Abstract

Kappa-opioid receptor (κ) antagonists are potential therapeutic agents for a range of psychiatric disorders. The feasibility of developing κ-antagonists has been limited by the pharmacodynamic properties of prototypic κ-selective antagonists; that is, they inhibit receptor signaling for weeks after a single administration. To address this issue, novel trans-(3R,4R)-dimethyl-4-(3-hydroxyphenyl) piperidine derivatives, based on JDTic, were designed using soft-drug principles. The aim was to determine if the phenylpiperidine-based series of κ-antagonists was amenable to incorporation of a potentially metabolically labile group, while retaining good affinity and selectivity for the κ-receptor. Opioid receptor binding affinity and selectivity of three novel compounds (BU09057, BU09058, and BU09059) were tested. BU09059, which most closely resembles JDTic, had nanomolar affinity for the κ-receptor, with 15-fold and 616-fold selectivity over μ- and δ-receptors, respectively. In isolated tissues, BU09059 was a potent and selective κ-antagonist (pA2 8.62) compared with BU09057 (pA2 6.87) and BU09058 (pA2 6.76) which were not κ-selective. In vivo, BU09059 (3 and 10 mg/kg) significantly blocked U50,488-induced antinociception and was as potent as, but shorter acting than, the prototypic selective κ-antagonist norBNI. These data show that a new JDTic analogue, BU09059, retains high affinity and selectivity for the κ-receptor and has a shorter duration of κ-antagonist action in vivo.
Keywords: kappa-opioid receptor, kappa antagonist, mu antagonist, norBNI, tail-withdrawal assay, CD-1 mouse
Kappa-opioid receptors (κ) are Gi/o-coupled receptors that are activated by the neuropeptide dynorphin.1 Both prodynorphin, the precursor for dynorphin peptides, and κ-receptor expression is high in the brain structures underlying emotional control and stress responses, including brain stem nuclei, amygdala, hippocampus, and cortical regions.2−4 κ-Agonists induce dysphoric responses in humans and aversive responses in rodents5−7 whereas κ-antagonists, κ-receptor gene deletion, or prodynorphin gene disruption block stress-induced behavioral responses.8,9 Hence, there is growing interest in κ-antagonists as potential therapeutic treatments for a variety of psychiatric diseases, including substance misuse and mood disorders.10,11
High affinity, selective κ-antagonists have an unusual pharmacodynamic property that is not well understood. A number of high affinity, selective, nonpeptidic κ-antagonists have been identified, including (3R)-7-hydroxy-N-[(1S)-1-[[(3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethyl-1-piperidinyl]methyl]-2-methylpropyl]-1,2,3,4-tetrahydro-3-isoquinoline-carboxamide (JDTic), 5′-guanidinonaltrindole (GNTI), and norbinaltorphimine (norBNI).12−14 However, these compounds all have very long-lasting effects in vivo. For example, a single dose of a selective κ-antagonist, such as JDTic, has peak effects at 7 days and long-lasting receptor blockade up to 21 days postinjection.15 Diverse mechanisms have been proposed to account for this unusual long duration of action of selective κ-antagonists.13 For example, it has been suggested that κ-antagonists are lipophilic and may form depots in the neuronal membranes which may lead to slow clearance of κ-antagonists from the brain. An alternative mechanism for the long-lasting action of κ-antagonists is that they are resistant to metabolic pathways or produce metabolites that are active at the receptors, although little is known about the metabolism of these compounds.13 More recently, κ-antagonists have been shown to produce ligand-directed signaling at the κ-receptor, with long-lasting κ-antagonists producing a c-Jun N terminal kinase (JNK) mediated inactivation of the κ-receptor.16,17 Whatever the mechanisms are that account for the long-duration of κ-antagonist action, such pharmacodynamic properties may not be ideal for therapeutic development. Recently, a phase 1, first in human clinical trial with JDTic was terminated due to undisclosed adverse effects (ClinicalTrials.gov identifier NCT01431586). Therefore, there remains a need to develop alternative, including shorter-acting, selective κ-antagonists.
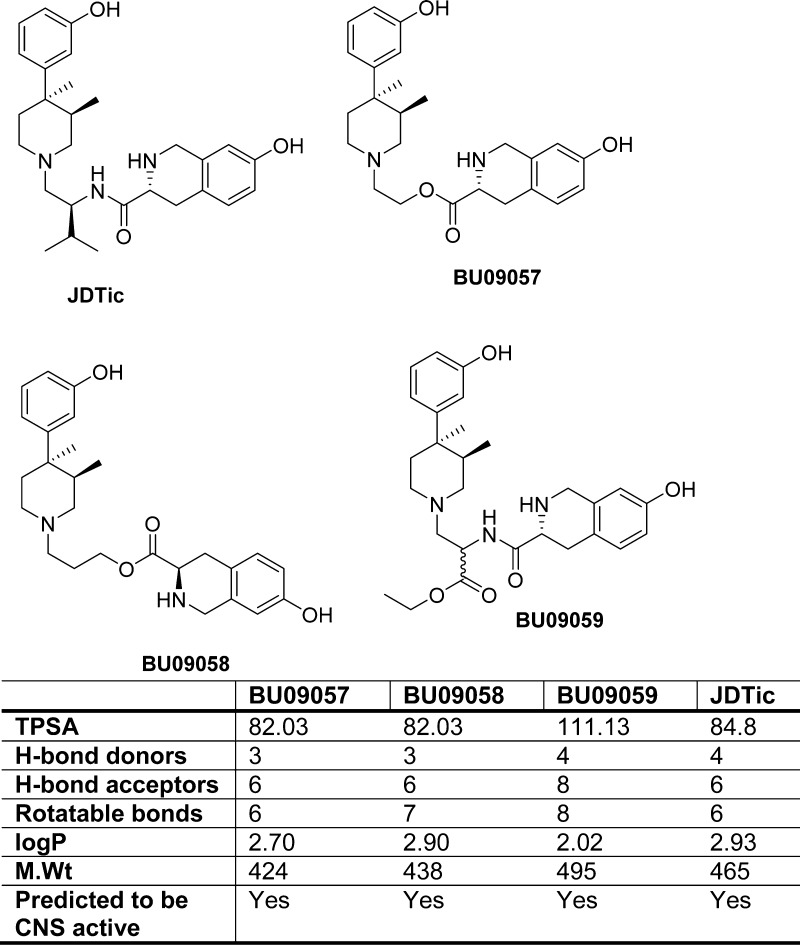
Our approach has been to investigate whether the phenylpiperidine series of opioid antagonists can be modified to produce high affinity, selective κ-antagonists with a shorter duration of action than the prototypic κ-antagonists. We have synthesized ester containing ligands BU09057, BU09058, and BU09059 (Figure 1). The ligands in this study retained the trans-3,4-dimethyl(3-hydroxyphenyl)piperidine moiety of JDTic, that serves as the message that recognizes all opioid receptors and the tetrahydroisoquinoline amino group that confers selectivity (address) for κ-receptors.18 The isopropyl side chain of JDTic has been omitted from two of the targets due to the synthetic challenge posed in incorporating it alongside the labile functionality. The amide is replaced by the isosteric, and potentially metabolically labile, ester group in BU09057 and BU09058. BU09059 can be considered the closest analogue to JDTic, with the side chain ester being used to mimic, as closely as possible, the isopropyl group present in JDTic. It was not clear whether changes of this magnitude could be tolerated while retaining affinity and selectivity for the κ-receptor. This paper describes the in vitro and in vivo characterization of BU09057, BU09058, and BU09059.
Figure 1.
Structures of BU09057, BU09058, and BU09059 alongside JDTic and predicted physicochemical properties (ACD/I-Lab-2, accessed via the Royal Society of Chemistry National Chemical Database Service) TPSA: topological polar surface area.
Results and Discussion
We have synthesized novel compounds with the aim of developing high affinity, selective κ-antagonists with a shorter duration of action than the prototypic κ-antagonists, such as norBNI. This is important for understanding the neurobiology of κ-receptors and also for the development of novel therapeutics that target κ-receptors. Novel ligands were designed using the structure of JDTic as a starting point (Figure 1). As far as possible, the critical structure–activity relationship (SAR) features identified by Thomas and co-workers18,19 to be essential for the high affinity and selectivity toward the κ-receptor were conserved. BU09057 and BU09058 both lack the isopropyl side chain found in JDTic, while in BU09059 an ester moiety is used to mimic the side chain. Potential sites of metabolism were designed within the chain connecting the message and address portions (BU09057, BU09058), or in a side chain off the connecting linker unit (BU09059). Like JDTic, each of the ligands has properties that would predict access to the brain and thus CNS activity (Figure 1). The synthesis and chemical characterization of the novel compounds is described in the Supporting Information. All compounds assayed were of ≥95% purity.
The affinities of BU09057, BU09058, BU09059, norBNI, and GNTI for the κ-, μ-, and δ-opioid receptors were determined using the competitive [3H]-diprenorphine binding assay (Table 1). Of the novel compounds tested, BU09059 had highest affinity for the κ-receptor (Ki 1.72 ± 4.38 nM) and showed promising selectivity for κ/μ (15-fold) in the range of the standard κ-opioid receptor antagonists norBNI and GNTI. Interestingly, the selectivity of BU09059 for κ/δ (616 fold) far exceeded that of norBNI and GNTI. In this study, norBNI and GNTI appeared to have greater binding affinities for μ and δ receptors than previously published data, thereby reducing the apparent selectivity of norBNI compared to the 169-fold κ/μ selectivity reported by Takemori et al.,20 for example. Here, values were obtained using competitive [3H]-diprenorphine binding, whereas others have used displacement of agonists such as [3H]-DAMGO, [3H]-DADLE, and [3H]-ethylketocyclazocine20 that may recognize different moieties in the receptor-binding pocket, and thus, different displacement curves might be produced and give rise to somewhat different affinity values. Among all the ligands tested, BU09057 appeared to have the lowest affinity for both the κ-receptor (Ki 158.0 ± 20.6 nM) and the μ-receptor (Ki 186.0 ± 110.8 nM) and was not selective for κ/μ and only weakly selective for κ/δ. BU09058 had the highest affinity for μ-receptors (Ki 6.55 ± 3.43 nM), rather than κ- or δ-receptors, and was weakly selective for μ/κ (∼4-fold) but rather had greater selectivity for μ/δ (∼37-fold). Interestingly, the novel test compounds showed lower affinity for the κ-receptor than the published reports for the parent compound JDTic21 (Table 1). However, this change in binding affinity might be predicted since increasing the length of this side chain in JDTic has previously been shown to reduce both the binding affinity and selectivity for the κ-receptor.22 The novel compounds were without agonist efficacy in the [35S]-GTPγS assay. At concentrations up to 10 μM, neither BU09057, BU09058, nor BU09059 produced stimulation of [35S]-GTPγS binding in C6-μ and CHO-κ cell membranes, indicating a lack of any agonist efficacy at κ- or μ-receptors (n = 3).
Table 1. Summary of Antagonist Affinity and Selectivity for κ-, μ-, and δ-Receptors in the Competitive [3H]-Diprenorphine Binding Assaya.
|
Ki (nM) |
K-selectivity |
||||
|---|---|---|---|---|---|
| ligand | κ | μ | δ | κ/μ | κ/δ |
| JDTicb | 0.41 ± 0.10 | 0.96 ± 0.0 | 29.6 ± 11.9 | 2 | 72 |
| NorBNI | 0.29 ± 0.02 | 1.99 ± 2.38 | 0.46 ± 0.09 | 14 | 2 |
| GNTI | 0.37 ± 0.16 | 4.74 ± 1.96 | 2.86 ± 1.45 | 13 | 8 |
| BU09057 | 158.6 ± 20.6 | 186.0 ± 110.8 | 475.0 ± 175.0 | 1 | 3 |
| BU09058 | 25.2 ± 8.2 | 6.55 ± 3.43 | 221.0 ± 137.0 | <1 | 9 |
| BU09059 | 1.72 ± 4.38 | 26.5 ± 8.4 | 1060.0 ± 320.0 | 15 | 616 |
Ki values were determined by competitive displacement of [3H]-diprenorphine (0.2 nmol·L–1) binding in CHO-κ, C6-μ, and C6-δ cell membranes. Values are the mean ± SEM of n = 3 in triplicate experiments.
Values previously reported and obtained using cloned human opioid receptors transfected into CHO cells.21
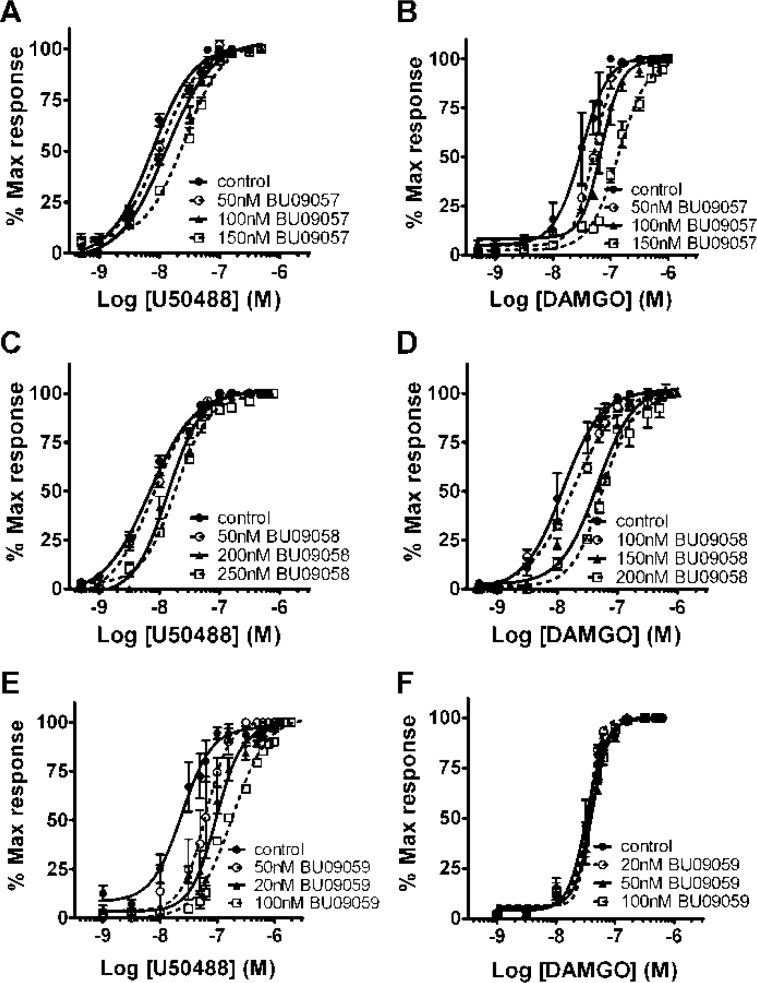
Antagonist action and potency was confirmed in isolated tissue preparations (Figures 2, 3). Electrically evoked twitches in the isolated guinea-pig ileum were inhibited by approximately 90% by maximal concentrations of the κ-agonist U50488 and the μ-agonist DAMGO. BU09057 (150 nM) produced a significant rightward shift in the U50,488 concentration–response curve (CRC) (control pEC50 = 7.63 (7.81–7.39, 95% CI); 150 nM BU09057 pEC50 = 7.30 (7.34–7.25, 95% CI); n = 4; P < 0.01) and of the DAMGO CRC (control pEC50 = 7.51 (7.95–7.07, 95% CI); 150 nM BU09057 pEC50 = 6.85 (7.05–6.64, 95% CI); n = 4; P < 0.05), without significant effects on the U50,488 or DAMGO Emax (Figure 2). Similarly, BU09058 significantly antagonized the effects of both U50,488 (control pEC50 = 8.23 (8.62–7.84, 95% CI); 200 nM BU09058 pEC50 = 7.79 (7.82–7.75, 95% CI); n = 4; P < 0.05) and DAMGO (control pEC50 = 8.06 (8.38–7.74, 95% CI); 100 nM BU09058 pEC50 = 7.74 (7.93–7.55, 95% CI); n = 4; P < 0.05) without affecting Emax (Figure 2). Thus, the κ- and μ-antagonist effects produced were surmountable with increasing concentrations of agonist. Antagonist potencies (pA2) were determined by Schild plot analysis. In each case, unity was within the 95% confidence limits of the slope of the Schild plot (Table 2). These experiments demonstrated that BU09057 and BU09058 are moderately potent antagonists at both the κ- and μ-receptors with no selectivity for either subtype.
Figure 2.
Cumulative concentration–response curves, in the guinea pig ileum for U50,488 and DAMGO (maximal agonist concentrations 1 μM), in the presence of increasing concentrations of BU09057 (A, B), BU09058 (C, D), and BU09059 (E, F). Apart from BU09059, all ligands tested caused rightward parallel shifts in the concentration response curves of both U50,488 and DAMGO. Results are expressed as the mean percentage of the maximum response induced by the agonist ± SEM, n = 4 tissues.
Figure 3.
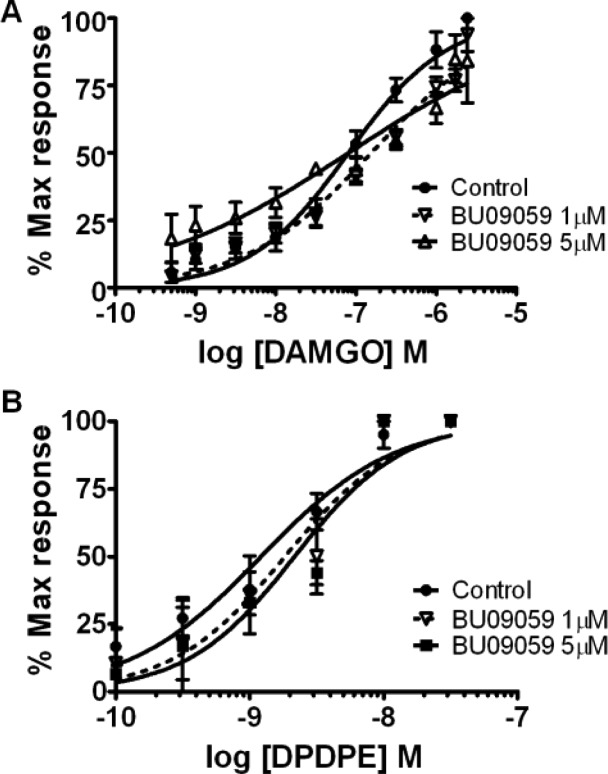
Cumulative concentration–response curves, in the mouse vas deferens, for DAMGO (maximal agonist concentration 2 μM) (A) and DPDPE (maximal agonist concentration 20 nM) (B), in the presence of high concentrations of BU09059. Results are expressed as the mean percentage of the maximum response induced by the agonist ± SEM, n = 4 tissues.
Table 2. Summary of Antagonist Potency at κ- and μ-Receptors in the Isolated Guinea Pig Ileum Preparationa.
| antagonist potency (pA2) |
||
|---|---|---|
| ligand | κ | μ |
| BU09057 | 6.87 (7.13–6.35) (134.9 nM) | 7.01 (7.89–6.79) (97.7 nM) |
| BU09058 | 6.76 (7.45–6.05) (173.8 nM) | 7.03 (7.32–6.90) (93 nM) |
| BU09059 | 8.62 (9.12–8.03) (2.39 nM) | |
| NorBNI | 8.30 (10.3–7.72) (5.0 nM) | |
pA2 values were determined by Schild plot analysis. Values are the mean and 95% confidence interval for n = 4 tissues. Values for the prototypic κ-antagonist norBNI were obtained from our previously published data.25 Mean values are also shown as nM.
In contrast, BU09059 was revealed to be a potent and selective κ-antagonist in the isolated guinea pig ileum. Increasing concentrations of BU09059 produced reversible progressive parallel rightward shifts of the U50,488 CRC (control pEC50 = 7.80 (8.50–7.09, 95% CI); 20 nM BU09057 pEC50 = 6.98 (7.13–6.83, 95% CI); n = 4; P < 0.05), without affecting Emax (Figure 2). Interestingly, BU09059 had no significant effects on the DAMGO CRC at concentrations up to 100 nM. The pA2 value for BU09059 at the κ-receptor was 8.62 (Table 2). To further investigate the selectivity of BU09059 for κ-receptors, the mouse vas deferens assay was used to examine effects at μ- and δ-receptors (Figure 3). Electrically evoked twitches of the mouse vas deferens were inhibited by almost 90% by maximal concentrations of the μ-agonist DAMGO and the δ-agonist DPDPE. At concentrations up to 5 μM, BU09059 was without significant effect on the DAMGO CRC (control pEC50 = 7.12 (7.22–6.98; 95% CI); 5 μM BU09059 pEC50 = 7.09 (7.36–6.83; 95% CI); n = 4; P > 0.05) or the DPDPE CRC (control pEC50 = 8.92 (9.06–8.77; 95% CI); 5 μM BU09059 pEC50 = 8.66 (8.89–8.44; 95% CI); n = 4; P > 0.05). There was no significant effect of BU09059 on Emax values for both DAMGO and DPDPE. Thus, BU09059 possessed negligible antagonist actions at μ- and δ-receptors.
BU09059 was considered the closest analogue to JDTic, with the ester side chain being slightly larger and electronically dissimilar to the isopropyl group of JDTic. Previously, a JDTic analogue with slightly increased bulk at this position has been reported to retain high affinity and selectivity for the κ-receptor,14,23 suggesting that introduction of the ester moiety in BU09059 might be well tolerated. Indeed, BU09059 demonstrated the highest affinity (Ki 1.72 nM), selectivity (κ/μ 15-fold; κ/δ 616-fold), and antagonist potency (pA2 8.62) for the κ-receptor among the novel phenylpiperidine-derived ligands tested. Furthermore, there were no detectable μ- or δ-receptor antagonist effects of BU09059 in isolated tissue preparations at concentrations up to 5 μM. Thus, introduction of the ester group appeared to cause only a modest disruption of the binding affinity for the κ-receptor in comparison to JDTic (Ki 0.41 nM in cloned human CHO-k cells).21 This change in binding affinity was expected, since Cueva et al.24 have shown that increasing the length of this side chain in JDTic (from an isopropyl to a sec-butyl group) reduces both the antagonist potency and selectivity for the κ-receptor by 13-fold (versus the μ-receptor) in the agonist stimulated [35S]-GTPγS binding assay. In addition, BU09059 appeared to be more selective in the functional assays than the binding assays and this mirrors the findings for JDTic, which had only 12-fold selectivity in binding assays but substantially greater selectivity in functional assays (discussed in (21)). Overall, BU09059 demonstrated a highly selective κ-receptor profile that made it particularly interesting for further testing in vivo.
Of the phenylpiperidine ligands tested, BU09057 was shown to have the lowest affinity for both the κ-receptor (Ki 158.6 nM) and the μ-receptor (Ki 475.0 nM) in the [3H]-diprenorphine binding assay. In the isolated guinea pig ileum, BU09057 appeared to demonstrate modest antagonist potency for both the κ- (pA2 6.87) and μ-receptor (pA2 7.01), indicating it was a nonselective mixed κ/μ antagonist. The substitution of the original amide containing chain in JDTic, with an ester in BU09057, coupled with loss of the isopropyl side chain, appeared to dramatically reduce the affinity for the κ-receptor (almost 100 fold compared with BU09059). Its closest analogue, BU09058, contained a one carbon longer ester linker, and, among the novel compounds, BU09058 had the highest affinity for the μ-receptor (Ki 6.55 nM) and the δ-receptor (Ki 221.0 nM), with a somewhat reduced affinity for the κ-receptor (Ki 25.2 nM). However, in the isolated guinea pig ileum, BU09058 had a very similar antagonist profile to that of BU09057 with modest antagonist potency at both the κ- (pA2 6.76) and μ-receptors (pA2 7.03). The major effect of the structural differences between BU09057 and BU09058 compared with BU09059 was loss of selectivity for the κ-receptor. The profile displayed by BU09057 and BU09058 may in itself prove interesting, as mixed μ/κ-receptor antagonists have recently been shown to have anxiolytic- and or antidepressant-like activity in preclinical studies.25 In addition, a combination of buprenorphine, a partial μ agonist and κ antagonist, and a selective μ antagonist (ALKS33BUP), together providing predominantly μ and κ antagonism, has completed phase II clinical trials in major depressive disorder patients (ClinicalTrials.gov identifier NCT1381107).
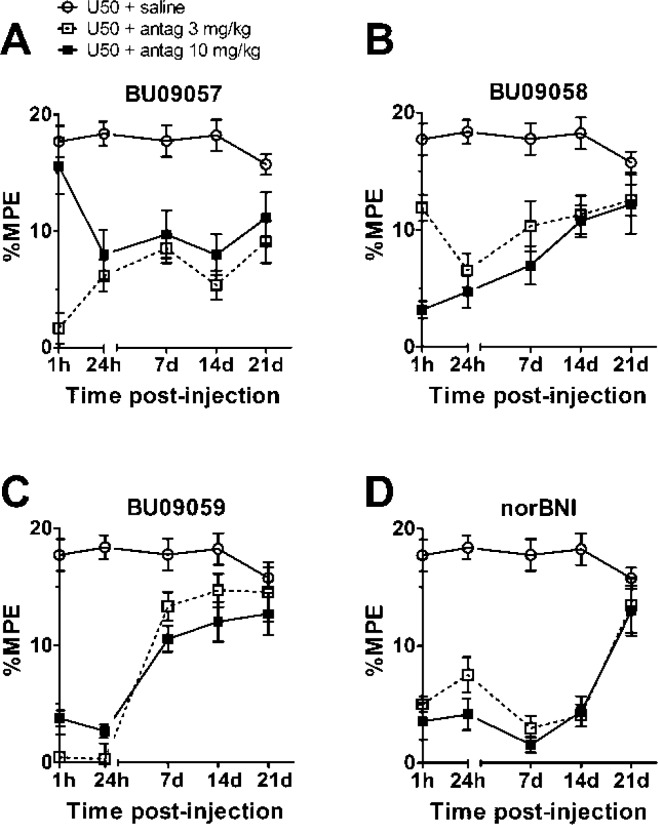
For in vivo studies, we first established that intraperitoneal injection in adult CD-1 mice of 1, 3.2, and 10 mg/kg of BU09057, BU09058, and BU09059 did not produce any adverse behaviors over a 48 h observation period (n = 3, per dose per drug). The ability of these novel phenylpiperidine derivatives to block κ-receptors in vivo was then examined using the blockade of U50,488-induced antinociception in the warm water tail-withdrawal assay in CD-1 mice (Figure 4). Tail-withdrawal responses were measured at 1 h, 24 h, 7 d, 14 d, and 21 d after a single injection of test ligand (3 or 10 mg/kg). A two-way repeated measures mixed model analysis revealed a significant main effect of drug treatment (F(8, 63) = 20.76, p < 0.001) and time (F(4, 252) = 20.62, p < 0.001). The interaction between treatment and time was also significant (F(32, 252) = 4.83, p < 0.001).
Figure 4.
Ability of the test compounds BU09057, BU09058, and BU09059 to block U50,488-induced antinociception in the tail-withdrawal test. Mice received a single injection of 0.9% (w/v) saline, BU09057 (A), BU09058 (B), BU09059 (C), and norBNI (D) at both 3 mg/kg (□) and 10 mg/kg (■), and tail-withdrawal latency measured at intervals up to 21 days post injection. U50,488 (10 mg/kg) was injected on each test occasion, 30 min prior to measuring tail-withdrawal latency. Data are expressed as mean percentage maximum possible effect (%MPE) ± SEM, n = 8 per treatment group.
Within-treatment post hoc pairwise comparisons revealed that, at 24 h postinjection, BU09057, BU09058, and BU09059 (3 and 10 mg/kg) significantly blocked U50,488-induced antinociception compared to saline (all p < 0.05), demonstrating functional κ-antagonist activity in vivo (Figure 4). However, BU09059 (3 mg/kg), displayed significantly greater blockade of U50,488 actions than did norBNI (3 mg/kg, p < 0.05), suggesting that it is more potent or has a more rapid onset of action than norBNI. BU09059 (3 and 10 mg/kg) significantly blocked U50,488-induced antinociception after 1 h postinjection, maintaining maximal peak until 24 h (compared to saline-controls, p < 0.05). At 7 and 14 d postinjection, norBNI continued to produce significant blockade of U50,488-induced antinociception (p < 0.05, compared to saline), consistent with its reported long duration of action in vivo.15 However, BU09059 (3 and 10 mg/kg) showed significantly diminished antagonist activity, compared to norBNI (at equivalent doses, all p < 0.05) at 7 and 14 d postinjection. Overall, these results show a different timecourse of effect for the novel phenylpiperidine derivatives compared to norBNI, with BU09059 particularly showing a more rapid onset of action and a more rapid reversal of effects, suggesting that this compound has a shorter duration of action than norBNI.
In vivo BU09059 was equipotent to the prototypic κ-antagonist norBNI but exhibited a different pharmacodynamic profile. Blockade of U50,488-induced antinociception in the tail- withdrawal assay by a single injection of norBNI, was effective 1 h postinjection, peaked at 7–14 d, and was significantly diminished by 21 d postinjection, consistent with previous reports in the literature.15,26 BU09059 had a similar rapid onset of blockade of U50,488-induced antinociception, effective 1 h postinjection. However, in contrast to the case of norBNI, peak effects of BU09059 were observed at 24 h postinjection and significantly reduced by 7 d. On the other hand, both BU09057 and BU09058 seemed to produce long-lasting κ-antagonism at days 7–14. These two compounds are the more distant analogues of JDTic, lacking the isopropyl side chain entirely. The modification in BU09059, where the isopropyl side chain is replaced with an ester, indicates that this connecting linker unit may be structurally important in determining the duration of action of κ-antagonists, as well as influencing κ-selectivity.
These data suggest that the insertion of the hypothetically metabolically labile ester side chain into BU09059 can reduce the duration of κ-antagonist action while maintaining high affinity and selectivity for the κ-receptor. This approach to achieving shorter-acting compounds by integrating hydrolyzable ester groups has been successful in a wide range of target drugs, including the μ-receptor analgesic remifentanil,27 beta-blockers,28,29 anticholinergics,30 and antiarrhythmic agents.31 The prolonged κ-receptor antagonist activity of high affinity, selective κ-antagonists such as JDTic has been seen as a limitation toward successful drug development for a number of therapeutic indications. A phase 1, first in human clinical trial with JDTic was indeed terminated in 2012 due to undisclosed adverse effects (ClinicalTrials.gov identifier NCT01431586). Therefore, there is a need to develop alternatives, including shorter-acting, selective κ-antagonists.
Several new κ-antagonists have recently been reported with a shorter duration of κ-antagonist activity.32−36 Different approaches have been adopted in the development of these ligands with some appearing to be shorter acting because they do not produce prolonged inactivation of the κ-receptor via JNK activation.17 It is not known to what extent a different metabolic profile contributes to their shorter duration of action. Until recently, very little was known about the metabolism of the long-acting selective κ-antagonists in mice. JDTic, norBNI and GNTI all bind reversibly to κ-receptors in vitro and do not form covalent bonds with the receptor. Thus, in vitro they are competitive antagonists producing surmountable antagonism, although in vivo they are pseudoirreversible antagonists.12 Following intraperitoneal administration, JDTic, norBNI, and GNTI (all 10 mg/kg) are rapidly absorbed and eliminated from the plasma.37 Clearly, plasma concentrations do not parallel the slow onset and long duration of κ-antagonism seen in vivo. These authors argue that their findings support the idea that selective κ-antagonists, with long duration of activity, act to produce an irreversible change in the κ-receptor, such as JNK-mediated inactivation. However, these authors also demonstrated that JDTic was only slowly eliminated from the brain, possibly because of accumulation in lysosomes, and that this effect might contribute to its long duration of action. Munro et al.37 also demonstrated that JDTic, norBNI, and GNTI were hydrophilic with a low affinity for brain homogenate suggesting that they were unlikely to form lipid depots or nonspecific tissue binding. More recently, norBNI has also been shown to be detectable in mouse brain homogenates isolated up to 21 days after intraperitoneal administration (10 mg/kg), which outlasted its apparent κ-antagonist activity in the tail-withdrawal assay.38 However, the levels of norBNI detected were proven to be below the threshold required to produce κ-antagonism, supporting the idea that the long duration of κ-antagonist action cannot be simply explained by the presence of antagonist in the brain and corresponding occupancy of κ-receptors. BU09059 has a shorter duration of action in vivo than norBNI, JDTic, and GNTI. While esters are a potential metabolic hotspot in a molecule, they are not necessarily easily metabolized, with some being metabolically stable. Furthermore, BU09059 lacks the isopropyl group present in JDTic and this may contribute to the shorter duration. Further determination of the pharmacokinetics of BU09059 in mouse brain and its potential metabolic products may provide insights regarding the shorter duration of activity of this novel compound. With its shorter duration of action, high selectivity, and potent κ-antagonist action in vivo, BU09059 will be a useful novel tool in understanding the functional role of κ-receptors and the chemistry of κ-receptor-drug interactions.
Methods
Materials
Cell culture reagents were from Gibco Life Sciences (Grand Island, NY). All other chemicals were analytical grade and purchased from Sigma-Aldrich except: guanosine-5′-O-(3-[35S]-thio)triphosphate ([35S]-GTPγS) and [3H]-diprenorphine (Perkin-Elmer, Waltham, MA), DAMGO and DPDPE from Abcam Biochemicals. Norbinaltorphimine dihydrochloride (norBNI), 5′-guanidinonaltrindole trifluoroacetic acid (GNTI), BU09057, BU09058, and BU09059 were synthesized (Figure 1) (>95% purity) in the Department of Pharmacy and Pharmacology, University of Bath.
Synthesis and Chemical Characterization of BU09057, BU09058, and BU09059
The compounds were prepared from (+)-(3R,4R)-3,4-dimethyl-4-(3-hydroxyphenyl)piperidine (purchased from Astatech Inc. and used as received) as described in the Supporting Information. 7-Hydroxy-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid was purchased from Acros Organics, and other reagents and solvents were from Sigma Aldrich. NMR spectra were obtained on a JEOL Delta-270 MHz instrument with TMS as internal standard. NMR spectra were obtained on a JEOL Delta-270 MHz instrument with TMS as internal standard; electrospray ionization mass spectrometry (ESIMS) was recorded using a microTOF (Bruker). Ligands were tested as their hydrochloride salts, formed by adding 5 N HCl in isopropanol to an ether solution of the compound until just acidic.
Animals
Experiments were performed in accordance with UK Home Office guidelines and the Animals (Scientific Procedures) Act 1986. Adult (8–9 weeks, 27–38 g) male CD-1 mice, from Charles River (Crl: CD1(ICR)) and bred at the University of Bath, were housed in groups of 3–4 in cages provided with a shelf, wood shavings, and nesting material. The colony rooms were held under a 12 h light/dark cycle (lights on at 07:00), at 20 ± 2 °C with ad libitum food and water. Adult, female, Dunkin Hartley guinea pigs (300–350 g, Harlan UK) were housed in open floor pens at 19 ± 2 °C, on 12 h light/dark cycle with ad libitum food and water.
Cell Membrane Preparation
Cell membranes were prepared from C6 rat glioma cells stably transfected with the rat μ-receptor (C6-μ)) or δ-receptor (C6-δ); and CHO cells stably expressing the human κ-receptor (CHO-κ).39−41
[3H]-Diprenorphrine Competitive Binding Assay
Membranes (20 μg) were incubated in 50 mM Tris-HCl, pH 7.4 with a saturating concentration of [3H]-diprenorphine (0.2–0.3 nM) in the absence or presence of test compounds (norBNI, GNTI, BU09057, BU09058, BU09059) with a concentration range of 0.1 pM to 1 μM, for 1 h, in a shaking water bath at 25 °C. Nonspecific binding was measured using 50 μM naloxone. Samples were filtered through GF/C glass-fiber filtermats mounted on a Brandel cell harvester and rinsed with 4 °C 50 mM Tris-HCl, pH 7.4 buffer. Filtermats were dried, and radioactivity in each sample area was counted. Three triplicate determinations were carried out for all compounds at each opioid receptor. Data were analyzed using Prism 5.0 (GraphPad Software, La Jolla, CA) to determine Ki values from the IC50 values using the Cheng-Prusoff equation.
[35S]-GTPγS Assay
As described previously,42 C6-μ/δ or CHO-k membranes (20 μg) were incubated in 20 mM Tris-HCl, pH 7.4 buffer containing (mM) 5 MgCl2, 100 NaCl, 2.2 dithiothreitol, 30 μM GDP, 0.1 nM [35S]-GTPγS, and 1 pM to 10 μM test compound (BU09057, BU09058, BU09059), 10 μM U69,593 or 10 μM DAMGO or Super Q H2O. The membranes were incubated for 60 min in a shaking water bath at 25 °C. Samples were filtered through GF/C glass-fiber filtermats and then processed as described above. [35S]-GTPγS stimulation by test compounds was expressed as a percentage of the stimulation by 10 μM U69,593 or DAMGO for the κ- and μ-receptor, respectively. The δ-receptor agonist activity of the novel compounds was not evaluated because of the very low affinity of the compounds for the δ-receptor compared to the κ- and μ-receptors. Data were analyzed using Prism 5.0 (GraphPad Software, La Jolla, CA).
Isolated Tissue Preparations
Guinea pigs were killed by CO2 euthanasia. Approximately 3 cm of ileum was mounted, under 1 g tension, in a 35 mL organ bath at 37 °C containing gassed (95%O2/5%CO2) Krebs solution (mM): 118 NaCl, 11.6 glucose, 25 NaHCO3, 4.7 KCl, 1.2 KH2PO4, 1.2 CaCl2·6H2O, 1.2 MgSO4·7H2O. Electrical field stimulation (100 V, 1 ms pulse duration, 0.033 Hz) via platinum wire electrodes (Grass S-D9 stimulator) was applied for 40 min prior to drug addition. Twitch contractions were recorded using an isotonic transducer connected to Powerlab/200 and Chart software (AD Instruments).
The vas deferens was isolated from CD-1 mice, killed by CO2 euthanasia. Tissues were mounted, under 0.5 g of tension via an isometric transducer, in a 5 mL organ bath containing gassed (95%O2/5%CO2) Mg2+-free Krebs solution at 37 °C. A grass S-88 electrostimulator was used to deliver a 25 ms train of pulses (3 pulses, 10 ms apart, 1 ms duration) at a frequency of 0.05 Hz. Twitches were recorded using a Powerlab 4/26 and Chart software (AD Instruments).
Cumulative concentration response curves (CRCs) were constructed for the κ-agonist U50,488 (1 nM to 1 μM) or the μ-agonist DAMGO (0.1 nM to 1 μM) or the δ-agonist DPDPE (0.1 nM to 0.1 μM), in the absence and presence of increasing concentrations of test ligands: BU09057, BU09058, and BU09059. Following each CRC, tissues were washed until twitch amplitudes returned to baseline. Agonist CRCs were repeated after incubation with antagonist for 30 min. An individual tissue was only exposed to one antagonist to avoid potential carryover effects. Agonist responses were calculated as percentage maximal response: (average baseline twitch – average agonist response twitch)/(average baseline twitch – average maximum agonist inhibition of twitch) × 100. Agonist potency was determined as the negative logarithm of the concentration required to produce 50% of the maximum response (pEC50). Shifts of the agonist concentration–response curve by the presence of a competing ligand (agonist pEC50 values, Emax values) were compared by a one-way ANOVA followed by Dunnett’s post hoc test. Probability p < 0.05 was taken to be statistically significant. The concentration ratio for the rightward shift of the agonist curve in the presence of antagonist was used to calculate the pA2 from a Schild linear regression plot.43 Values are reported as mean ± SEM or mean ±95% confidence interval (CI) for each treatment group.
Establishing Nontoxic Doses in Vivo
Drugs were dissolved in 0.9% w/v saline solution and administered via intraperitoneal injection at volumes of 10 mL/kg. Toxicity of BU09057, BU09058, and BU09059 was assessed in naïve mice using a stepwise minimal numbers approach, starting at a low dose (1 mg/kg) and monitoring of behaviors (including respiration rate, posture, locomotor behavior, pilo erection).44 If no toxicity was seen, higher doses up to 10 mg/kg were administered in stepwise increments.
Warm Water Tail-Withdrawal Test
In vivo studies commenced at 10:00 am. Mice were positioned vertically, and ∼2 cm of the tail placed into a beaker of warm water (50 °C). The control tail-withdrawal latency was measured 30 min after saline injection.45 Subsequently, the κ-agonist U50,488 (10 mg/kg) was administered and the test latency measured 30 min later. A cutoff time of 15 s was used to prevent tissue damage. Antinociception was calculated as percent maximum possible effect (% MPE) = (test latency – control latency)/(15 s – control latency) × 100. Mice were pretreated with 0.9% w/v saline, norBNI, BU09057, BU09058, and BU09059 (3 and 10 mg/kg) to assess their ability to block U50,488 induced antinociception in the tail-withdrawal test at 1 h, 24 h, 7 d, 14 d, and 21 d postinjection. Data were analyzed using a two-way repeated measures mixed model approach, with treatment factor “Treatment” and repeated factor “Time” using InVivoStat software.46 Planned pairwise comparisons were made, and Bonferroni’s correction for multiple comparisons applied. Values are reported as mean ± SEM or mean ±95% confidence interval (CI) for each treatment group.
Glossary
Abbreviations
- ANOVA
analysis of variance
- CRCs
concentration response curves
- δ
delta opioid receptor
- GNTI
5′-guanidinonaltrindole
- JDTic
(3R)-7-hydroxy-N-((1S)-1-[[(3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethyl-1-piperidinyl]methyl]-2-methylpropyl)-1,2,3,4-tetrahydro-3-isoquinoline carboxamide
- JNK
c-Jun N terminal kinase
- norBNI
norbinaltorphimine
- κ
kappa opioid receptor
- μ
mu opioid receptor
- % MPE
percent maximum possible effect
- U50,488
(trans)-3,4-dichloro-N-methyl-N-[2-(1-pyrrolidinyl)-cyclohexyl]benzeneacetamide
Supporting Information Available
Details of synthesis and chemical characterization of the novel compounds. This material is available free of charge via the Internet at http://pubs.acs.org
This work is supported by the University of Bath, the Royal Society (S.J.B.), and NIDA DA07315 (S.M.H.).
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Schwarzer C. (2009) 30 years of dynorphins—New insights on their functions in neuropsychiatric diseases. Pharmacol. Ther. 123, 353–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour A.; Fox C. A.; Burke S.; Meng F.; Thompson R. C.; Akil H.; Watson S. J. (1994) Mu, delta, and kappa opioid receptor mRNA expression in the rat CNS: an in situ hybridization study. J. Comp. Neurol. 350, 412–438. [DOI] [PubMed] [Google Scholar]
- Kitchen I.; Slowe S. J.; Matthes H. W. D.; Kieffer B. (1997) Quantitative autoradiographic mapping of μ-, δ- and κ-opioid receptors in knockout mice lacking the μ-opioid receptor gene. Brain Res. 778, 73–88. [DOI] [PubMed] [Google Scholar]
- Lin S.; Boey D.; Lee N.; Schwarzer C.; Sainsbury A.; Herzog H. (2006) Distribution of prodynorphin mRNA and its interaction with the NPY system in the mouse brain. Neuropeptides 40, 115–123. [DOI] [PubMed] [Google Scholar]
- Pfeiffer A.; Brantl V.; Herz A.; Emrich H. M. (1986) Psychotomimesis mediated by kappa opiate receptors. Science 233, 774–776. [DOI] [PubMed] [Google Scholar]
- Todtenkopf M. S.; Marcus J. F.; Portoghese P. S.; Carlezon W. A. Jr. (2004) Effects of kappa-opioid receptor ligands on intracranial self-stimulation in rats. Psychopharmacology 172, 463–470. [DOI] [PubMed] [Google Scholar]
- Carlezon W. A. Jr.; Beguin C.; DiNieri J. A.; Baumann M. H.; Richards M. R.; Todtenkopf M. S.; Rothman R. B.; Ma Z.; Lee D. Y.; Cohen B. M. (2006) Depressive-like effects of the kappa-opioid receptor agonist salvinorin A on behavior and neurochemistry in rats. J. Pharmacol. Exp. Ther. 316, 440–447. [DOI] [PubMed] [Google Scholar]
- McLaughlin J. P.; Marton-Popovici M.; Chavkin C. (2003) Kappa opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses. J. Neurosci. 23, 5674–5683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mague S. D.; Pliakas A. M.; Todtenkopf M. S.; Tomasiewicz H. C.; Zhang Y.; Stevens W. C. Jr.; Jones R. M.; Portoghese P. S.; Carlezon W. A. Jr. (2003) Antidepressant-like effects of kappa-opioid receptor antagonists in the forced swim test in rats. J. Pharmacol. Exp. Ther. 305, 323–330. [DOI] [PubMed] [Google Scholar]
- Carlezon W. A.; Béguin C.; Knoll A. T.; Cohen B. M. (2009) Kappa-opioid ligands in the study and treatment of mood disorders. Pharmacol. Ther. 123, 334–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas M. R.; Land B. B.; Chavkin C. (2010) The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res. 1314, 44–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf M.; Coop A. (2005) Kappa opioid antagonists: Past successes and future prospects. AAPS J. 7, E704–E722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beguin C., and Cohen B. M. (2009) Medicinal Chemistry of Kappa Opioid Receptor Antagonists. In Opiate Receptors and Antagonists From Bench to Clinic; Dean R. L. III, Bilsky E. J., and Negus S. S., Eds.), pp 99–118, Humana Press, Totawa, NJ. [Google Scholar]
- Carroll F. I.; Carlezon W. A. (2013) Development of κ Opioid Receptor Antagonists. J. Med. Chem. 56, 2178–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll I.; Thomas J. B.; Dykstra L. A.; Granger A. L.; Allen R. M.; Howard J. L.; Pollard G. T.; Aceto M. D.; Harris L. S. (2004) Pharmacological properties of JDTic: a novel kappa-opioid receptor antagonist. Eur. J. Pharmacol. 501, 111–119. [DOI] [PubMed] [Google Scholar]
- Bruchas M. R.; Chavkin C. (2010) Kinase cascades and ligand-directed signaling at the kappa opioid receptor. Psychopharmacology 210, 137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melief E. J.; Miyatake M.; Carroll F. I.; Beguin C.; Carlezon W. A. Jr.; Cohen B. M.; Grimwood S.; Mitch C. H.; Rorick-Kehn L.; Chavkin C. (2011) Duration of action of a broad range of selective kappa-opioid receptor antagonists is positively correlated with c-Jun N-terminal kinase-1 activation. Mol. Pharmacol. 80, 920–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. B.; Fix S. E.; Rothman R. B.; Mascarella S. W.; Dersch C. M.; Cantrell B. E.; Zimmerman D. M.; Carroll F. I. (2004) Importance of phenolic address groups in opioid kappa receptor selective antagonists. J. Med. Chem. 47, 1070–1073. [DOI] [PubMed] [Google Scholar]
- Thomas J. B.; Atkinson R. N.; Rothman R. B.; Fix S. E.; Mascarella S. W.; Vinson N. A.; Xu H.; Dersch C. M.; Lu Y. F.; Cantrell B. E.; Zimmerman D. M.; Carroll F. I. (2001) Identification of the First trans-(3R,4R)- Dimethyl-4-(3-hydroxyphenyl)piperidine Derivative To Possess Highly Potent and Selective Opioid κ Receptor Antagonist Activity. J. Med. Chem. 44, 2687–2690. [DOI] [PubMed] [Google Scholar]
- Takemori A. E.; Ho B. Y.; Naeseth J. S.; Portoghese P. S. (1988) Nor-binaltorphimine, a highly selective kappa-opioid antagonist in analgesic and receptor binding assays. J. Pharmacol. Exp. Ther. 246, 255–258. [PubMed] [Google Scholar]
- Thomas J. B.; Atkinson R. N.; Vinson N. A.; Catanzaro J. L.; Perretta C. L.; Fix S. E.; Mascarella S. W.; Rothman R. B.; Xu H.; Dersch C. M.; Cantrell B. E.; Zimmerman D. M.; Carroll F. I. (2003) Identification of (3R)-7-Hydroxy-N-((1S)-1-{[(3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethyl-1-piperidinyl]methyl}-2-methylpropyl)-1,2,3,4-tetrahydro-3-isoquinolinecarboxamide as a Novel Potent and Selective Opioid κ Receptor Antagonist. J. Med. Chem. 46, 3127–3137. [DOI] [PubMed] [Google Scholar]
- Thomas J. B.; Fall M. J.; Cooper J. B.; Rothman R. B.; Mascarella S. W.; Xu H.; Partilla J. S.; Dersch C. M.; McCullough K. B.; Cantrell B. E.; Zimmerman D. M.; Carroll F. I. (1998) Identification of an Opioid κ Receptor Subtype-Selective N-Substituent for (+)-(3R,4R)-Dimethyl-4-(3-hydroxyphenyl)piperidine. J. Med. Chem. 41, 5188–5197. [DOI] [PubMed] [Google Scholar]
- Cai T. B.; Zou Z.; Thomas J. B.; Brieaddy L.; Navarro H. A.; Carroll F. I. (2008) Synthesis and In Vitro Opioid Receptor Functional Antagonism of Analogues of the Selective Kappa Opioid Receptor Antagonist (3R)-7-Hydroxy-N-((1S)-1-{[(3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethyl-1-piperidinyl]methyl}-2-methylpropyl)-1,2,3,4-tetrahydro-3-isoquinolinecarboxamide (JDTic). J. Med. Chem. 51, 1849–1860. [DOI] [PubMed] [Google Scholar]
- Cueva J. P.; Cai T. B.; Mascarella S. W.; Thomas J. B.; Navarro H. n. A.; Carroll F. I. (2009) Synthesis and In Vitro Opioid Receptor Functional Antagonism of Methyl-Substituted Analogues of (3R)-7-Hydroxy-N-[(1S)-1-{[(3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethyl-1-piperidinyl]methyl}-2-methylpropyl]-1,2,3,4-tetrahydro-3-isoquinolinecarboxamide (JDTic). J. Med. Chem. 52, 7463–7472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal-Dominguez J. J.; Clark M.; Traynor J. R.; Husbands S. M.; Bailey S. J. (2013) In vivo and in vitro characterization of naltrindole-derived ligands at the κ-opioid receptor. J. Psychopharmacol. 27, 192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endoh T.; Matsuura H.; Tanaka C.; Nagase H. (1992) Nor-binaltorphimine: a potent and selective kappa-opioid receptor antagonist with long-lasting activity in vivo. Arch. Int. Pharmacodyn. Ther. 316, 30–42. [PubMed] [Google Scholar]
- Glass P. S. A.; Hardman D.; Kamiyama Y.; Quill T. J.; Marton G.; Donn K. H.; Grosse C. M.; Hermann D. (1993) Preliminary Pharmacokinetics and Pharmacodynamics of an Ultra-Short-Acting Opioid: Remifentanil (GI87084B). Anesth. Analg. 77, 1031–1040. [DOI] [PubMed] [Google Scholar]
- Erhardt P. W.; Woo C. M.; Anderson W. G.; Gorczynksi R. J. (1982) Ultra-short-acting beta-adrenergic receptor blocking agents. 2. (Aryloxy)propanolamines containing esters on the aryl function. J. Med. Chem. 25, 1408–1412. [DOI] [PubMed] [Google Scholar]
- Yang H. S.; Wu W. M.; Bodor N. (1995) Soft drugs. XX. Design, synthesis, and evaluation of ultra-short acting beta-blockers. Pharm. Res. 12, 329–336. [DOI] [PubMed] [Google Scholar]
- Hammer R. H.; Wu W. M.; Sastry J. S.; Bodor N. (1991) Short acting soft mydriatics. Curr. Eye Res. 10, 565–570. [DOI] [PubMed] [Google Scholar]
- Chorvat R. J.; Black L. A.; Ranade V. V.; Barcelon-Yang C.; Stout D. M.; Brown B. S.; Stampfli H. F.; Quon C. Y. (1993) Mono- and bis(aminomethyl)phenylacetic acid esters as short-acting antiarrhythmic agents. 2. J. Med. Chem. 36, 2494–2498. [DOI] [PubMed] [Google Scholar]
- Verhoest P. R.; Sawant Basak A.; Parikh V.; Hayward M.; Kauffman G. W.; Paradis V.; McHardy S. F.; McLean S.; Grimwood S.; Schmidt A. W.; Vanase-Frawley M.; Freeman J.; Van Deusen J.; Cox L.; Wong D.; Liras S. (2011) Design and Discovery of a Selective Small Molecule kappa Opioid Antagonist (2-Methyl-N-((2′-(pyrrolidin-1-ylsulfonyl)biphenyl-4-yl)methyl)propan-1-amine, PF-4455242. J. Med. Chem. 54, 5868–5877. [DOI] [PubMed] [Google Scholar]
- Grimwood S.; Lu Y.; Schmidt A. W.; Vanase-Frawley M. A.; Sawant-Basak A.; Miller E.; McLean S.; Freeman J.; Wong S.; McLaughlin J. P.; Verhoest P. R. (2011) Pharmacological characterization of 2-methyl-N-((2′-(pyrrolidin-1-ylsulfonyl)biphenyl-4-yl)methyl)propan-1-amine (PF-04455242), a high-affinity antagonist selective for kappa opioid receptors. J. Pharmacol. Exp. Ther. 339, 555–566. [DOI] [PubMed] [Google Scholar]
- Peters M. F.; Zacco A.; Gordon J.; Maciag C. M.; Litwin L. C.; Thompson C.; Schroeder P.; Sygowski L. A.; Piser T. M.; Brugel T. A. (2011) Identification of short-acting κ-opioid receptor antagonists with anxiolytic-like activity. Eur. J. Pharmacol. 661, 27–34. [DOI] [PubMed] [Google Scholar]
- Beardsley P. M.; Pollard G. T.; Howard J. L.; Carroll F. I. (2010) Effectiveness of analogs of the kappa opioid receptor antagonist (3R)-7-hydroxy-N-((1S)-1-{[(3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethyl-1-piperidinyl ]methyl}-2-methylpropyl)-1,2,3,4-tetrahydro-3-isoquinolinecarboxamide (JDTic) to reduce U50,488-induced diuresis and stress-induced cocaine reinstatement in rats. Psychopharmacology 210, 189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eans S. O.; Ganno M. L.; Reilley K. J.; Patkar K. A.; Senadheera S. N.; Aldrich J. V.; McLaughlin J. P. (2013) The macrocyclic tetrapeptide [D-Trp]CJ-15,208 produces short-acting κ opioid receptor antagonism in the CNS after oral administration. Br. J. Pharmacol. 169, 426–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro T.; Berry L.; Van’t Veer A.; Beguin C.; Carroll F.; Zhao Z.; Carlezon W.; Cohen B. (2012) Long-acting kappa opioid antagonists nor-BNI, GNTI and JDTic: pharmacokinetics in mice and lipophilicity. BMC Pharmacol. 12, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patkar K. A.; Wu J.; Ganno M. L.; Singh H. D.; Ross N. C.; Rasakham K.; Toll L.; McLaughlin J. P. (2013) Physical Presence of Nor-Binaltorphimine in Mouse Brain over 21 Days after a Single Administration Corresponds to Its Long-Lasting Antagonistic Effect on κ-Opioid Receptors. J. Pharmacol. Exp. Ther. 346, 545–554. [DOI] [PubMed] [Google Scholar]
- Emmerson P. J.; Clark M. J.; Mansour A.; Akil H.; Woods J. H.; Medzihradsky F. (1996) Characterization of opioid agonist efficacy in a C6 glioma cell line expressing the mu opioid receptor. J. Pharmacol. Exp. Ther. 278, 1121–1127. [PubMed] [Google Scholar]
- Lee K. O.; Akil H.; Woods J. H.; Traynor J. R. (1999) Differential binding properties of oripavines at cloned mu- and delta-opioid receptors. Eur. J. Pharmacol. 378, 323–330. [DOI] [PubMed] [Google Scholar]
- Clark M. J.; Emmerson P. J.; Mansour A.; Akil H.; Woods J. H.; Portoghese P. S.; Remmers A. E.; Medzihradsky F. (1997) Opioid efficacy in a C6 glioma cell line stably expressing the delta opioid receptor. J. Pharmacol. Exp. Ther. 283, 501–510. [PubMed] [Google Scholar]
- Traynor J. R.; Nahorski S. R. (1995) Modulation by mu-opioid agonists of guanosine-5′-O-(3-[35S]thio)triphosphate binding to membranes from human neuroblastoma SH-SY5Y cells. Mol. Pharmacol. 47, 848–854. [DOI] [PubMed] [Google Scholar]
- Kenakin T. P. (2009) A pharmacology primer: theory, applications and methods, Elsevier Academic Press, San Diego. [Google Scholar]
- Irwin S. (1968) Comprehensive observational assessment: Ia. A systematic, quantitative procedure for assessing the behavioral and physiologic state of the mouse. Psychopharmacologia 13, 222–257. [DOI] [PubMed] [Google Scholar]
- Burke T. F.; Woods J. H.; Lewis J. W.; Medzihradsky F. (1994) Irreversible opioid antagonist effects of clocinnamox on opioid analgesia and mu receptor binding in mice. J. Pharmacol. Exp. Ther. 271, 715–721. [PubMed] [Google Scholar]
- Clark R. A.; Shoaib M.; Hewitt K. N.; Stanford S. C.; Bate S. T. (2012) A comparison of InVivoStat with other statistical software packages for analysis of data generated from animal experiments. J. Psychopharmacol. 26, 1136–1142. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.