size of the 4-substituent is increased. This is mirrored in our result: Starting from 4-methyl (7b), there is a drop in activity going from ethyl (8b) to propyl (9b) with the N-(2-hydroxybenzyl) substituent. In the N-(2,3-methylendioxybenzyl) series, the trend is similar; in the 4-thioalkyl series, 10d–11d–12, where 11d is the most potent of the three; see Figure 7. Thus, it appears that the substituent on the N-benzyl somehow influences the interaction of the 4-substituent as well.
Figure 7.
Structure and in vitro pharmacological profile of 7b–9b and 10d–12d.
In conclusion, we have investigated the structure activity relationship of 48 closely related N-benzyl phenethylamines as 5-HT2A/2C agonists. From that study, several interesting compounds emerged. Several compounds displayed affinities and potencies in the picomolar range with varying levels of selectivity. Although the structure activity relations of the 48 ligands were erratic, the effect of the cyano substituent in the 4-position and the general trend of the N-(2,3-methylenedioxybenzyl) substituted phenethylamines on the selectivity is an interesting new observation. The most selective compound (when taking both binding and functional data into account) was 6b being 100- and 90-fold selective; see Figure 8. In 2013, Juncosa et al.31 published a conformationally restrained N-benzyl phenethylamine with more than 100-fold selectivity for the 5-HT2A receptor; see Figure 8. We are currently pursuing ligands where these structural motifs are merged in the search for even more selective 5-HT2A agonists.
Figure 8.
Comparison between 5d and 5-HT2A selective agonist published by Juncosa et al. in 2013.
Methods
Synthesis of Secondary Amines
Et3N (1.0 equiv) was added to a suspension of the phenethylamine hydrochloride (1.0 mmol) and aldehyde (1.1 equiv) in EtOH (10 mL), and the reaction was stirred until formation of the imine was complete according to TLC or GC (30 min to 3 h). NaBH4 (2.0 mmol) was added, and the reaction was stirred for another 30 min. The reaction mixture was concentrated under reduced pressure and partitioned between CH2Cl2/H2O (30 mL, 1:1). The organic layer was isolated, and the aqueous layer was extracted with CH2Cl2 (2 × 15 mL). The combined organic extracts were dried (Na2SO4), filtered, and evaporated under reduced pressure. The residue was purified by flash chromatography (CH2Cl2/MeOH/NH3 98:2:0.04). The purified free base was dissolved in EtOH (2 mL), there was added ethanolic HCl (1M, 2 mL), and the solution was diluted with Et2O until crystals formed. The crystals were collected by filtration and dried under reduced pressure.
Functional Pharmacology
Cell culturing, transfection, and inositol phosphate turnover assay were adopted from a previously published procedure.36
Cell Culture and Transfections
tsA201 cells (a transformed HEK293 cell line) were cultured in GlutaMAX-I Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% dialyzed fetal bovine serum, penicillin (100 U mL–1), and streptomycin (100 mg mL–1) at 37 °C in a humidified atmosphere of 95% air and 5% CO2. Constructs encoding human 5-HT2A and 5-HT2C in pcDNA3.1 were obtained from the Missouri S&T cDNA Resource Center (www.cdna.org) and transiently transfected into cells using PolyFect according to the manufacturer’s protocol (Qiagen, West Sussex, U.K.).
Inositol Phosphate (IP) Turnover Assay
The day after transfection, tsA201 cells were split into poly-d-lysine-coated 96-well tissue culture plates in inositol-free DMEM supplemented with 10% dialyzed fetal bovine serum, penicillin (100 U mL–1), streptomycin (100 mg mL–1), and 4 μCi mL–1myo-[2-3H]inositol (GE Healthcare, Buckinghamshire, U.K.). Two days after transfection, cells were washed with assay buffer 1 (Hanks’ balanced saline solution (HBSS) containing 20 mM HEPES, 1 mM CaCl2, 1 mM MgCl2, and 1 mg mL–1 BSA, pH 7.4) and preincubated in 100 μL assay buffer 1 for 4 h at 37 °C, where the buffer was replaced after 2 h. The cells were then washed and subsequently incubated in 50 μL of assay buffer 2 (HBSS containing 1 mM CaCl2, 1 mM MgCl2, and 20 mM LiCl) for 30 min at 37 °C. Following this incubation, the cells were stimulated with 50 μL of the indicated agonists in assay buffer 2 for 30 min at 37 °C.
The reactions were stopped by exchanging the buffer with 50 μL ice-cold 10 mM formic acid and incubating the cells at 4 °C for at least 30 min. Yttrium silicate scintillation proximity assay beads (GE Healthcare, Buckinghamshire, U.K.) were used for measuring radioactivity from generated [3H]-IP essentially as previously described.37 In brief, 20 μL of the formic acid cell extracts were transferred to white 96-well plates, and 1 mg of yttrium silicate scintillation proximity assay beads suspended in 80 μL water added to each well. The plates were sealed, shaken vigorously for 1 h, and centrifuged at 1500 rpm for 5 min. The radioactivity was quantified in a Wallac Microbeta scintillation counter, and responses read as counts per minute (CPM). All experiments were performed in triplicate and repeated in at least three independent experiments.
Acknowledgments
Radioligand competition binding assay for affinity at human 5-HT2A receptors and rat 5-HT2C receptors using displacement of antagonist radioligands [3H]Ketanserin and [3H]Mesulergine was generously provided by the National Institute of Mental Health’s Psychoactive Drug Screening Program, Contract # HHSN-271-2008-00025-C (NIMH PDSP). The NIMH PDSP is Directed by Bryan L. Roth MD, PhD at the University of North Carolina at Chapel Hill and Project Officer Jamie Driscol at NIMH, Bethesda MD.
Supporting Information Available
NMR-data, including copies of 1H and 13C NMR spectra and full details on the pharmacological characterization. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
J.K., M.H., M.B., and H.B.O. conceived the experiments. M.H. and J.S.P. synthesized the compounds. M.H. and S.L.P. characterized the compounds. K.P. and H.B.O. performed the functional assays. M.H., S.L.P., and J.L.K. prepared the manuscript.
The Lundbeck foundation, the Danish Ministry of Science, Innovation, and Higher Education and the The A. P. Møller Foundation for the Advancement of Medical Sciences are gratefully acknowledged for financial support.
The authors declare no competing financial interest.
Supplementary Material
References
- Berger M.; Gray J. A.; Roth B. L. (2009) The Expanded Biology of Serotonin. Annu. Rev. Med. 60, 355–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs T. S.; Johansen P. Ø. (2012) Lysergic acid diethylamide (LSD) for alcoholism: meta-analysis of randomized controlled trials. J. Psychopharmacol. 26(7), 994–1002. [DOI] [PubMed] [Google Scholar]
- Vollenweider F. X.; Vollenweider-Scherpenhuyzen M. F.; Babler A.; Vogel H. (1998) Psilocybin induces schizophrenia-like psychosis in humans via a serotonin- 2 agonist action. NeuroReport 9(17), 3897–3902. [DOI] [PubMed] [Google Scholar]
- Adams K. H.; Hansen E. S.; Pinborg L. H.; Hasselbalch S. G.; Svarer C.; Holm S.; Bolwig T. G.; Knudsen G. M. (2005) Patients with obsessive-compulsive disorder have increased 5-HT2A receptor binding in the caudate nuclei. Int. J. Neuropsychopharmacol. 8(3), 391–401. [DOI] [PubMed] [Google Scholar]
- Moreno F. A.; Wiegand C. B.; Taitano E. K.; Delgado P. L. (2006) Safety, tolerability and efficacy of psilocybin in 9 patients with obsessive compulsive-compulsive disorder. J. Clin. Psychiatry 67(11), 1735–1740. [DOI] [PubMed] [Google Scholar]
- Celada P.; Puig M. V.; Amargós-Bosch M.; Adell A.; Artigas F. (2004) The therapeutic role of 5-HT1A and 5-HT2A receptors in depression. J. Psychiatry Neurosci. 29(4), 252–265. [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris R. L.; Erritzoe D.; Williams T.; Stone J. M.; Reed L. J.; Colasanti A.; Tyacke R. J.; Leech R.; Malizia A. L.; Murphy K.; Hobden P.; Evans J.; Feilding A.; Wise R. G.; Nutt D. J. (2012) Neural correlates of the psychedelic state as determined by fMRI studies with psilocybin. Proc. Natl. Acad. Sci. U.S.A. 109(6), 2138–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K.; Imbe H.; Kimura A.; Donishi T.; Tamai Y.; Senba E. (2007) Activation of central 5HT2A receptors reduces the craniofacial nociception of rats. Neuroscience 147(4), 1090–1102. [DOI] [PubMed] [Google Scholar]
- Nau F. Jr.; Yu B.; Martin D.; Nichols C. D. (2013) Serotonin 5-HT2A Receptor Activation Blocks TNF-a Mediated Inflammation In Vivo. PLoS One 8(10), e75426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel E. (2007) Serotonin and migraine: biology and clinical implications. Cephalalgia 27(11), 1293–1300. [DOI] [PubMed] [Google Scholar]
- Sewell R. A.; Halpern J. H.; Pope H. G. Jr. (2006) Response of cluster headache to psilocybin and LSD. Neurology 66(12), 1920–1922. [DOI] [PubMed] [Google Scholar]
- Griffiths R. R.; Richards W. A.; McCann U.; Jesse R. (2006) Psilocybin can occasion mystical-type experiences having substantial and sustained personal meaning and spiritual significance. Psychopharmacology 187(3), 268–283. [DOI] [PubMed] [Google Scholar]
- Griffiths R.; Richards W.; Johnson M.; McCann U.; Jesse R. (2008) Mystical-type experiences occasioned by psilocybin mediate the attribution of personal meaning and spiritual significance 14 months later. J. Psychopharmacol. 22(6), 621–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths R. R.; Johnson M. W.; Richards W. A.; Richards B. D.; McCann U.; Jesse R. (2011) Psilocybin occasioned mystical-type experiences: immediate and persisting dose-related effects. Psychopharmacology 218, 649–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollenweider F. X.; Geyer M. A. (2001) A systems model of altered consciousness: integrating natural and drug-induced psychoses. Brain Res. Bull. 56(5), 495–507. [DOI] [PubMed] [Google Scholar]
- Nichols D. E. (2004) Hallucinogens. Pharmacol. Ther. 2, 131–81. [DOI] [PubMed] [Google Scholar]
- Karst M.; Halpern J. H.; Bernateck M.; Passie T. (2010) The non-hallucinogen 2-bromo-lysergic acid diethylamide as preventative treatment for cluster headache: an open, non-randomized case series. Cephalalgia 30(9), 1140–1144. [DOI] [PubMed] [Google Scholar]
- Weiner D. M.; Burstein E. S.; Nash N.; Croston G. E.; Currier E. A.; Vanover K. E.; Harvey S. C.; Donohue E.; Hansen H. C.; Andersson C. M.; Spalding T. A.; Gibson D. F.; Krebs-Thomson K.; Powell S. B.; Geyer M. A.; Hacksell U.; Brann M. R. (2001) 5-hydroxytryptamine 2A receptor inverse agonists as antipsychotics. J. Pharmacol. Exp. Ther. 299(1), 268–276. [PubMed] [Google Scholar]
- Nichols D. E. (2012) Structure–activity relationships of serotonin 5-HT 2A agonists. WIREs Membr. Transp. Signaling 1, 559–579. [Google Scholar]
- Schardl C. L.; Panaccione D. G.; Tudzynski P. (2006) Ergot alkaloids – biology and molecular biology. Alkaloids: Chem. Biol. 63, 45–86. [DOI] [PubMed] [Google Scholar]
- Tfelt-Hansen P.; Saxena P. R.; Dahlöf C.; Pascual J.; Láinez M.; Henry P.; Diener H.; Schoenen J.; Ferrari M. D.; Goadsby P. J. (2000) Ergotamine in the acute treatment of migraine: a review and european consensus. Brain 123(1), 9–18. [DOI] [PubMed] [Google Scholar]
- Reichmann H.; Bilsing A.; Ehret R.; Greulich W.; Schulz J. B; Schwartz A.; Rascol O. (2006) Ergoline and non-ergoline derivatives in the treatment of Parkinson’s disease. J Neurol. 253(4), 36–38. [DOI] [PubMed] [Google Scholar]
- Colao A.; Abs R.; Bárcena D. G.; Chanson P.; Paulus W.; Kleinberg D. L. (2008) Pregnancy outcomes following cabergoline treatment: extended results from a 12-year observational study. Clin. Endocrinol. 68(1), 66–71. [DOI] [PubMed] [Google Scholar]
- Glennon R. A.; Raghupathi R. K.; Bartyzel P.; Teitler M.; Leonhardt S. (1992) Binding of phenylalkylamine derivatives at 5-HT1C and 5-HT2 serotonin receptors: evidence for a lack of selectivity. J. Med. Chem. 35, 734–740. [DOI] [PubMed] [Google Scholar]
- Parker M. A.; Marona-Lewicka D.; Lucaites V. L.; Nelson D. L.; Nichols D. E. (1998) A novel (benzodifuranyl)aminoalkane with extremely potent activity at the 5-HT2A receptor. J. Med. Chem. 41(26), 5148–5149. [DOI] [PubMed] [Google Scholar]
- McLean T. H.; Parrish J. C.; Braden M. R.; Marona-Lewicka D.; Gallardo-Godoy A.; Nichols D. E. (2006) 1-Aminomethylbenzocycloalkanes: conformationally restricted hallucinogenic phenethylamine analogues as functionally selective 5-HT2A receptor agonists. J. Med. Chem. 49(19), 5794–5803. [DOI] [PubMed] [Google Scholar]
- Nelson D. L.; Lucaites V. L.; Wainscott D. B.; Glennon R. A. (1999) Comparisons of hallucinogenic phenylisopropylamine binding affinities at cloned human 5-HT2A, -HT(2B) and 5-HT2C receptors. Naunyn-Schmiedeberg's Arch. Pharmacol. 359(1), 1–6. [DOI] [PubMed] [Google Scholar]
- Ho B. T.; Tansey L. W.; Balster R. L.; An R.; McIsaac W. M.; Harris R. T. (1970) Amphetamine analogs. II. Methylated phenethylamines. J. Med. Chem. 13(1), 134–135. [DOI] [PubMed] [Google Scholar]
- Heim R.Synthese und Pharmakologie potenter 5-HT2A-Rezeptoragonisten mit N-2-Methoxybenzyl-Partialstruktur. Entwicklung eines neuen Struktur-Wirkungskonzepts. Ph.D. Thesis, Freie Universität Berlin, Berlin, Germany, 2003. [Google Scholar]
- Halberstadt A. L.; Geyer M. A. (2013) Effects of the hallucinogen 2,5-dimethoxy-4-iodophenethylamine (2C-I) and superpotent N-benzyl derivatives on the head twitch response. Neuropharmacology 77, 200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juncosa J. I. Jr.; Hansen M.; Bonner L. A.; Cueva J. P.; Maglathlin R.; McCorvy J. D.; Marona-Lewicka D.; Lill M. A.; Nichols D. E. (2013) Extensive rigid analogue design maps the binding conformation of potent N-benzylphenethylamine 5-HT2A serotonin receptor agonist ligands. ACS Chem. Neurosci. 4(1), 96–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettrup A.; Hansen M.; Santini M. A.; Paine J.; Gillings N.; Palner M.; Lehel S.; Herth M. M.; Madsen J. (2011) Radiosynthesis and in vivo evaluation of a series of substituted 11C-phenethylamines as 5-HT2A agonist PET tracers. Eur. J. Nucl. Med. Mol. Imaging 38(4), 681–693. [DOI] [PubMed] [Google Scholar]
- Ettrup A.; Holm S. R.; Hansen M.; Wasim M.; Santini M. A.; Palner M.; Madsen J.; Svarer C.; Kristensen J. L.; Knudsen G. M. (2013) Preclinical Safety Assessment of the 5-HT2A Receptor Agonist PET Radioligand [11C]Cimbi-36. Mol. Imaging Biol. 15(4), 376–383. [DOI] [PubMed] [Google Scholar]
- Blaazer A. R.; Smid P.; Kruse C. (2008) G.Structure-activity relationships of phenylalkylamines as agonist ligands for 5-HT(2A) receptors. ChemMedChem 3(9), 1299–1309. [DOI] [PubMed] [Google Scholar]
- Isberg V.; Balle T.; Sander T.; Jorgensen F.; Gloriam D. (2011) G Protein- and Agonist-Bound Serotonin 5-HT2A Receptor Model Activated by Steered Molecular Dynamics Simulations. J. Chem. Inf. Model. 51, 315–325. [DOI] [PubMed] [Google Scholar]
- Christiansen B.; Hansen K. B.; Wellendorph P.; Bräuner-Osborne H. (2007) Pharmacological characterization of mouse GPRC6A, an L-α-amino acid receptor with ability to sense divalent cations. Br. J. Pharmacol. 150, 798–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandish P. E.; Hill L. A.; Zheng W.; Scolnick E. M. (2003) Scintillation proximity assay of inositol phosphates in cell extracts: high-throughput measurement of G-protein-coupled receptor activation. Anal. Biochem. 313(2), 311–318. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.