Abstract
This study examined the relationship of γ-aminobutyric acid (GABA) and glutamate levels from the anterior and posterior cingulates (AC and PC) with cerebral blood flow (CBF) at rest. 1H magnetic resonance spectroscopy measurements in the AC and PC and pseudo-continuous arterial spin labeling data were acquired from 10 healthy controls. GABA levels from the AC were strongly inversely correlated with global (whole-brain) CBF (r =−0.91, p=0.0015). GABA levels from the PC and glutamate levels from both regions were not significantly correlated with CBF. We hypothesize that GABA-mediated inhibition of AC activation of the locus coeruleus-norepinephrine pathway may influence global CBF.
Keywords: arterial spin labeling, cerebral blood flow, GABA, magnetic resonance spectroscopy, anterior cingulate
Introduction
Determining the relationship between neurotransmitters and cerebral blood flow (CBF) has important implications for understanding the function of both the healthy and pathological brain. Neurotransmitters are known to modulate CBF through flow-metabolism coupling, where synaptic activity influences the release of chemical messengers that regulate vasodilation, and through neurogenic regulation, where blood vessels can be stimulated directly by innervation and indirectly through astrocytic processes [1, 2]. The main excitatory and inhibitory neurotransmitters of the brain, glutamate (Glu) and γ-aminobutyric acid (GABA), have been implicated in CBF regulation [1–4]. However, the relationship between these neurotransmitters and CBF has not been systematically studied using magnetic resonance (MR) techniques in human subjects. Only one MR study previously examined the relationship between GABA levels and CBF in humans, and it focused exclusively on the visual cortex [5]. Investigation of this relationship in healthy controls in the anterior and posterior cingulates (AC and PC), which are implicated in several brain disorders, would provide information that may be of use in understanding these diseases. Therefore, this study examined the relationship of GABA and Glu levels from the AC and PC with CBF.
Materials and Methods
All studies were performed under a University of Maryland Baltimore IRB approved protocol, and informed consent was obtained for each study participant. Ten healthy participants (mean age: 26.1 ± 9 years: 4 males, 6 females) completed the study. To be included in this study, participants had to be free of any major medical or psychiatric illness, not pregnant, and able to have an MRI scan. Imaging was conducted on a 3T Siemens TIM Trio MR scanner (Siemens Medical Solutions, Inc., Erlangen, Germany) with a 32-channel phased array head coil while participants were at rest. T1 anatomical images were acquired using a 3-D MP-RAGE sequence. Spectroscopic voxels were prescribed in two regions of interest: AC and PC (Figure 1A, 1B). For shimming, the Siemens “Advanced” shimming algorithm was used and manual adjustments were made when necessary. All spectroscopic data were acquired using phase rotation STEAM: TR/TM/TE = 2000/10/6.5-ms, VOI ~ 6-cm3 in the AC and PC, NEX=256, 2.5-kHz spectral width, 2048 complex points, and phases: φ1=135°, φ2=22.5°, φ13=112.5°, φADC=0° [6]. A water reference (NEX=16) was also acquired for phase and eddy current correction as well as quantification. A basis set of 19 metabolites was simulated using the GAVA software package: alanine, aspartate, creatine, GABA, glucose, Glu, glutamine, glutathione, glycine, glycerophosphocholine, lactate, myo-Inositol, N-acetylaspartate, N-acetylaspartylglutamate, phosphocholine, phosphocreatine, phosphoroylethanolamine, scyllo-Inositol, and taurine [7]. The basis set was imported into LCModel (6.3-0I) and used for quantification [8]. Correction for the proportion of the gray matter, white matter, and cerebrospinal fluid (CSF) within each spectroscopic voxel was performed using in-house Matlab code [9]. Only metabolites with Cramer Rao lower bounds (CRLB) less than 20% were included in statistical analyses. Spectra with LCModel reported linewidths (LW) greater than 0.1 Hz and signal-to-noise ratio (SNR) less than 10 were excluded from further analyses. Metabolite levels are reported in institutional units.
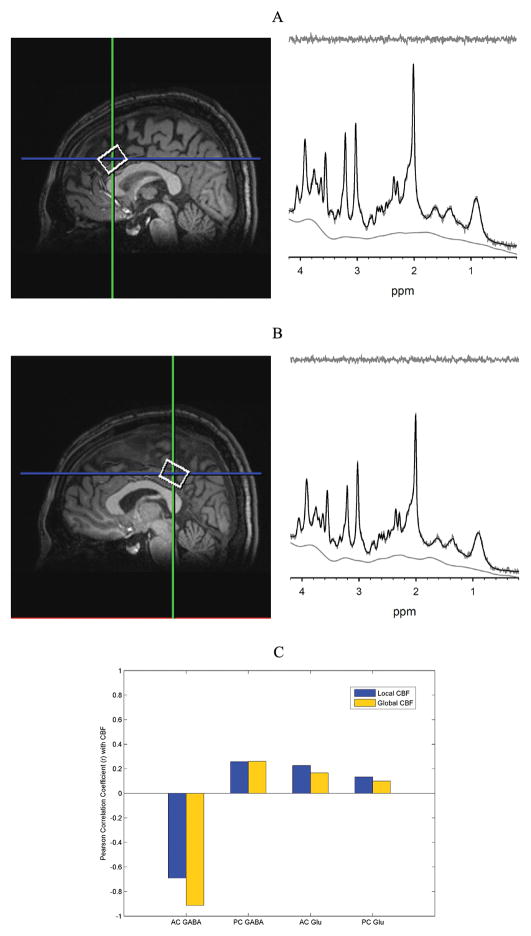
Figure 1.
Spectroscropic voxel locations from the anterior cingulate (AC) (A) and posterior cingulate (PC) (B). Representative spectra (−) and LCModel fits (−) from the AC and PC show excellent fits to the data as evidenced by the lack of signal in residual shown above. Pearson correlation coefficients were calculated for GABA and Glu levels in the AC and PC with local CBF (within the same spectroscopic voxel) and global (whole-brain) CBF using linewidth and SNR as co-variates (C).
Psuedo-Continuous Arterial Spin Labeling (pCASL) was applied with the following parameters: TR/TE = 4000/16ms, FOV = 220×220mm, Number of slices = 23, Slice thickness = 5 mm, Voxel size = 3.4×3.4×5.0 mm3, Bandwidth=1594 Hz/pixel, 136 measurements, labeling offset = 90 mm, labeling duration of 1.85 s, post labeling delay of 0.93s [10]. ASL data were processed with pCASL MATLAB scripts (http://www.mccauslandcenter.sc.edu/CRNL/tools/asl) with SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/) using ASL tbx [11]. Labeled and unlabeled ASL images were independently motion-corrected, and a combined mean image was computed and coregistered to the T1-weighted anatomical image. The ASL images were resliced to match the mean image and spatially smoothed with a 6 mm full-width half-maximum Gaussian kernel. CBF at each image voxel was determined by subtraction, resulting in a mean CBF image. Absolute CBF quantification was calculated in native space from the mean CBF images masked to exclude voxels with a combined grey matter and white matter probability of < 0.5 from the SPM8 segmented T1 images. The T1 images were normalized using SPM8’s unified segmentation-normalization, and these parameters were used to reslice the CBF images and T1 image to standard space. SPM8’s default brain mask was then used to mask the normalized CBF images (with a 50% threshold).
Pearson product moment correlations were calculated for Glu and GABA levels from the AC and PC with local CBF within the spectroscopic voxel and with global (whole-brain) CBF. To control for individual differences between spectra, spectral LW and SNR were added as co-variates in the correlation analyses. The significance level was set to p = 0.00625 to control for multiple tests of 2 neurotransmitters from 2 locations with both local and global CBF using Bonferroni correction (0.05/8 =0.00625). The relationship between AC GABA and global CBF was further explored to determine if the effect was widespread or driven by specific regions by performing correlations between AC GABA and regional CBF (rCBF) from 116 different regions of interest (ROIs) defined by the Automated Anatomical Labeling (AAL) atlas [12]. To control for the 116 multiple correlation tests, Benjamini-Hochberg correction was applied and corrected p values of < 0.05 were considered statistically significant [13].
Results
The mean global CBF among the whole group was 52.2 ± 11.3 ml/100g/min, which is within the range of previous reports [14]. The CBF for the AC spectroscopic voxel (Figure 1A) was 80.7 ± 12.4 ml/100g/min, whereas the CBF for the PC spectroscopic voxel (Figure 1B) was 91.8 ± 20.6 ml/100g/min.
Representative spectra, LCModel fits, and residuals from the AC and PC are shown in Figure 1A and Figure 1B, respectively. Supplementary Figures 1 and 2 show all spectra acquired for the AC and PC, respectively. No data were rejected based on our criteria for CRLB, LW, or SNR. In the AC, mean Glu and GABA levels were 15.5 ± 0.4 and 3.72 ± 0.2, respectively. Mean CRLBS for AC Glu and GABA were 3.5 ± 0.5% and 12.7 ± 0.9%. Glu and GABA levels from the PC were 11.4 ± 0.9 and 3.02 ± 0.4, respectively. Mean CRLBs for Glu and GABA in the PC were 4.2 ± 0.4% and 13.7 ± 1.9%.
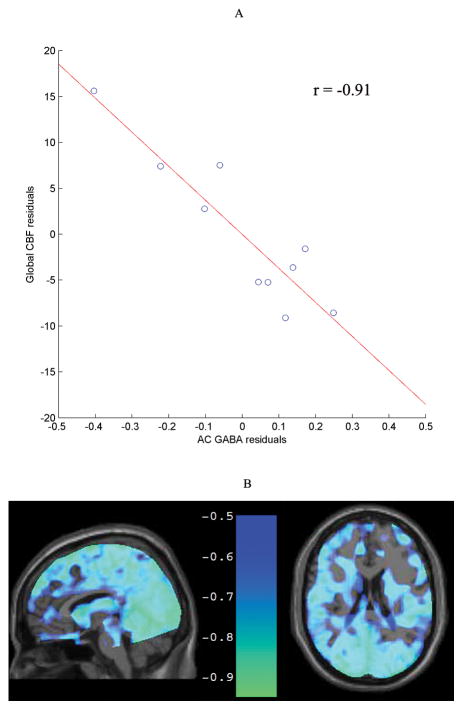
Figure 1C shows correlation coefficients for Glu and GABA levels from the AC and PC with local CBF within each spectroscopic voxel and with global CBF. The correlation between AC GABA and global CBF was statistically significant (r =−0.91, p=0.0015) (Figure 2A). The correlation between AC GABA and local CBF within the same AC spectroscopic voxel was moderate but did not reach statistical significance (r =−0.69, p =0.06). PC GABA was not significantly correlated with local CBF (r=0.26, p=0.53) or global CBF (r=0.26, p=0.54). There were no statistically significant correlations between CBF and Glu in either region (all p-values > 0.5).
Figure 2.
The relationship between AC GABA and global CBF with linewidth and SNR as covariates was inversely correlated (r = −0.91, p = 0.0015) (A). The widespread nature of this relationship is apparent in a voxel-wise analysis (at a threshold r ≤ −0.5), shown in normalized sagittal and axial images (B).
A secondary correlation analysis between AC GABA and rCBF in AAL atlas ROIs revealed that AC GABA was negatively correlated with rCBF in 97% of the ROIs (median strength was r=−0.84). This correlation was significant at the p < 0.05 Benjamini-Hochberg corrected level in 68% of the ROIs indicating that this relationship was widespread throughout the brain. A voxel-wise correlation analysis also illustrated the global nature of this relationship (Figure 2B). Supplementary Table 1 summarizes the r and p values of the secondary correlation analysis.
Discussion
This study examined the relationship of GABA and Glu from the AC and PC with CBF, both locally within the spectroscopic voxel and globally throughout the brain. A strong inverse relationship was found between AC GABA and global CBF. The global nature of this relationship was supported by the strong correlations between AC GABA and CBF in ROIs throughout the brain selected from the AAL atlas. Consistent with the AC GABA global CBF relationship, AC GABA was moderately inversely correlated with local AC CBF but did not reach statistical significance. There were no statistically significant relationships between PC GABA levels and CBF, or between Glu levels from both regions and CBF. The exclusivity of the relationship between AC GABA and global CBF suggests that the AC may have an important role in mediating CBF throughout the brain.
While causation cannot be established from the relationship between AC GABA levels and CBF, this finding provides insight into the AC’s involvement in cortical CBF regulation. In this study, the lack of a relationship between PC GABA and CBF, combined with a previous finding that occipital lobe GABA was positively correlated with local CBF [5], suggests that the AC’s relationship with global CBF is different from that of other cortical regions. Evidence of AC involvement in autonomic regulation that may be mediated through brainstem connections [15] led us to hypothesize that the AC may regulate cortical CBF through its projections to the locus coeruleus (LC). Retrograde tracers were previously used to show that the LC receives prominent inputs from neurons near the rostral/caudal border of the AC [16], which corresponds with the AC spectroscopic voxel in this study. AC activation of the LC-norepinephrine (NE) pathways appears to play an important role in mediating levels of arousal [16], which could potentially lead to increased energy demands throughout the brain. Additionally, activation of the LC-NE pathway causes large increases in cortical CBF in rats, a finding that is dependent on K+ flux and metabolism of arachidonic acid [17]. Thus, AC GABA may play an inhibitory role in AC innervation of the LC-NE pathway and down-regulate CBF throughout the cortex.
Several possible limitations of this study should be considered. First, it is possible that the calculated CBFs may have been influenced by between-subject differences in arterial transit time (ATT), resulting in differences in CBF estimates. However, to minimize potential ATT effects, only young healthy subjects were enrolled in the study, all scans were performed while the subjects were at rest, and a post-labeling delay was utilized. Thus, it is unlikely that differences in ATT contributed to the significant inverse relationship between AC GABA and CBF in this study. Second, when using a very short TE spectroscopic sequence, contributions from the macromolecule background are more pronounced. To minimize these effects on quantification, all data were processed in LCModel, which parameterizes the macromolecule background to remove its influence on metabolite values. Third, it is possible that the GABA levels derived from our very short TE technique are contaminated by metabolites with resonances that overlap with the three main multiplets of GABA. Currently, spectral editing is the gold standard for measurement of GABA in vivo; however, most editing studies report a measurement of “GABA+” which includes the 40–50% contamination from macromolecules. Two recent studies by Near et al. and Wijtenburg et al. using very short TE acquisition techniques at 3T have demonstrated the feasibility and reproducibility of detecting GABA without spectral editing [18, 19]. Small residuals from LCModel and low CRLBs from Wijtenburg et al. for GABA as well as from the metabolites with overlapping resonances, such as N-acetylaspartate, Glu, glutamine, total creatine, and glutathione, suggest that contamination of the GABA levels reported here are most likely minimal. Thus, an inherent advantage of a very short TE technique over a spectral editing technique is that multiple metabolites can be quantified from one experiment. Fourth, it is possible that head motion can influence measurements of CBF and GABA levels. Head motion was minimized by providing padding around each participant’s head to prevent movement. Linewidth, which is greatly affect by patient motion, and GABA levels were not significantly correlated in the AC (r=0.25, p=0.493) or the PC (r=−0.096, p=0.791). The spectral quality of the each participant’s data is excellent (narrow LW and high SNR), and the CRLBs for GABA are low. The CBFs reported are within ranges of previously reported studies and the peripheral ring of elevated CBF values that is common with head motion [20] is also not observed. Thus, head motion is unlikely to have contributed to the strong inverse correlation between AC GABA and global CBF. Fifth, the number of subjects enrolled in this study results in limited statistical power; thus, a replication study with a larger sample size is needed.
Conclusions
While the findings of this study alone cannot lead to definitive conclusions on mechanisms, this study highlights the value of investigating the relationship between neurotransmitter levels and CBF and presents a novel set of methods for doing so. This is the first study to examine the relationship between historically difficult to quantify GABA and Glu and CBF in the AC and PC. Additionally, this study acquired spectroscopic data at a very short TE, which enables quantification of coupled spin systems, since it minimizes the effects of J-coupling and T2 relaxation. Our findings also suggest that the role of AC GABA should be considered in models of CBF regulation. Understanding how the cortex regulates blood flow and addresses its metabolic needs has important implications for studying disorders that involve altered CBF or GABAergic pathways. Future research will use the techniques from this study to examine similar relationships in other brain regions and investigate the hypothesized role of the AC-LC pathway in mediating CBF.
Supplementary Material
Highlights.
Anterior cingulate GABA levels were inversely correlated with cerebral blood flow.
Posterior cingulate GABA levels were not related to CBF.
Glutamate levels were not related to CBF.
GABA-mediated inhibition from the AC may influence CBF throughout the brain.
Acknowledgments
The authors give special thanks to Dr. Chris Rorden of the University of South Carolina for providing the pCASL data processing MATLAB scripts.
The authors would like to acknowledge NIH for support to: T32MH067533 (SAW) and R01MH094520 (LMR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Peterson EC, Wang Z, Britz G. Regulation of cerebral blood flow. Int J Vasc Med. 2011;2011:823525. doi: 10.1155/2011/823525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–243. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matthew E, Andreason P, Pettigrew K, Carson RE, Herscovitch P, Cohen R, King C, Johanson CE, Greenblatt DJ, Paul SM. Benzodiazepine receptors mediate regional blood flow changes in the living human brain. Proc Natl Acad Sci U S A. 1995;92:2775–2779. doi: 10.1073/pnas.92.7.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iliff JJ, D’Ambrosio R, Ngai AC, Winn HR. Adenosine receptors mediate glutamate-evoked arteriolar dilation in the rat cerebral cortex. Am J Physiol Heart Circ Physiol. 2003;284:H1631–1637. doi: 10.1152/ajpheart.00909.2002. [DOI] [PubMed] [Google Scholar]
- 5.Donahue MJ, Near J, Blicher JU, Jezzard P. Baseline GABA concentration and fMRI response. Neuroimage. 2010;53:392–398. doi: 10.1016/j.neuroimage.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 6.Wijtenburg SA, Knight-Scott J. Very short echo time improves the precision of glutamate detection at 3T in 1H magnetic resonance spectroscopy. J Magn Reson Imaging. 2011;34:645–652. doi: 10.1002/jmri.22638. [DOI] [PubMed] [Google Scholar]
- 7.Soher BJ, Young K, Bernstein A, Aygula Z, Maudsley AA. GAVA: spectral simulation for in vivo MRS applications. J Magn Reson. 2007;185:291–299. doi: 10.1016/j.jmr.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- 9.Gasparovic C, Song T, Devier D, Bockholt HJ, Caprihan A, Mullins PG, Posse S, Jung RE, Morrison LA. Use of tissue water as a concentration reference for proton spectroscopic imaging. Magnetic resonance in medicine: official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2006;55:1219–1226. doi: 10.1002/mrm.20901. [DOI] [PubMed] [Google Scholar]
- 10.Wu WC, Fernandez-Seara M, Detre JA, Wehrli FW, Wang J. A theoretical and experimental investigation of the tagging efficiency of pseudocontinuous arterial spin labeling. Magn Reson Med. 2007;58:1020–1027. doi: 10.1002/mrm.21403. [DOI] [PubMed] [Google Scholar]
- 11.Wang Z, Aguirre GK, Rao H, Wang J, Fernandez-Seara MA, Childress AR, Detre JA. Empirical optimization of ASL data analysis using an ASL data processing toolbox: ASLtbx. Magn Reson Imaging. 2008;26:261–269. doi: 10.1016/j.mri.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 13.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a new and powerful approach to multiple testing. J R Statist Soc B. 1995;57:289–300. [Google Scholar]
- 14.Calamante F, Thomas DL, Pell GS, Wiersma J, Turner R. Measuring cerebral blood flow using magnetic resonance imaging techniques. J Cereb Blood Flow Metab. 1999;19:701–735. doi: 10.1097/00004647-199907000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Critchley HD, Mathias CJ, Josephs O, O’Doherty J, Zanini S, Dewar BK, Cipolotti L, Shallice T, Dolan RJ. Human cingulate cortex and autonomic control: converging neuroimaging and clinical evidence. Brain. 2003;126:2139–2152. doi: 10.1093/brain/awg216. [DOI] [PubMed] [Google Scholar]
- 16.Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- 17.Toussay X, Basu K, Lacoste B, Hamel E. Locus coeruleus stimulation recruits a broad cortical neuronal network and increases cortical perfusion. J Neurosci. 2013;33:3390–3401. doi: 10.1523/JNEUROSCI.3346-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Near J, Andersson J, Maron E, Mekle R, Gruetter R, Cowen P, Jezzard P. Unedited in vivo detection and quantification of gamma-aminobutyric acid in the occipital cortex using short-TE MRS at 3 T. NMR Biomed. 2013 doi: 10.1002/nbm.2960. [DOI] [PubMed] [Google Scholar]
- 19.Wijtenburg SA, Gaston FE, Spieker EA, Korenic SA, Kochunov P, Hong LE, Rowland LM. Reproducibility of phase rotation STEAM at 3T: focus on glutathione. Magn Reson Med. 2013 doi: 10.1002/mrm.24959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deibler AR, Pollock JM, Kraft RA, Tan H, Burdette JH, Maldjian JA. Arterial spin-labeling in routine clinical practice, part 1: technique and artifacts. AJNR Am J Neuroradiol. 2008;29:1228–1234. doi: 10.3174/ajnr.A1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.