Abstract
Rationale
A rigorously investigated model of stress and antidepressant administration during pregnancy is needed to evaluate possible effects on the mother.
Objective
The objective of this study was to develop a model of clinically relevant prenatal exposure to an antidepressant and stress during pregnancy to evaluate the effects on maternal care behavior.
Results
Female rats implanted with 28 day osmotic minipumps delivering the SSRI escitalopram throughout pregnancy had serum escitalopram concentrations in a clinically observed range (17-65 ng/mL). A separate cohort of pregnant females exposed to a chronic unpredictable mild stress paradigm on gestational days 10-20 showed elevated baseline (305 ng/mL), and acute stress-induced (463 ng/mL), plasma corticosterone concentrations compared to unstressed controls (109 ng/mL). A final cohort of pregnant dams were exposed to saline (control), escitalopram, stress, or stress and escitalopram to determine the effects on maternal care. Maternal behavior was continuously monitored over the first 10 days post parturition. A reduction of 35% in maternal contact and 11% in nursing behavior was observed due to stress during the light cycle. Licking and grooming behavior was unaffected by stress or drug exposure in either the light or dark cycle.
Conclusions
These data indicate that: 1) clinically relevant antidepressant treatment during human pregnancy can be modeled in rats using escitalopram; 2) chronic mild stress can be delivered in a manner that does not compromise fetal viability; and 3) neither of these prenatal treatments substantially altered maternal care post parturition.
Keywords: animal models, developmental pharmacology, hypothalamic-pituitary-adrenal axis, maternal care, pharmacokinetics, pregnancy, selective serotonin reuptake inhibitor, stress
Introduction
Pregnancy expands a woman's health considerations beyond herself to include her unborn child. Approximately 10-20% of pregnant women experience depression (Gavin et al. 2005) and pharmacological intervention may be indicated in a substantial proportion of these women. Women who discontinue antidepressant treatment during pregnancy are twice as likely to relapse (Cohen et al. 2006). Therefore, clinicians are faced with a difficult dilemma: continue treating women to avoid relapse or discontinue treatment and risk a depressive episode. Data exist that shows that untreated depression during pregnancy leads to untoward effects on the infant (vide infra). This study aims to develop a clinically-relevant rat model of antidepressant exposure and stress during pregnancy to determine the effects on the offspring and maternal care.
Elucidating the effects of antidepressants or other psychotropic medications on the fetus must rely on a dosing and exposure strategy that approximates clinically relevant human exposure. This study will focus on the selective-serotonin reuptake inhibitor (SSRI) escitalopram that has been purported to have superior efficacy and fewer discontinuations compared to other second-generation antidepressants (Cipriani et al. 2009) and is commonly prescribed to pregnant women at the Emory Women's Mental Health Program (Emory WMHP, http://www.emorywomensprogram.org/). Typical treatment of pregnant women with major depressive disorder results in steady state trough serum concentrations ranging from 17-65 ng/mL of escitalopram, and hereby designated the “Clinically Observed Range” (N = 60, ZN Stowe, unpublished observations). Laboratory animals typically metabolize xenobiotics at a much faster rate than humans which necessitates consideration of pharmacokinetic differences between species when developing a dosing paradigm. In rats for example, escitalopram's half-life can be shortened to 15-20% of that in humans due to rapid metabolism and elimination (Bundgaard et al. 2007; Rao 2007). Consequently, classic single or multiple daily dosing regimens of most antidepressants are unlikely to achieve steady state in a clinically relevant range and, therefore, may not provide the requisite data germane to expanding our understanding of human exposure. We hypothesize that continuous drug delivery with an osmotic minipump will more closely model clinically observed escitalopram concentrations.
Modeling all aspects of human depression in animals is not possible; however, chronic variable stress has been used as a proxy. Characterizing stress during pregnancy is problematic due to altered endocrine baselines and stress responsivity associated with pregnancy (Williams et al. 1999). The detrimental effects of stress to human infants has been investigated (Cottrell and Seckl 2009; Davis and Sandman 2010). In humans and animals, depression or stress (animals) during pregnancy can also have effects in the postpartum period by disrupting maternal care of the infant (Lovejoy et al. 2000; Newport et al. 2002; Baker et al. 2008). We hypothesize that exposure to a chronic stress model during pregnancy can be verified by induction of an HPA axis response characterized by elevated basal corticosterone and/or an exaggerated response to a stressor.
The importance of the maternal-fetal serotonin system in development has been recently elucidated (Bonnin et al. 2011) but the concurrent examination of prenatal stress and antidepressants exposures has yet to be explored. In animal models, there are studies that have examined prenatal stress (Newport et al. 2002; Mueller and Bale 2006; Mueller and Bale 2008) and some studies that have examined antidepressant-exposed pregnant rats from the perspective of the offspring (Henderson and McMillen 1993; Cabrera and Battaglia 1994; Cabrera-Vera et al. 1997; Cabrera-Vera and Battaglia 1998; Forcelli and Heinrichs 2008). To our knowledge, prenatal stress and antidepressant exposure have not been examined concurrently to investigate the effects on maternal care. This study provides a clinically-relevant model of antidepressant exposure and/or prenatal stress as well as investigates the impact of these exposures on maternal care.
Materials and Methods
Animals
Sprague-Dawley male retired breeders and nulliparous females weighing 200-225 grams were purchased from Charles River Laboratories (Charles River, Wilmington, MA). Rats were kept on a 12:12 light: dark cycle (lights on at 7:00 AM) in a humidity (60%) and temperature (20°C-23°C) controlled facility. Rodent diet 5001 chow (Purina Mills, Richmond, IN) and water were available ad libitum throughout the study. After two weeks at the Emory University animal facility, female rats were paired with male retired breeders in a breeding cage. Gestational day 0 (G0) was designated by the presence of a sperm plug and pregnant females were single-housed after breeding. Pregnancy was confirmed post-decapitation with a cesarean section for the catheter studies.
All animal protocols were approved by the Emory University Institutional Animal Care and Use Committee (IACUC protocol number 079-2010) and carried out in accordance with the Guide for the Care and Use of Laboratory Animals (Institute for Laboratory Animal Resources 1996) as adopted and promulgated by the U.S. National Institutes of Health. All steps were taken to minimize animal suffering at each stage of the study.
Escitalopram Administration and Quantification (Cohort One)
For the pharmacokinetic (elimination half-life) experiment, nulliparous females (N = 3-5 rats/group) were injected subcutaneously or intraperitoneally with 12.2 mg/kg escitalopram oxalate (29.44 μmol/kg) dissolved in 0.9% saline (1 mL/kg). For chronic dosing studies, female rats (N = 7-10 rats) were implanted subcutaneously with Alzet 28-day osmotic minipumps (Alzet, Cupertino, CA) slightly posterior to the scapulae. Osmotic pumps delivered an average dose of 12.2 mg/kg/day escitalopram oxalate in 0.9% saline based upon the predicted final weight on embryonic day 21 of 440 grams (17.3 mg/kg/day escitalopram oxalate after pump was initially implanted). Three days after pump implantation, female rats were bred. Six days after breeding, jugular catheters were implanted. Blood collected from catheters was spun down at 1,800 × g and the plasma fraction was collected. Sample extraction was accomplished using a standard protein crash (0.1 mL of sample + 10uL of mobile phase A + 0.2 mL of the internal standard of deuterated citalopram in methanol). The assay was performed on a Waters Inc, Acquity ultra-performance liquid chromatography system with a triple quadrapole detector in the multiple reaction monitoring mode employing electrospray positive ionization (Waters, Milford, MA). The mobile phases were (A) 2mM ammonium acetate and 0.1% formic acid in water and (B) 2 mM ammonium acetate and 0.1% formic acid in methanol. The flow rate was 0.6 mL/min and the chromatography was developed using a gradient from 25% B to 75% B over 3.5 minutes on an Acquity ultra-performance liquid chromatography, C-18 column (1.7 μM, 2.1 × 50 mm). 5 μL of extract was injected. A seven point standard curve with two levels of quality control were processed in each run. The method is linear from 0.2 to 2000 ng/mL. The limit of detection is 0.05 ng/mL and the limit of quantification is 0.2 ng/mL (10%). The method exhibits no matrix effects by the method of Matuszewski and colleagues (Matuszewski et al. 2003). Absolute recoveries range from 88.9% to 119.6% and inter-assay imprecisions range from 3 to 13% at levels of 75 and 300 ng/mL. The method compares favorably with the high performance liquid chromatography with ultraviolet detection methods used previously in our laboratory. Extraction and quantification were carried out at the Emory Clinical Translational Research Laboratory. The method to calculate half life is through the following equation:
Jugular Catheter Implantation
Jugular catheters were implanted according to Thrivikraman et al. (2002). Briefly, animals were anesthetized with a preparation of ketamine:xylazine:acepromazine and assessed for reaction to a painful stimuli prior to surgery. The jugular vein was implanted with a catheter to allow for repeated blood sampling and to prevent a stress response elicited by other sampling methods (i.e. tail nick). Animals were given three days to recover before initiation of stress or dosing models. Blood samples were collected (200 μL) and an equal volume of sterile 0.9% saline was injected to replace the blood volume lost. Catheters were flushed with sterile gentamicin (120 μg/mL, 150 μL) after sampling to prevent infection.
Chronic Unpredictable Mild Stress Model (Cohort Two)
A separate cohort of animals (N = 5-11 rats/group) were implanted with jugular catheters and exposed to the chronic unpredictable mild stress model. On gestational day 10 (G10), the chronic unpredictable mild stress (CMS) model of depression began and consisted of restraint three times a day for 45 minutes in a clear acrylic cylinder designed to minimize movement (2.25 in diameter × 6 in long, Harvard Apparatus, Holliston, MA), a 23.5° cage tilt, damp bedding (450 mL of water), noise (adjusted to 95 dB of intermittent noise), cage changes, or overnight illumination (Table 1). G10 was selected to begin the stress paradigm because the fetal central nervous system begins substantial development at this point (Clancy et al. 2001) and to minimize premature termination of the pregnancy as a result of excessive stress. Baseline corticosterone concentrations were assessed by jugular catheter blood sampling which took place at 9:00 AM, the nadir of the circadian cycle of corticosterone. After baseline sampling, animals in the CMS group were exposed to the stressor for that day (45 minutes of restraint on G10, G14, G17, or damp bedding on G12 and G19). At 9:45 AM, a second jugular catheter blood sampling was taken to determine stress-induced increases in plasma corticosterone. The CMS ended following the G20 stress to prevent premature parturition. During the noise and overnight illumination stressors, animals were housed overnight in a separate temperature and humidity controlled room from the unstressed animals. Otherwise, all animals were kept in the same housing room and subjected to identical handling and cage change procedures.
Table 1.
Summary of the chronic unpredictable mild stress model.
| Gestational Day | Stress |
|---|---|
| G0 | Breeding |
| G7 | Implant catheters |
| G10 | Restraint (3×45 minute sessions) |
| G11 | Cage Tilt (24 hours) |
| G12 | Damp bedding (24 hours) |
| G13 | New cage; Noise (24 hours) |
| G14 | Restraint (3×45 minute sessions) |
| G15 | Overnight illumination |
| G16 | New cage |
| G17 | Restraint (3×45 minute sessions) |
| G18 | Overnight illumination |
| G19 | Damp bedding (24 hours) |
| G20 | New cage; Noise (24 hours) |
| G21 | Amniotic fluid collection |
Maternal Care Behavior (Cohort Three)
In a separate experiment from the jugular catheter blood sampling experiments, groups were exposed to stress and/or escitalopram in pregnancy (N = 7-11 mothers/group). The control group experienced no stress with saline minipump (Saline). Experimental groups consisted of no stress with an escitalopram minipump (Escitalopram), prenatal stress with a saline minipump(Stress-Saline), and prenatal stress with an escitalopram minipump (Stress-Escitalopram). Maternal care behavior was monitored by video cameras continuously for 10 days postpartum, and the videotape record was later scored by observers to assess maternal behavior. The videotape record was scored by three observers (their scores were later averaged) who were blinded to treatment conditions and who determined the presence or absence of three behaviors by the dam: licking/grooming, no maternal contact, and nursing. Licking/grooming was defined as the mother's mouth in contact with at least one pup for at least one second. No maternal contact was defined as the mother not engaged in contact with any pup for at least one second. Nursing was defined as at least one pup suckling from the mother for at least one second. Prior to the present study, other studies had been undertaken to determine the how frequently the video record needed to be scored to accurately represent maternal behavior taking place in a 24 hour period. These studies examined the entire 24 hour period followed by successive blocks of shortened sampling time. It was determined that a sampling frequency equal to one hour of monitoring during the light phase and one hour during the dark phase accurately characterizes maternal care behavior throughout the entire 24 hour period. Light and dark phase behavior was analyzed separately and then combined into a single measure of maternal care behavior which was expressed as the percentage of time the dam spent performing the indicated behavior. No culling occurred during the maternal care behavioral assessment.
Corticosterone Assays
Plasma corticosterone was assayed using the ImmuChem 125I Corticosterone RIA kit with a sensitivity of 1 ng/mL (MP Biomedicals, Orangeburg, NY).
Statistical Analyses
In all figures, values are expressed as mean ± SEM. GraphPad Prism 4.0 (GraphPad Software, La Jolla, CA) was used to conduct statistical analyses. Experimental differences were analyzed with a 2-way ANOVA followed by a Bonferroni Post Hoc Test. Maternal care behavior was analyzed with a 3-way ANOVA (Time × Drug × Stress) in SPSS 17.0 (IBM, Armonk, NY). Differences were considered significant if p < 0.05 except where noted.
Results
Comparison of Escitalopram Delivery Methods (Cohort One)
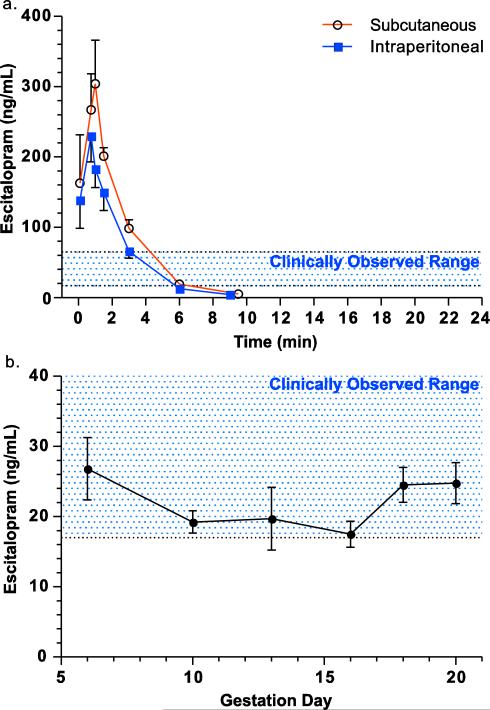
Initial studies were conducted to determine the appropriate clinically relevant dose during pregnancy. To examine daily, acute drug exposure models, 12.2 mg/kg escitalopram oxalate was injected subcutaneously or intraperitoneally. Intraperitoneal injection resulted in a lower area under the curve compared to subcutaneous injection (785 ng/mL/24 hours for subcutaneous, 565 ng/mL/24 hours for intraperitoneal) (Fig. 1a). The terminal half-life was calculated as 1.27 hours for both routes of administration. The time in the clinically observed range was calculated as 2.46 hours after a single injection and the drug was not detected in serum after 9 hours (Fig 1a). Osmotic minipumps were implanted in pregnant females and designed to deliver escitalopram oxalate at a mean dose of 12.2 mg/kg/day based on the animal's actual weight of 440 grams on embryonic day 21 (mean dose range: 10.76-13.13 ng/mL). A dose of 12.2 mg/kg/day was used for all studies since this dose produced serum concentrations in pregnant rats within the clinically observed range of 17-65 ng/mL observed in human pregnant women (17.3 mg/kg/day escitalopram oxalate after pump was initially implanted). A separate cohort of catheterized, pregnant dams implanted with osmotic minipumps showed a consistent concentration of escitalopram in the serum over the entire course of pregnancy (Fig. 1b). The area under the curve was calculated for chronic dosing to yield an average serum concentration of 21 ng/mL for the osmotic pump administration (AUC: 298 ng/mL/14 days or 510 ng/mL/24 hours for osmotic pump administration).
Fig. 1.
Serum drug concentrations following escitalopram administration. To determine the clearance of escitalopram in acute dosing models, an acute subcutaneous or intraperitoneal dose of 12.2 mg/kg of escitalopram oxalate was injected into catheterized females and serum was collected over 9 hours (a, N = 3-5 rats/group). Pregnant catheterized females were implanted with osmotic minipumps delivering 12.2 mg/kg/day of escitalopram oxalate (17.3 mg/kg/day escitalopram oxalate after pump was initially implanted). Blood was collected on gestational days 6, 10, 13, 16, 18, and 20 via a jugular vein catheter and analyzed for serum escitalopram (b, N = 7-10 rats). Shaded areas reflect the clinically range observed in patients at the Emory Women's Mental Health Program (17-65 ng/mL; N = 60).
Comparison of Chronic Unpredictable Mild Stress (Cohort Two) and Chronic Restraint Stress Models
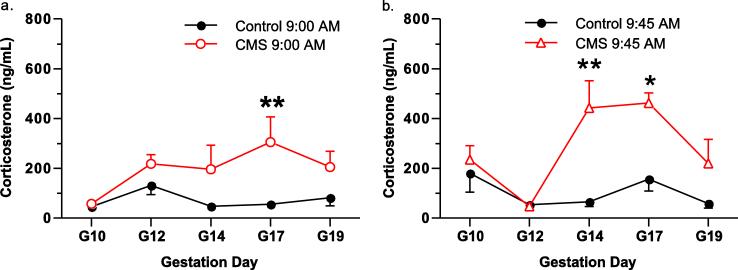
Catheters were implanted in the jugular vein to take plasma samples over the course of pregnancy and measure baseline (9:00 AM) and stress-induced (9:45 AM) changes in plasma corticosterone. Plasma corticosterone samples were analyzed with a 2-way ANOVA (chronic stress × gestational day) Baseline (9:00 AM) measurements of plasma corticosterone concentrations in controls compared to CMS animals showed that the stressed dams displayed an increase in baseline plasma corticosterone due to the stress model (F(1,70) = 17.0; p < 0.001) (Fig. 2a). Baseline increases in plasma corticosterone had the largest difference on gestational day 17: plasma corticosterone concentrations in control animals were 55.6 ng/mL compared to 305.2 ng/mL in stressed animals (t11 = 3.5; p > 0.05) (Fig. 2a).
Fig. 2.
Corticosterone measurements during the chronic unpredictable mild stress model. Pregnant females implanted with jugular catheters were sampled over the course of pregnancy at 9:00 and 9:45. Baseline corticosterone in plasma was measured in control and stress (CMS) animals (a). The plasma measurement of corticosterone at 9:45 was compared between control and CMS animals to illustrate the corticosterone response compared to controls at the same time of plasma measurement (b). Data are mean ± SEM, N = 5-11 rats/group. * p < 0.05, ** p < 0.01 with a Bonferroni post hoc Test.
A second blood draw was taken at 9:45 AM to determine acute stress-induced increases in plasma corticosterone. CMS rats showed an increase in corticosterone due to the daily stressor compared to unstressed animals at 9:45 AM (F(1,58) = 16.6; p < 0.001) (Fig. 2b). Stress-induced increases in plasma corticosterone concentrations were maximal on gestational day 14 (670% difference; t9 = 3.7; p < 0.01) and gestational day 17 (296% difference; t9 = 3.0; p < 0.05) (Fig. 2b). Anesthetized animals were analyzed on G21 but showed no differences between treatment groups for plasma corticosterone (Control = 256.6 ± 60.5, Stress = 310.9 ± 60.15; t15 = 0.6; p > 0.05) or amniotic fluid corticosterone (Control = 173.5 ± 32.4, Stress = 155.5 ± 32.0; t16 = 0.4; p > 0.05).
Maternal Care Behavior
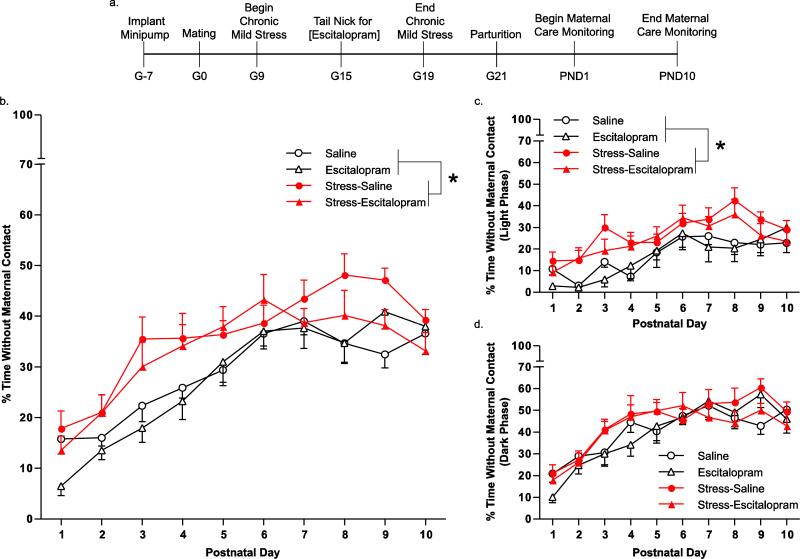
A combination of stress and escitalopram exposure during pregnancy (Fig. 3a) was used to determine alterations in maternal care behavior. The control group experienced no stress and had a saline minipump (Saline). Experimental groups consisted of no stress with an escitalopram minipump (Escitalopram), prenatal stress with a saline minipump (Stress-Saline), and prenatal stress with an escitalopram minipump (Stress-Escitalopram). Maternal care behavior was analyzed with a 3-way ANOVA with repeated measures on one factor to determine changes due to stress or escitalopram exposure over time. Time without maternal contact was increased by 35% due to stress (F(1,32) = 8.0; p < 0.01) but not due to escitalopram (F(1,32) = 0.9; p > 0.05). Over the postnatal period, time without maternal contact increased steadily (F(9,288) = 37.4; p < 0.001) (Fig. 3b). Stress increased the time without maternal contact only in the light phase, which is characterized by reduced activity in rats (F(1,32) = 8.70; p < 0.01). Stress did not alter time without maternal contact in the dark phase, which is the active phase in a 24 hour period (F(1,32) = 1.3; p > 0.05).
Fig. 3.
Maternal contact behavior following chronic unpredictable mild stress and escitalopram administration during pregnancy. The timeline of the experiment is summarized including the chronic unpredictable stress model, escitalopram administration, and endpoints (a). Maternal care behavior was videotaped continuously for 10 days and sampled for one hour during the light and the dark cycle of each day. Time spent without maternal contact was increased due to stress when measured over a 24 hour period for ten days (b). When analyzed separately, the time spent without maternal contact was reduced in the light cycle when the mother is less active (c) but not the dark cycle when the mother is more active (d). Groups were based on treatment to the pregnant dam: no stress with saline minipump (Saline), no stress with escitalopram minipump(Escitalopram), prenatal stress with saline minipump (Stress-Saline), prenatal stress with escitalopram minipump (Stress-Escitalopram). Data are mean ± SEM, N = 7-11 mothers/group. * p < 0.05, main effect due to stress in a 3-way ANOVA (Stress × Escitalopram × Time).
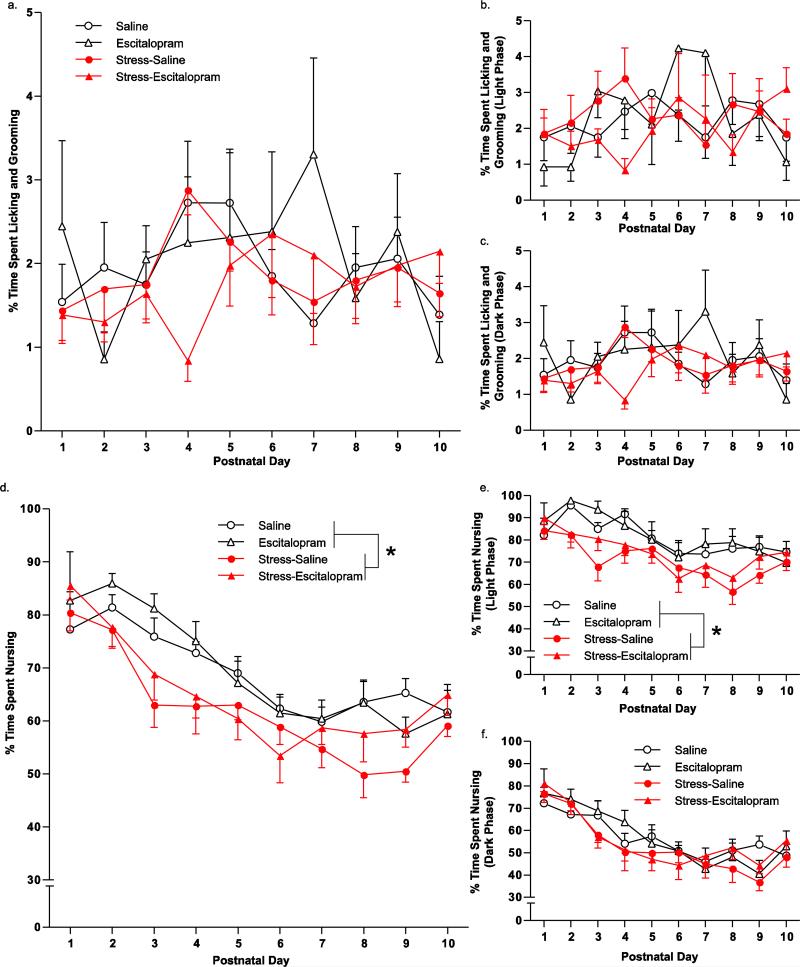
Licking and grooming was not affected due to time (F(9,288) = 1.5; p > 0.05), stress (F(1,32) = 0.3; p > 0.05) or escitalopram exposure (F(1,32) = 0.0; p > 0.05) (Fig. 4a). There was an interaction with time × drug (F(9,288) = 2.2; p < 0.05), but no other interactions reached statistical significance. Licking and grooming was also unaffected by time, stress, or escitalopram in both the light cycle (p > 0.05) (Fig. 4b) and in the dark cycle (p > 0.05) (Fig. 4c).
Fig. 4.
Maternal licking and grooming behavior and nursing behavior. Maternal care behavior was videotaped continuously for ten days and sampled for one hour each day during the light and dark phases. The percent of time spent licking and grooming was unaffected over a 24 hour period for ten days (a), during the light cycle (b), or during the dark cycle (c). The percent of time spent nursing was reduced due to stress over a 24 hour period for ten days (d). Reduction in nursing due to stress was only observed in the light cycle (e) but not the dark cycle (f). Groups were based on treatment to the pregnant dam: no stress with saline minipump (Saline), no stress with escitalopram minipump (Escitalopram), prenatal stress with saline minipump (Stress-Saline), prenatal stress with escitalopram minipump (Stress-Escitalopram). Data are mean ± SEM, N = 7-11 mothers/group. * p < 0.05, main effect due to stress in a 3-way ANOVA (Stress × Escitalopram × Time).
There was a significant effect of stress on maternal nursing behavior (F(1,32) = 5.9; p < 0.05) but no effect due to escitalopram (F(1,32) = 16.6; p < 0.001). This resulted in an overall reduction in nursing due to stress by 11%. There was no interaction between these exposures (F(1,32) = 0.6; p > 0.05). Over time, mothers spent less time nursing their pups (F(9,288) = 27.6; p < 0.001). There was a significant interaction between time × stress (F(9,288) = 1.9; p < 0.001) (Fig. 4d). The reduction in nursing due to stress was only observed in the light cycle (F(1,32) = 7.3; p < 0.05) (Fig. 4e) but not in the dark cycle (F(1,32) = 0.8; p > 0.05) (Fig. 4f). Similarly, the interaction of time and stress observed over a 24 hour period was entirely due to the effect in the light cycle (F(9,288) = 2.1; p < 0.05) (Fig. 4e) while there was no interaction in the dark cycle (F(9,288) = 1.6; p > 0.05) (Fig. 4f).
Maternal Endpoints After Chronic Unpredictable Mild Stress
Measurements from pregnant dams showed that on G15, there was escitalopram exposure that resulted in serum concentrations within the clinically observed range (F(1,32) = 309; p < 0.0001) (Table 2, top). Pregnant dams exposed to stress had a trend towards a decreased rate of weight gain (F(1,32) = 4.2; p = 0.050) although this did not reach statistical significance (Table 2, middle). Pregnancy duration, litter size, and litter rate of weight gain were unaffected by any exposure (p > 0.05), (Table 2, bottom).
Table 2.
Maternal and litter endpoints.
| Saline | Escitalopram | Stress-Saline | Stress-Escitalopram | |
|---|---|---|---|---|
| Maternal Drug Conc (ng/mL) | ±0.5 ± 0.07 | 40.9 ± 3.38 | ±0.5 ± 0.23 | 35.1 ± 2.70 |
| G5-19 Maternal Rate of Weight Gain (g/day) | 7.01 ± 0.33 | 7.32 ± 0.74 | 6.16 ± 0.47 # | 6.23 ± 0.39 # |
| Pregnancy Duration (days) | 21.00 ± 0.24 | 21.00 ± 0.00 | 20.89 ± 0.11 | 21.20 ± 0.20 |
| # of Pups Born | 12.8 ± 0.7 | 12.1 ± 1.0 | 13.3 ± 0.7 | 12.9 ± 0.5 |
| PND 1-21 Litter Rate of Weight Gain (g/day) | 20.1 ± 0.8 | 19.3 ± 1.0 | 20.0 ± 1.0 | 20.7 ± 0.8 |
Pregnant rats were measured for rate of weight gain, serum escitalopram at G15, and number of pups born (N = 7-11 pregnant rats/group). Maternal rate of weight gain was decreased in dams exposed to stress, although this did not quite reach statistical significant (# p = 0.05). Groups were based on treatment to the pregnant dam: no prenatal stress with saline minipump (Saline), no prenatal stress with escitalopram minipump (Escitalopram), prenatal stress with saline minipump (Stress-Saline), prenatal stress with escitalopram minipump (Stress-Escitalopram). The culled litter weight normalized to pup number was used to calculate the prepubescent rate of weight gain. Data are mean ± SEM, N = 7-11 litters/group.
Discussion
The purpose of the present study was to develop and characterize a clinically-relevant exposure to an antidepressant, escitalopram, and/or stress during pregnancy as a putative animal model of the treatment of maternal depression during pregnancy. Escitalopram oxalate has excellent aqueous solubility properties (>70 mg escitalopram oxalate/mL 0.9% saline; unpublished observations) and we found that continuous escitalopram administration via an osmotic minipump resulted in an accurate representation of human exposure. Chronic mild and variable stress during pregnancy was found to increase plasma corticosterone concentrations at baseline and in response to the daily stressor as well as slightly alter maternal care in the dam. Chronic stress and/or antidepressant exposure during pregnancy did not have any effect on indices of litter viability, indicating that neither maternal stress nor maternal escitalopram treatment overtly interfered with infant vitality
Chronic dosing of many psychotropic drugs in small animals that approximates human exposure is complicated due to issues with rapid metabolism. We have previously examined fluoxetine and norfluoxetine and found rapid clearance of parent compounds from the system, although the persistence of norfluoxetine can allow for clinically relevant exposure (Capello et al. 2011). We have estimated the half-life of the antidepressant paroxetine to be 8.0 hours in the rat (unpublished observations). Daily injections are problematic due to the generally short half-life of antidepressants in animals which will prevent the compound from achieving and maintaining clinically relevant drug concentrations. This half-life effect was demonstrated in the subcutaneous and intraperitoneal dosing in this study: females demonstrated rapid metabolism of escitalopram that provided less than two hours in the clinically observed range. To approximate human exposure with injections, animals would theoretically have to be dosed approximately once an hour with a lower dose. While this is impractical from a logistic standpoint, much of the extant literature examining chronic antidepressant exposure during pregnancy currently uses a daily injection model to investigate effects to the pregnant animal and fetus. Osmotic minipump dosing results in an invariable exposure once steady state is reached, in comparison to peak and trough plasma drug concentrations during typical dosing in a human. However, drug exposure via minipump in rats is a much closer approximation to the clinical scenario than single daily injections due to the bolus effect and hastened clearance in rodents.
Daily injections are also problematic due to the bolus effect. A single injection results in a rapid peak of serum escitalopram concentrations. This peak was approximately five times higher than the upper range of clinically observed serum concentrations from human pregnant mothers medicated with escitalopram. These high serum concentrations can logically result in transient toxic effects and produce a false positive effect in the pregnant rat or fetus for studies examining what are thought to be standard or moderate exposure. The majority of the animal literature, which is not limited to the antidepressant area, is based on a daily injection model that may plausibly induce this transient toxic effect.
Current animal dosing paradigms include daily injections, adding the drug in drinking water or food pellets, and implantation of an osmotic minipump for continuous drug delivery. Adding the drug to food and drinking water can be unreliable because the animals eat in a pulsatile manner and the medications may have an undesirable taste leading to a decreased fluid consumption. When the physical properties of the drug are amenable, osmotic minipumps can deliver a steady concentration of drug over a period of 4 weeks and do not subject the animal to repeated stress for an injection. Proper administration in pregnant animals which approximates human exposure is key to investigating any potential long-term effects of antidepressants. Our model utilizing minipumps demonstrated that escitalopram serum concentrations were stable during the entire course of pregnancy. The serum concentrations were consistently within the clinically observed range in the Emory WMHP and as reported elsewhere for trough concentrations associated with therapeutically effective doses in patients with major depressive disorder (6-21 ng/ml; Sogaard et al. 2005). SERT occupancy of ~80% has been consistently associated with therapeutic doses of SRIs in patients with major depressive disorder (Meyer et al. 2001; 2004) and 80% SERT occupancy measured in rodents is obtained at serum concentrations of 18 ng/ml (Kreilgaard et al. 2008; Capello et al. 2011). While humans have some peak to trough fluctuations during dosing intervals that vary according to each drug, they are much more closely reflected by continuous minipump exposure.
An animal model of stress exposure in the pregnant rat with a well-established endocrine endpoint has not been established, although it has been investigated. Some groups have characterized the rat's stress response during pregnancy with “high-intensity” stressors typical of experiencing a traumatic event (Barlow et al. 1975; Takahashi et al. 1998). Other groups have examined the HPA response during a rat's pregnancy with a “low-intensity” stressor (Ward and Weisz 1984; Léonhardt et al. 2007) showing that stress-induced corticosterone release can be measured during this period. The current study's model was capable of producing an increase in basal corticosterone and a further increase of corticosterone in response to a stressor. Although terminal plasma and amniotic fluid corticosterone was unchanged in chronically stressed dams compared to controls, it is likely that the anesthesia administered may have confounded these results (Fromm et al. 1983). While others have shown that classic rises in maternal cortisol are offset by increases in cortisol-binding globulin such that the mother and fetal brains are protected (Ballard et al. 1982; Coe et al. 1986), the effects of stress on maternal care or possible effects on other pathways in the fetus may cause altered neonatal development. Future studies will determine if prenatal stress-induced changes in the offspring are offset by developmental antidepressant exposure, as others have demonstrated (Ishiwata et al. 2005; Rayen et al. 2011).
Chronic stress during pregnancy in our study produced a minor disruption in maternal care by reducing nursing time and increasing the time spent away from the infant during the inactive phase of the light-dark cycle. This did not affect the rate of weight gain of the pups (Table 2). Analysis of the light cycle or dark cycle separately showed that the persistent effects in the 24 hour period were confined to the light cycle when the animal is least active. While our scoring system accurately extrapolates the behavior over the 24 hour period, this is an important distinction to note as stress did not affect maternal care during the most active phase of the day. Other groups have found that stress during a rat's pregnancy results in disrupted maternal care, leading to long-term changes in the offspring (Smith et al. 2004; Champagne et al. 2003; Champagne and Meaney 2006). However, these studies also selectively breed for maternal care behavior to determine the interaction of stress and trait-associated licking and grooming behavior. We found that stress itself did not disrupt licking and grooming behavior. Therefore, the limited effects of stress in the face of clear increases in endocrine measures of stress exposure in this study and the extant literature suggest that other factors, such as genetics, play important roles in maternal care behavior. Based on the work of Meaney and colleagues, a gene × environment interaction must be considered when examining maternal care behavior in the rodent (Meaney 2010), however laboratory animals that have have been commercially bred for decades for research purposes are, arguably, selected for being good at providing maternal care.
Maternal care was altered due to prenatal treatments but licking and grooming behavior was not affected. Previous studies have shown that licking and grooming behavior have profound changes on the offspring's development and produces altered gene expression in adulthood (Weaver et al. 2005; Weaver et al. 2006). These studies of Weaver and colleagues breed the licking and grooming trait selectively in the animals to alter maternal care. Another study utilizing minipump exposure to fluoxetine alone during pregnancy increased arched-back nursing of pups (Pawluski et al. 2013). In our study, treatment with escitalopram alone did not change nursing behavior compared to saline controls; therefore, we conclude that gestational stress was marginally disruptive to maternal care and that treatment with escitalopram did not offset this deficit nor have independent effects alone.
Our model of stress and antidepressant exposure provides a reproducible endpoint in the pregnant dam. The stress model resulted in alterations in the HPA axis, small but statistically significant decrease in weight gain, and produced modest alterations in maternal care. Minipump administration of escitalopram oxalate resulted in clinically relevant serum concentrations not possible from acute dosing. When these methods were combined, they resulted in no substantial alterations in pup viability at parturition, growth rates between postnatal days 1-21, or maternal care behavior. These experiments provide a model for studies seeking to elucidate detailed investigations of antidepressant and/or stress exposure during pregnancy, as a model of maternal depression and its treatment, on offspring.
Acknowledgements
We would like to thank Dr. James Ritchie and Bailey Glover from the department of Pathology and Laboratory Medicine at Emory University for running the serum escitalopram assay. We would also like to thank Dr. K.V. Thrivikraman for help with the jugular catheterization surgery. Escitalopram oxalate was a generously provided by Lundbeck Research USA (Paramus NJ). All experiments comply with the current laws of the United States of America.
Funding
This work was supported by the National Institutes of Health National Institute of Mental Health [Grant P50 MH-77928] (ZNS and MJO) and the National Institute of Environmental Health Sciences [Grant 12870] (CHB), the National Center for Research Resources [Grant 012870] (CHB), the Howard Hughes Medical Institute [Grant 5600672] (CHB), and in part by the Emory Biomarker Service Center.
ZNS has received research support from NIH, GSK, Pfizer and Wyeth, has served on speakers or advisory boards for Pfizer, Eli Lilly, Wyeth, BMS, and GSK, and has received honoraria from Eli Lilly, GSK, Pfizer, and Wyeth. MJO has research grants from NIH, Eli Lilly, Lundbeck A/S, Cyberonics, Ortho-McNeil Janssen, AstraZeneca, Dainippon Sumitomo Pharma, Sunovion, and SK Life Sciences. He is a consultant for H. Lundbeck A/S, Takeda and RJ Reynolds. He has a patent entitled: “Method of assessing antidepressant drug therapy via transport inhibition of monoamine neurotransmitters” (US 7,148,027 B2).
Abbreviations
- CMS
chronic unpredictable mild stress
- CRF
corticotropin-releasing factor
- GN
gestational day N
- PNDN
postnatal day N
- SSRI
selective serotonin reuptake inhibitor
Footnotes
Conflicts of Interest
CHB, CFC, SMR, MLY, KAB, and JMW have no conflicts to disclose.
References
- Baker S, Chebli M, Rees S, Lemarec N, Godbout R, Bielajew C. Effects of gestational stress: 1. Evaluation of maternal and juvenile offspring behavior. Brain Res. 2008;1213:98–110. doi: 10.1016/j.brainres.2008.03.035. [DOI] [PubMed] [Google Scholar]
- Ballard PL, Kitterman JA, Bland RD, Clyman RI, Gluckman PD, Platzker AC, Kaplan SL, Grumbach MM. Ontogeny and regulation of corticosteroid binding globulin capacity in plasma of fetal and newborn lambs. Endocrinology. 1982;110:359–66. doi: 10.1210/endo-110-2-359. [DOI] [PubMed] [Google Scholar]
- Barlow SM, Morrison PJ, Sullivan FM. Effects of acute and chronic stress on plasma corticosterone levels in the pregnant and non-pregnant mouse. J Endocrinol. 1975;66:9O–9. doi: 10.1677/joe.0.0660093. [DOI] [PubMed] [Google Scholar]
- Bonnin A, Goeden N, Chen K, Wilson ML, King J, Shih JC, Blakely RD, Deneris ES, Levitt P. A transient placental source of serotonin for the fetal forebrain. Nature. 2011;21:347–50. doi: 10.1038/nature09972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundgaard C, Jørgensen M, Larsen F. Pharmacokinetic modelling of blood-brain barrier transport of escitalopram in rats. Biopharm Drug Dispos. 2007;28:349–60. doi: 10.1002/bdd.562. [DOI] [PubMed] [Google Scholar]
- Cabrera TM, Battaglia G. Delayed decreases in brain 5-hydroxytryptamine2A/2C receptor density and function in male rat progeny following prenatal fluoxetine. J Pharmacol Exp Ther. 1994;269:637–645. [PubMed] [Google Scholar]
- Cabrera-Vera TM, Battaglia G. Prenatal exposure to fluoxetine (Prozac) produces site-specific and age-dependent alterations in brain serotonin transporters in rat progeny: evidence from autoradiographic studies. J Pharmacol Exp Ther. 1998;286:1474–1481. [PubMed] [Google Scholar]
- Cabrera-Vera TM, Garcia F, Pinto W, Battaglia G. Effect of prenatal fluoxetine (Prozac) exposure on brain serotonin neurons in prepubescent and adult male rat offspring. J Pharmacol Exp Ther. 1997;280:138–145. [PubMed] [Google Scholar]
- Capello CF, Bourke CH, Ritchie JC, Stowe ZN, Newport DJ, Nemeroff A, Owens MJ. Serotonin transporter occupancy in rats exposed to serotonin reuptake inhibitors in utero or via breast milk. J Pharmacol Exp Ther. 2011;339:275–285. doi: 10.1124/jpet.111.183855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA, Francis DD, Mar A, Meaney MJ. Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiol Behav. 2003;79:359–71. doi: 10.1016/s0031-9384(03)00149-5. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Meaney MJ. Stress during gestation alters postpartum maternal care and the development of the offspring in a rodent model. Biol Psychiatry. 2006;59:1227–1235. doi: 10.1016/j.biopsych.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Cipriani A, Furukawa TA, Salanti G, Geddes JR, Higgins JP, Churchill R, Watanabe N, Nakagawa A, Omori IM, McGuire H, Tansella M, Barbui C. Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet. 2009;373:746–758. doi: 10.1016/S0140-6736(09)60046-5. [DOI] [PubMed] [Google Scholar]
- Clancy B, Darlington RB, Finlay BL. Translating developmental time across mammalian species. Neuroscience. 2001;105:7–17. doi: 10.1016/s0306-4522(01)00171-3. [DOI] [PubMed] [Google Scholar]
- Coe CL, Murai JT, Wiener SG, Levine S, Siiteri PK. Rapid cortisol and corticosteroid-binding globulin responses during pregnancy and after estrogen administration in the squirrel monkey. Endocrinology. 1986;118:435–40. doi: 10.1210/endo-118-1-435. [DOI] [PubMed] [Google Scholar]
- Cohen LS, Altshuler LL, Harlow BL, Nonacs R, Newport DJ, Viguera AC, Suri R, Burt VK, Hendrick V, Reminick AM, Loughead A, Vitonis AF, Stowe ZN. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA. 2006;295:499–507. doi: 10.1001/jama.295.5.499. [DOI] [PubMed] [Google Scholar]
- Cottrell EC, Seckl JR. Prenatal stress, glucocorticoids and the programming of adult disease. Front Behav Neurosci. 2009;3:19. doi: 10.3389/neuro.08.019.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EP, Sandman CA. The timing of prenatal exposure to maternal cortisol and psychosocial stress is associated with human infant cognitive development. Child Dev. 2010;81:131–48. doi: 10.1111/j.1467-8624.2009.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forcelli PA, Heinrichs SC. Teratogenic effects of maternal antidepressant exposure on neural substrates of drug-seeking behavior in offspring. Addict Biol. 2008;13:52–62. doi: 10.1111/j.1369-1600.2007.00078.x. [DOI] [PubMed] [Google Scholar]
- Fromm M, Oelkers W, Hegel U. Time course of aldosterone and corticosterone plasma levels in rats during general anaesthesia and abdominal surgery. Pflugers Arch. 1983;399:249–54. doi: 10.1007/BF00652747. [DOI] [PubMed] [Google Scholar]
- Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G, Swinson T. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol. 2005;106:1071–1083. doi: 10.1097/01.AOG.0000183597.31630.db. [DOI] [PubMed] [Google Scholar]
- Henderson MG, McMillen BA. Changes in dopamine, serotonin and their metabolites in discrete brain areas of rat offspring after in utero exposure to cocaine or related drugs. Teratology. 1993;48:421–30. doi: 10.1002/tera.1420480506. [DOI] [PubMed] [Google Scholar]
- Institute for Laboratory Animal Resources . Guide for the Care and Use of Laboratory Animals. 7th ed Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council; Washington DC: 1996. [Google Scholar]
- Ishiwata H, Shiga T, Okado N. Selective serotonin reuptake inhibitor treatment of early postnatal mice reverses their prenatal stress-induced brain dysfunction. Neuroscience. 2005;133:893–901. doi: 10.1016/j.neuroscience.2005.03.048. [DOI] [PubMed] [Google Scholar]
- Kreilgaard M, Smith DG, Brennum LT, Sanchez C. Prediction of clinical response based on pharmacokinetic/pharmacodynamic models of 5-hydroxytryptamine reuptake inhibitors in mice. Br J Pharmacol. 2008;155:276–284. doi: 10.1038/bjp.2008.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Léonhardt M, Matthews SG, Meaney MJ, Walker C-D. Psychological stressors as a model of maternal adversity: diurnal modulation of corticosterone responses and changes in maternal behavior. Horm Behav. 2007;51:77–88. doi: 10.1016/j.yhbeh.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Lovejoy MC, Graczyk PA, O'Hare E, Neuman G. Maternal depression and parenting behavior: a meta-analytic review. Clin Psychol Rev. 2000;20:561–92. doi: 10.1016/s0272-7358(98)00100-7. [DOI] [PubMed] [Google Scholar]
- Matuszewski BK, Constanzer ML, Chavez-Eng CM. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Anal Chem. 2003;75(13):3019–30. doi: 10.1021/ac020361s. [DOI] [PubMed] [Google Scholar]
- Meaney MJ. Epigenetics and the biological definition of gene x environment interactions. Child Dev. 2010;81:41–79. doi: 10.1111/j.1467-8624.2009.01381.x. [DOI] [PubMed] [Google Scholar]
- Meyer JH, Wilson AA, Ginovart N, Goulding V, Hussey D, Hood K, Houle S. Occupancy of serotonin transporters by paroxetine and citalopram during treatment of depression: a [(11)C]DSAB PET imaging study. Am J Psychiat. 2001;158:1843–1849. doi: 10.1176/appi.ajp.158.11.1843. [DOI] [PubMed] [Google Scholar]
- Meyer JH, Wilson AA, Sagrati S, Hussey D, Carella A, Potter WZ, Ginovart N, Spencer EP, Cheok A, Houle S. Serotonin transporter occupancy of five selective serotonin reuptake inhibitors at different doses: an [11C]DSAB positron emission tomography study. Am J Psychiat. 2004;161:826–835. doi: 10.1176/appi.ajp.161.5.826. [DOI] [PubMed] [Google Scholar]
- Mueller BR, Bale TL. Impact of prenatal stress on long term body weight is dependent on timing and maternal sensitivity. Physiol Behav. 2006;88:605–14. doi: 10.1016/j.physbeh.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. J Neurosci. 2008;28:9055–9065. doi: 10.1523/JNEUROSCI.1424-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newport DJ, Stowe ZN, Nemeroff CB. Parental depression: animal models of an adverse life event. Am J Psychiatry. 2002;159:1265–83. doi: 10.1176/appi.ajp.159.8.1265. [DOI] [PubMed] [Google Scholar]
- Pawluski JL, Charlier TD, Fillet M, Houbart V, Crispin HT, Steinbusch HW, van den Hove DL. Chronic fluoxetine treatment and maternal adversity differentially alter neurobehavioral outcomes in the rat dam. Behav Brain Res. 2013;228(1):159–68. doi: 10.1016/j.bbr.2011.11.043. [DOI] [PubMed] [Google Scholar]
- Rao N. The clinical pharmacokinetics of escitalopram. Clin Pharmacokinet. 2007;46:281–90. doi: 10.2165/00003088-200746040-00002. [DOI] [PubMed] [Google Scholar]
- Rayen I, van den Hove DL, Prickaerts J, Steinbusch HW, Pawluski JL. Fluoxetine during development reverses the effects of prenatal stress on depressive-like behavior and hippocampal neurogenesis in adolescence. PLoS ONE. 2011;6:e24003. doi: 10.1371/journal.pone.0024003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogaard B, Mengel H, Rao N, Larsen F. The pharmacokinetics of escitalopram after oral and intravenous administration of single and multiple doses to healthy subjects. J Clin Pharmacol. 2005;45:1400–1406. doi: 10.1177/0091270005280860. [DOI] [PubMed] [Google Scholar]
- Smith JW, Seckl JR, Evans AT, Costall B, Smythe JW. Gestational stress induces post-partum depression-like behaviour and alters maternal care in rats. Psychoneuroendocrinology. 2004;29:227–244. doi: 10.1016/s0306-4530(03)00025-8. [DOI] [PubMed] [Google Scholar]
- Takahashi LK, Turner JG, Kalin NH. Prolonged stress-induced elevation in plasma corticosterone during pregnancy in the rat: implications for prenatal stress studies. Psychoneuroendocrinology. 1998;23:571–581. doi: 10.1016/s0306-4530(98)00024-9. [DOI] [PubMed] [Google Scholar]
- Thrivikraman K, Huot R, Plotsky P. Jugular vein catheterization for repeated blood sampling in the unrestrained conscious rat. Brain Research Protocols. 2002;10:84–94. doi: 10.1016/s1385-299x(02)00185-x. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Meaney MJ, Szyf M. Maternal care effects on the hippocampal transcriptome and anxiety-mediated behaviors in the offspring that are reversible in adulthood. Proc Natl Acad Sci U S A. 2006;103:3480–3485. doi: 10.1073/pnas.0507526103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward IL, Weisz J. Differential effects of maternal stress on circulating levels of corticosterone, progesterone, and testosterone in male and female rat fetuses and their mothers. Endocrinology. 1984;114:1635–1644. doi: 10.1210/endo-114-5-1635. [DOI] [PubMed] [Google Scholar]
- Weaver ICG, Champagne FA, Brown SE, Dymov S, Sharma S, Meaney MJ, Szyf M. Reversal of maternal programming of stress responses in adult offspring through methyl supplementation: altering epigenetic marking later in life. J Neurosci. 2005;25:11045–54. doi: 10.1523/JNEUROSCI.3652-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MT, Davis HN, McCrea AE, Long SJ, Hennessy MB. Changes in the hormonal concentrations of pregnant rats and their fetuses following multiple exposures to a stressor during the third trimester. Neurotoxicol Teratol. 1999;21:403–414. doi: 10.1016/s0892-0362(98)00060-9. [DOI] [PubMed] [Google Scholar]