Abstract
Aging and degeneration of the intervertebral disk are accompanied by decreases in water and proteoglycan contents, and structural alterations. The aim of this study was to determine the impact of compositional changes on the material properties of intervertebral disk tissues. Confined compression stress-relaxation experiments were applied to bovine caudal annulus fibrosus and nucleus pulposus tissue specimens that were separated into three experimental groups: in situ, free-swelling control (PBS), and digestion (chondroitinase-ABC).Measurements of glycosaminoglycan (GAG) and water content, as well as nonlinear finite deformation biphasic theory and multiple linear regression analyses were performed. The compressive modulus HA0 and permeability k0 of in situ specimens were 0.37±0.06 MPa and 0.49±0.08×10−15 m4 N−1 s−1 for nucleus, and 0.74±0.13 MPa and 0.42±0.05×10−15 m4 N−1 s−1 for annulus, respectively. There was a larger effect of swelling and digestion on the material properties and biochemical composition of nucleus pulposus than for annulus fibrosus. Alterations in proteoglycan and water content affected the compressive modulus and permeability, although the permeability was somewhat more strongly affected by water content than by proteoglycan content. Correlation coefficients r≤0.75 for the multiple regression indicated water and GAG content can moderately predict material properties, however other compositional and structural factors must be considered.
Keywords: Confined compression, Intervertebral disk, Annulus fibrosus, Nucleus pulposus, Hydration, Proteolytic digestion, Compressive modulus, Hydraulic permeability
INTRODUCTION
The intervertebral disk consists of two clearly distinct regions, the annulus fibrosus rich in collagen, and the nucleus pulposus rich in proteoglycans. Collagen represents about 15–20% of the nucleus- and 65–70% of the annulus-dry weight whereas proteoglycans represent approximately 50% of the nucleus- and 10–20% of the annulus-dry weight.2,6,7,12,21 As intervertebral disks degenerate, the nucleus becomes more consolidated and fibrous, and is less clearly demarcated from the annulus. With more advanced degeneration, focal defects appear and there is a decrease in the number of layers of the annulus with an increase in the thickness and spacing of the collagen fibers.9,28 Compositionally, degeneration results in a decrease in proteoglycan and water content in both annulus and nucleus, whereas the collagen content remains relatively constant in the nucleus and decreases in the annulus with a change in the distribution of types I and II collagen.2,6,7,21,25,26 Understanding the relationship between composition and material properties of disk tissue is a priority.
The intervertebral disk tissuemay be considered a biphasic material with a porous, permeable, and fiber-reinforced solid phase, and a fluid phase of water mixed with ions. It is the composition and structure of the solid matrix that is responsible for providing the compressive, tensile, and shear properties of disk tissue. The dramatic changes in composition and structure that occur in the intervertebral disk with aging and degeneration are accompanied by specific changes in material properties including an alteration of the compressive modulus,18 hydraulic permeability, 14 and tensile properties of the annulus,1 and an alteration of the elastic modulus,29 shear modulus,19 and swelling pressure4,16 of the nucleus. It is a goal of this study to evaluate the correlation between disk composition and material properties by first measuring the compressive modulus and hydraulic permeability of normal annulus and nucleus tissue and then comparing it to tissue with altered water and glycosaminoglycan (GAG) contents.
Evaluating the role of composition in defining the material properties will improve our understanding of how compositional and structural alterations with degeneration affect load carriage mechanisms in the intervertebral disk. The biomechanical response of annulus fibrosus to in vitro compressive loading has been described with some attention to structure–function relationships,5,18,20,30 however multiple regressions between material properties, water, and proteoglycan content that include mechanical characterization of nucleus pulposus in confined compression have not been previously reported.
We hypothesized that both annulus and nucleus tissue subjected to free-swelling and enzymatic digestion would alter the water and GAG composition of the disk resulting in measurable changes in tissue mechanical properties. Furthermore, we hypothesized that significant correlations exist between the biochemical properties and the biomechanical properties for both nucleus and annulus tissue. Thus, the first aim of this study was to determine the effects of free-swelling and proteoglycan digestion on the compressive modulus, hydraulic permeability, and water and proteoglycan-content of both nucleus and annulus tissue. The second aim of this study was to assess the correlation between the biochemical and material properties in order to evaluate the predictability of mechanical properties from biochemical composition.
MATERIALS AND METHODS
Bovine (~2–4 years old) tails (n=15) were obtained from an abattoir within 4 h of death, and caudal disks (levels C2–3 and C3–4, approximately 20–25 mm in diameter) were dissected. Bovine caudal disks were used because they have been demonstrated to have similar composition, resting stress, and height to diameter ratio as human lumbar disks.10,24 Annulus and nucleus samples were harvested with a scalpel, frozen at −80°C and microtomed to approximately 1.5 mm thickness. Annulus specimens were obtained in the radial direction from the outermost layers of annulus in order to control for the previously reported radial variation in material properties while nucleus specimens were obtained in the axial direction from the center of the disk. Both nucleus and annulus tissues were separated into three experimental groups: in-situ, free-swelling control, and digestion. In-situ specimens were stored at −80°C until the day of testing. Digestion samples were digested in 0.125 units/ml chondroitinase ABC (Sigma, St. Louis, MO) solution in Dubelcco’s phosphate-buffered saline (PBS) under slight agitation (pH=8.0 for 24 h at 25°C). Chondroitinase ABC specifically degrades glycosaminoglycans found on proteoglycans, namely the chondroitin and dermatan sulfate chains, and its catabolic effects on the nucleus were previously evaluated.26,27 Free-swelling control samples were submerged in PBS and allowed to swell for 1 h at 25°C in order to control for the change in water content that occurs in the digestion group. Pilot studies indicated there was no difference in water content between specimens that swelled for 1 h vs. 24 h. Consequently, a 1 h swelling protocol was chosen to minimize potential GAG leaching with time and therefore maximize differences in GAG content between free-swelling and digestion groups. Free-swelling and digestion samples were stored at −80°C until the day of testing. The 30 annulus and 30 nucleus specimens (n=10 per group) were microtomed again to ~1.5 mm thickness for AF and 1.3 mm thickness for NP. This difference between AF and NP samples is because more tissue needed to be removed to obtain parallel surfaces for the NP samples. On the day of testing, a cylindrical test sample was obtained using a 5 mm biopsy punch, weighed (for wet weight), and placed in the cylindrical chamber for the confined compression stress-relaxation experiment. In addition, a similar- sized piece of adjacent tissue was taken for biochemical assays.
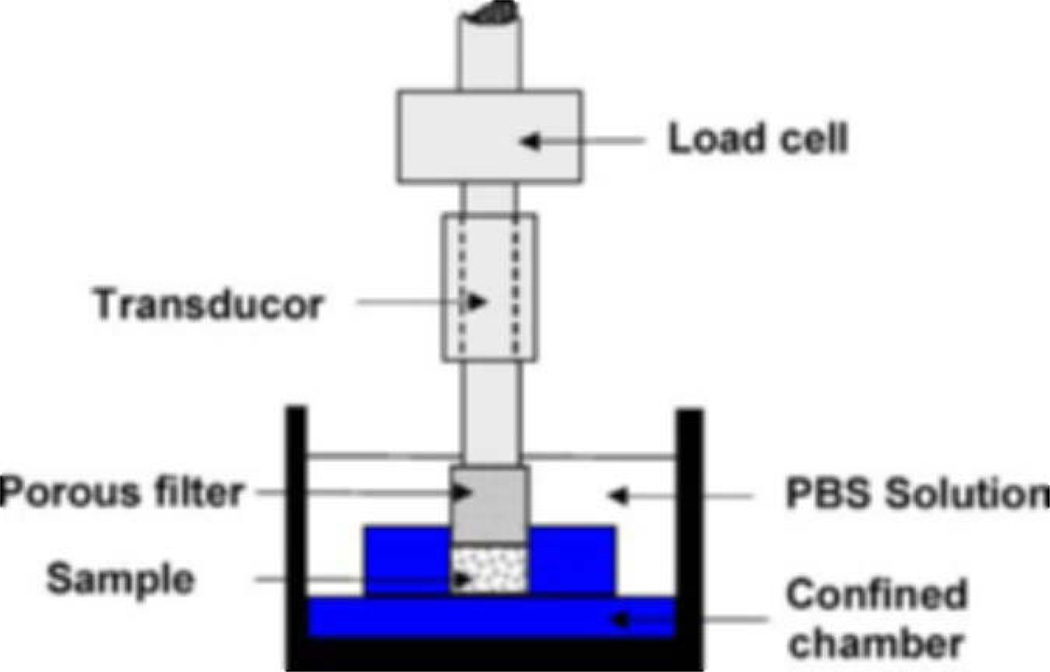
The confined compression apparatus was composed of a lower nonporous chamber and an upper rigid and porous platen of sintered steel. The upper platen was connected in series to a load cell and operated under displacement control using a testing machine (ELF-3200, Enduratec, Minnetonka, MN). After the tissue sample was placed in the chamber, the upper platen was lowered until a stable force of 0.1 N was recorded, indicating that the platen was in contact with the top of the specimen. The tissue thickness was deduced from the displacement at this 0.1 N force and used for all subsequent strain calculations (1.52±0.05 mm for the annulus, 1.32±0.08 mm for the nucleus). PBS solution was then added to the chamber, and the tissue was left under constant displacement until force equilibrium was reached (for 3600–4000 s). The equilibrium swelling force was used to calculate a swelling pressure, by dividing by cross-sectional area, and was then subtracted from subsequent force measurements taken during stress relaxation tests. Four successive stress-relaxation tests were applied using 5% strain increments. The dwell periods ranged from 400 to 6000 s depending on the experimental group and the strain level, and were chosen based on the time to reach stress-relaxation equilibrium in pilot experiments. The strain rate was 0.0001 s−1 for all the annulus groups and the in situ nucleus group, and 0.001 s−1 for the free-swelling control and digestion nucleus groups. No significant effects of strain rate (0.0001 s−1 vs. 0.001 s−1) were detected for compressive modulus (p=0.94, t-test) or hydraulic permeability (p=0.59, t-test) for nucleus tissue in extensive preliminary tests. The higher strain rate was used to increase the magnitude of the transient stress response for the free-swelling and digestion nucleus specimens. The 5% strain increments provided peak and equilibrium stresses that were large magnitudes to insure good sensitivity for parameters’ estimation using the chosen nonlinear mathematical model while also being consistent with disk height losses reported in the literature.23
The biphasic model3,17,22 with nonlinear elasticity and strain-dependent permeability was used to fit the stress-relaxation data using methods previously described.3,18 This model assumes a porous and permeable solid matrix saturated with inviscid interstitial fluid, and viscoelastic effects are attributed to momentum exchange between the solid and fluid phases due to frictional drag. The parameters describing the elastic behavior of the solid matrix (zero-strain compressive modulus HA0 and nonlinear stiffening coefficient β) were determined by nonlinear curve fit of Eq. (1) to the equilibrium stress–stretch data. Stress (σ) was calculated from the recorded force divided by the cross-sectional area of the specimen. Stretch (λ) was calculated as the deformed specimen height divided by its original height. Using the determined values of HA0 and β, the parameters describing the hydraulic permeability (zero-strain permeability k0 and nondimensional nonlinear permeability coefficient M, Eq. (2)), were determined by nonlinear curve fit of the numerical solution for Eq. (3) to the experimental stress-relaxation data (Fig. 1). This two-step curve fit was insensitive to a wide range of initial guesses and we are confident that we obtained a unique set of physiologically relevant material parameters with our methods. However, some imprecisions in the curve-fitting procedure due to relatively low sensitivity of the stress-relaxation and creep behaviors to large variations of M in the permeability function were previously noted.3 All numerical calculations were performed using custom code written in MATLAB (Mathworks, Natick, MA).
| (1) |
| (2) |
| (3) |
where σe is the equilibrium stress, λ is the stretch, and ϕ0 is the initial solid volume ratio.
Figure 1.
Experimental setup for the mechanical test.
Adjacent tissue from specimens in the in-situ, free-swelling control, and digested groups was lyophilized to obtain dry weight, and the water content was determined as the difference between the wet and dry tissue weights. The dry tissue was assayed for total sulfated glycosaminoglycans (GAG, µg GAG/mg dry tissue) as a measure of proteoglycan content using the dimethylmethylene blue dye binding colorimetric assay.13 For the correlation analysis, it was important to measure biochemical content on adjacent tissue rather than test specimens because adjacent tissue most closely represents the specimens’ initial GAG and water contents which correspond most closely to the “zero-strain” material properties evaluated in this study. The final (i.e., posttesting) biochemical compositions had decreased water content due to compression and some leaching of GAG due to soaking in buffer.
Statistical tests were performed on the annulus fibrosus and nucleus pulposus separately. One-way ANOVA was used to evaluate the effects of test condition (in situ, freeswelling control, digestion) on the mechanical properties, followed by a Student’s t-test or Mann–Whitney Rank sum tests (depending on the success or failure of the normality and equal variance tests) to isolate the groups that significantly differed and to calculate the corresponding p-values. Multiple linear regressions were performed with the mechanical properties HA0 and k0 as the dependent variables and the water and GAG content as the independent variables. The relative importance of one of the independent variables on the dependent variables was evaluated by comparing both standardized coefficients β and the corresponding p-values. All statistical tests were performed with SigmaStat (Jandel Scientific, San Rafael, CA) and significance was considered for p≤0.05 with a power >0.8. All data is expressed as Mean±SEM.
RESULTS
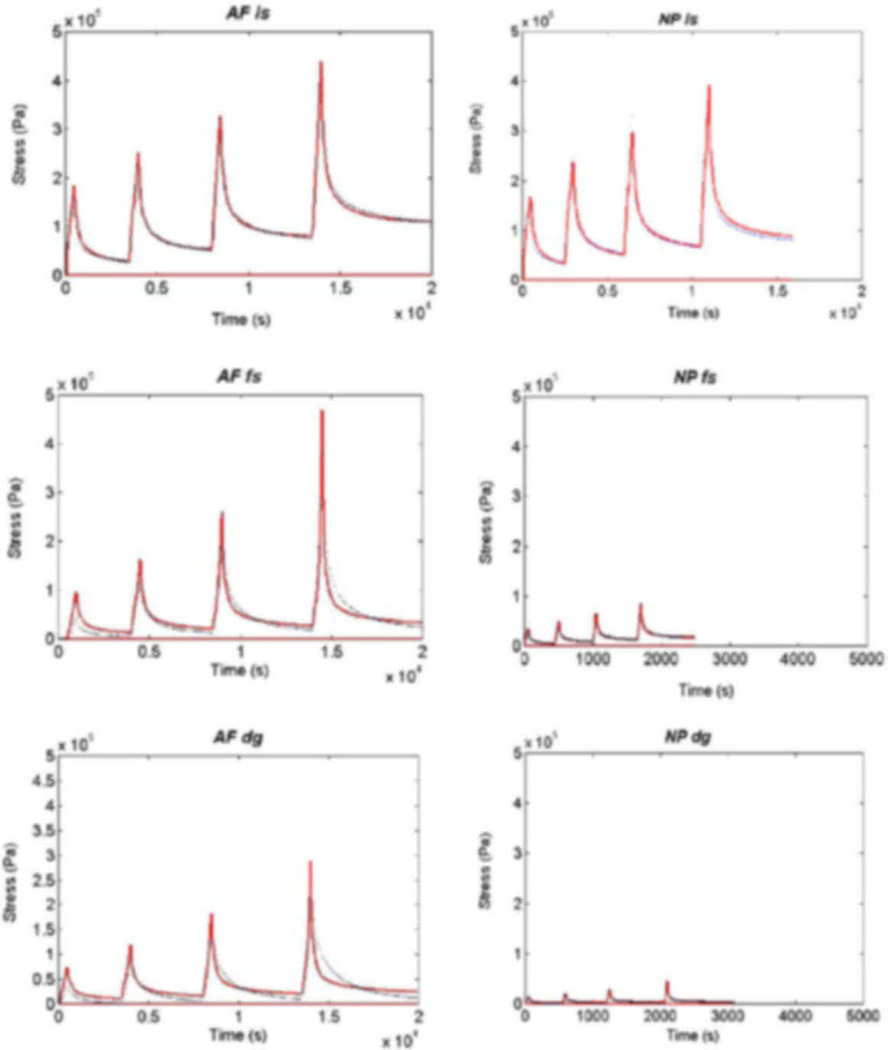
Swelling pressure for the in situ annulus and nucleus specimens were 0.05 ± 0.01 and 0.08 ± 0.01 MPa, respectively. The swelling pressures for all free-swelling control and digested specimens were negligible. Annulus and nucleus specimens exhibited a stress-relaxation response characteristic of most biological materials (Fig. 2). For all groups, the equilibrium stress versus stretch relationship was nearly linear. During the constant displacement stages, equilibrium was approached more rapidly for nucleus than annulus and for the digestion specimens compared to free swelling control and in situ specimens. The equilibrium and peak stresses at 5, 10, 15, and 20% were greatest for the in situ followed by the free-swelling and digestion groups.
Figure 2.
Transient stress relaxation response for in-situ (is), control free-swelling (fs), and digested (dg) annulus (AF) and nucleus (NP) groups. A curve fit between experimental data (dotted line) and the numerical solution (solid line) of Eq. (3) was used to find the hydraulic permeability k0 and the strain dependent permeability parameter M.
The transient stress-relaxation response exhibited good agreement with the finite difference solution of the biphasic model for all loading groups but was best for the in situ and free-swelling groups. Specifically, the coefficient of determination R2 was 0.94 ± 0.016, 0.93 ± 0.013, and 0.87 ± 0.015 for the annulus, and 0.87±0.032, 0.86±0.027, and 0.76±0.046 for the nucleus, for the in situ, free-swelling, and digestion groups, respectively.
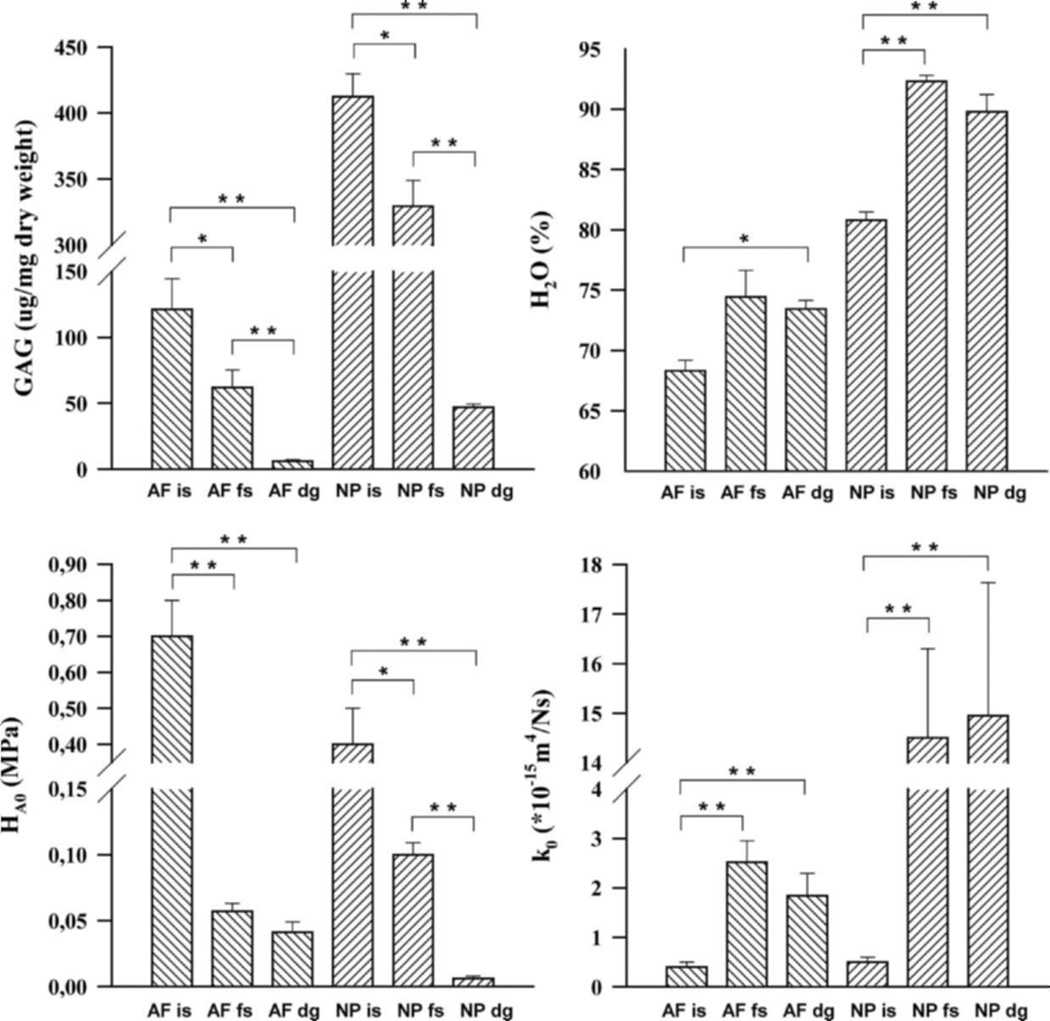
The biochemical contents (water and GAG) and mechanical properties (compressive modulus and hydraulic permeability) obtained for both annulus and nucleus from the three groups are summarized in Fig. 3. For the annulus specimens, the experimental group (in situ, free-swelling, and digestion) influenced both mechanical (p < 0.0001 for HA0 and k0) and biochemical (p < 0.0001 for GAG, p = 0.02 for water) properties. For the nucleus specimens, the experimental group also influenced both mechanical (p < 0.0001 for HA0 and k0) and biochemical (p < 0.0001 for water and GAG) properties. For the annulus, the nonlinear stiffening coefficient β was 0.02±0.02, 0.75±0.35, and 0.25±0.17, while the strain-dependent permeability coefficient M was 2.38±0.89, 2.81±1.70, and 1.26 ± 0.55, for the in situ, free-swelling, and digestion groups, respectively. For the nucleus, the nonlinear stiffening coefficient β and strain-dependent permeability coefficient M were zero except for the free-swelling group which had a value of M = 1.81 ± 0.36.
Figure 3.
Mean ± SEM water content (%), GAG content (µg/mg dry weight), compressive modulus (MPa), and hydraulic permeability (*10−15m4N−1s−1) for the in-situ (is), control free-swelling (fs), and digestion (dg) groups for both annulus (AF) and nucleus (NP). Significant differences between the experimental groups were indicated by *p < 0.05 or **p < 0.0001. Annulus and nucleus groups were not compared.
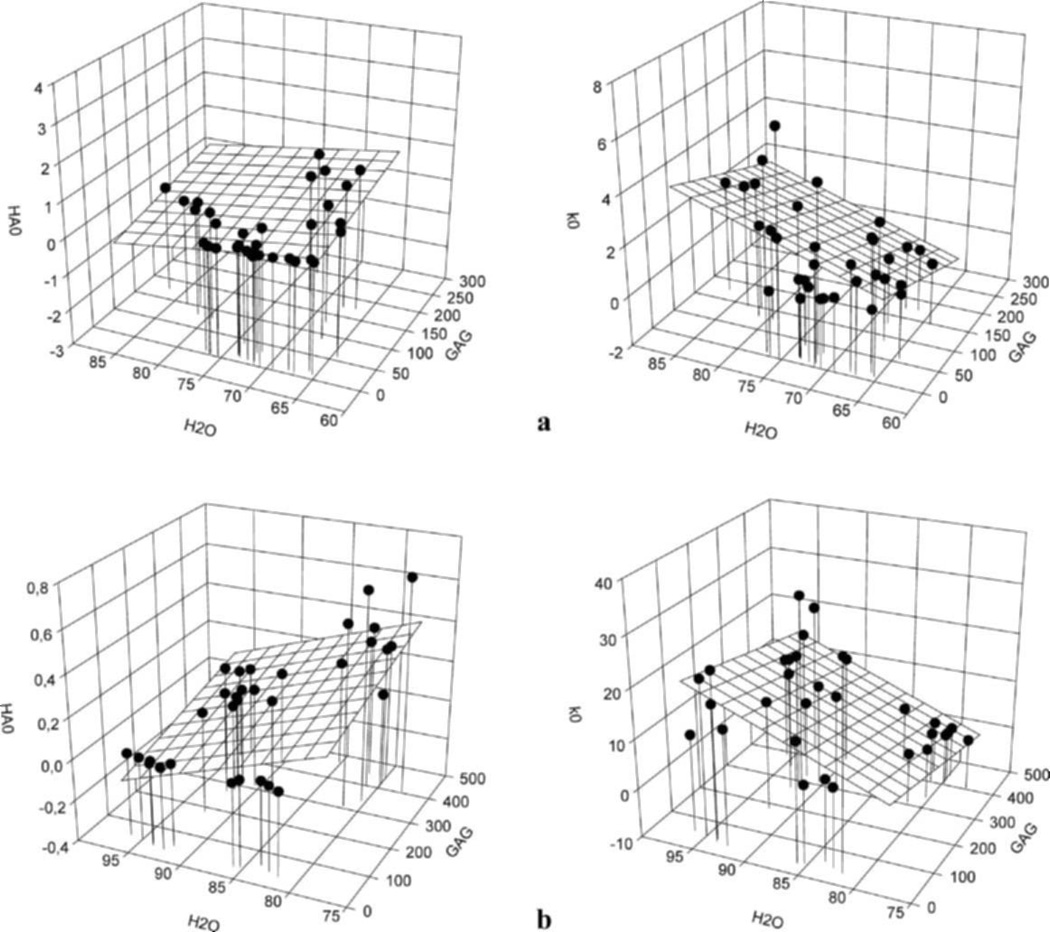
All regression analyses provide an adjusted R2 value as well as β and p values for each coefficient (GAG and H2O) allowing for determination of relative predictive ability of the equation as well as relative importance of the coefficients. Three primary findings were made for both annulus and nucleus (Fig. 4, Eqs. (4–7)). First, water and GAG were somewhat better at predicting HA0 than k0 as given by greater values for . Second, HA0 was predicted by both H2O and GAG since β and p-values were similar for H2O and GAG. Third, k0 was somewhat more predicted by H2O than GAG since for H2O β was greater and p < 0.01 whereas for GAG β was lower and p < 0.05.
Figure 4.
Results of the multiple regression analysis for the annulus fibrosus (a) and nucleus pulposus (b) with HA0 (MPa) and k0(*10−15m4N−1s−1) expressed as a function of GAG (µg/mg dry weight) and H2O (%) contents. Equations (4)–(7) refer to these graphs.
Annulus:
| (4) |
| (5) |
Nucleus:
| (6) |
| (8) |
with HA0 in MPa, k0 in * 10−15m4/Ns, H2O in %, and GAG in µg/mg dry weight.
DISCUSSION
Bovine annulus fibrosus and nucleus pulposus specimens were tested in confined compression using stress-relaxation experiments. In situ, free-swelling, and digestion experimental groups were used to investigate the relationship between water and proteoglycan content, and disk material properties. Significant and specific effects of free-swelling and enzymatic digestion on the material properties and biochemical composition were detected, consistent with our first hypothesis. Significant correlations were detected between biochemical and material properties consistent with our second hypothesis. Specifically, the compressive modulus was inversely related to water content and directly related to GAG content while the permeability was directly related to water content and inversely related to GAG content. Groupwise comparisons and multiple linear regression analyses demonstrated that both GAG and water content had large effects on the compressive modulus while water content had a slightly larger impact on permeability than GAG content for both annulus and nucleus tissue.
This is one of very few studies to report multiple regression results between mechanical (dependent) and biochemical (independent) variables for either the annulus fibrosus or nucleus pulposus, and we believe this technique is an effective way to define structure–function relationships. However, there were some limitations to this study.
Values for R2 ≤ 0.6 for the multiple regression suggested that water and GAG content alone can only moderately predict material properties and that additional compositional and structural factors must be considered for more accurate material property determinations.
We assumed that the 0.1 N contact force caused negligible initial tissue compression. This was justified by the measure of the tissue thickness with this contact force (1.52 ± 0.05 mm for the annulus, 1.32 ± 0.08 mm for the nucleus), which corresponded to the thickness measured with themicrotome when we prepared the tissue (≈1.5mm for the annulus, ≈1.3 mm for the nucleus). The application of the 0.1 N tare force is required to insure contact and appropriate seating of the tissue in the chamber, yet the force and the hypothesis that the samples are in a zero strain condition does represent a limitation to this experiment.
Biochemical data on the tested tissue indicated a small amount of GAG leached from the tissue during the mechanical test. While this may introduce some errors in the measurements of mechanical parameters, the effects are anticipated to be small. Furthermore, the analytical model does account for alterations in water content due to volume changes under large strain conditions, which does have a large effect but does not influence the curve-fit parameters.
The finite difference approximation scheme for the solution of the finite deformation biphasic theory with nonlinear elasticity and strain dependent permeability provided a good fit to the experimental stress-relaxation behavior of bovine nucleus and annulus specimens. However, the goodness of fit was the best for the in situ group, with a slightly higher error for the digestion group. The digested groups had substantially lower steady-state stress values than for the in situ groups but maintained relatively high peak stresses (particularly for the annulus) and took a long time to reach steady state at each strain increment. Future modifications to describe such behavior may involve the addition of intrinsic matrix viscoelasticity, yet were not used in this study to maintain consistency in parameter estimation across groups.
There is some evidence that interdigitation of cartilage tissue into the pores of the filters in confined compression may underestimate the values for permeability.8 While it is possible that our permeability calculation was affected by interdigitation, the relative comparisons (between annulus and nucleus and between experimental groups) as well as our conclusions would remain unchanged. In our study, the diameter of the confining wall and tare loading conditions were optimized to give a tight fit for the annulus and nucleus specimens and no discontinuities or initial jump in the measured force was observed upon loading. Moreover, a low strain rate was used to decrease the friction between the tissue and the confining wall. The durations of all tests were determined based on extensive pilot testing to determine equilibrium times where there was less than 0.1 N change in force over 600 s. In some tests, steady state force values were approached but not achieved, yet we anticipate this to be a modest effect on material parameters which would not affect our conclusion due to the large effects measured in this study.
In this study, we focused on digestion and measurement of GAG because of the large loss of proteoglycan reported with degeneration, and we used chondroitinase ABC digestion because of its specificity for glycosaminoglycans. However, in order to achieve adequate GAG depletion, specimens had to be soaked (unconfined) in the enzyme bath. This “free swelling” digestion resulted in an increase in water content and therefore the second control group (free-swelling control) was included to help differentiate the effect of GAG depletion from the accompanying change in water content despite the fact that the increase in water content is not consistent with decreases in water content observed with human disk degeneration. In our study, we felt it was important to correlate the in situ water content with the zero-strain compressive modulus and permeability values to arrive at a relationship between material constants at our chosen reference configurations with their reference composition rather than to define relationships between porosity and permeability with tissue strain which has been well-described for soft-tissues in the literature.15 The 10-fold decrease in HA0 and 5-fold increase in k0 observed in this study were consistent with 4-fold decrease and 2-fold increase reported in trypsin-treated porcine annulus tissue.30 The difference in the magnitude of the changes may be due to a larger amount of GAG depleted by chondroitinase ABC digestion than by trypsin treatment, and also to the initial tissue compression used (40% for Yao et al., 2002; 0% in our study). Our results also agree with Best et al.,5 who found significant correlation between compressive modulus and water content (r= −0.5, p < 0.002) for human annulus fibrosus.
The nucleus pulposus had lower values of compressive modulus and higher values of permeability than the annulus fibrosus, consistent with the trends for water content, but in contrast to the trends with GAG content. This observation supported our finding that water content was generally a stronger determinant of material properties than GAG. The relative lack of nonlinearities in nucleus pulposus tissue as compared to annulus fibrosus and hyaline cartilage is notable. The relatively high proteoglycan content and low collagen content of nucleus tissue may suggest that material nonlinearities are associated with tissues with more organized collagenous networks, even when loaded in confined compression where strains are purely compressive.
While bovine caudal disks are considered a good model for the study of intervertebral disks due to their relatively large aspect ratio,24 some differences in biomechanical properties between lumbar and caudal levels has been reported in rodent models.11 The large range in water and GAG content used in this study, along with the finite deformation test provided an understanding of disk mechanical behaviors beyond the physiological range which is interesting in the context of tissue engineering constructs that often have greater water and lower proteoglycan contents than native tissue.
CONCLUSION
Confined compression stress-relaxation experiments and a finite deformation biphasic theory allowed us to evaluate the effects of GAG depletion and swelling on the mechanical properties of nucleus pulposus and annulus fibrosus tissues. This was also the first systematic series of studies on the influence of water and proteoglycan contents on nucleus pulposus tissue properties. We concluded that changes in both water and GAG content affected the compressive modulus and permeability, although alterations in water content appeared to have a greater influence on the permeability of disk tissue. The values of R2 ≤0.6 for multiple linear regressions demonstrated a moderate predictive ability of compressive modulus and permeability from water and GAG contents alone, but also underscored the need for additional biochemical and structural properties to more accurately predict these biomechanical parameters. It is hoped that improved understanding of the relationship between mechanical properties and parameters such as GAG and water content is an initial step in being able to evaluate structural and mechanical integrity of the intervertebral disk from in-vivo disk composition measured using noninvasive techniques such as MRI.
ACKNOWLEDGMENTS
Supported by NIH grant 1K01AR02078 and Whitaker Foundation grant RG-03-0030.
REFERENCES
- 1.Acaroglu ER, Iatridis JC, Setton LA, Foster RJ, Mow VC, Weidenbaum M. Degeneration and aging affect the tensile behavior of human lumbar anulus fibrosus. Spine. 1995;20:2690–2701. doi: 10.1097/00007632-199512150-00010. [DOI] [PubMed] [Google Scholar]
- 2.Antoniou J, Steffen T, Nelson F, Winterbottom N, Hollander AP, Poole RA, Aebi M, Alini M. The human lumbar intervertebral disc: Evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J. Clin. Invest. 1996;98:996–1003. doi: 10.1172/JCI118884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ateshian GA, Warden WH, Kim JJ, Grelsamer RP, Mow VC. Finite deformation biphasic material properties of bovine articular cartilage from confined compression experiments. J. Biomech. 1997;30:1157–1164. doi: 10.1016/s0021-9290(97)85606-0. [DOI] [PubMed] [Google Scholar]
- 4.Bernick S, Walker JM, Paule WJ. Age changes to the annulus fibrosus in human intervertebral discs. Spine. 1991;16:520–524. doi: 10.1097/00007632-199105000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Best BA, Guilak F, Setton LA, Zhu W, Saed-Nejad F, Ratcliffe A, Weidenbaum M, Mow VC. Compressive mechanical properties of the human anulus fibrosus and their relationship to biochemical composition. Spine. 1994;19:212–221. doi: 10.1097/00007632-199401001-00017. [DOI] [PubMed] [Google Scholar]
- 6.Brickley-Parsons D, Glimcher MJ. Is the chemistry of collagen in intervertebral discs an expression of Wolff’s Law? A study of the human lumbar spine. Spine. 1984;9(2):148–163. doi: 10.1097/00007632-198403000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Buckwalter JA. Aging and degeneration of the human inter-vertebral disc. Spine. 1995;20(11):1307–1314. doi: 10.1097/00007632-199506000-00022. [DOI] [PubMed] [Google Scholar]
- 8.Buschmann MD, Soulhat J, Shirazi-Adl A, Jurvelin JS, Hunziker EB. Confined compression of articular cartilage: Linearity in ramp and sinusoidal tests and the importance of interdigitation and incomplete confinement. J. Biomech. 1998;31:171–178. doi: 10.1016/s0021-9290(97)00124-3. [DOI] [PubMed] [Google Scholar]
- 9.Cassidy JJ, Hiltner A, Baer E. Hierarchical structure of the intervertebral disc. Connect. Tissue Res. 1989;23(1):75–88. doi: 10.3109/03008208909103905. [DOI] [PubMed] [Google Scholar]
- 10.Demers CN, Antoniou J, Mwale F. Value and limitations of using the bovine tail as a model for the human lumbar spine. Spine. 2004;29(24):2793–2799. doi: 10.1097/01.brs.0000147744.74215.b0. [DOI] [PubMed] [Google Scholar]
- 11.Elliott DM, Sarver JJ. Young investigator award: Validation of the mouse and rat disc as mechanical models of the human lumbar disc. Spine. 2004;29(7):713–722. doi: 10.1097/01.brs.0000116982.19331.ea. [DOI] [PubMed] [Google Scholar]
- 12.Eyre DR, Muir H. Quantitative analysis of types I and II collagens in human intervertebral discs at various ages. Biochim. Biophys. Acta. 1977;492:29–42. doi: 10.1016/0005-2795(77)90211-2. [DOI] [PubMed] [Google Scholar]
- 13.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim. Biophys. Acta. 1986;883:173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 14.Gu WY, Mao XG, Foster RJ, Weidenbaum M, Mow VC, Rawlins BA. The anisotropic hydraulic permeability of human lumbar annulus fibrosus. Influence of age, degeneration, direction, and water content. Spine. 1999;24:2449–2455. doi: 10.1097/00007632-199912010-00005. [DOI] [PubMed] [Google Scholar]
- 15.Gu WY, Yao H. Effects of hydration and fixed charge density on fluid transport in charged hydrated soft tissues. Ann. Biomed. Eng. 2003;31:1162–1170. doi: 10.1114/1.1615576. [DOI] [PubMed] [Google Scholar]
- 16.Happey F, Pearson CH, Naylor A, Turner RL. The ageing of the human intervertebral disc. Gerontologia. 1969;15:174–188. doi: 10.1159/000211685. [DOI] [PubMed] [Google Scholar]
- 17.Holmes MH, Mow VC. The nonlinear characteristic of soft gels and hydrated connective tissues in ultrafiltration. J. Biomech. 1990;23:1145–1156. doi: 10.1016/0021-9290(90)90007-p. [DOI] [PubMed] [Google Scholar]
- 18.Iatridis JC, Setton LA, Weidenbaum M, Mow VC. Alterations in the mechanical behavior of the human lumbar nucleus pulposus with degeneration and aging. J. Orthop. Res. 1997;15:318–322. doi: 10.1002/jor.1100150224. [DOI] [PubMed] [Google Scholar]
- 19.Iatridis JC, Setton LA, Foster RJ, Rawlins BA, Weidenbaum M, Mow VC. Degeneration affects the anisotropic and nonlinear behaviors of human annulus fibrosus in compression. J. Biomech. 1998;31(6):535–544. doi: 10.1016/s0021-9290(98)00046-3. [DOI] [PubMed] [Google Scholar]
- 20.Klisch SM, Lotz JC. A special theory of biphasic mixtures and experimental results for human annulus fibrosus tested in confined compression. J. Biomech. Eng. 2000;122:180–188. doi: 10.1115/1.429640. [DOI] [PubMed] [Google Scholar]
- 21.Lyons G, Eisenstein SM, Sweet MB. Biochemical changes in intervertebral disc degeneration. Biochim. Biophys. Acta. 1981;673(4):443–453. doi: 10.1016/0304-4165(81)90476-1. [DOI] [PubMed] [Google Scholar]
- 22.Mow VC, Kuei SC, Lai WM, Armstrong CG. Biphasic creep and stress relaxation of articular cartilage in compression: Theory and experiments. J. Biomech. Eng. 1980;102:73–84. doi: 10.1115/1.3138202. [DOI] [PubMed] [Google Scholar]
- 23.Natarajan RN, Williams JR, Andersson GB. Recent advances in analytical modeling of lumbar disc degeneration. Spine. 2004;29(23):2733–2741. doi: 10.1097/01.brs.0000146471.59052.e6. [DOI] [PubMed] [Google Scholar]
- 24.Oshima H, Ishihara H, Urban JP, Tsuji H. The use of coccygeal discs to study intervertebral disc metabolism. J. Orthop. Res. 1993;11(3):332–338. doi: 10.1002/jor.1100110304. [DOI] [PubMed] [Google Scholar]
- 25.Pearce RH, Grimmer BJ, Adams ME. Degeneration and the chemical composition of the human lumbar intervertebral disc. J. Orthop. Res. 1987;5(2):198–205. doi: 10.1002/jor.1100050206. [DOI] [PubMed] [Google Scholar]
- 26.Roughley PJ, White RJ, Magny MC, Liu J, Pearce RH, Mort JS. Non-proteoglycan forms of biglycan increase with age in human articular cartilage. Biochem. J. 1993;295:421–426. doi: 10.1042/bj2950421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takahashi T, Kurihara H, Nakajima S, Kato T, Matsuzaka S, Sekiguchi T, Onaya M, Miyauchi S, Mizuno S, Horie K. Chemonucleolytic effects of chondroitinaseABCon normal rabbit intervertebral discs. Course of action up to 10 days postinjection and minimum effective dose. Spine. 1996;21:2405–2411. doi: 10.1097/00007632-199611010-00001. [DOI] [PubMed] [Google Scholar]
- 28.Thompson JP, Pearce RH, Schechter MT, Adams ME, Tsang IK, Bishop PB. Preliminary evaluation of a scheme for grading the gross morphology of the human intervertebral disc. Spine. 1990;15(5):411–415. doi: 10.1097/00007632-199005000-00012. [DOI] [PubMed] [Google Scholar]
- 29.Umehara S, Tadano S, Abumi K, Katagiri K, Kaneda K, Ukai T. Effects of degeneration on the elastic modulus distribution in the lumbar intervertebral disc. Spine. 1996;21(7):811–819. doi: 10.1097/00007632-199604010-00007. [DOI] [PubMed] [Google Scholar]
- 30.Yao H, Justiz MA, Flagler D, Gu WY. Effects of swelling pressure and hydraulic permeability on dynamic compressive behavior of lumbar annulus fibrosus. Ann. Biomed. Eng. 2002;30(10):1234–1241. doi: 10.1114/1.1523920. [DOI] [PubMed] [Google Scholar]