Abstract
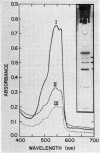
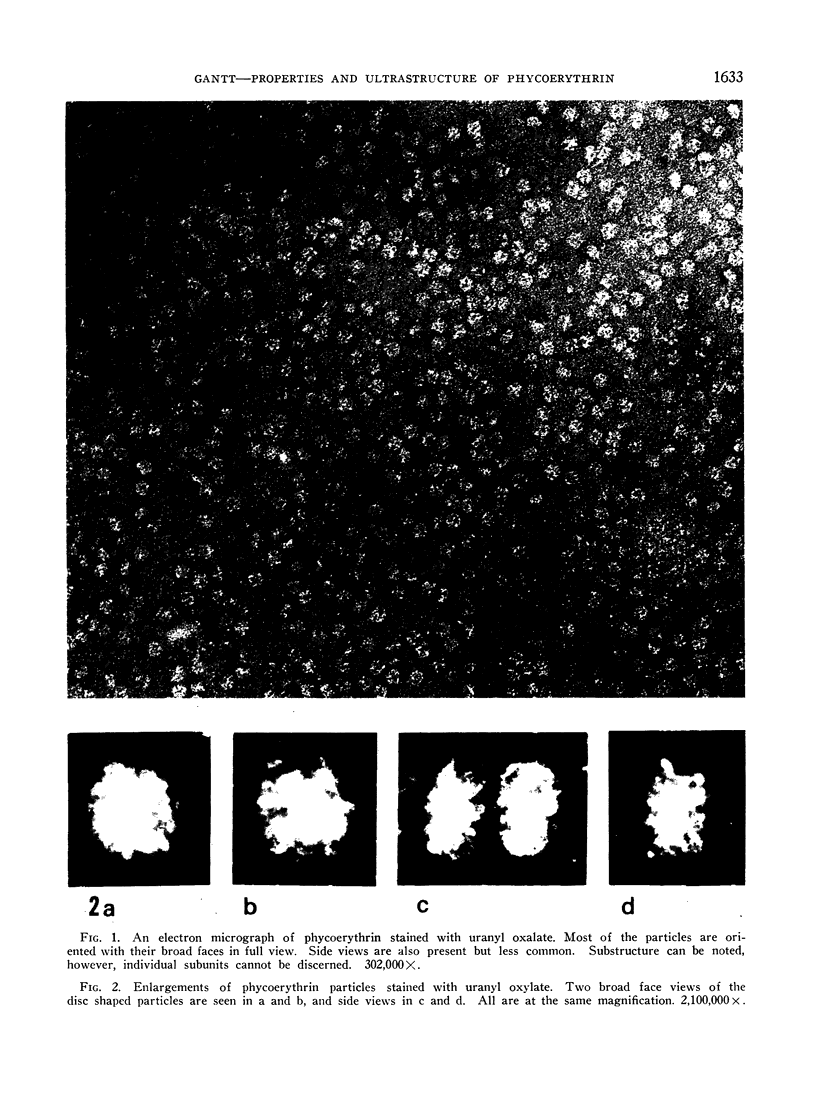
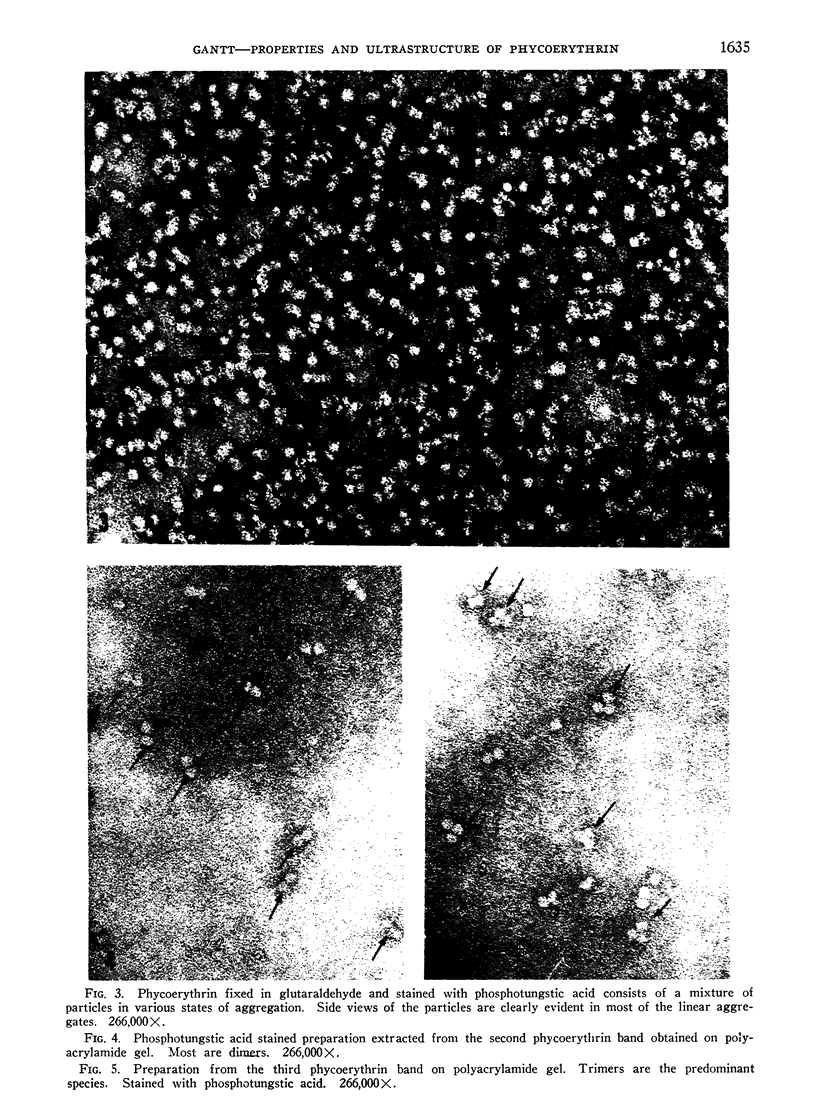
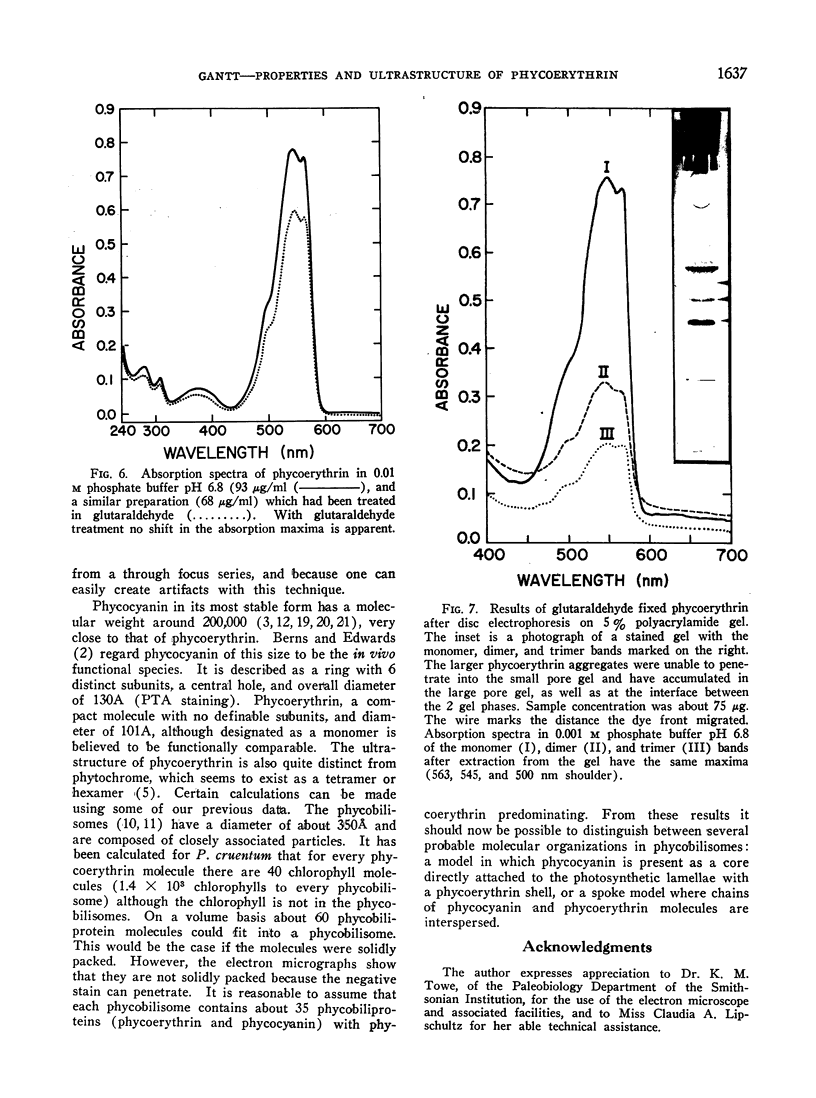
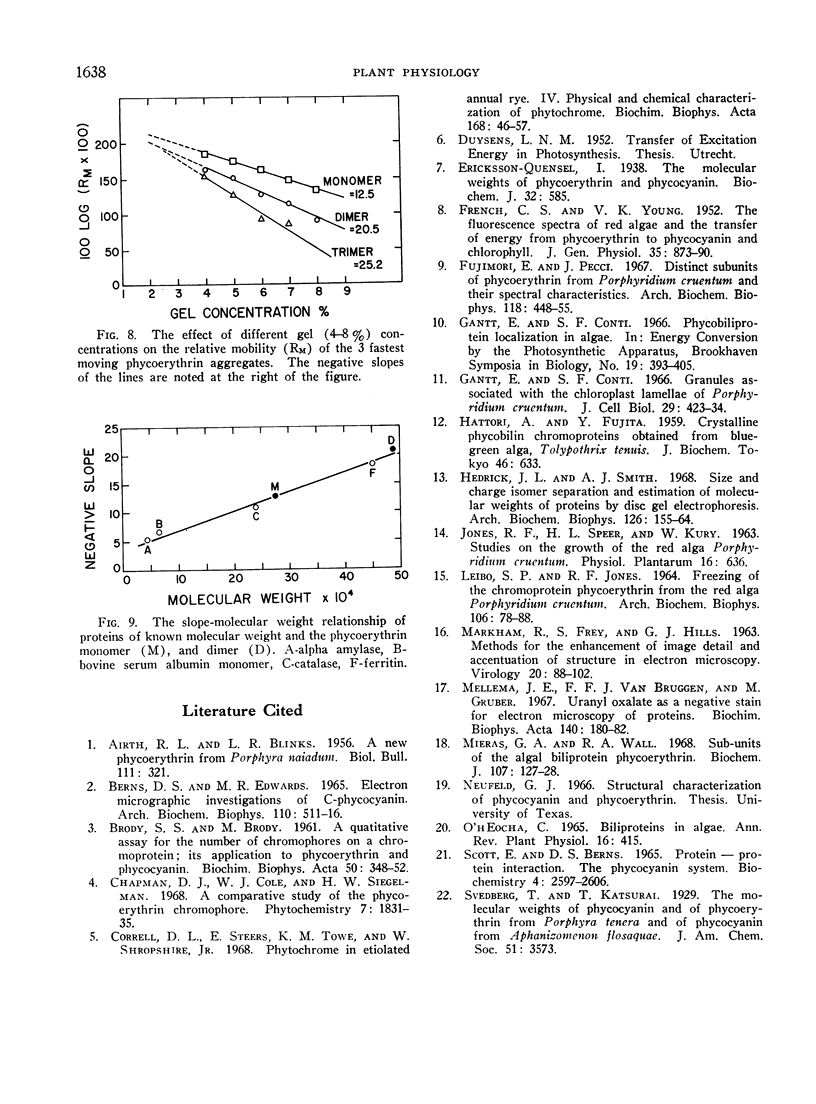
Phycoerythrin, a photosynthetic accessory pigment, was isolated from Porphyridium cruentum and examined by electron microscopy and disc gel electrophoresis. The absorption monomer, with maxima at 563, 545, and a shoulder at 500 nm, has a molecular weight of about 300,000. With phosphotungstic acid staining it appears as a tightly structured disc-shaped particle possessing a mean diameter of 101 ± 0.4Ä and height of 54 ± 0.7Å. The absorption maxima remained the same in glutaraldehyde fixed material, and in dimer and trimer aggregates. Treatment with sodium dodecyl sulfate caused a breakdown into smaller units accompanied by a loss of the 563 nm peak. It is suggested that this absorption monomer is the in vivo functional species and comparable to the phycocyanin hexamer, but structurally distinguishable at the ultrastructural level. It has been calculated that about 35 phycobiliprotein molecules can be contained within each phycobilisome. There are 1.4 × 103 chlorophyll molecules per phycobilisome, but not contained within it.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berns D. S., Edwards M. R. Electron micrographic investigations of C-phycocyanin. Arch Biochem Biophys. 1965 Jun;110(3):511–516. doi: 10.1016/0003-9861(65)90444-3. [DOI] [PubMed] [Google Scholar]
- Correll D. L., Steers E., Jr, Towe K. M., Shropshire W., Jr Phytochrome in etiolated annual rye. IV. Physical and chemical characterization of phytochrome. Biochim Biophys Acta. 1968 Sep 10;168(1):46–57. doi: 10.1016/0005-2795(68)90232-8. [DOI] [PubMed] [Google Scholar]
- Eriksson-Quensel I. B. The molecular weights of phycoerythrin and phycocyan. I. Biochem J. 1938 Mar;32(3):585–589. doi: 10.1042/bj0320585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRENCH C. S., YOUNG V. K. The fluorescence spectra of red algae and the transfer of energy from phycoerythrin to phycocyanin and chlorophyll. J Gen Physiol. 1952 Jul;35(6):873–890. doi: 10.1085/jgp.35.6.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimori E., Pecci J. Distinct subunits of phycoerythrin from Porphyridium cruentum and their spectral characteristics. Arch Biochem Biophys. 1967 Feb;118(2):448–455. doi: 10.1016/0003-9861(67)90373-6. [DOI] [PubMed] [Google Scholar]
- Gantt E., Conti S. F. Granules associated with the chloroplast lamellae of Porphyridium cruentum. J Cell Biol. 1966 Jun;29(3):423–434. doi: 10.1083/jcb.29.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick J. L., Smith A. J. Size and charge isomer separation and estimation of molecular weights of proteins by disc gel electrophoresis. Arch Biochem Biophys. 1968 Jul;126(1):155–164. doi: 10.1016/0003-9861(68)90569-9. [DOI] [PubMed] [Google Scholar]
- LEIBO S. P., JONES R. F. FREEZING OF THE CHROMOPROTEIN PHYCOERYTHRIN FROM THE RED ALGA PORPHYRIDIUM CRUENTUM. Arch Biochem Biophys. 1964 Jul 20;106:78–88. doi: 10.1016/0003-9861(64)90159-6. [DOI] [PubMed] [Google Scholar]
- Mieras G. A., Wall R. A. Sub-units of the algal biliprotein phycoerythrin. Biochem J. 1968 Mar;107(1):127–128. doi: 10.1042/bj1070127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott E., Berns D. S. Protein-protein interaction. The phycocyanin system. Biochemistry. 1965 Dec;4(12):2597–2606. doi: 10.1021/bi00888a008. [DOI] [PubMed] [Google Scholar]