Abstract
Rationale
The heart is exquisitely sensitive to mechanical stimuli in order to rapidly adapt to physiological demands. In muscle lacking dystrophin, such as Duchenne muscular dystrophy (DMD), increased load during contraction triggers pathological responses thought to worsen the disease. The relevant mechano-transducers and therapies to target them remain unclear.
Objectives
We tested the role of transient receptor potential canonical channels TRPC3 and TRPC6 and their modulation by protein kinase G in controlling cardiac systolic mechano-sensing, and determined their pathophysiological relevance in an experimental model of DMD.
Methods and Results
Contracting isolated papillary muscles and/or cardiomyocytes from controls and mice genetically lacking either TRPC3 or TRPC6 were subjected to auxotonic load to induce stress-stimulated contractility (SSC, gradual rise in force and intracellular Ca2+). Incubation with cGMP (PKG activator) markedly blunted SSC in controls and Trpc3−/−; whereas in Trpc6−/−, the resting SSC response was diminished and cGMP had no impact. In DMD myocytes (mdx/utrophin deficient), the SSC was excessive and arrhythmogenic. Gene deletion or selective drug blockade of TRPC6, or cGMP/PKG activation, all reversed this phenotype. Chronic PDE5A inhibition also normalized abnormal mechano-sensing while blunting progressive chamber hypertrophy in DMD mice.
Conclusion
PKG is a potent negative-modulator of cardiac systolic mechano-signaling that requires TRPC6 as the target effector. In dystrophic hearts, excess SSC and arrhythmia are coupled to TRPC6 and are ameliorated by its targeted suppression or PKG activation. These results highlight novel therapeutic targets for this disease.
Keywords: TRPC6, Duchenne muscular dystrophy, PKG, muscle contraction, mechanotransduction, cell physiology
INTRODUCTION
The working heart rapidly adjusts to changes in mechanical load in order to adapt to physiological demand. A primary example is the augmentation of contractility that ensues when a heart is subjected to higher afterload as occurs with increased systemic resistance. This stress-stimulated contractility (SSC) response, often termed the Anrep effect 1, 2, allows the heart to provide similar cardiac output despite higher load. The mechanisms are thought to involve mechano-stimulated proteins that ultimately result in a rise of intracellular calcium [Ca2+]i. Candidates for these transducers revealed in passively-stretched non-contracting cells include stretch-activated G-protein coupled receptors such as the angiotensin-type 1 receptor 3, members of the transient receptor potential (TRP) superfamily of cation channels 4–7, and the recently described piezo (1 and 2) proteins 8. The SSC response, however, involves stress imposed during contraction, and here data identifying the relevant transducers remains scant. Understanding this signaling maybe particularly important to disorders such as Duchenne muscular dystrophy, where a lack of the cytoskeletal protein dystrophin results in pathologically augmented responses to systolic load, which are thought to be a core mechanism for progressive muscle disease.
Insight into this signaling pathway maybe gleaned from studies of more sustained pressure-overload that identified both TRPC3 and TRPC6 as modulators of the pathological cardiac response 9–11. TRPC6 is also a putative mechano-sensitive channel in non-contracting cells 6, 7, though this remains somewhat controversial 12. Another feature shared by TRPC3 and TRPC6 is their post-translational modification by the serine/threonine kinase, protein kinase G (PKG) at analogous residues in their intracellular N-terminus (T11 and S263 for TRPC3; T70 and S322 for TRPC6; human gene) 13–17. This modification reduces channel conductance in vitro, and for TRPC6, has been shown to suppress activation of a calcineurin/NFAT signaling pathway and its associated hypertrophy 14, 16, 17. Whether PKG modification of these channels also impacts mechano-sensing is unknown. Intriguingly, strategies to stimulate PKG are currently being studied in experimental 18–21 and human dystrophinopathy 22 spawned initially by its potential impact on vasomotor function in skeletal muscle 18, 22, and some early evidence supports cardiac benefits 20, 21.
Accordingly the present study tested the role of TRPC3 and TRPC6 in the modulation of systolic mechano-sensing in cardiac muscle and intact myocytes, and whether their influence is regulated by PKG. Secondly, we tested whether this pathway is altered in experimental cardiac muscular dystrophy and if so, if it is ameliorated by acute and/or chronic PKG activation. We reveal PKG to be a very potent negative-modulator of the SSC via a TRPC6-dependent mechanism. Further, we show TRPC6 mechano-signaling is excessive in dystrophin deficient muscle and/or cells leading to abnormal calcium entry, excess force and arrhythmia. All of these are blocked by targeted TRPC6 suppression, and by acute or chronic PKG activation.
METHODS
An expanded Methods section is available in the Online Data Supplement at http://circres.ahajournals.org. All studies were conducted in accordance with NIH Guidelines for the Care and Use of Animals and reviewed by the Institutional Animal Care and Use Committee at Johns Hopkins University, where the work was performed.
Animals
TRPC3 knockout (KO) (Trpc3−/−) and TRPC6 KO (Trpc6−/−) mice were generated as previously described23, 24. Each TRPC KO mouse was backcrossed for at least 5 generations into a C57BL6/J background, and their cross yielded the combined TRPC3/TRPC6 double-knockout (dKO) (Trpc3−/−/6−/−) mouse. Heterozygous mice were crossed to yield KO and littermate controls. Both KO models displayed selectivity for the TRPC channel involved, preserving normal levels of expression for other TRPC channels (Online Figure I). For all studies, age-matched gene−/− and gene+/+ littermate controls at 4–6 months of age were used. Female mice lacking dystrophin (mdx, Jackson Laboratories) were crossed with mice with a heterozygous deletion of utrophin (utrn, Jackson Laboratories), and studies performed in either mdx/utrn+/− at 4±1 months of age or in a few instances, mdx/utrn−/− animals at 6–10 weeks of age due to their early mortality. Male mdx/utrn+/− were crossed with female Trpc6−/− to generate male utrn+/−/Trpc6+/− and female mdx+/−/utrn+/−/Trpc6+/−, and their cross yielded male mdx/utrn+/−/Trpc6−/−. Studies were performed at 4±1 months of age. Only male mice were studied, with some controls provided by male C57BL6/J mice as well.
Pharmaceuticals
A new selective dual TRPC3/TRPC6 small molecule inhibitor GSK2833503A (GSK503A, also denoted as Example 19) 25, 26 was provided by GlaxoSmithKline Pharmaceuticals. GSK503A has an IC50 = 21 nM for TRPC3 and 3 nM for TRPC6. Corresponding IC50 for Cav1.2, hERG, Nav1.5, TRPV1, and TRPV4 are 10,000, >50,000, 3300, 6,300, and 12,500 respectively. Cell permeable cGMP-analog (8-pCPT-cGMP) and cAMP-analog (8-Br-cAMP) were obtained from Sigma. For in vivo studies, sildenafil citrate (Revatio, Pfizer) was compressed into soft rodent chow (Transgenic Dough Diet, Bio-Serv) and provided at a dose of 200mg/kg/day for 2 months as described previously 27. This dose yields a free plasma concentration in the range of 30–50 nM, well within the selective range for PDE5A.
Papillary muscle studies
Papillary muscle studies were performed as previously described 28. Briefly, hearts were rapidly excised and placed in modified Krebs-Henseleit (KH) solution containing 30 mmol/L 2,3-butadione monoxime (BDM). The KH solution contained (in mmol/L) 141 NaCl, 50 Dextrose, 25 NaHCO3, 5 HEPES, 5 KCl, 1.2 NaH2PO4, 1 MgSO4 and 2.0 CaCl, pH adjusted to 7.35, and bubbled with 95% O2, 5% CO2. Thin papillary muscle strips with chordae tendineae were dissected from the right ventricle. The muscle was connected to a force transducer (Scientific Instruments GmbH, Heidelberg, Germany) at one end and mechanical anchor at the chorda tendinae end, allowing the muscle to contract auxotonically.
Calcium was measured by Fura-2AM (340 nm and 380 nm excitation, 510 nm emission). Fluorescence was collected by photomultiplier tube (R1527, Hamamatsu, Japan) with background recorded before dye loading. Fura-2AM (50 μg) was dissolved in 25 μL dimethyl sulfoxide (DMSO) and 25 μL Pluronic to which 2.755 mL Krebs-Henseleit, 4.3 mg/L TPEN, and 5.0 mg/L cremophor were added. Muscles were loaded with this solution for 30 minutes. The muscle length (Lmax) generating maximal developed force (max-min force; ΔF) was determined and then length reduced to 92% this value.
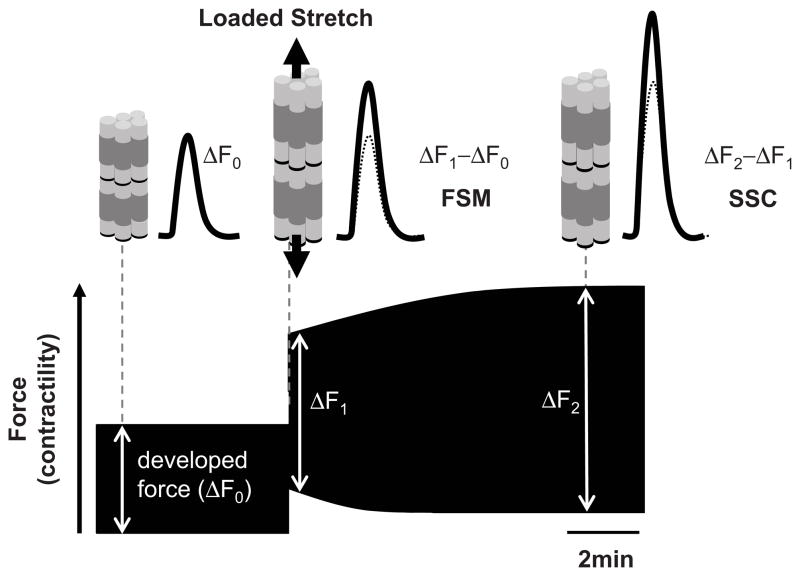
Upon achieving steady state developed force (ΔF0), muscles were stretched to 98% Lmax, and force and Ca2+ recorded for 10 minutes to assess the SSC. Figure 1 displays a schematic for the SSC response and its analysis. Developed force rose immediately upon stretch (ΔF1, Frank-Starling Mechanism, FSM) and was indexed by ΔF1–ΔF0. The subsequent more gradual rise in developed force (ΔF2 − ΔF1) assessed SSC.
Figure 1. Stress-stimulated contractility (SSC).
Immediately following papillary muscle or cardiomyocyte auxotonic stretch there is rapid calcium-independent rise in developed force (ΔF1−ΔF0, Frank-Starling Mechanism, FSM) followed by a more gradual rise that is associated with an intracellular calcium increase (ΔF2−ΔF1). The latter is termed stress-stimulated contractility (SSC), calculated by the augmentation in developed force after the acute FSM change.
Isolated cardiac myocyte studies
SSC analysis was also performed in isolated loaded cardiac myocytes. Cell isolation was performed as described 28 (cf. Online Supplemental Methods). Myocytes were incubated for 15 min with 1 μmol/L Indo1-AM (Invitrogen, Molecular Probes, Carlsbad CA) in Tyrodes (1.0 mmol/L Ca2+). The ratio of fluorescence emitted at 405 nm and 485 nm measured [Ca2+]i. Myocytes were then mounted on a custom force-length control system as previously described 29. Rod-shaped quiescent single cardiomyocytes were selected and a pair of carbon or glass fibers (6 μm at one end, 20 μm at the other) coated with a biological adhesive (MyoTak) 30 was attached to both ends using micromanipulators. The thin fiber was compliant and its position digitally controlled by a piezoelectric translator (Physik Instrumente, P-841-80) while the other fiber was rigid and served as a mechanical anchor. Cells were electrically stimulated at 0.15-0.2 Hz with 15-ms pulses. Cardiomyocyte sarcomere length and fiber tip displacements were recorded at 120 Hz and analyzed in real time using IonOptix (MA) equipment and software. Cells were then stretched to increase sarcomere length by ~4%, and active and passive force (F) was determined from fiber bending moment given by F = K(ΔLP−ΔLF), where K is the effective fiber stiffness, ΔLF the change in distance between the two fibers, and ΔLP the displacement of piezoelectric translator. Components of the SSC were determined as for papillary muscle data.
Acute trans-aortic constriction in vivo studies
Adult Trpc6−/− or littermate control mice were anesthetized with 3% isoflurane, the chest opened between ribs 2–4, and a 26G needle placed on the transverse aorta. A micro-pressure volume catheter (Millar Instruments, TX) was inserted through a left ventricular apical stab, and positioned so the distal tip lay in the proximal aortic root 31. Instantaneous pressure-volume loops were recorded using custom developed hardware and software (WinPVAN). After obtaining rest data, the aorta was constricted with a 6.0 suture around the aorta and 26G needle to increase ventricular afterload. Pressure-volume data were recorded during the initial 15 minutes following aortic banding.
Statistical analysis
Statistical analyses utilized 1-way or 2-way ANOVA or ANCOVA for normally distributed data with equal variance among groups. For other data, we used a Kruskal-Wallis test. Post-hoc analysis used a Tukey-Kramer test, or Mann-Whitney-U test as appropriate. Analysis was performed using SigmaStat Ver 13 and Systat Ver 10 software.
RESULTS
cGMP/PKG activation markedly suppresses stress-stimulated contractility (SSC)
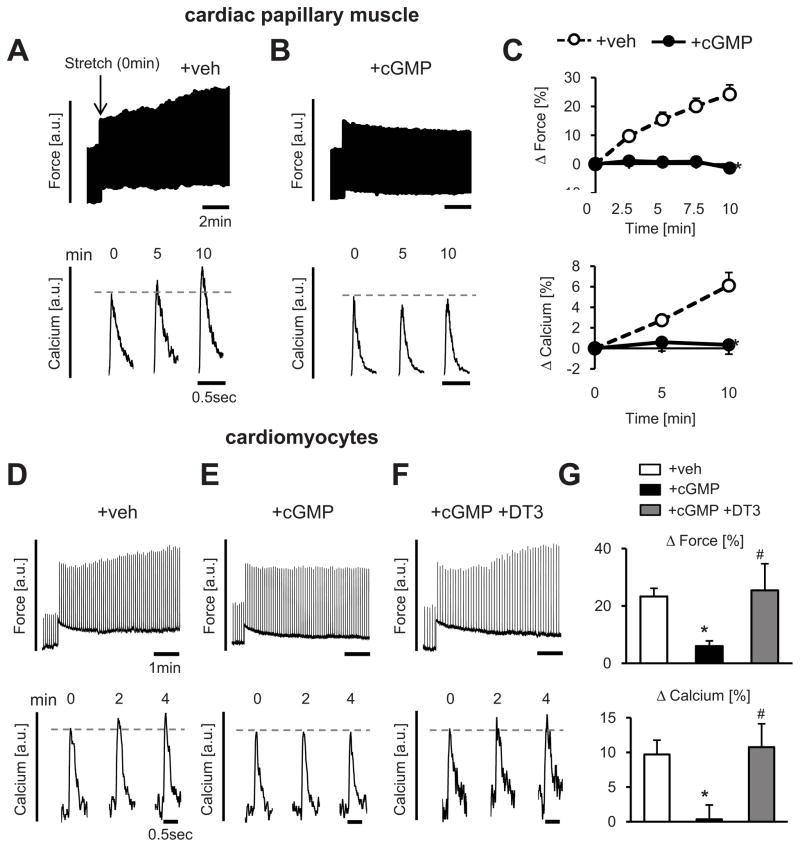
To test whether PKG modifies systolic mechano-transduction in heart muscle, SSC was first measured in isolated cardiac papillary muscles with or without incubation with membrane permeable cGMP. Upon exposure to 6% auxotonic stretch, muscles exhibited an immediate rise in developed force reflecting length-dependent activation (FSM), followed by a gradual 20–30% rise in contractility (SSC) accompanied by high peak [Ca2+]i transients that occurred over the ensuing 10 minutes (Figure 2A). The FSM was unaltered whereas the SSC response was markedly suppressed by pre-incubation with 8-pCPT-cGMP (1.0 mmol/L) (Figure 2B and 2C).
Figure 2. Cyclic GMP/PKG stimulation blocks cardiac muscle or cell contractility enhancement with stress increase.
A–C) Force and calcium transients in isolated cardiac muscle pre-treated with or without 8-pCPT-cGMP; Right panels: Summary data for SSC response (n=12–14/group, *-p<0.05 vs +veh by ANCOVA). D–G) Force and calcium in auxotonically contracting cardiomyocytes. The SSC was suppressed by exposure to cGMP, but this was prevented by inhibition of PKG (DT3, 0.2 μM, n=5–24/group, *-p<0.05 vs +veh, #-p<0.05 vs +cGMP).
Cyclic GMP-PKG can also target vascular cells and fibroblasts potentially influencing the muscle response; therefore, we tested if its regulation of the SSC was myocyte autonomous. Isolated myocytes were attached to carbon or glass microfibers, and the same protocol performed. With a rise in auxotonic stress, we again observed an immediate rise in developed force (FSM) followed by SSC (Figure 2D). Incubation with cGMP (0.1 mmol/L) did not alter the FSM, but markedly depressed the SSC (Figure 2E). When myocytes were pretreated with the PKG-inhibitor DT3 (0.2 μmol/L), the SSC response (both force and Ca2+) was normal despite subsequent exposure to cGMP (Figure 2F; summary data Figure 2G). This was confirmed using an alternative PKG-inhibitor Rp-8-CPT-cGMP (10 μmol/L) (Online Figure II). Thus, Ca2+-associated SSC induced by systolic stress is cardiac myocyte autonomous and can be potently suppressed by cGMP-PKG modulation.
SSC modulation by cGMP requires TRPC6 and is independent of TRPC3
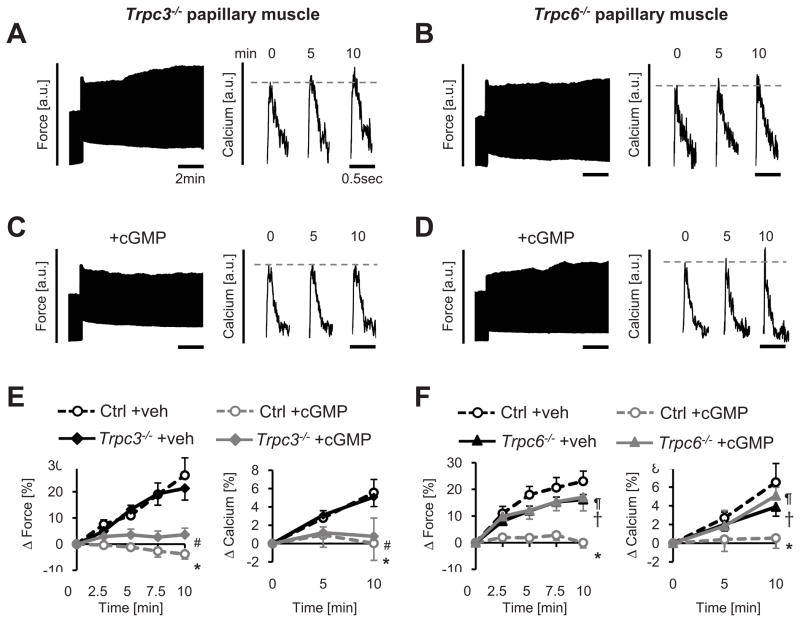
To test the role of TRPC channels to the SSC and its suppression by PKG, papillary muscles from mice genetically lacking either Trpc3−/− or Trpc6−/− and respective littermate controls were studied. Cardiac muscle lacking Trpc3 exhibited behavior identical to controls (Figure 3A), whereas Trpc6−/− muscle had a significantly blunted force and Ca2+ SSC response (Figure 3B). The rapid FSM response was similar in all groups (Online Figure III). In Trpc3−/− muscle, incubation with 8-pCPT-cGMP also still profoundly inhibited the SSC as in littermate controls (Figure 3C; summary data Figure 3E). However, in Trpc6−/− mice, the SSC response remained unaltered despite cGMP incubation (Figure 3D) whereas littermate controls displayed marked suppression (Figure 3F). Thus, TRPC6, not TRPC3, contributes to the SSC response, and is required for its suppression by PKG.
Figure 3. TRPC6 not TRPC3 contributes to SSC and is required for SSC suppression by cGMP/PKG.
A, B) Force time-tracings and calcium transient examples from cardiac trabeculae exposed to mechanical stress from mice lacking either Trpc3 or Trpc6. C, D) Similar tracing examples in muscle exposed to 8Br-pCPT-cGMP. Exposure to cGMP depressed the SSC response in Trpc3−/− but not Trpc6−/−muscle. E, F) Summary results for SSC force or calcium temporal response (n=4–13/group). Unlike Trpc3−/−, Trpc6−/− muscle responses in both parameters were not significantly altered by the addition of cGMP (both p>0.05). Significant differences between responses based on ANCOVA are denoted by the symbols: Panel E: *-p<0.05 Ctrl+veh, #-p<0.05 vs Trpc3−/−+veh. Panel F: *-p<0.01 vs Ctrl+veh, †-p<0.05 Trpc6−/−+veh vs Ctrl+veh, ¶-p<0.05 Trpc6−/−+cGMP vs Ctrl+cGMP.
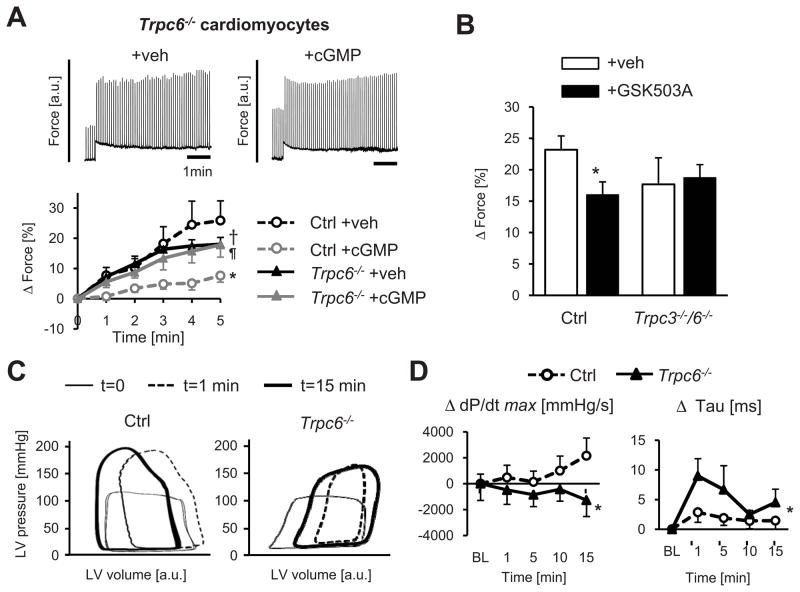
Since vascular cells and fibroblasts also express TRPC6, we again tested its role in isolated cardiomyocytes. Controls showed a robust SSC response that was cGMP-inhibited, whereas Trpc6−/− cells displayed a blunted SSC with no change in the response after cGMP incubation (Figure 4A). As an alternative to gene deletion, we also tested the role of TRPC6 with a new selective small molecule TRPC3/6 blocker (GSK503A, 5 μmol/L) 26. Given that the SSC was unaltered by TRPC3 gene deletion, GSK503A effects were interpreted as dependent on TRPC6. GSK503A depressed the SSC by ~30%, similar to results from Trpc6−/− cells (Figure 4B). As a negative control, we tested GSK503A in cardiac myocytes lacking both Trpc3 and Trpc6 and found no SSC effect, supporting its selectivity (Figure 4B).
Figure 4. Genetic deletion or pharmacological inhibition of TRPC6 blocks cardiac cell and whole heart SSC response.
A) Trpc6−/− cardiomyocytes display a similar SSC response despite cGMP exposure in contrast to controls (n=6–8/group, *-p<0.001 vs Ctrl+veh, †-p<0.05 Trpc6−/−+veh vs Ctrl+veh, ¶-p<0.001 Trpc6−/−+cGMP vs Ctrl+cGMP by ANCOVA). B) TRPC3/6 inhibitor (GSK503A) blocks SSC in WT but not Trpc3−/−/6−/− cells (n=7–24/group, *-p<0.05 vs Ctrl+veh). C) Intact heart response to acute pressure-overload depicted by pressure-volume loops. Trpc6−/− mice had a depressed inotropic response versus controls. See text for details. D) Summary results for maximal peak rate of pressure rise (dP/dtmax, contractility) and relaxation (time contstant, Tau) normalized to baseline; n=5–8/group, *-p<0.05 vs Ctrl by ANCOVA.
Lastly, we tested the relevance of TRPC6 mechano-sensing in the intact heart. Hearts in situ were subjected to 15 minutes of increased afterload induced by proximal aortic constriction, and pressure-volume loops recorded (Figure 4C). In both groups, the rise in afterload was first manifest by loops rapidly becoming taller and narrower and shifting rightward (shown by the response after 1 minute). In controls, the loops then gradually shifted leftward reflecting increased contractility countering the persistently high afterload. However, this latter response was reduced in Trpc6−/− hearts. Mean data for maximal rate of pressure rise (dP/dtmax) and relaxation time constant (tau) are shown in Figure 4D. Controls with a rise in contractility and little delay in relaxation following high afterload contrasted to Trpc6−/− mice that displayed a fall in contractility and delayed relaxation. Taken together, these results indicate that TRPC6 is an important mechano-transducer in cardiomyocytes and the in vivo heart, and is required for the modulation of cGMP-PKG suppression of afterload stress-stimulated contractility.
Hyperactive SSC and arrhythmia in myocytes lacking dystrophin/utrophin are related to TRPC6 and suppressed by PKG activation
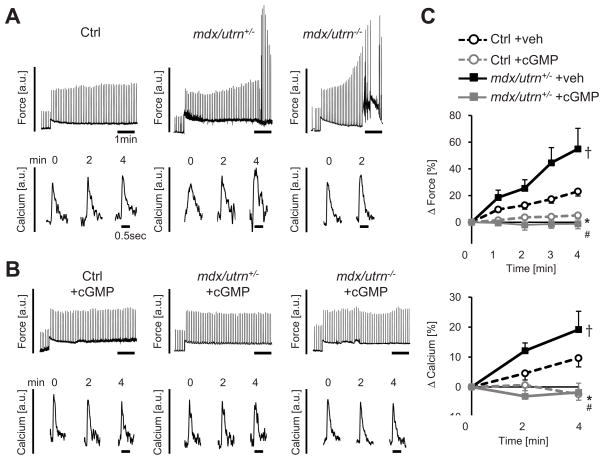
We next asked whether TRPC6-dependent systolic mechano-stimulation was abnormal in cells lacking dystrophin and utrophin (mdx/utrn+/−), and whether this too could be modulated by PKG activation. The combined mutation model was used as absence of dystrophin alone produces a mild phenotype in mice in part due to compensatory upregulation of utrophin. Mice fully lacking both genes display a severe skeletal and cardiac pathology with early mortality (6–8 weeks) 32, whereas partial utrophin deletion still leads to earlier and more prominent heart disease (Online Figure IV), but can be more easily studied. In mdx/utrn+/− (and mdx/utrn−/−) cells, the SSC force and corresponding [Ca2+]i transient increase were markedly amplified over controls (Figure 5A). Stressed cells frequently displayed arrhythmia minutes after load was increased, in some instances leading to cell demise. This exacerbated SSC response in DMD myocytes was fully blocked by pre-incubation with cGMP (Figures 5B and 5C).
Figure 5. Dystrophin/utrophin deficiency amplifies SSC (force and Ca2+) and associated arrhythmia; all are suppressed cGMP.
A) Example force and calcium transients from control, mdx/utrn+/− and mdx/utrn−/− myocytes after stress-increase. B) Abnormal force/calcium response is prevented in both normal and DMD myocytes by cGMP. C) Summary data; n=5–24/group, *-p<0.001 Ctrl+cGMP vs Ctrl+veh, #-p<0.001 mdx/utrn+/−+cGMP vs mdx/utrn+/−+veh, †-p<0.01 mdx/utrn+/−+veh vs Ctrl+veh by ANCOVA.
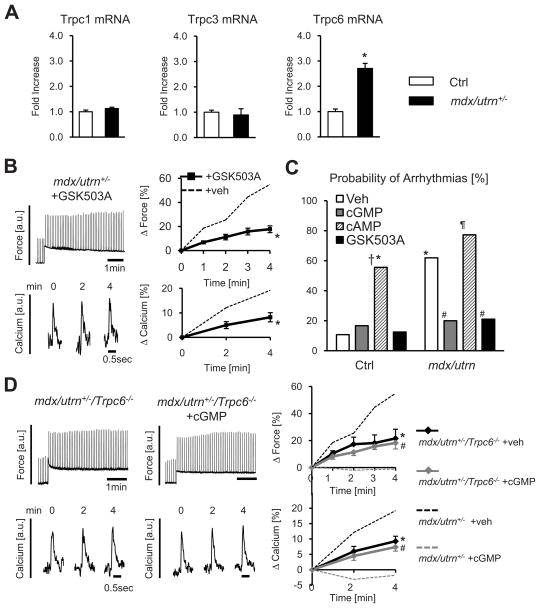
In MD cells, gene expression for Trpc6 was elevated ~3-fold over controls (Figure 6A), whereas expression of Trpc1 and Trpc3 were unchanged. Poor signal/noise and antibody specificity combined with low expression levels precluded detection of protein by immunoblot; however, evidence for a functional role of TRPC6 was obtained by incubating DMD cells with GSK503A. This reversed amplified force and Ca2+ SSC responses to control levels (Figure 6B).
Figure 6. Abnormal SSC and stress-induced arrhythmia are suppressed by TRPC6 antagonist.
A) Quantitative PCR analysis TPRC channel expression from WT or mdx/utrn+/− heart (n=6–9/group,*-p<0.05 vs Ctrl). B) GSK503A suppresses SSC in mdx/utrn+/− myocytes; summary data; n=7–15/group, *-p<0.01 vs mdx/utrn+/−+veh. C) Arrhythmia prevalence following myocyte stress-increase is greater in DMD cells and blocked by cGMP or GSK503A but not cAMP (n=12–28/group, *-p<0.002 vs Ctrl+veh, #-p<0.02 vs mdx/utrn+veh, †-p=0.06 vs Ctrl+cGMP, ¶-p<0.001 vs mdx/utrn+cGMP). D) Myocyte SSC response (±cGMP) in myocytes from mdx/utrn+/−/Trpc6−/− hearts. Genetic deletion of Trpc6 in mdx/utrn+/−reversed amplified SSC responses present in mdx/utrn+/− alone (dashed lines, from Fig 5C), and eliminated sensitivity of SSC to cGMP. Data from n=6–10/group, *-p<0.01 vs mdx/utrn+/−+veh, #-p<0.01 vs mdx/utrn+/−+cGMP by ANCOVA.
Figure 6C summarizes the impact of cGMP or GSK503A incubation on stress-induced arrhythmia. In DMD cells, arrhythmia prevalence was 6-times higher than control, but was restored to normal by cGMP or the TRPC3/6 blocker. The impact of cGMP was opposite that from cAMP (8-Br-cAMP, 0.1 mmol/L) which increased stress-stimulated arrhythmia incidence in control and DMD cells, the latter approaching ~80% of cells studied. This highlights the selective impact of cGMP-PKG as a negative modulator of this signaling.
To more directly test if TRPC6 was required for abnormal SSC responses in DMD myocytes, we crossed mdx/utrn+/− with Trpc6−/− mice and subjected isolated myocytes from the triple-mutant hearts (mdx/utrn+/−/Trpc6−/−) to the cell-stress protocol. The SSC force and [Ca2+]i transient responses in these cells behaved like healthy controls (Figure 6D). Furthermore, cGMP exposure had no effect on the SSC in these cells. Collectively, these results strongly support a major role of TRPC6 to abnormal mechano-stimulation in cardiac DMD, and its importance to the amelioration of this pathophysiology by cGMP-PKG stimulation.
Acute and chronic treatment of dystrophic myocytes and hearts by PDE5A inhibition blocks hyperactive SSC and arrhythmia
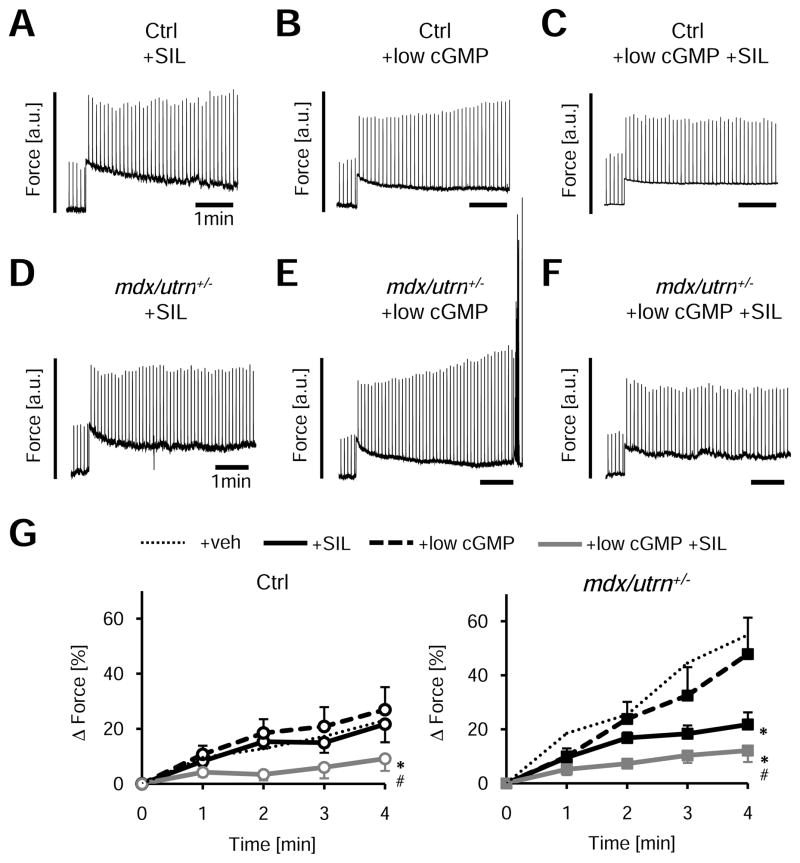
Activation of PKG can be pharmacologically achieved in vivo by stimulating cGMP synthesis (nitrates or natriuretic peptides) or by blocking its hydrolysis by phosphodiesterases such as PDE5A. The latter is more amenable to chronic therapy (e.g. sildenafil, SIL), and has been shown to improve skeletal muscle fatigue 18 and blunt progressive cardiac dysfunction in mdx mice 20. Incubation of normal cells with SIL alone (1 μmol/L for 10 min) did not alter the SSC (Figure 7A). However, the efficacy of PDE5A inhibition is itself dependent upon how much cGMP is present, and resting isolated cells have low levels. We therefore next identified a low cGMP dose (1/10th that used previously, e.g. 0.01 mmol/L) that had no impact itself on the SSC (Figure 7B); however, when combined with SIL, the SSC was suppressed (Figure 7C; summary data Figure 7G). In mdx/utrn+/−, SIL alone was effective (Figure 7D), potentially due to higher basal PDE5A activity in the model (3.5±0.5 fold over controls (p<0.001, Figure 8E). Combining SIL with low-dose cGMP further suppressed the SSC (Figure 7F, 7G).
Figure 7. Adverse mechano-response in dystrophinopathy is blunted by acute PDE5A inhibition.
A–F) Example tracing for the effects of sildenafil (SIL) ± a sub-effective low-dose cGMP on SSC in cardiac myocytes from normal or DMD hearts. Data can be further compared with DMD results with no treatment (Fig 5A). G) Summary results for these experiments; n=6–11/group, *-p<0.01 vs +low-dose cGMP, #-p<0.05 vs+low-dose cGM+SIL by ANCOVA). Low dose cGMP had no effect itself on the SSC (non-treated control data shown by gray dashed lines), but when combined with SIL suppressed the SSC. SIL alone was effective in DMD cells.
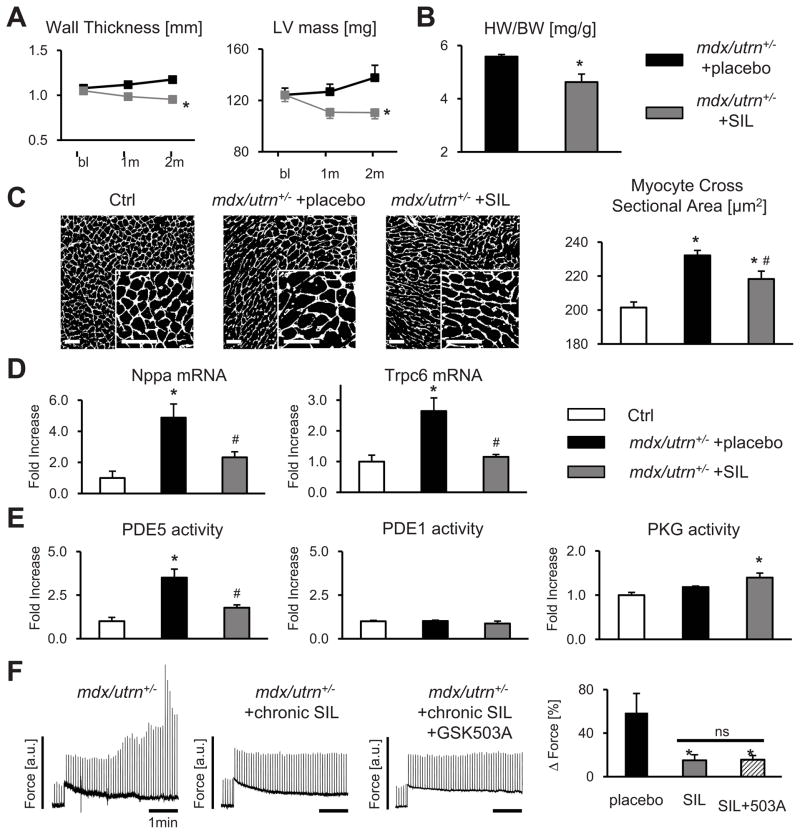
Figure 8. Amplified mechano-response in dystrophinopathy is blunted by chronic PDE5A inhibition.
A) Progressive cardiac hypertrophy (serial echocardiography) in DMD mice was blunted by sildenafil (SIL) treatment (n=14–17/group, *-p<0.05 vs placebo by ANCOVA), and these data confirmed by heart weight/body weight (HW/BW) at terminal study (B; n=5–8/group, *-p<0.05 vs placebo). C) Myocyte cross-sectional area by WGA staining, representative images and summary data, scale bar=50 μm; n=4 mice/group (>1000 cells/mouse), *-p<0.05 vs Ctrl, #-p<0.05 vs placebo. D) mRNA expression of A-type natriuretic peptide (Nppa) and Trpc6 (n=3–8/group, *-p<0.05 vs Ctrl, #-p<0.05 vs placebo). E) PDE5, PDE1 and PKG activity for each group (n=5/group, *-p<0.01 vs Ctrl, #-p<0.01 vs placebo). F) Amplified SSC in mdx/utrn+/− is normalized after chronic SIL treatment, and unaltered further by the addition of GSK503A (n=6–12/group, *-p<0.05 vs placebo).
To test if chronic PDE5A inhibition also ameliorated abnormal myocyte mechano-responses in DMD, mdx/utrn+/− mice were treated for 2 months with SIL (200 mg/kg/d po). This therapy blunted progressive chamber hypertrophy (Figure 8A and 8B) and reduced myocyte cross sectional area (Figure 8C). Cardiac function (fractional shortening) also declined slightly in the placebo group but was maintained in SIL treated mice (Online Figure V). Fetal gene markers of pathological hypertrophy (Nppa) and Trpc6 gene expression were elevated in DMD myocardium and were significantly reduced with SIL treatment (Figure 8D). Changes in Trpc6 were associated with modest directionally similar changes in calcineurin protein expression, though regulator of calcineurin (Rcan-1) gene expression was similar in all groups (Online Figure VI). DMD mice displayed ~4-fold higher myocardial PDE5A activity compared to controls and this declined with SIL treatment accompanied by a modest rise in PKG activity (Figure 8E). By contrast, PDE1 activity was similar to controls and unaltered by SIL (Figure 8E).
Figure 8F displays the effects of chronic SIL treatment on cellular mechano-stimulation, the primary focus of this study. SIL treated cells displayed an essentially normal SSC response as compared with those receiving placebo. When further incubated with GSK503A, the SSC response was unaltered in cells from SIL-treated DMD hearts. This is consistent with the notion that chronic SIL-therapy had already reversed TRPC6 hyperactivity.
DISCUSSION
This investigation reveals TRPC6 as a modulator of systolic load-induced contractility increases in cardiomyocytes, muscle, and intact hearts, and that it is also required for PKG-mediated suppression of this mechanical response. The normally adaptive mechanism is pathologically amplified in heart muscle lacking dystrophin/utrophin, resulting in dysregulated force and [Ca2+]i and arrhythmogenicity. Hyperactive mechano-sensing in DMD is greatly suppressed by inhibiting or genetically deleting TRPC6, or by activating the cGMP/PKG pathway so long as TRPC6 is present. These results provide new insight into normal stress-induced contractility adaptations, and reveal a novel therapeutic target for the dystrophic heart.
The SSC (or Anrep) response has been recognized for over a century, yet its mechanisms have remained uncertain. Some attribute it to stretch activated Gq-coupled receptors (GqPCRs, e.g. angiotensin-II and endothelin-1) which via reactive oxygen species 33 generation induce ERK1/2 activation of the Na+/H+ exchanger (NHE1). The latter in turn results in higher [Ca2+]i via reverse mode Na+/Ca2+ exchange (NCX) 2. Other studies support activation of stretch-activated channels (SACs) 34 that themselves conduct Na+ and/or Ca2+, with [Ca2+]i being further enhanced by the NCX, NHE1, or Ca2+ released from internal stores 35. The current results help reconcile these mechanisms by placing TRPC6 as an important upstream mechano-sensor. TRPC6 is stimulated by GqPCRs, and in other cell types, can also activate ERK1/2 36, 37 upstream of NHE1 2, and conduct Na+ that in turn triggers Ca2+ entry via the NCX 38–40.
The mechano-sensing capacity of TRPC6 was first revealed by Spassova et al. 7 in studies of smooth muscle cells exposed to osmotic or direct membrane passive stretch. TRPC6 mechano-sensing was later reported in quiescent adult cardiac myocytes subjected to plasma membrane shear stress 6. Some, however, have questioned this role and attributed earlier data to stretch-activated GqPCRs and/or artifacts from TRPC6 overexpression in heterologous systems 12. The present data combines results from physiologically loaded myocytes, muscle, and intact hearts, and employs gene deletion and/or selective TRPC3/6 blockade, to support the role of TRPC6 as a mechano-sensor in both the normal heart and particularly hearts lacking dystrophin. The experiments did not involve artificial overexpression models, and while more proximal GqPCR stretch activation could still play a role, gene deletion and targeted pharmacological blockade studies strongly implicates TRPC6 as a critical node in the stress response.
Modulation of TRPC channel function by PKG was first reported for TRPC3 in HEK293 cells 13 and later in TRPC6 in similar cells 15 and cardiac myocytes 14, 16. For TRPC6, this modification not only reduces cation conductance but also blunted associated signaling via calcineurin/NFAT activation triggered by Gq-coupled agonists 14, 16. Phospho-silenced or phospho-mimetic TRPC6 mutants display gain or loss of channel function, respectively, with respect to conductance and associated NFAT signaling 14, 16. The current data adds mechano-signaling to the list of TRPC6-mediated responses modified by cGMP/PKG, and this influence appears separable from effects on hypertrophic signaling. First, we identified its role in normal cells exposed to rapid stress that is unlikely to involve hypertrophic cascades. Second, we found TRPC6 modulation of the SSC was greatly amplified in DMD despite only modest evidence of calcineurin/NFAT activation in the same hearts. Lastly, TRPC3 was uninvolved with mechano-activation or PKG modulation of SSC, yet it has been previously linked to calcineurin/NFAT activation and hypertrophy in chronic pressure-overloaded hearts 10, 11, 41.
Abnormal mechano-responses in DMD skeletal muscle 42, 43 have long been considered fundamental to the disease. Stretch imposed on contracting DMD muscle induces enhanced [Ca2+]i and a decline in tetanic force, and these abnormalities are reduced by lowering Ca2+ in the bathing solution or by blocking SACs. TRPC1 has been proposed as a relevant SAC 44, 45, though TRPC6 is also expressed and may be involved 46. The current study focusing on the heart reveals a key role of TRPC6 to altered mechano-sensing. Afterload imposed on the heart during systole has a parallel with stretch imposed during skeletal muscle contraction. The consequential amplified rise in [Ca2+]i is thought to stimulate myocardial damage. This rise has been linked to plasma membrane defects 47, 48 and/or altered SR calcium handling 49, 50, and the current study adds TRPC6 hyperactivity to this list.
The current results counter the notion that DMD myocytes are subject to stress-induced membrane tears that non-selectively leak cations, since SSC force/Ca2+ responses were both normalized by selectively blocking TRPC6 or activating PKG. However, membrane instability stemming due to a lack of dystrophin could well trigger TRPC6 hyperactivity, and surfactants 48 that can stabilize the plasma membrane may also suppress such hyperactivity. Interestingly, muscle cell permeability of Evans Blue in mdx declines from treatment with a SAC inhibitor (streptomycin 43) or with chronic sildenafil therapy 21.
Benefits in DMD skeletal muscle from PKG activation have been reported, with most data reporting on improved vascular perfusion 18, 21, 51. While some benefit has also been observed in the heart 20, mechanisms have remained unclear. While long-term studies remain to be performed, the present data support a PKG-TRPC6 targeting mechanism is likely involved. The findings that SSC was near fully blocked by cGMP/PKG so long as Trpc6 was expressed yet more modest suppression was observed when TRPC6 was inhibited or genetically deleted suggests phosphorylated TRPC6 may interact and impair other proteins as well. This would be consistent with the observation that TRPC channels often form heterotetramers. The specific nature of such interactions remains to be elucidated.
There are several limitations of the current study. First, we were unable to directly measure TRPC6 phosphorylation by PKG in adult myocardium or isolated myocytes so as to relate this modification with the physiological behavior. As noted, this was due to very low expression levels of the channel (even with enhanced gene expression) and poor antibody signals for both total and particularly phospho-TRPC6 from these tissues. Indeed, published data stem almost exclusively from HEK293 cells overexpressing the protein 17. Nonetheless, such data confirm a strong influence of PKG-phosphorylation on the channel, suppressing GqPCRs or DAG-stimulated conductance and hypertrophy signaling 14, 16, 17. The failure of PKG to modulate the SSC response in control or DMD myocytes without Trpc6 strongly supports this interaction. The chronic SIL study also supports targeting, as cells from PKG treated DMD hearts displayed no further impact from TRPC3/6 blockade. Another limitation resides with the new small molecule TRPC3/6 inhibitor that could not be tested in vivo, even for acute studies, due to high plasma binding and extremely rapid metabolism 26. This compound has no known influence on TRPC6 phosphorylation, and suppressed GqPCR-signaling in cells expressing a TRPC6 phosphomimetic (Trpc6T70E,S322E), indicating independence from this modification.
In conclusion, we identify TRPC6 as a cardiac systolic mechano-sensor, primary target of PKG modulation of SSC, and a major contributor to mechano-stimulation pathology in the DMD heart. Combined, Duchenne and Becker muscle diseases involving the absence or deficiency of dystrophin, account for 80% of all muscular dystrophies. While the major focus of prior research has been on often catastrophic skeletal muscle disease, the heart is potently involved as well and heart failure is now a major cause of mortality 52. The present results identify a novel and important role of TRPC6 and its modulation by PKG that may provide a new therapeutic avenue for these diseases.
Supplementary Material
Novelty and Significance.
What Is Known?
Stretch of contracting cardiac muscle increases calcium entry and augments force.
Calcium entry from muscle stretch increases in dystrophin-deficient forms of muscular dystrophy (DMD), leading to cell damage.
Mechano-sensitive transient receptor potential canonical (TRPC) channels are expressed in heart, and their hormone receptor-activated conductance is blocked by protein kinase G (PKG).
What New Information Does This Article Contribute?
PKG potently blocks stress-stimulated contractility in a TRPC6-dependent manner.
Increased mechano-stimulated force and arrhythmia in DMD cells is prevented by genetic or pharmacological suppression of TRPC6, or by PKG activation.
Chronic PKG activation in DMD hearts by PDE5-inhibition reverses hyper-active mechano-stimulation in DMD myocytes.
Understanding stress-stimulated contractility in the heart is important as it plays a role in normal adaptations to afterload, and is also potentially relevant to forms of muscular dystrophy in which dystrophin is effectively absent. In the latter case, membrane instability is thought to augment stress-induced myocyte calcium entry, contributing to disease progression. Here we show that increases in intracellular calcium and force that follow a rise in mechanical stress imposed on contracting heart muscle is blocked by PKG activation. This modulation requires the presence of TRPC6. In a mouse model of DMD, we find these responses are hyperactivated and often lead to arrhythmia. Either TRPC6 inhibition, using gene deletion or selective pharmacological inhibition, or acute PKG activation blocks this pathological behavior. We also found that chronic treatment with sildenafil, a cGMP-selective phosphodiesterase type 5 inhibitor, improved measures of cardiac structural disease and molecular stress signaling, and reversed abnormal mechano-stimulation responses. These findings reinforce the potential utility of PKG activation strategies for DMD treatment and the development of novel TRPC6 antagonists.
Acknowledgments
We thank GlaxoSmithKline Heart Failure Discovery Performance Unit (King of Prussia, PA) for providing GSK2833503A, and Wei Dong Gao, Jonathan A. Kirk, and Brian O’Rourke (Johns Hopkins University) for their technical assistance.
SOURCE OF FUNDING
This research was supported by a grant from Muscular Dystrophy Association (186454), Fondation Leducq TransAtlantic Network of Excellence, and National Institutes of Health Grant - HL 59480 (DAK), American Heart Association Fellowship Grant (KS), Max Kade Fellowship, Austrian Academy of Sciences (PPR), Intramural Research Program of the NIH (Project Z01-ES-101864, (LB) and the Abraham and Virginia Weiss Professorship and Peter Belfer Endowment (DAK).
Nonstandard Abbreviations and Acronyms
- cAMP
cyclic adenosine monophosphate
- cGMP
cyclic guanosine monophosphate
- Ctrl
control
- dKO
double knockout
- DMD
Duchenne muscular dystrophy
- ERK
extracellular signal regulated kinase
- FSM
Frank-Starling Mechanism
- KO
knockout
- GqPCR
Gq protein-coupled receptor
- NCX
sodium-calcium exchanger
- NFAT
nuclear factor of activated T-cells
- NHE
sodium-hydrogen exchanger
- PDE
phosphodiesterase
- PKG
protein kinase G
- Rcan
regulator or calcineurin
- SAC
stretch-activated channel
- SIL
sildenafil
- SSC
stress-stimulated contractility
- TRPC
transient receptor potential canonical
- veh
vehicle
- WT
wild-type
Footnotes
DISCLOSURES
None
References
- 1.von Anrep G. On the part played by the suprarenals in the normal vascular reactions of the body. J Physiol. 1912;45:307–317. doi: 10.1113/jphysiol.1912.sp001553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cingolani HE, Perez NG, Cingolani OH, Ennis IL. The anrep effect: 100 years later. Am J Physiol Heart Circ Physiol. 2013;304:H175–182. doi: 10.1152/ajpheart.00508.2012. [DOI] [PubMed] [Google Scholar]
- 3.Zou Y, Akazawa H, Qin Y, et al. Mechanical stress activates angiotensin ii type 1 receptor without the involvement of angiotensin ii. Nat Cell Biol. 2004;6:499–506. doi: 10.1038/ncb1137. [DOI] [PubMed] [Google Scholar]
- 4.Friedrich O, Wagner S, Battle AR, Schurmann S, Martinac B. Mechano-regulation of the beating heart at the cellular level--mechanosensitive channels in normal and diseased heart. Prog Biophys Mol Biol. 2012;110:226–238. doi: 10.1016/j.pbiomolbio.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 5.Maroto R, Raso A, Wood TG, Kurosky A, Martinac B, Hamill OP. Trpc1 forms the stretch-activated cation channel in vertebrate cells. Nat Cell Biol. 2005;7:179–185. doi: 10.1038/ncb1218. [DOI] [PubMed] [Google Scholar]
- 6.Dyachenko V, Husse B, Rueckschloss U, Isenberg G. Mechanical deformation of ventricular myocytes modulates both trpc6 and kir2.3 channels. Cell Calcium. 2009;45:38–54. doi: 10.1016/j.ceca.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Spassova MA, Hewavitharana T, Xu W, Soboloff J, Gill DL. A common mechanism underlies stretch activation and receptor activation of trpc6 channels. Proc Natl Acad Sci U S A. 2006;103:16586–16591. doi: 10.1073/pnas.0606894103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, Dubin AE, Patapoutian A. Piezo1 and piezo2 are essential components of distinct mechanically activated cation channels. Science. 2010;330:55–60. doi: 10.1126/science.1193270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuwahara K, Wang Y, McAnally J, Richardson JA, Bassel-Duby R, Hill JA, Olson EN. Trpc6 fulfills a calcineurin signaling circuit during pathologic cardiac remodeling. J Clin Invest. 2006;116:3114–3126. doi: 10.1172/JCI27702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakayama H, Wilkin BJ, Bodi I, Molkentin JD. Calcineurin-dependent cardiomyopathy is activated by trpc in the adult mouse heart. FASEB J. 2006;20:1660–1670. doi: 10.1096/fj.05-5560com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Onohara N, Nishida M, Inoue R, Kobayashi H, Sumimoto H, Sato Y, Mori Y, Nagao T, Kurose H. Trpc3 and trpc6 are essential for angiotensin ii-induced cardiac hypertrophy. EMBO J. 2006;25:5305–5316. doi: 10.1038/sj.emboj.7601417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gottlieb P, Folgering J, Maroto R, Raso A, Wood TG, Kurosky A, Bowman C, Bichet D, Patel A, Sachs F, Martinac B, Hamill OP, Honore E. Revisiting trpc1 and trpc6 mechanosensitivity. Pflugers Arch. 2008;455:1097–1103. doi: 10.1007/s00424-007-0359-3. [DOI] [PubMed] [Google Scholar]
- 13.Kwan HY, Huang Y, Yao X. Regulation of canonical transient receptor potential isoform 3 (trpc3) channel by protein kinase g. Proc Natl Acad Sci U S A. 2004;101:2625–2630. doi: 10.1073/pnas.0304471101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koitabashi N, Aiba T, Hesketh GG, Rowell J, Zhang M, Takimoto E, Tomaselli GF, Kass DA. Cyclic gmp/pkg-dependent inhibition of trpc6 channel activity and expression negatively regulates cardiomyocyte nfat activation novel mechanism of cardiac stress modulation by pde5 inhibition. J Mol Cell Cardiol. 2010;48:713–724. doi: 10.1016/j.yjmcc.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takahashi S, Lin H, Geshi N, Mori Y, Kawarabayashi Y, Takami N, Mori MX, Honda A, Inoue R. Nitric oxide-cgmp-protein kinase g pathway negatively regulates vascular transient receptor potential channel trpc6. J Physiol. 2008;586:4209–4223. doi: 10.1113/jphysiol.2008.156083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kinoshita H, Kuwahara K, Nishida M, et al. Inhibition of trpc6 channel activity contributes to the antihypertrophic effects of natriuretic peptides-guanylyl cyclase-a signaling in the heart. Circ Res. 2010;106:1849–1860. doi: 10.1161/CIRCRESAHA.109.208314. [DOI] [PubMed] [Google Scholar]
- 17.Nishida M, Watanabe K, Sato Y, Nakaya M, Kitajima N, Ide T, Inoue R, Kurose H. Phosphorylation of trpc6 channels at thr69 is required for anti-hypertrophic effects of phosphodiesterase 5 inhibition. J Biol Chem. 2010;285:13244–13253. doi: 10.1074/jbc.M109.074104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kobayashi YM, Rader EP, Crawford RW, Iyengar NK, Thedens DR, Faulkner JA, Parikh SV, Weiss RM, Chamberlain JS, Moore SA, Campbell KP. Sarcolemma-localized nnos is required to maintain activity after mild exercise. Nature. 2008;456:511–515. doi: 10.1038/nature07414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khairallah M, Khairallah RJ, Young ME, Allen BG, Gillis MA, Danialou G, Deschepper CF, Petrof BJ, Des RC. Sildenafil and cardiomyocyte-specific cgmp signaling prevent cardiomyopathic changes associated with dystrophin deficiency. Proc Natl Acad Sci U S A. 2008;105:7028–7033. doi: 10.1073/pnas.0710595105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adamo CM, Dai DF, Percival JM, Minami E, Willis MS, Patrucco E, Froehner SC, Beavo JA. Sildenafil reverses cardiac dysfunction in the mdx mouse model of duchenne muscular dystrophy. Proc Natl Acad Sci U S A. 2010;107:19079–19083. doi: 10.1073/pnas.1013077107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Percival JM, Whitehead NP, Adams ME, Adamo CM, Beavo JA, Froehner SC. Sildenafil reduces respiratory muscle weakness and fibrosis in the mdx mouse model of duchenne muscular dystrophy. J Pathol. 2012;228:77–87. doi: 10.1002/path.4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin EA, Barresi R, Byrne BJ, Tsimerinov EI, Scott BL, Walker AE, Gurudevan SV, Anene F, Elashoff RM, Thomas GD, Victor RG. Tadalafil alleviates muscle ischemia in patients with becker muscular dystrophy. Sci Transl Med. 2012;4:162ra155. doi: 10.1126/scitranslmed.3004327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hartmann J, Dragicevic E, Adelsberger H, Henning HA, Sumser M, Abramowitz J, Blum R, Dietrich A, Freichel M, Flockerzi V, Birnbaumer L, Konnerth A. Trpc3 channels are required for synaptic transmission and motor coordination. Neuron. 2008;59:392–398. doi: 10.1016/j.neuron.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dietrich A, Mederos YSM, Gollasch M, et al. Increased vascular smooth muscle contractility in trpc6−/− mice. Mol Cell Biol. 2005;25:6980–6989. doi: 10.1128/MCB.25.16.6980-6989.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Washburn DG, Holt DA, Dodson J, et al. The discovery of potent blockers of the canonical transient receptor channels, trpc3 and trpc6, based on an anilino-thiazole pharmacophore. Bioorg Med Chem Lett. 2013;23:4979–4984. doi: 10.1016/j.bmcl.2013.06.047. [DOI] [PubMed] [Google Scholar]
- 26.Seo K, Rainer PP, Shalkey-Hahn V, Lee DI, Jo SH, Andersen A, Liu T, Xu X, Willette RN, Lepore JJ, Marino JP, Birnbaumer L, Schnackenberg CG, Kass DA. Combined trpc3 and trpc6 blockade by selective small molecule or genetic deletion inhibits pathological cardiac hypertrophy. Proc Natl Acad Sci U S A. 2014 doi: 10.1073/pnas.1308963111. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagayama T, Hsu S, Zhang M, Koitabashi N, Bedja D, Gabrielson KL, Takimoto E, Kass DA. Sildenafil stops progressive chamber, cellular, and molecular remodeling and improves calcium handling and function in hearts with pre-existing advanced hypertrophy caused by pressure overload. J Am Coll Cardiol. 2009;53:207–215. doi: 10.1016/j.jacc.2008.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cingolani OH, Kirk JA, Seo K, Koitabashi N, Lee DI, Ramirez-Correa G, Bedja D, Barth AS, Moens AL, Kass DA. Thrombospondin-4 is required for stretch-mediated contractility augmentation in cardiac muscle. Circ Res. 2011;109:1410–1414. doi: 10.1161/CIRCRESAHA.111.256743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nishimura S, Yasuda S, Katoh M, Yamada KP, Yamashita H, Saeki Y, Sunagawa K, Nagai R, Hisada T, Sugiura S. Single cell mechanics of rat cardiomyocytes under isometric, unloaded, and physiologically loaded conditions. Am J Physiol Heart Circ Physiol. 2004;287:H196–202. doi: 10.1152/ajpheart.00948.2003. [DOI] [PubMed] [Google Scholar]
- 30.Prosser BL, Ward CW, Lederer WJ. X-ros signaling: Rapid mechano-chemo transduction in heart. Science. 2011;333:1440–1445. doi: 10.1126/science.1202768. [DOI] [PubMed] [Google Scholar]
- 31.Georgakopoulos D, Mitzner WA, Chen CH, Byrne BJ, Millar HD, Hare JM, Kass DA. In vivo murine left ventricular pressure-volume relations by miniaturized conductance micromanometry. Am J Physiol. 1998;274:H1416–1422. doi: 10.1152/ajpheart.1998.274.4.H1416. [DOI] [PubMed] [Google Scholar]
- 32.Deconinck AE, Rafael JA, Skinner JA, Brown SC, Potter AC, Metzinger L, Watt DJ, Dickson JG, Tinsley JM, Davies KE. Utrophin-dystrophin-deficient mice as a model for duchenne muscular dystrophy. Cell. 1997;90:717–727. doi: 10.1016/s0092-8674(00)80532-2. [DOI] [PubMed] [Google Scholar]
- 33.Caldiz CI, Garciarena CD, Dulce RA, Novaretto LP, Yeves AM, Ennis IL, Cingolani HE, Chiappe de Cingolani G, Perez NG. Mitochondrial reactive oxygen species activate the slow force response to stretch in feline myocardium. J Physiol. 2007;584:895–905. doi: 10.1113/jphysiol.2007.141689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ward ML, Williams IA, Chu Y, Cooper PJ, Ju YK, Allen DG. Stretch-activated channels in the heart: Contributions to length-dependence and to cardiomyopathy. Prog Biophys Mol Biol. 2008;97:232–249. doi: 10.1016/j.pbiomolbio.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 35.Fanchaouy M, Polakova E, Jung C, Ogrodnik J, Shirokova N, Niggli E. Pathways of abnormal stress-induced ca2+ influx into dystrophic mdx cardiomyocytes. Cell Calcium. 2009;46:114–121. doi: 10.1016/j.ceca.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chiluiza D, Krishna S, Schumacher VA, Schlondorff J. Gain-of-function mutations in transient receptor potential c6 (trpc6) activate extracellular signal-regulated kinases 1/2 (erk1/2) J Biol Chem. 2013;288:18407–18420. doi: 10.1074/jbc.M113.463059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yao H, Peng F, Fan Y, Zhu X, Hu G, Buch SJ. Trpc channel-mediated neuroprotection by pdgf involves pyk2/erk/creb pathway. Cell Death Differ. 2009;16:1681–1693. doi: 10.1038/cdd.2009.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poburko D, Fameli N, Kuo KH, van Breemen C. Ca2+ signaling in smooth muscle: Trpc6, ncx and lnats in nanodomains. Channels (Austin, Tex) 2008;2:10–12. doi: 10.4161/chan.2.1.6053. [DOI] [PubMed] [Google Scholar]
- 39.Poburko D, Liao CH, Lemos VS, Lin E, Maruyama Y, Cole WC, van Breemen C. Transient receptor potential channel 6-mediated, localized cytosolic [na+] transients drive na+/ca2+ exchanger-mediated ca2+ entry in purinergically stimulated aorta smooth muscle cells. Circ Res. 2007;101:1030–1038. doi: 10.1161/CIRCRESAHA.107.155531. [DOI] [PubMed] [Google Scholar]
- 40.Louhivuori LM, Jansson L, Nordstrom T, Bart G, Nasman J, Akerman KE. Selective interference with trpc3/6 channels disrupts ox1 receptor signalling via ncx and reveals a distinct calcium influx pathway. Cell Calcium. 2010;48:114–123. doi: 10.1016/j.ceca.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 41.Wu X, Eder P, Chang B, Molkentin JD. Trpc channels are necessary mediators of pathologic cardiac hypertrophy. Proc Natl Acad Sci U S A. 2010;107:7000–7005. doi: 10.1073/pnas.1001825107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yeung EW, Whitehead NP, Suchyna TM, Gottlieb PA, Sachs F, Allen DG. Effects of stretch-activated channel blockers on [ca2+]i and muscle damage in the mdx mouse. J Physiol. 2005;562:367–380. doi: 10.1113/jphysiol.2004.075275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whitehead NP, Streamer M, Lusambili LI, Sachs F, Allen DG. Streptomycin reduces stretch-induced membrane permeability in muscles from mdx mice. Neuromuscul Disord. 2006;16:845–854. doi: 10.1016/j.nmd.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 44.Vandebrouck C, Martin D, Colson-Van Schoor M, Debaix H, Gailly P. Involvement of trpc in the abnormal calcium influx observed in dystrophic (mdx) mouse skeletal muscle fibers. J Cell Biol. 2002;158:1089–1096. doi: 10.1083/jcb.200203091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matsumura CY, Taniguti AP, Pertille A, Santo Neto H, Marques MJ. Stretch-activated calcium channel protein trpc1 is correlated with the different degrees of the dystrophic phenotype in mdx mice. Am J Physiol Cell Physiol. 2011;301:C1344–1350. doi: 10.1152/ajpcell.00056.2011. [DOI] [PubMed] [Google Scholar]
- 46.Kruger J, Kunert-Keil C, Bisping F, Brinkmeier H. Transient receptor potential cation channels in normal and dystrophic mdx muscle. Neuromuscul disord. 2008;18:501–513. doi: 10.1016/j.nmd.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 47.Townsend D, Turner I, Yasuda S, Martindale J, Davis J, Shillingford M, Kornegay JN, Metzger JM. Chronic administration of membrane sealant prevents severe cardiac injury and ventricular dilatation in dystrophic dogs. J Clin Invest. 2010;120:1140–1150. doi: 10.1172/JCI41329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yasuda S, Townsend D, Michele DE, Favre EG, Day SM, Metzger JM. Dystrophic heart failure blocked by membrane sealant poloxamer. Nature. 2005;436:1025–1029. doi: 10.1038/nature03844. [DOI] [PubMed] [Google Scholar]
- 49.Goonasekera SA, Lam CK, Millay DP, Sargent MA, Hajjar RJ, Kranias EG, Molkentin JD. Mitigation of muscular dystrophy in mice by serca overexpression in skeletal muscle. J Clin Invest. 2011;121:1044–1052. doi: 10.1172/JCI43844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bellinger AM, Reiken S, Carlson C, Mongillo M, Liu X, Rothman L, Matecki S, Lacampagne A, Marks AR. Hypernitrosylated ryanodine receptor calcium release channels are leaky in dystrophic muscle. Nat Med. 2009;15:325–330. doi: 10.1038/nm.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martin EA, Barresi R, Byrne BJ, Tsimerinov EI, Scott BL, Walker AE, Grurudevan SV, Anene F, Elashoff RM, Thomas GD, Victor RG. Tadalafil alleviates muscle ischemia in patients with becker muscular dystrophy. Sci Transl Med. 2012;4:162ra155. doi: 10.1126/scitranslmed.3004327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Romfh A, McNally EM. Cardiac assessment in duchenne and becker muscular dystrophies. Curr Heart Fail Rep. 2010;7:212–218. doi: 10.1007/s11897-010-0028-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.