Abstract
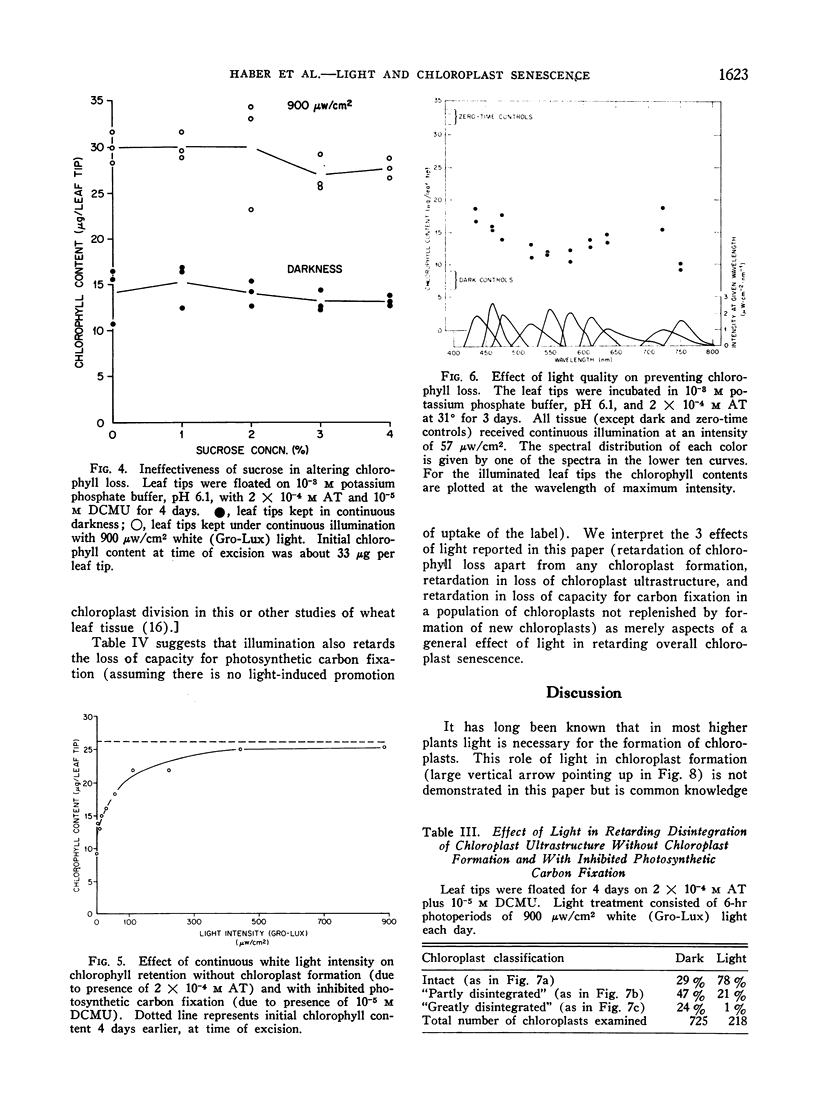
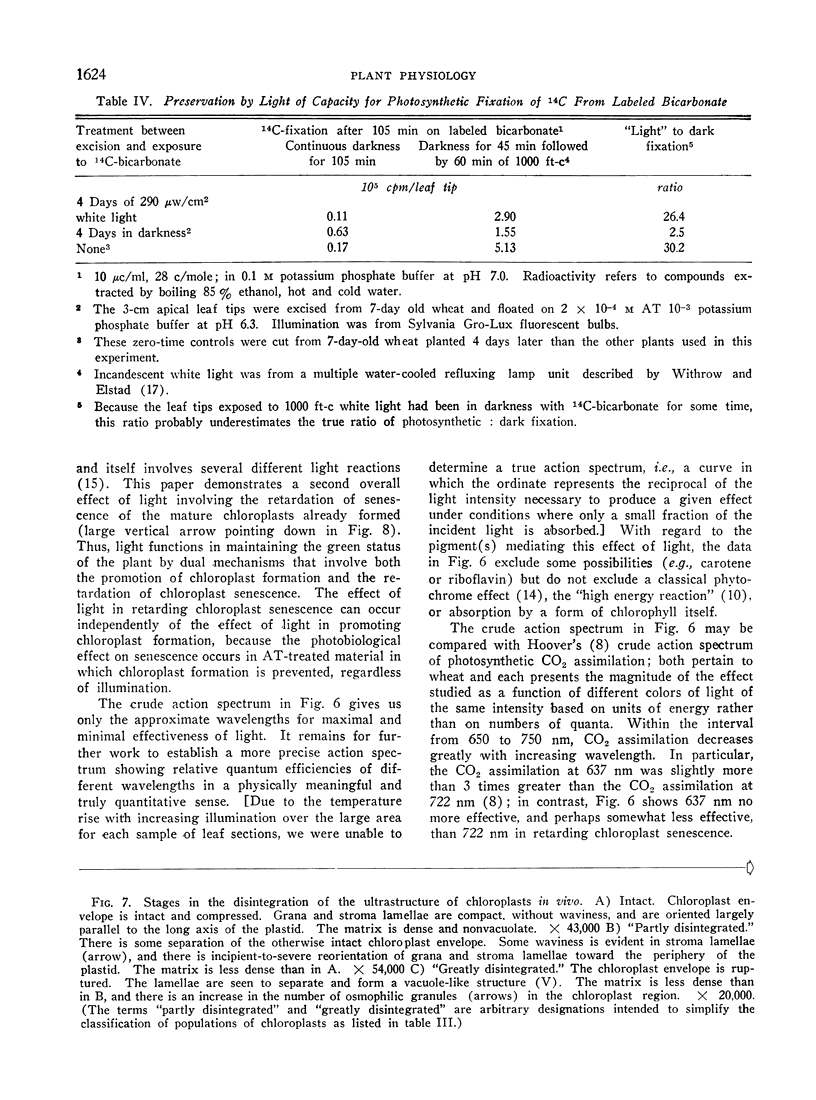
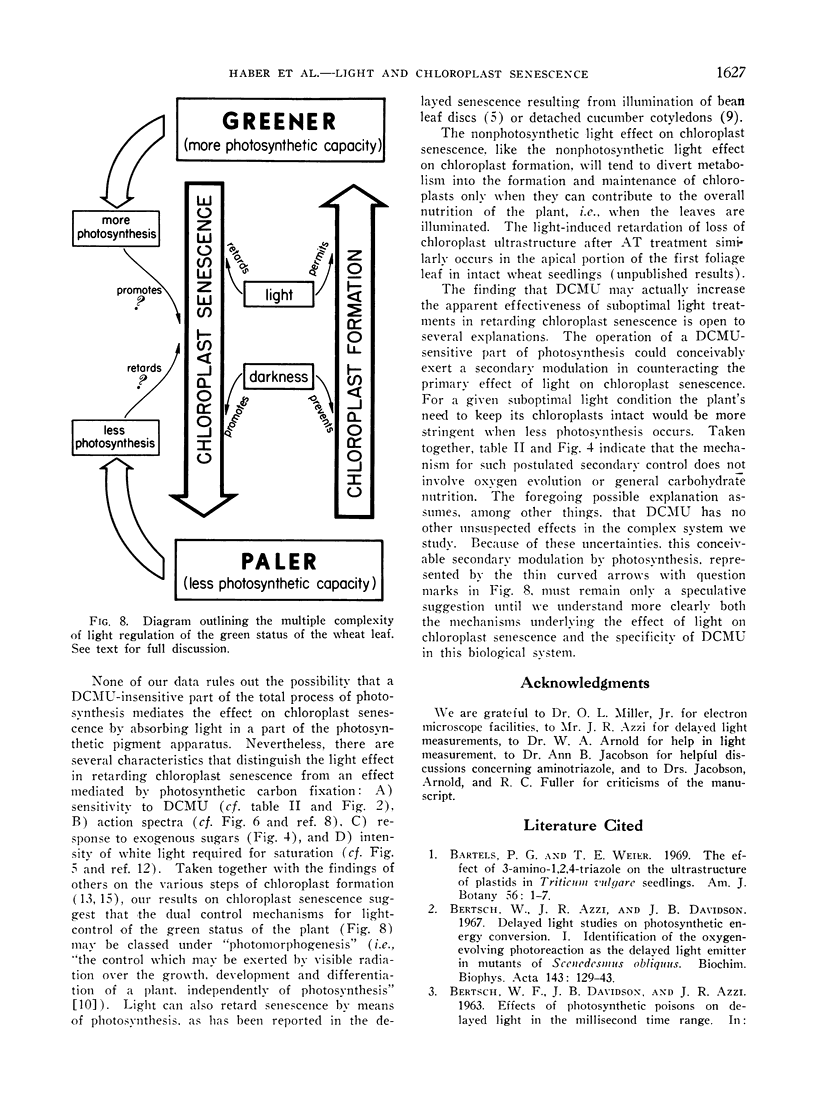
Excised apical portions of green wheat leaf sections were treated with aminotriazole to prevent formation of new chloroplasts. Illumination retarded the decline in chlorophyll content per leaf section, the disintegration of chloroplast ultrastructure, and the loss of capacity for photosynthetic carbon fixation. We interpret these 3 effects of illumination as facets of a single light effect in retarding chloroplast senescence. This light effect in retarding chloroplast senescence has features differing from characteristics of photosynthetic carbon fixation. For example, A) application of the photosynthetic inhibitor 3-(3,4-dichlorophenyl)-1, 1-dimethylurea did not decrease, and may have even slightly increased, the effectiveness of light; B) although the action spectrum contains peaks in the blue and red regions, it differs from the action spectrum for photosynthetic CO2 assimilation in wheat; C) in nonphotosynthesizing tissue, application of sugars did not retard chloroplast senescence; D) light saturation was achieved by only a few hundred microwatts/cm2. Considered together with the well-known light requirement for chloroplast formation, our results indicate that light has a dual, photomorphogenetic control in maintaining the green status of the plant by also exerting a second effect: retarding of senescence of chloroplasts already present.
Full text
PDF

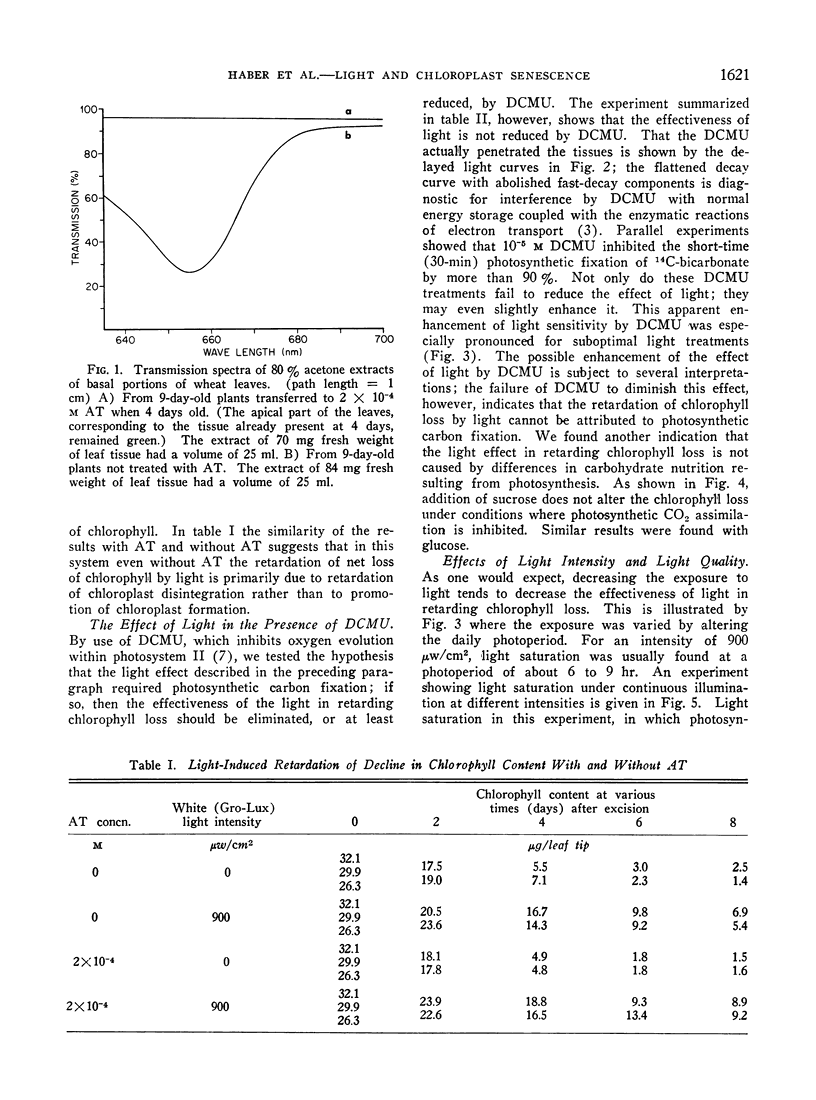


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertsch W., Azzi J. R., Davidson J. B. Delayed light studies on photosynthetic energy conversion. I. Identification of the oxygen-evolving photoreaction as the delayed light emitter in mutants of Scenedesmus obliquus. Biochim Biophys Acta. 1967 Jul 5;143(1):129–143. doi: 10.1016/0005-2728(67)90116-8. [DOI] [PubMed] [Google Scholar]
- Goldthwaite J. J., Laetsch W. M. Regulation of senescence in bean leaf discs by light and chemical growth regulators. Plant Physiol. 1967 Dec;42(12):1757–1762. doi: 10.1104/pp.42.12.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOLLENHAUER H. H. PLASTIC EMBEDDING MIXTURES FOR USE IN ELECTRON MICROSCOPY. Stain Technol. 1964 Mar;39:111–114. [PubMed] [Google Scholar]
- Schiff J. A., Zeldin M. H., Rubman J. Chlorophyll Formation and Photosynthetic Competence in Euglena During Light-Induced Chloroplast Development in the Presence of 3, (3,4-dichlorophenyl) 1,1-Dimethyl Urea (DCMU). Plant Physiol. 1967 Dec;42(12):1716–1725. doi: 10.1104/pp.42.12.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withrow R. B., Elstad V. Water-cooled Lamp Systems with Refluxing Aqueous Filters. Plant Physiol. 1953 Apr;28(2):334–338. doi: 10.1104/pp.28.2.334. [DOI] [PMC free article] [PubMed] [Google Scholar]